Abstract
Background and aims
Rice is prone to Cd uptake under aerobic soil conditions primarily due to the OsNramp5 Mn transport pathway. Unlike Cd, Mn availability in rice paddies decreases as redox potential increases. We tested whether increasing Mn concentrations in solution would decrease Cd accumulation in rice through competition between Mn and Cd for uptake and/or suppression of OsNramp5 expression.
Methods
Rice was grown to maturity under Mn concentrations that spanned three orders of magnitude (0.30 to 37 μM) that corresponded to free Mn2+ activities of 10–7.9 to 10–5.0 M while free Cd2+ activity was held as constant as achievable (10–10.2 to 10–10.4 M). Plant biomass and elemental concentrations were measured in roots and shoots at each stage. Fold changes in the expression of OsNramp5, OsCd1, OsHMA3, OsCCX2, and OsYSL6 genes in vegetative and grain-filling stages of rice plants were determined.
Results
Competition between Mn and Cd for root uptake and accumulation in shoots was observed at the highest concentration of Mn tested. OsNramp5 expression was significantly higher in rice plants at the vegetative stage compared to the grain-filling stage, while OsCd1 and OsHMA3 showed the opposite. Solution Mn concentrations previously thought to be tolerable by rice grown to the vegetative stage led to Mn toxicity as plants matured.
Conclusions
Mn competes with Cd during uptake into rice with OsNramp5 expression unaffected. Different translocation paths may occur for Mn and Cd within the rice plant and over the rice life cycle, with OsCCX2 correlated with shoot Cd concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is a globally important staple food and can take up Cd from the soil solution and accumulate it in shoots and grain. Cadmium is a highly toxic metal and known carcinogen found naturally in the earth’s crust, usually at low concentrations ranging from 0.1 to 1 mg kg−1, with concentrations up to 1000 mg kg−1 in contaminated sites (Buchauer 1973; Grant & Sheppard 2008; Morrow 2010). Cadmium is less toxic to plants than to humans; therefore, plant Cd levels can be toxic to humans without affecting plants (Clemens et al. 2013). Once absorbed into the blood, Cd is excreted very slowly and can accumulate to harmful levels, causing adverse effects including increased oxidative stress and impaired pulmonary function (ATSDR 2002; NTP 2021). Nearly all human Cd exposure comes from eating or smoking contaminated plant materials (ATSDR 2002). Rice consumption is the primary Cd exposure source for many people (Jin et al. 2002; Nordberg et al. 1997; Tsukahara et al. 2003; Watanabe et al. 2000, 1996). Therefore, understanding how Cd is transported to edible rice grain is crucial to protecting human health.
Cadmium chemically resembles other divalent metal nutrients such as Fe2+, Zn2+, and Mn2+, and Cd2+ is transported into roots and within rice plants through divalent metal transporters, with Mn transporters playing a major role. For example, OsNramp1 is an Fe transporter induced by Fe deficiency, and was reported to contribute to Cd uptake and translocation in rice but not under the Cd2+ or Fe2+ levels representative of field conditions (Chang et al. 2020; Takahashi et al. 2011); thus, OsNramp1 may only have a negligible contribution to Cd uptake under field conditions. OsCd1 was also reported to transport Cd in rice roots, and the natural variation of this gene was found to be responsible for the different Cd accumulation capacity between indica (higher Cd genotype) and japonica (lower Cd genotype) rice subspecies (Yan et al. 2019). Researchers suggested that OsHMA3 can sequester Cd into vacuoles during Cd transport through roots, contributing to lower Cd in rice grain (Miyadate et al. 2011; Ueno et al. 2010). After transport across the root endodermis, Cd must be loaded into the xylem for shoot transport. This xylem loading is a major step in determining the eventual Cd translocation into rice grain (Uraguchi et al. 2009). A node-expressed transporter gene, OsCCX2, was reported to be involved in grain Cd accumulation; this gene was reported to be mainly expressed in the xylem region of vascular tissues at rice nodes (Hao et al. 2018). Although a few divalent cation transporters were reported to transport Cd into rice roots under manipulated experimental conditions (Chang et al. 2020; Nakanishi et al. 2006; Takahashi et al. 2011), the Mn transporter OsNramp5 has been shown to be the primary transporter of Cd into rice roots under field conditions (Ishikawa et al. 2012; Sasaki et al. 2012). When OsNramp5 was knocked out or knocked down, 21 d old rice shoots and roots accumulated significantly and substantially less Mn and Cd than the wildtype (Sasaki et al. 2012). Some extremely low Cd accumulators with defects in OsNramp5 were found in experiments with ion-beam irradiated mutant rice plants (Ishikawa et al. 2012). In addition, OsNramp5 was reported to play a crucial role in Mn root-to-shoot transport and is expressed in other aboveground tissues (Yang et al. 2014). While OsNramp5 is clearly important for Cd uptake in rice, other transporters may also play a role in the distribution of Cd within the plant, particularly as plants age. Much of what is known regarding Mn and Cd antagonism has been gleaned from short-term hydroponic studies, so it remains unresolved how Mn and Cd antagonism might change over the rice life cycle.
