Abstract
Background and aims
Weeds are a major biotic stressor impacting crop production. Improving the competitiveness of wheat (Triticum aestivum L.) could provide a useful tool in integrated weed management. While wheat typically exhibits conservative early growth, early vigour has been increased through long-term recurrent selection for greater early biomass and leaf area. However, the influence of integrating such vigour into breeding lines for improving competitive ability remains to be explored.
Methods
In replicated controlled environment experiments, the effect of breeding early shoot vigour on root development and below-ground competitiveness was carefully examined. Physical and chemical characteristics of wheat vigour lines were assessed and compared with commercial cultivars in hydroponics and field soil experiments. Measurements included early root growth, rhizosheath size and growth responses in the presence of annual ryegrass, a major weed in wheat production.
Results
The vigorous lines exhibited larger leaf widths, increased cell file number, increased total root length and larger rhizosheaths compared with commercial parents. Numerous secondary metabolites with known allelopathic effects on weeds were detected in the roots and the rhizosphere, and significant allelochemical level differences observed between distilled water and soil water extract-treated plants. Although the vigour lines were significantly more competitive than the commercial cultivars against ryegrass, they produced similar levels of phytotoxic secondary metabolites.
Conclusion
Competition below-ground was strongly suppressive of ryegrass for the more vigorous genotypes suggesting that breeding with shoot vigour had pleiotropic effects on key root traits for below-ground wheat competitiveness.
Similar content being viewed by others
Introduction
Competition from weeds represents the single largest biotic stress for wheat (Triticum aestivum L.) with a potential loss in production of approximatively 25% (Oerke 2006). The large potential for loss occurs because the wheat crop and weeds compete for the same pool of resources (Gallandt and Weiner 2015). Increases in the harvest index of wheat, a major priority for modern breeders, were achieved by reducing plant height which reduced lodging and allowed a greater investment of carbon in floret fertility to increase grain number (Youssefian et al. 1992). However, shorter wheat ideotypes, as described by Donald (1968,) with smaller canopies were less competitive against neighbouring weeds (Cousens et al. 2003b). Indeed, decreased competitiveness has been associated with the introduction of the semi-dwarf genes central to the yield increases of the “Green Revolution” (Reynolds et al. 1994). Weed control required more intensive management practices that combined primary tillage with more regular herbicide applications (Evers and Bastiaans 2016). These methods introduced several challenges. Tillage is laborious and energy intensive (Morris et al. 2010; Soane et al. 2012), and can lead to soil erosion, compaction and loss of soil structure (Lowry and Smith 2018). Intensive herbicide use led to increased occurrence of herbicide resistance (Délye et al. 2013; Heap 2018), negative environmental impacts and food safety concerns (Lemerle et al. 2001a; Stoate et al. 2009). The rise in herbicide-resistant weeds, the paucity of new herbicides with novel modes of action (Heap 2014), together with other economic, environmental and social challenges associated with excessive use of chemical herbicides (Colas et al. 2020; Wezel et al. 2013) highlight the need for integrated weed management strategies. One sustainable technique involves the development of crops that are inherently more competitive against weed infestation. Indeed, enhancing the competitiveness of wheat is considered to be one of the most promising strategies for controlling weeds compared with other integrated farming practices (Gaba et al. 2018; Debaeke et al. 2024).