In slightly acidic rice paddy soil, redox conditions can significantly impact Mn and Cd mobility in soil and uptake into rice. Trade-off relations between plant-available Mn2+ and Cd2+ were found across changing soil pH, Eh, and pe + pH induced by flooding and drainage (Wang et al. 2022a, b). When the rice paddy is flooded, soil redox potential decreases and Mn (III/IV) oxides can be reduced to Mn2+, leading to elevated Mn2+ concentration in soil solution (Ponnamperuma 1972). Mean soil porewater Mn2+ concentration was reported to be 500 to 1000 μM in a continuously flooded rice paddy in Arkansas, U.S. (Maguffin et al. 2020), and the soil porewater Mn2+ was reported to reach ~ 1560 μM in a flooded Mn-rich paddy soil in Fujiang, China (Xu et al. 2017). Rice has a high root-to-shoot Mn translocation efficiency and can tolerate up to 1000 to 4000 mg kg−1 Mn concentrations in shoots (Tanaka and Navasero 1966b). The Mn-nicotianamine transporter OsYSL6 was suggested to be involved in the detoxication of excess Mn in rice plants, and higher OsYSL6 expression was found in older leaves (Sasaki et al. 2011). In contrast to Mn, Cd availability decreases in slightly acidic flooded soil to nearly non-detectable levels because of three geochemical processes: 1) formation of sparingly-soluble CdS under anaerobic conditions (Arao et al. 2009; de Livera et al. 2011), 2) soil pH increase toward near neutral (Wang et al. 2019), and 3) elevated free Mn2+ in soil solution, which promotes Cd binding with Fe–Mn (oxyhydr)oxides (Wang et al. 2022a, b). Therefore, in uncontaminated soil, Cd availability to rice grown under flooded conditions is typically low because of low solution-phase Cd and high solution-phase Mn, which can compete for root uptake and translocation (Yang et al. 2014).
Recently, water management such as alterate wetting and drying or furrow irrigation has been adopted, which can decrease the accumulation of As but increase Cd accumulation in some soils (Arao et al. 2009; Honma et al. 2016; Seyfferth et al. 2019). The elevated plant Cd levels under more oxidized soil conditions are likely due to a combination of geochemical and plant molecular factors. Under oxidizing conditions solution-phase Cd concentrations increase while Mn concentrations are low. Under this scenario, less Mn is available in solution to compete for plant uptake into roots, and the low Mn possibly increases the expression of Mn transporters. These soil and plant factors would promote more Cd uptake under oxidized soil conditions. In a four-week hydroponic study with a gradient in Mn and Cd solution concentrations, competition between Mn and Cd was found in both rice roots and shoots, while expression of OsNramp5 was not affected (Yang et al. 2014). If increased Mn supply can decrease grain Cd accumulation, it may provide a feasible Cd mitigation method in rice production particularly under non-flooded water management. However, it remains unclear how increasing Mn supply will impact rice shoot and grain Cd accumulation when plants are grown to maturity and if transporter expression changes as plants age.
We hypothesized that increasing the Mn supply would decrease plant Cd due to decreased expression of Mn transporters and competition between Mn and Cd for uptake. We further hypothesized that the expression of Mn transporters would change over the plant life cycle and impact shoot accumulation of Cd. To test our hypotheses, a hydroponic experiment was conducted in which rice was grown to grain maturity under a range of culture solution Mn2+ activities and environmentally-relevant Cd2+ activity (Parker & Norvell 1999), as plant uptake of metal nutrients were suggested to correlate best with free metal ion activities (Chaney 1988; Halvorson and Lindsay 1977). Chelator-buffered nutrient solutions (Chaney et al. 1989; Parker et al. 1995) were used to control micronutrient cation activities in hydroponic culture solutions, and hydroxyethylethylenediaminetriacetic acid (HEDTA) was selected as the chelator using GEOCHEM-EZ (Shaff et al. 2010) to calculate chemical species equilibrium distribution in solution. While others have mostly grown rice to vegetative stages in such experiments, here we measured Mn and Cd concentration and quantity in rice plants at vegetative, grain filling and grain maturity rice growth stages and the expression of key transporters involved in Mn and Cd uptake, transfer, and detoxification in vegetative and grain-filling stage plants.
Materials and methods
Rice hydroponic experiment
The hydroponic experiment was conducted in a growth chamber (14 h day, 28/26 °C day/night temperature, 60% humidity) with an average light intensity of 412 μmol m−2 s−1 delivered by fluorescent bulbs. Hybrid rice (cv. CLXL745, RiceTec, USA) seeds were surface sterilized in a diluted bleach solution and germinated on germination paper (day 1). After germination for seven days, seedlings were transferred from germination paper to four 16-L high-density polyethylene (HDPE) nursery chambers with four treatment solutions and 35 rice seedlings per nursery chamber. Four treatment solutions contained the same basal solution formula (Table S1) but four different Mn concentrations and activities (pMn2+ of 7.9, 7.4, 6.5, and 5.0) and with similar free Cd2+ activity (pCd2+ of 10.2 to 10.4) (Table 1). All treatment solutions contained 100 μM HEDTA to keep Fe3+, Zn2+, Cu2+, and Ni2+ fully chelated and maintain Mn2+ and Cd2+ activity at the desired level. HEDTA has much stronger chelation with Fe3+, Cu2+, Zn2+ and Cd2+ compared to chelation with Mn, and, therefore, can obtain the desired Mn activity range while maintaining other metal activities at very low levels (Table 1). According to our pilot experiments, rice grown in HEDTA + Fe3+ solution only had minor Fe/Mn plaque formation in contrast to rice grown in Ferrozine + Fe2+ solution, which had significant amount of Fe/Mn plaque formation on rice roots and can interfere with this mechanistic Cd uptake experiment. MES buffer (2 mM) was also used to buffer the pH to 5.9—6.0 during plant growth. After one week of growth in the nursery chamber, rice plants of similar height were transferred to 8 L opaque HDPE pots, one plant per pot and nine pots per Mn treatment. All containers (nursey chambers and HDPE pots) used in the experiment were acid-washed before use. Nutrient solutions were changed every four to seven days with more frequent solution changes as plants grew larger. Nutrient solution samples were taken before and after each solution change, and nutrient element concentrations were analyzed via inductively coupled plasma-optical emission spectroscopy (ICP-OES). Throughout the experiment, rice plants experienced slight fluctuations in solution concentrations of basal nutrients and the targeted Mn and Cd levels (Figure S2). Therefore, all solution concentrations are reported as the average concentrations that the rice experienced throughout its growth stage, and pMn2+ and pCd2+ were calculated based on these average component concentrations (Table 1 and Table S1).
Sample collection and processing
Rice plants were destructively harvested at vegetative (day 73), grain filling (day 97), and grain maturity (day 113) stages; three replicate pots per treatment were sampled at each sampling stage. For vegetative and grain filling stage sampling, one to two whole leaves (leaf immediately below the top leaf), three to four nodes (nodes above the basal nodes), and five to 10 whole roots were collected from each plant, respectively, for gene expression analysis. These tissue samples were flash-frozen in liquid N2 and stored in -80 °C freezer until RNA extraction and quantitative real-time polymerase chain reaction (qPCR) analysis. The remaining plant shoots (without panicles) were oven dried at 50 °C for 60 h, and the remaining roots were triple washed in DI water and air-dried at room temperature (25 °C). Dry biomass of roots, shoots, and grain was recorded. Dried whole roots were further processed with the cold dithionite citrate bicarbonate (DCB) extraction (Taylor and Crowder 1983) to remove the oxides layer on the root surface, with roots of a single plant fully immersed in DCB solution (30 mL 0.3 M sodium citrate + 3.75 mL 1 M sodium bicarbonate + 2.25 g sodium dithionite) and shaken for 3 h. Extraction solutions, including root-rinsed solutions, were transferred to 100 ml volumetric flasks, brought to volume, and analyzed for Mn and Cd accumulation in root plaques via inductively coupled plasma-mass spectrometry (ICP-MS, Thermo iCap-TQ). For pots sampled at the grain maturity stage, panicles were separated from shoots, air-dried, and ripe grain was separated from the panicles. Ripe grain was further separated into husk and brown rice in a rice huller (Nongao, NA.JLG-2018), and brown rice was then polished into powdered bran and white rice in a rice milling machine (XinFeng, JNMJ3). All plant samples (root, shoot, husk, bran, polished rice) were ground into fine powders with a stainless-steel grinder and stored in air-tight HDPE vials until elemental analysis.
Rice tissue elemental quantification analyses
Elemental concentrations in all ground rice tissue samples (root, shoot, husk, bran, and polished grain) were quantified by ICP-MS (Thermo iCap-TQ) after microwave-assisted acid digestion following established protocols (Seyfferth et al. 2016; Teasley et al. 2017). Briefly, rice tissue (0.100 to 0.400 g dry tissue) was weighed into a Teflon digestion vessel (MARS 6, CEM Corp., USA), and 7 mL concentrated trace-metal grade HNO3 was added for microwave digestion (MARS 6, CEM Corp., USA). After digestion, all elements except Si were dissolved in the acid fraction, and Si gel was settled down after centrifugation and separated from the acid fraction. To ensure data quality, NIST 1568b rice flour, NIST 1570a spinach leaves, and NIST WEPAL IPE 188 oil palm were included in each digestion run as certified standards (Table S2). Acid solutions were diluted to 4% HNO3 matrix before ICP-MS analysis. Digestion method blanks and check standards were also included in the ICP-MS analysis. The quantity of elements in rice tissue was obtained by multiplying the tissue element concentration by the tissue biomass for each pot.
Gene expression analyses
To quantify the expression of Mn/Cd transporter genes, RNA was extracted from the frozen roots, leaves, and node samples. For this, flash-frozen root and leaf samples were ground in liquid nitrogen with a ceramic mortar and pestle that had been sterilized and autoclaved. In contrast, flash-frozen node samples were ground in a mixer mill (Retsch, GmbH MM301) using sterilized and RNase-free vessels. RNA was extracted from the ground rice tissue samples using a RNeasy Plant Mini Kit (QIAGEN). Extracted RNA (1 μg) was reverse transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time polymerase chain reaction (qPCR) was performed with 50 ng cDNA in each reaction cell to determine fold change expression of OsNramp5, OsCd1, OsYSL6, and OsHMA3 in root tissue, and OsNramp5, OsYSL6, and OsCCX2 in nodes and leaves (see Table S3 for primer sequences and references). Expression levels were analyzed using the ΔΔCT method (Livak and Schmittgen 2001) and are expressed relative to the same tissue in one rice plant harvested at the grain filling stage in the pMn2+ 6.5 treatment. We attempted quantitative real-time polymerase chain reaction analyses of OsLCT1 and OsHMA2 in nodes and leaves and OsNramp1 in roots, nodes, and leaves but these had large threshold amplification cycle numbers (Ct > 36) or failed amplification, indicating very low or no expression. The rice eEF-1a gene was used as the housekeeping gene. Primer sequences used in the qPCR reaction are listed in Table S3. PowerUp SYBR Green Master Mix (Applied Biosystems) was used as the reaction substrate, and the reaction was conducted in QuantStudio 3 Real-Time PCR System (Applied Biosystems) using the comparative ΔΔCT method in fast mode.
Statistical analyses
Elemental concentration and transporter expression results were analyzed with 2-way ANOVA followed by Tukey HSD tests. Two-way ANOVAs were performed using the plant growth stage (sampling stage) and hydroponic solution Mn activity as factors. The logarithm of plant Mn concentrations and quantities were used in the two-way ANOVA analyses. Linear regression analyses were performed among plant elemental concentrations and fold change in transporter expression. All statistical analyses were performed with JMP (v. Pro 16) software. No significant interaction was observed between solution Mn activity and sampling stage in any measured parameters except in shoot Cd quantity; however, differences were observed due to Mn activity and growth stage (Table S4).
Results
Rice biomass was affected by the growth stage and Mn activity. Rice sampled in the vegetative stage had significantly lower biomass than in the grain filling and grain maturity stages for both roots (p < 0.0001) and shoots (p < 0.0001) (Figure S1). Shoot biomass was significantly lower at the highest solution Mn tested (pMn2+ of 5.0) for all growth stages (Figure S1B, p = 0.003), but there was no significant effect of Mn activity on the biomass of root (Figure S1A) and grain (Figure S1C) among treatments. Frequent nutrient solution changes maintained the desired solution concentrations at target levels throughout the experiments (Figure S2).