The competitive ability of wheat has been defined as the sum of its tolerance to the presence of weeds without significantly affecting yield, and the suppression of weed growth (Jordan 1993). Above-ground, crop-weed competition typically is for space and light (Kaur et al. 2018). The quantity of photosynthetically active radiation intercepted by the crop is negatively correlated with weed fecundity (Lemerle et al. 1996) and the emergence of weed seeds (Gaba et al. 2017). Therefore, physiological traits that affect the amount (Colbach et al. 2020; Page et al. 2010) and quality (Liu et al. 2009) of light available to weeds will contribute to the competitive ability of wheat (Petit et al. 2018). Competition also occurs below ground and the presence of neighbouring plants can affect the early development of wheat well before canopy closure (Finch et al. 2017). Meta-analyses comparing the contributions of above and below-ground competition to overall growth inhibition concluded that below-ground competition intervened earlier and had a greater effect on growth than above-ground interactions in a majority of the studies analysed (Kiær et al. 2013; Wilson 1988). Below-ground crop competition represents a synergy of physical and allelopathic interaction (Einhellig 1996). The ability of crops to quickly and efficiently access nutrients (Giehl et al. 2014) or to “pre-empt” the nutrients (Craine 2006) will contribute to their competitive ability (Craine and Dybzinski 2013). Overall, the volume of soil a crop plant can explore will impact directly on the resources it can capture (Lynch et al. 2021).
Above-ground early vigour has been described as the early rapid growth and spread of a crop (Richards 1989). Early vigour in wheat is characterised by increased length and width of the first two leaves and a greater leaf area index (Rebetzke and Richards 1999; Zhang et al. 2015). Multiple studies have found that early shoot vigour benefits the competitiveness of wheat (Bhaskar et al. 2019; Hendriks et al. 2022a), and has the added advantage of increasing competitiveness without reducing harvest index (Zerner et al. 2016). Early shoot vigour significantly varies between wheat cultivars (Rebetzke et al. 2004, 2008) with recurrent selection for shoot vigour resulting in substantial increase in leaf width and area (Zhang et al. 2015).
Above- and below-ground growth in wheat are isometrically related (Maydup et al. 2012; Qin et al. 2012; Van Noordwijk and de Willigen 1987). Consequently, wheat cultivars with high rates of shoot growth tend to have increased rates of root growth (Van Den Boogaard et al. 1996). Consistent with these observations, wheat cultivars with greater early shoot vigour also produced greater root biomass (Duggan et al. 2005; Liao et al. 2006; Palta et al. 2011; Palta and Yang 2014; Watt et al. 2005), longer root systems (Palta et al. 2011), increased branching (Liao et al. 2006; Palta et al. 2007) and deeper root growth (Palta et al. 2011) than less vigorous commercial wheat cultivars. More recently, we showed that the selection for greater shoot vigour in wheat also increased root hair length (Hendriks et al. 2022b). However, these lines were solely selected on their early shoot vigour and not on any other agronomic characteristics (Zerner et al. 2008). In order to assess the agronomic potential of early vigour wheat more thoroughly, the vigorous genotypes developed previously were top-crossed with commercial cultivars (Coleman et al. 2001; Rebetzke et al. 2018). Resulting lines were examined in more detail and their competitive ability assessed (Hendriks et al. 2022a; Rebetzke et al. 2018).
The rapid growth and establishment of a root system enables a greater volume of soil to be explored which could improve competitiveness and reduce reliance on herbicides (White et al. 2013). Root traits such as total root length (Rogers and Benfey 2015), root angle (Manschadi et al. 2006) and root growth at depth (Lilley and Kirkegaard 2011; Wasson et al. 2012) are important traits for improving soil exploration. Likewise, several studies have linked branching patterns and root hairs to the uptake of water (Ahmed et al. 2018), nitrogen (Liao et al. 2004, 2006), phosphorus (Gahoonia et al. 1997; James et al. 2016; Ryan et al. 2015) and potassium (Jungk 2001). Chemical defence, or allelopathy is another mechanism of below-ground competition, which occurs when one plant, through its living or decaying tissue, interferes with the growth of another plant via production of allelochemicals (Zimdahl 2018). Wheat produces hydroxamic acids called benzoxazinoids (BX). Two molecules of interest are 2,4-dihydroxy-(2H)-1,4-benzoxazin-3(4H)-one (DIBOA) and 2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) (Niemeyer 1988; Sue et al. 2011). These secondary metabolites are key players in the allelopathic interference (Pratley 1996; Schandry and Becker 2019). Wu et al. (2000) suggested that allelopathy should be distinguished from competition. Where allelopathy by a plant adds allelochemicals to the soil, direct competition removes resources from another plant to reduce its growth (Wu et al. 2000). Nevertheless, these processes act in synergy in the field, and a small allelopathic effect may change the final competitive balance (Einhellig 1996). Therefore, separating the competitive and allelopathic effects on the competitor is difficult (Inderjit and Moral 1997; Thijs et al. 1994). Indeed, most studies either do not separate physical and chemical interactions or provide little evidence for the presence of allelopathic interaction in the field (Mahé et al. 2022).