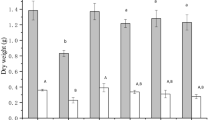
Rice plant Mn and root plaque Mn accumulation were more affected by Mn activity than the growth stage (Figs. 1 and 2 and S3A). Concentrations and quantities of Mn in rice plant tissues generally increased with increasing Mn activity in the hydroponic solution (Figs. 1, A, and 2C). However, similar root Mn levels between the two lowest Mn treatments (pMn2+ of 7.9 and 7.4) were observed (Fig. 1A and C). Root Mn concentrations were significantly higher at the grain maturity stage compared to the vegetative and grain filling stages (Fig. 1A, p = 0.02), and root Mn quantities significantly increased at later sampling stages (Fig. 1C, p < 0.0001). Shoot Mn concentrations were similar across vegetative, grain filling and grain maturity stages (Fig. 1B), while lower shoot Mn quantities, due to lower shoot biomass, were obtained at the vegetative stage (Fig. 1D, p < 0.0001).
Rice root (A) and shoot (B) Mn concentration, and root (C) and shoot (D) Mn quantity at all sampling stages when rice plants were grown hydroponically with four Mn activities and a relatively consistent Cd activity. Bars and error bars represent mean concentration or quantity and standard deviation, respectively (n = 3). Capital letters above bars denote the statistical difference among sampling stages, and lower-case letters denote the statistical difference among Mn treatments. Levels not connected by the same letter are significantly different. V, vegetative; GF, grain filling; GM, grain maturity
Mn (A) and Cd (B) concentrations of polished grain, bran, and husk and grain Mn (C) and Cd (D) quantity when rice plants were grown hydroponically with four Mn activities and a relatively consistent Cd activity. Bars and error bars represent mean concentration and standard deviation, respectively (n = 3). Lower-case letters denote the statistical difference among Mn treatments. Levels not connected by the same letter are significantly different
Treatments affected cadmium in shoots, roots, and root plaque but not in grain (Figs. 2B, D and 3 and S3B). Treatment with the highest Mn activity in solution resulted in the lowest root Cd concentration (Fig. 3A, p = 0.0007) and quantity (Fig. 3C, p < 0.0001), while lower activities were statistically similar. Root Cd concentrations showed no statistical difference across sampling stages (Fig. 3A). In contrast, vegetative stage roots had significantly lower Cd quantities, due to lower biomass, compared to roots at grain filling and grain maturity stages (Fig. 3C, p < 0.0001). Shoot Cd generally increased with increasing Mn activity at low Mn levels before decreasing at the highest Mn activity, and this pattern was most evident at later growth stages (Fig. 3B and D). In particular, treatment with pMn2+ of 6.5 had significantly higher shoot Cd concentrations compared to treatments with pMn2+ of 7.4 and 7.9, with shoot Cd concentrations of pMn2+ of 5.0 treatment in between pMn2+ of 6.5 and pMn2+ of 7.4 or 7.9 treatments (Fig. 3B, p = 0.001). Shoots sampled at the grain maturity stage had significantly higher Cd concentrations than vegetative and grain filling stages (Fig. 3B, p = 0.0006). Shoot Cd quantity was significantly affected by treatment solution Mn activity (p < 0.0001), sampling stage (p < 0.0001), and the interaction between these two factors (p = 0.0005).
Rice root (A) and shoot (B) Cd concentration, and root (C) and shoot (D) Cd quantity at all sampling stages when rice plants were grown hydroponically with four Mn activities and a relatively consistent Cd activity. Bars and error bars represent mean concentration or quantity and standard deviation, respectively (n = 3). Capital letters above bars denote the statistical difference among sampling stages, and lower-case letters denote the statistical difference among Mn treatments. Levels not connected by the same letter are significantly different. V, vegetative; GF, grain filling; GM, grain maturity
We examined the expression of Mn transporters in roots, leaves, and nodes at the vegetative and grain-filling stages across the four Mn treatments, and the growth stage had more of an impact on transporter expression than Mn treatments. Mn treatments did not have a significant effect on the expression of OsNramp5, OsCd1, or OsHMA3 expression in rice roots (Fig. 4) or OsNramp5 in rice nodes (Figure S4A) and leaves (Figure S4C). OsNramp5 expression was significantly higher at the vegetative stage than the grain filling stage in roots (Fig. 4A, p = 0.02), nodes (Figure S4A, p < 0.0001), and leaves (Figure S4C, p = 0.02). In contrast, OsCd1 (Fig. 4B, p < 0.0001) and OsHMA3 (Fig. 4C, p < 0.0001) had significantly higher expression in the grain filling stage compared to the vegetative stage.
Relative expression level (relative to the roots of one rice plant at grain filling stage in pMn2+ 6.5 treatment) of OsNramp5 (A), OsCd1 (B), and OsHMA3 (C) in vegetative (V) and grain filling (GF) stage rice root. Bar and error bars represent the mean expression level and standard deviation, respectively (n = 3). Capital letters above bars denote the statistical difference among sampling stages. Lower-case letters denote the statistical difference among Mn treatments. Levels not connected by the same letter are significantly different
The expression of OsCCX2 was affected by Mn treatment and the growth stage (Figure S4 and 5). The expression of OsCCX2 in nodes was significantly higher for plants grown in the pMn2+ 6.5 treatment than the other treatments (p = 0.0001) and was significantly higher for all Mn treatments at the grain filling stage than the vegetative stage (p < 0.0001) (Figure S4B). OsCCX2 expression level in leaves significantly increased from pMn2+ 7.9 to 6.5 treatments but then slightly decreased in the pMn2+ 5.0 treatment (p = 0.01), and there was no significant difference in expression between sampling stages (Figure S4D). A significant positive correlation was found between OsCCX2 expression in nodes and shoot Cd accumulation (Fig. 5, p < 0.0001).