Early vigour and allelopathy have been identified as targets for improved crop competitiveness (Bertholdsson 2005) and aid in integrated weed management (Lowry and Smith 2018). Nonetheless, little is known of how breeding with shoot vigour to improve cultivar competitiveness affects root system architecture and below-ground competitiveness. The present study evaluated the early root growth and below-ground competitive interactions of progeny from top-crosses between germplasm from the third cycle of recurrent selection for vigour and two Australian commercial cultivars, Yitpi and Wyalkatchem. Experiments performed in hydroponics and field soil examined root length, rhizosheath size, root angles and the presence of exuded phytotoxic secondary metabolites on plants grown under controlled environments. Competitive interactions were then evaluated in root pouches and in field soil in the presence of perennial ryegrass (Lolium perenne L. cv Kidman) as a weed competitor.
Materials and methods
Germplasm
Breeding lines were produced with vigour donors from the third cycle (C3) of a recurrent selection program for early shoot vigour as described in Wilson et al. (2015). Briefly, the C3 vigour donors were crossed with parental lines containing alternative dwarfing genes Rht12 in W400203 and W470201 or Rht13 in W010709 and W670704, and the resulting F1 plants were top-crossed (TC) with commercial cultivars, Wyalkatchem and Yitpi, both of which possess the Rht-D1b semi-dwarf allele. A top cross enables certain target traits to be combined from each parent. Up to five top-cross F1 plants were sown from each commercial parent and allowed to self-pollinate to produce a number of TC1F2. These TC lines were subsequently self-pollinated through single-seed descent until the F4:5 generation whereupon they were assessed in the field for plant height at maturity. Semi-dwarf lines were identified and retained. The present study examined two independent vigour lines derived from C3 and Wyalkatchem (W010709 and W670704) and two lines independent vigour lines derived from C3 and Yitpi (W400203 and W470201) (Hendriks et al. 2022a). These TC vigour lines were compared with the original lines from the third cycle of the recurrent selection for shoot vigour (C3), the two commercial parental cultivars (Wyalkatchem and Yitpi), a wheat cultivar previously identified as vigorous and weed suppressive (Condo), and a less vigorous and uncompetitive cultivar (Mace), a heritage cultivar (Federation), and triticale (× Triticosecale, cv Chopper). The last two genotypes were considered highly vigorous and competitive (Hendriks et al. 2022a). Unless mentioned otherwise, these genotypes were used in all the experiments described below.
Leaf assessments
For leaf vein and cell file number assessments, 2 cm long sections of the third leaf were taken 2 cm distance from the point of attachment to the pseudo-stem. Samples were collected when the leaves were fully elongated, fixed in 70% ethanol for 24 hours and transferred to 1% w/v bleach (White King© sodium hypochlorite 42 g/L) for clearing (Botwright et al. 2005). Leaf pieces were mounted whole in water using glass slides and 0.17 mm thick cover slips. Brightfield images were obtained using a Zeiss AxioImager Z1 microscope equipped with a Zeiss Axiocam 712 colour CCD camera (Carl Zeiss Micro-imaging GmbH, Jena, Germany) and plan-apochromat 10x (NA = 0.3) objective. Tiled images were captured to record epidermal cell position along with cell length and width measurements using Zeiss ZEN V2.6 (Carl Zeiss Micro-imaging GmbH, Jena, Germany).
Root angle assessment - clear-pot method
A clear-pot, root angle assessment technique was adapted as described by Richard et al. (2018). Fifteen seeds per genotype were weighed individually within a range of 47 ± 2 mg. The seeds were treated with fungicide by soaking them for 3 minutes in Thyram (1.4 g/L), and then germinated on wet paper placed in Petri dishes in the cold (4 °C) for three days and then at room temperature for 24 h. Six germinated seeds (roots approx. 3 mm) were selected per genotype and sown in transparent pots (4 L, 200 mm diameter, 190 mm height from Anovapot Pty Ltd., Brisbane, QLD, Australia). The seeds were sown vertically, along the pot wall with the embryo facing downwards against the edge of the transparent wall, every 60 degrees along the circumference of a pot filled with a 50:50 compost:sand potting mix. The transparent pots were then placed in opaque black pots (4 L, Anovapot, 200 mm diameter, 190 mm height) to avoid exposing roots to light. Position of the pots was randomised on the bench in the greenhouse. Three days after sowing (DAS) the seeds had germinated, and shoots emerged. The seminal roots were identified through the transparent pot-wall and the first pair, when visible, was traced on the pot. Photographs of the plants at 1-leaf stage were taken 12 d after sowing with a digital camera at a standard height and distance from the pots. Pictures were analysed with Fiji software (version 1.53 s; imagej.net/software/fiji/downloads), using the “Angle tool” option, to calculate the angle between the first pair of seminal roots.
Rhizosheath measurements
This experiment was conducted in a cropping soil obtained from the Ginninderra Experimental Station (35°17’S, 149°05′E; ACT, Australia). The topsoil was collected to a depth of 10 cm and passed through a 4 mm sieve to remove larger sized organic matter and small stones. The soil, classified as an acidic (pH = 4.3) Podzol soil, was limed with CaCO3 (2 g kg−1 of soil) to achieve a pH of 7. At harvest, plants were removed from each pot and the three first seminal roots were excised at the seed leaving the residual soil attached to the root (Delhaize et al. 2012). Root length and root fresh weight (including attached soil) were measured. Rhizosheath sizes (positively correlated with root hair length) were then expressed as the ratio of the fresh weight to length of the three seminal roots. The roots were then washed to remove excess soil and stored in 50% (v/v) ethanol.
Competitive experiments: root pouches
For the hydroponic experiments, sheets (25.4 × 38.1 cm) of Anchor Seed Germination Paper (Hoffman Manufacturing Inc., Oregon, USA) were used. The seeds were placed between two water-moistened sheets, embryo facing downwards, 25 mm from the top of the germination paper and held in place with paperclips. The pouch was attached to a steel rod with two paper clips, on the upper edge of the pouch and hung vertically in purpose built opaque Perspex boxes (48 × 26 × 28 cm). Two blue blotting papers were used to stop the roots contacting the side of the boxes, and one empty pouch was placed at either end of the box. Water was added to 2 cm from the bottom of each box and maintained at this level for the duration of the experiment. To ensure consistent germination, the boxes were stored for three days in the dark at +4 °C. Subsequently, the boxes were placed in a growth chamber with a 12 hour day/night (600 μmol.m−2.s−1) and 20 °C /15 °C day/night cycle. After 12 days in the growth chamber, the experiment was terminated as the plants reached the second leaf stage (Zadoks et al. 1974). Five pouches were used per genotype and randomly allocated across the Perspex boxes for root length assessments, and the experiment was repeated thrice. Similarly, ten pouches per genotype were set up for the extraction of secondary metabolites. The pouches were isolated from each other with clingwrap to avoid the transfer of phytochemicals from one pouch to another. Two Perspex boxes were used, each containing five pouches of every genotype. In the first box, distilled water was used to irrigate the pouches, while the second box was irrigated with an aqueous soil extract prepared by percolating distilled water through topsoil (0–0.1 m) collected from the Ginninderra Experimental Station, Australian Capital Territory (35°17’S, 149°05′E) (2 L of water for 500 g of soil). The aim was to include the complement of microflora from this soil into the extract. The aqueous samples were stored in the dark at 4 °C.