Because we observed lower shoot biomass at the highest free Mn2+ activity, we sought to understand how Mn treatments affected the expression of OsYSL6. OsYSL6 expression was significantly affected by the treatment and sampling stage in nodes but not in leaves. The highest OsYSL6 expression level was found in nodes of plants grown under the pMn2+ 6.5 treatment, while the lowest expression occurred in plants grown in pMn2+ 7.4 and 5.0 treatment solutions (p = 0.004). Moreover, the grain filling stage had significantly higher OsYSL6 expression than the vegetative stage in the nodes (p = 0.047) (Figure S5A). In leaves, OsYSL6 had statistically similar expression levels regardless of treatment or sampling stage (Figure S5B) but was substantially lower in the highest Mn activity treatment at the grain-filling stage.
Discussion
Mn and Cd compete for uptake into rice
Understanding the mechanisms of Cd translocation and their interaction with micronutrient Mn in rice is critically important to help develop Mn-based strategies to protect human health from Cd intake via rice ingestion. We hypothesized that a higher Mn supply would decrease Cd uptake and accumulation in rice due to Mn-Cd antagonism and possibly suppression of Cd transporters. Our results partly supported our hypothesis. Significantly lower rice root Cd concentration and quantity were found in the highest solution Mn treatment of pMn2+ 5.0, and there was a trend of lower shoot and grain mean Cd concentration and quantity in that treatment, despite the slightly higher Cd activity (pCd2+ of 10.2) compared to other three lower Mn treatments (pCd2+ 10.3 to 10.4) (Figs. 2B and D, 3, and Table 1). These data support that competition between Mn and Cd uptake occurred; however, this competition was not enough to affect grain Cd levels at environmentally relevant concentrations. In addition, increasing Mn supply did not suppress the expression of OsNramp5, OsCd1, or OsHMA3.
Our Mn and Cd antagonism and transporter expression data aligns with previous reports. An antagonistic relationship between Mn and Cd was reported by Yang et al. (2014), who tested a Mn range of 0.08 to 800 μM with 2 nM background Cd in 4-week rice experiments. However, in our study of rice grown for 16 weeks, this antagonistic relationship was only observed when pMn2+ was beyond 6.5, representing Mn concentration > 6.6 μM (Fig. 3A, C and D) with pCd2+ of 10.2–10.3 (0.10 to 0.55 μM Cd). Similarly, in a 4-week experiment with similar Cd activity to the present study, Wang et al. (2022b) only observed elevated rice Cd under Mn deficiency (pMn2+ 8.2 or 0.032 μM Mn) or decreased rice Cd at toxic Mn supply level (pMn2+ 4.8 or 31.6 μM Mn), under which rice toxicity symptoms were observed, with no distinct antagonistic Mn/Cd relation observed in the range of pMn2+ 5.3 to 7.7 (0.1–10 μM Mn). The differences observed may be because OsNramp5 has a higher affinity for Cd than for Mn, as the half-saturation constant Km for Cd and Mn uptake by rice through OsNramp5 is 0.38 μM and 1.08 μM, respectively (Sasaki et al. 2012), or because there are other transporters involved in Mn uptake. OsNramp5 is reported to be a major transporter responsible for both Mn and Cd uptake into rice (Sasaki et al. 2012), while OsCd1 (Yan et al. 2019) can transport Cd but not Mn. Therefore, the competition between Mn and Cd for the OsNramp5 transporter may only be observed at extreme Mn levels (deficiency or toxicity) and may not be sufficiently manipulated to control Cd accumulation.
Mn and Cd translocation pathways inside rice differ
Although Mn and Cd share the same major transporter OsNramp5 for uptake into rice roots, translocation pathways within the rice plant seem to differ for Mn and Cd. Results of our study suggest that Mn was preferentially translocated to shoots (Fig. 1) while Cd accumulated preferentially in roots (Fig. 3). Shoot Mn concentrations did not significantly increase during the three sampled growth stages, with mean shoot Mn concentration in pMn2+ 7.9 treatment even slightly decreased from the vegetative to grain filling sampling stage (Fig. 1B). In contrast, shoot Cd concentrations increased as rice plants aged and were significantly higher at the grain maturity stage (Fig. 3B, p = 0.03). Our results suggest different root-to-shoot translocation pathways for Mn and Cd within the rice plant that may change over the rice life cycle.
With rice grown to grain maturity, our data indicated that different transporters are involved to differing extents in Mn and Cd uptake and translocation at different growth stages, which could not be seen in previous short-term studies. In our study, OsNramp5 had higher expression in roots at the vegetative stage (Fig. 4A), while OsCd1 had higher expression in the grain filling stage (Fig. 4B). This observation suggests that OsCd1 may have played an important role in determining grain Cd levels in rice, as grain forms later in the rice life cycle. Natural variation in OsCd1 activity among rice cultivars was suggested previously to be responsible for differentiating grain Cd accumulation capacity (Yan et al. 2019), indicating the important role of OsCd1 in Cd uptake during the grain filling stage. After uptake into rice roots, OsHMA3 plays a role of vacuolar sequestration of Cd and perhaps other elements (e.g., Mn and Zn) (Miyadate et al. 2011). Higher OsHMA3 expression was found in roots at the grain-filling stage (Fig. 4C). This may be because of higher Cd detoxification needs in older rice plants, as plants at later stages have higher concentrations of Cd in the tissues (Fig. 3C and D).