Another five pouches were set-up for each genotype along with ryegrass as a competitor to assess the below-ground interaction with the different wheat genotypes. Each box contained 56 pouches, and a total of four boxes were used for this experiment. The pouch position was randomised in each box. Ryegrass seeds were placed less than 5 mm above the wheat seed. The number of weed plants varied. The first box was set up as a competition free negative control with no ryegrass. In the second box each wheat pouch contained wheat and two ryegrass seed; each wheat pouch in the 3rd box contained four ryegrass seeds; and wheat pouches in the 4th box contained six ryegrass seeds. Root pouches with only ryegrass (no wheat) used as free negative control for ryegrass growth.
Root systems of the wheat plants were photographed under fluorescent light and analysed with FIJI (Schindelin et al. 2012) to quantify the total root length. The shoot length was also measured using this technique. Roots and shoots were oven dried (70 °C for 3 days) and weighed.
Competition experiment: soil experiment
To compare data from the competitive root pouch experiment with a soil-based assay, Yitpi-derived vigour line and Wyalkatchem-derived vigour line their commercial cultivar parents and germplasm from the third cycle of the recurrent selection of vigour were grown in 20 cm polyvinylchloride (PVC) tubes (9 cm diameter) using soil collected from the Graham Centre field research site in Wagga Wagga (35°03 S, 147°36 E; NSW, Australia). The topsoil (0–0.1 m, pH 6.4), which was classified as a fine red clay-loam kandosol, was sieved through a 4 mm mesh. The tubes were each filled with 1.7 kg of this soil (density of 1.34 g/cm3).
A single wheat seed was sown at 2 cm depth, and the tubes were established with zero, four or eight ryegrass seeds (equivalent infestation of approximatively 0, 625 and 1250 ryegrass plants per square meter, respectively). Three levels of competition were assessed: only below-ground competition where plants were kept from competing above-ground by using a white plastic divider (width 10 cm height 25 cm; Plastic Creations, Fyshwick, Australia) (Fig. 1A); full competition with above and below-ground competition (Fig. 1B); absence of competition where only a single wheat plant was present in the tube as a negative control (Fig. 1C).
Setup of the field soil competitive experiment. Wheat (green and light brown) and two, four or eight ryegrass (orange and purple) plants were allowed to interact A) only below-ground with the above-ground growth kept separated by a white acrylic sheet, B above and below-ground, C a no competition control tube with either only wheat, D no competition control tube with only two, four or eight ryegrass plants
The plants were grown in a controlled environment at 12 h day/night (600 μmol.m−2 s−1) and 21 °C /16 °C day/night cycle. Plants were harvested 30 days after sowing at mid tillering (GS 25). The tubes were emptied, roots washed out and roots of ryegrass and wheat were separated. Plants were dissected, shoot and roots were oven dried (70 °C for 3 days) and weighed.
Collection and analysis of secondary metabolites
For this experiment one the vigour germplasm parent was replaced by a germplasm from the sixth cycle of the recurrent selection (C6) due to the limited availability of viable C3 seed. Samples were collected at early vegetative growth corresponding to second leaf stage. The shoots were dissected and were oven dried (70 °C for 3 days) and weighed. The roots were dipped in 20 ml solution (99% methanol and 1% of acetic acid) to extract the metabolites present in the rhizosphere. Roots were weighed fresh and immediately placed post-sampling in an insulated cooler with ice for transportation to the laboratory and then stored at −80 °C until extraction.