Mn root-to-shoot and shoot-to-grain translocation in rice has been reported to be related to OsNramp5 and OsNramp3 (Yamaji et al. 2013; Yang et al. 2014), while Cd translocation involves OsHMA3, OsCCX2, OsHMA2, OsZIP7, OsCAL1 and OsLCT1 (Hao et al. 2018; Luo et al. 2018; Takahashi et al. 2012; Tan et al. 2019; Ueno et al. 2010; Uraguchi et al. 2011). Significantly higher OsNramp5 expression was observed in the vegetative node and leaf compared to the grain filling stage in our study, indicating higher Mn transport activity via OsNramp5 in younger rice plants. Although not tested in our study, OsNramp3 was reported to be highly expressed in nodes and to preferentially transport Mn to younger rice tissue and panicle when Mn is insufficient. The OsNramp3 protein degrades within hours in response to excess Mn, inhibiting the Mn translocation to young rice tissue. Under these conditions, excess Mn would be allocated to older tissues, such as older leaves with larger surface areas and higher transpiration rates (Yamaji et al. 2013). OsHMA2 was suggested to be involved in rice root to shoot Cd translocation via xylem (Satoh-Nagasawa et al. 2012; Takahashi et al. 2012), and OsLCT1 was reported to be involved in Cd phloem transport to grain (Uraguchi et al. 2011, 2014). However, in our study, OsHMA2 and OsLCT1 had very low or no detectable expression in rice nodes and leaves at the vegetative and grain filling stages (data not shown). Instead, OsCCX2 was found to be expressed in both nodes and leaves, and the pMn2+ 6.5 treatment had the highest OsCCX2 expression (Figure S4B and D) and the highest shoot Cd levels (Fig. 3B and D), particularly as plants aged. A significant positive correlation was also found between OsCCX2 expression in the nodes and shoot Cd quantity (Fig. 5), suggesting a contribution of OsCCX2 in Cd node transport, especially during the grain-filling stage. Although OsCCX2 was reported to be preferentially expressed in rice nodes (Hao et al. 2018), comparable OsCCX2 expression was also observed in rice leaves in our study, which may indicate the active role of OsCCX2 in the distribution of Cd to leaves (Hao et al. 2018).
Mn tolerance of rice plants
Rice seems not to strictly regulate the amount of Mn uptake into roots and efficiently transports Mn to shoots (Fig. 1). Instead, rice develops a strategy to preferentially distribute Mn to younger and reproductive tissue when Mn concentrations are growth limiting or to preferentially load Mn to older tissue as an internal detoxification strategy to deal with excess Mn (Sasaki et al. 2011; Yamaji et al. 2013). Rice plants can accommodate a wide range of Mn concentrations from 20 to 4000 mg kg−1 in shoot tissue (Tanaka and Navasero 1966a) and maintain normal biophysical function. In our study, the highest Mn treatment, which corresponded to 37 µM in solution, had shoot Mn concentrations exceeding 4500 mg kg−1 and significantly lower shoot biomass than the other treatments (Fig. 1B and S1B). Therefore, we hypothesized that disruption of OsYSL6 expression, which is critical to tolerate excess Mn concentration in shoots (Sasaki et al. 2011), may have occurred in that treatment. Indeed, we observed the lowest expression of OsYSL6 in the highest Mn treatment (Figure S5). The shoot Mn levels we found were higher than the suggested 2500 mg kg−1 Mn toxicity concentration threshold (Tanaka and Navasero 1966b). This finding supports our hypothesis that Mn toxicity occurred in the highest Mn treatment and led to low shoot biomass (Figure S1B).
The tolerable Mn level in hydroponic culture solution may be lower for rice grown to maturity than previously observed in short-term rice experiments. Rice plants growing in our hydroponic study to grain maturity showed stunted growth in the highest Mn treatment (pMn2+ of 5.0 or 37 μM Mn), one of the symptoms of Mn toxicity. We also grew rice in the same basal solution as in this study with higher Mn (100 μM), which showed brown chlorosis in rice leaves as well as stunted growth after the grain-filling stage but not in younger plants (data not shown). While short-term (≤ 5 weeks) rice studies did not report Mn toxicity with solution Mn up to ~ 1800 μM (Yang et al., Tanaka and Navasero 1966a), our data suggest that plants cannot tolerate such high Mn levels in solution culture for longer periods (i.e., grown to maturity). Previously, 182 μM was suggested as the upper Mn level that rice can maintain normal growth (Tanaka and Navasero 1966b); however, this value may be too high for rice grown to maturity in solution culture. In our study, only up to the 37 μM Mn treatment allowed plants to complete the rice growth cycle.
Rice plants seem to tolerate higher Mn solution phase concentrations in soil environments than in hydroponic cultures. In contrast to rice grown in solution culture, rice can grow in paddy soils with 500 and even up to ~ 1500 μM Mn in pore waters (Maguffin et al. 2020; Xu et al. 2017). High soil solution Mn concentrations observed in bulk paddy pore waters are likely attenuated by iron plaque in the rhizosphere (Colmer 2003), or by soil organic matter chelation (Himes and Barber 1957), limiting the plant-available Mn pool. Fe was reported to show an antagonistic effect on rice Mn uptake (Tanaka and Navasero 1966a), and Fe concentration was reported to be in the range of 800 to 1600 μM in flooded rice paddy soil pore water (Seyfferth et al. 2019); these Fe concentrations are much higher than the 56 μM Fe used in our hydroponic study. Likely, the formation of Fe and Mn oxide minerals at the rice root surface acts to prevent excess rice root uptake of Fe and Mn in soil environments by providing adsorption sites (Suda and Makino 2016). Therefore, evaluating the ‘actual’ solution-phase Mn levels observed by rice roots growing in soil is complex. Future studies that target the competition between Mn and Cd uptake should manipulate Mn supply by increasing rice paddy soil redox potential and evaluate how this elevated Mn exposure impacts Cd uptake into rice studies that last longer than 4 weeks.
Conclusions
Our data call into question whether increasing the Mn supply can decrease the grain concentrations of Cd via competition for Mn transporters in rice. We observed that higher Mn supply (pMn2+ > 6.5 in this study) significantly decreased Cd concentration in rice roots, but not grain, through competition for transporter OsNramp5 when plants were young, but as plants aged, OsCd1 may have contributed significantly to root Cd uptake as well. Cadmium translocation inside rice plants seems less related to Mn translocation but more related to other metal transporters such as OsHMA3 and OsCCX2. However, the hydroponic solution cannot fully represent the actual environment in which rice is grown, because we observed Mn toxicity symptoms in older plants at solution levels up to 50 uM, which has been reported as purportedly “safe” for rice in short-duration experiments. Future studies with rice growing in soil are needed to evaluate the impact of Mn on Cd mobility in soil and uptake into rice.
Data availability
The data supporting the findings of this study are available within the supplementary materials of this article or can be obtained by contacting the corresponding author.
References
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol 43(24):9361–9367. https://doi.org/10.1021/es9022738
ATSDR (2002). Toxicological profile for cadmium. In: Agency for toxic substances and disease registry’s toxicological profiles. https://doi.org/10.1201/9781420061888_ch48
Buchauer MJ (1973) Contamination of soil and vegetation near a zinc smelter by zinc, cadmium, copper, and lead. Environ Sci Technol 7(2):131–135. https://doi.org/10.1021/es60074a004
Chaney RL (1988) Metal speciation and interaction among elements affect trace element transfer in agricultural and environmental food-chains. In: Kramer JR, Allen HE (eds) Metal speciation: theory, analysis, and application. Lewis Publishers Inc, pp 219–260
Chaney RL, Bell PF, Coulombe BA (1989) Screening strategies for improved nutrient uptake and utilization by plants. HortScience 24(4):565–572. https://doi.org/10.21273/HORTSCI.24.4.565
Chang J, Huang S, Yamaji N, Ma JF, Zhao F (2020) OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant, Cell Environ 43:2476–2491. https://doi.org/10.1111/pce.13843
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18(2):92–99. https://doi.org/10.1016/j.tplants.2012.08.003
Colmer TD (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Botany 91(SPEC. ISS. JAN.), 301–309. https://doi.org/10.1093/aob/mcf114
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409(8):1489–1497. https://doi.org/10.1016/j.scitotenv.2010.12.028
Grant CA, Sheppard SC (2008) Fertilizer impacts on cadmium availability in agricultural soils and crops. Hum Ecol Risk Assess 14(2):210–228. https://doi.org/10.1080/10807030801934895
Halvorson AD, Lindsay WL (1977) The critical Zn2+ concentration for corn and the nonabsorption of chelated zinc. Soil Sci Soc Am J 41:531–534
Hao X, Zeng M, Wang J, Zeng Z, Dai J, Xie Z, Yang Y, Tian L, Chen L, Li D (2018) A node-expressed transporter OsCCX2 is involved in grain cadmium accumulation of rice. Front Plant Sci 9:476. https://doi.org/10.3389/fpls.2018.00476
Himes FL, Barber SA (1957) Chelating ability of soil organic matter. Soil Sci Soc Am J 21(4):368–373. https://doi.org/10.2136/sssaj1957.03615995002100040005x
Honma T, Ohba H, Kaneko-Kadokura A, Makino T, Nakamura K, Katou H (2016) Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ Sci Technol 50(8):4178–4185. https://doi.org/10.1021/acs.est.5b05424
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA 109(47):19166–19171. https://doi.org/10.1073/pnas.1211132109
Jin T, Nordberg M, Frech W, Dumont X, Bernard A, Ye TT, Kong Q, Wang Z, Li P, Lundström NG, Li Y, Nordberg GF (2002) Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 15(4):397–410. https://doi.org/10.1023/A:1020229923095
Livak KJ, Schmittgen, TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Luo J, Huang J, Zeng D, Peng J, Zhang G, Ma H, Guan Y, Yi H, Fu Y, Han B, Lin H, Qian Q, Gong J (2018) A defensin-like protein drives cadmium efflux and allocation in rice. Nat Commun 9(645):1–9. https://doi.org/10.1038/s41467-018-03088-0
Maguffin SC, Abu-ali L, Tappero RV, Pena J, Rohila JS, Mcclung AM, Reid MC (2020) Influence of manganese abundances on iron and arsenic solubility in rice paddy soils. Geochim Cosmochim Acta 276(2020):50–69. https://doi.org/10.1016/j.gca.2020.02.012
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, Satoh-Nagasawa N, Watanabe A, Fujimura T, Akagi H (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189(1):190–199. https://doi.org/10.1111/j.1469-8137.2010.03459.x
Morrow H (2010) Cadmium and cadmium alloys. In Kirk-Othmer encyclopedia of chemical technology. https://doi.org/10.1002/0471238961.0301041303011818.a01.pub3
Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK (2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52(4):464–469. https://doi.org/10.1111/j.1747-0765.2006.00055.x
Nordberg GF, Jin T, Kong Q, Ye T, Cai S, Wang Z, Zhuang F, Wu X (1997) Biological monitoring of cadmium exposure and renal effects in a population group residing in a polluted area in China. Sci Total Environ 199(1–2):111–114. https://doi.org/10.1016/S0048-9697(97)05486-7
NTP (2021) The Report on Carcinogens 15th Edition. https://ntp.niehs.nih.gov/whatwestudy/assessments/cancer/roc. Accessed 1 Oct 2023
Parker DR, Chaney RL, Norvell WA (1995) Chemical equilibrium models: applications to plant nutrition research. In: Loeppert RH, Schwab AP, Goldberg S (eds) Chemical equilibrium and reaction models. Soil Science Society of America Journal, pp 163–200
Parker DR, Norvell WA (1999) Advances in solution culture methods nutrition. Adv Agron 65:151–213
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24(C):29–96. https://doi.org/10.1016/S0065-2113(08)60633-1
Sasaki A, Yamaji N, Xia J, Ma JF (2011) OsYSl6 is involved in the detoxification of excess manganese in rice. Plant Physiol 157(4):1832–1840. https://doi.org/10.1104/pp.111.186031
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24(5):2155–2167. https://doi.org/10.1105/tpc.112.096925
Satoh-Nagasawa N, Mori M, Nakazawa N, Kawamoto T, Nagato Y, Sakurai K, Takahashi H, Watanabe A, Akagi H (2012) Mutations in rice (oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol 53(1):213–224. https://doi.org/10.1093/pcp/pcr166
Seyfferth AL, Amaral D, Limmer MA, Guilherme LRG (2019) Combined impacts of Si-rich rice residues and flooding extent on grain As and Cd in rice. Environ Int 128:301–309. https://doi.org/10.1016/j.envint.2019.04.060
Seyfferth AL, Morris AH, Gill R, Kearns KA, Mann JN, Paukett M, Leskanic C (2016) Soil incorporation of silica-rich rice husk decreases inorganic arsenic in rice grain. J Agric Food Chem 64(19):3760–3766. https://doi.org/10.1021/acs.jafc.6b01201
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330(1):207–214. https://doi.org/10.1007/s11104-009-0193-9
Suda A, Makino T (2016) Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: a review. Geoderma 270:68–75. https://doi.org/10.1016/j.geoderma.2015.12.017
Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK (2011) The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot 62(14):4843–4850. https://doi.org/10.1093/jxb/err136
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant, Cell Environ 35(11):1948–1957. https://doi.org/10.1111/j.1365-3040.2012.02527.x
Tan L, Zhu Y, Fan T, Peng C, Wang J, Sun L, Chen C (2019) OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn / Cd to grain in rice. Biochem Biophys Res Commun 512(1):112–118. https://doi.org/10.1016/j.bbrc.2019.03.024
Tanaka A, Navasero SA (1966a) Interaction between iron and manganese in the rice plant. Soil Sci Plant Nutr 12(5):29–33. https://doi.org/10.1080/00380768.1966.10431958
Tanaka A, Navasero SA (1966b) Manganese content of the rice plant under water culture conditions. Soil Sci Plant Nutr 12(2):21–26. https://doi.org/10.1080/00380768.1966.10431185
Taylor GJ, Crowder AA (1983) Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70(8):1254–1257. https://doi.org/10.2307/2443295
Teasley WA, Limmer MA, Seyfferth AL (2017) How rice (Oryza sativa L.) responds to elevated As under different Si-rich soil amendments. Environ Sci Technol 51(18):10335–10343. https://doi.org/10.1021/acs.est.7b01740
Tsukahara T, Ezaki T, Moriguchi J, Furuki K, Shimbo S, Matsuda-Inoguchi N, Ikeda M (2003) Rice as the most influential source of cadmium intake among general Japanese population. Sci Total Environ 305(1–3):41–51. https://doi.org/10.1016/S0048-9697(02)00475-8
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107(38):16500–16505. https://doi.org/10.1073/pnas.1005396107
Uraguchi S, Kamiya T, Clemens S, Fujiwara T (2014) Characterization of OsLCT1, a cadmium transporter from indica rice (Oryza sativa). Physiol Plant 151(3):339–347. https://doi.org/10.1111/ppl.12189
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA 108(52):20959–20964. https://doi.org/10.1073/pnas.1116531109
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60(9):2677–2688. https://doi.org/10.1093/jxb/erp119
Wang J, Wang P, Gu Y, Kopittke PM, Zhao F, Wang P (2019) Iron − Manganese (Oxyhydro)oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems. Environ Sci Technol 53:2500–2508. https://doi.org/10.1021/acs.est.8b06863
Wang M, Chen S, Shi H, Liu Y (2022a) Redox dependence of manganese controls cadmium isotope fractionation in a paddy soil-rice system under unsteady pe + pH conditions. Sci Total Environ 806(Part 2):150675. https://doi.org/10.1016/j.scitotenv.2021.150675
Wang M, Chen W, Ma W, Chaney RL, Green CE (2022b) Effects of Mn 2 + on Cd accumulation and ionome in rice and spinach. J Environ Qual 51:890–898. https://doi.org/10.1002/jeq2.20358
Watanabe T, Zhang ZW, Moon CS, Shimbo S, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K, Ikeda M (2000) Cadmium exposure of women in general populations in Japan during 1991–1997 compared with 1977–1981. Int Arch Occup Environ Health 73(1):26–34. https://doi.org/10.1007/PL00007934
Watanabe T, Shimbo S, Moon CS, Zhang ZW, Ikeda M (1996) Cadmium contents in rice samples from various areas in the world. Sci Total Environ 184(3):191–196. https://doi.org/10.1016/0048-9697(96)05100-5
Xu X, Chen C, Wang P, Kretzschmar R, Zhao F (2017) Control of arsenic mobilization in paddy soils by manganese and iron. Environ Pollut 231(2017):37–47. https://doi.org/10.1016/j.envpol.2017.07.084
Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF (2013) A node-based switch for preferential distribution of manganese in rice. Nat Commun 4:1–11. https://doi.org/10.1038/ncomms3442
Yan H, Xu W, Xie J, Gao Y, Wu L, Sun L, Feng L, Chen X, Zhang T, Dai C, Li T, Lin X, Zhang Z, Wang X, Li F, Zhu X, Li J, Li Z, Chen C, … He Z (2019) Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat Commun 10(1):1–12. https://doi.org/10.1038/s41467-019-10544-y
Yang M, Zhang Y, Zhang L, Hu J, Zhang X, Lu K, Dong H, Wang D, Zhao FJ, Huang CF, Lian X (2014) OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J Exp Bot 65(17):4849–4861. https://doi.org/10.1093/jxb/eru259
Acknowledgements
This research was supported by funding to A.L.S. and M.A.L by the National Science Foundation (Grant No. 1930806). We thank Chin Chen Kuo at the Advanced Materials Characterization Laboratory at the University of Delaware for analytical assistance, undergrad student Sara D'Appolonia for assistance in rice hydroponic experiment, William Bartz and Rodney Dempsey at Fisher Greenhouse at the University of Delaware for rice growth chamber facility support, Katherine Evans for assistance in grinding rice samples, Nicole Donofrio and Adam Ginn for providing qPCR technique training and support, and David Parker for fruitful discussions.
Funding
This research was supported by funding to A.L.S. and M.A.L by the National Science Foundation (Grant No. 1930806).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Rice hydroponic experiment, sample collection and analyses, data collection and analysis were performed by RH, MAL assisted in DCB extraction sample testing, data collection and analysis. The first draft of the manuscript was written by RH with contributions from ALS, and all authors reviewed and edited versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The Authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Jian Feng Ma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, R., Limmer, M.A. & Seyfferth, A.L. How manganese affects rice cadmium uptake and translocation in vegetative and mature plants. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06669-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06669-9