Metabolic profiling of benzoxazinoids and targeted metabolic profiling of aminophenoxazinones in root dips and extracts was conducted as described by Mwendwa et al. (2021). In brief, liquid chromatography was performed using an Agilent 1290 Infinity UHPLC coupled to an Agilent 6530 quadrupole time-of-flight (QTOF) mass spectrometer (MS) with an Agilent Dual Jet Stream electrospray ionisation source (Agilent Technologies, Australia). Nitrogen was used as the drying gas at a flow of 9 L min−1, and nebuliser pressure was 35 psig. Chromatographic separation was performed using a Synergi™ Polar-RP column (2.0 mm × 30 mm, 2.5 μm particle size) (Phenomenex, Torrance, CA USA) preceded by a guard column (2.0 × 3.0 mm, 2.5 μm particle size) of the same phase. Every 11 samples, a blank injection and quality control sample were analysed. For the profiling of the aminophenoxazinones a standard mixture of aminophenoxazinone metabolites (APO, AMPO, AAPO and AMPO obtained from the Fomsgaard laboratory at Aarhus University (Denmark)) was injected at the beginning and the end of the entire sample set. Abundance of benzoxazinoinds was expressed in units of detector response as purified standards were generally unavailable for the majority of analytes recovered. The abundance of aminophenoxazinones was expressed in units of detector response. The molecules detected and quantified are describes in Supplementary Table 1.
The target metabolite compounds were annotated based on matching molecular features extracted from chromatograms with Agilent MassHunter Qualitative Analysis (v. B.07) with a personal library of molecular formulas of benzoxazinoids constructed previously (Agilent PCDL Manager v. B.08.00), with reference to elution order based on similar analyses performed by Mwendwa et al. (2016) or by comparison with authentic standards of BOA and MBOA purchased from Sigma-Aldrich (Castle Hill, NSW).
Multiple reaction mode (MRM) analyses were used to quantitate the phenoxazinone metabolites (APO, AAPO, AMPO and AAMPO) using purified standards (kindly provided by I. Fomsgaard, Aarhus University) using positive ion mode. APO was quantified by tracking the transition 213➔185 m/z, AMPO using the transition 243➔228 m/z, AMPO using the transition 255➔213 m/z and AAMPO using the transition 285➔ 243 m/z.
Statistical analyses
A mixed linear model was fitted containing random components that identified the structure of the experimental design for each experiment: (i) pot position (row, column); and (ii) soil type. The analysis of variance and estimation of least squares means was conducted considering genotypes as fixed effects using the function lm in “emmeans” (Lenth et al. 2021) in the R (4.03) (R Core Team 2020) software package. Pairwise comparisons between genotype means were obtained using the “emmeans” package using the Benjamini-Hochberg procedure (Haynes 2013).
Metabolite abundance data were analysed using several methods. The effects of genotype and water treatment were analysed with factorial analysis of variance (ANOVA) after transforming data with log10 transformation (Statistix 10, Analytical Software, Tallahassee, FL USA). Associations between abundance of benzoxazinoids and aminophenoxazinones were assessed and visualised with heatmaps generated by MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) after 1) filtering by interquartile range, 2) estimating missing values using Limit of Detection method where all the missing values are replaced with small values (i.e. 1/5 of the minimum positive value of each metabolite), and 3) transforming data with log10 transformation.
Results
Above-ground vigour
Above-ground shoot vigour was assessed by measuring the width of the third leaf of various genotypes (Fig. 2A). The C3 genotypes representing the third cycle of recurrent selection for early shoot vigour had significantly wider leaves than other wheat entries. Similarly, topcross-derived (TC) vigour lines typically had wider leaves than their respective commercial cultivar parents. The vigorous wheat cultivar control, cv. Condo, exhibited significantly wider leaves than the low vigour cultivar control, cv. Mace. All vigour lines were significantly wider than the commercial control for by up to 20%.
Shoot vigour assessment on the third leaf. Data show (A) leaf width of the third leaf (n = 36 for C3 and n = 24 for the other entries), B number of cell files over the width of the third leaf of the wheat entries. The error bars represent standard errors (n = 24). Significant differences were determined with a one-way ANOVA. Letters identify significant differences between means (P ≤ 0.05) as determined by pairwise comparisons with the Benjamini-Hochberg (BH) procedure
Detailed microscopic examination indicated that genotypes with wider leaves also had significantly greater cell file numbers across the third leaf (Fig. 2B) while increased cell file number was associated with greater vein numbers across the width of the leaf (Supplementary data Fig. 1A). The vigorous lines (parent C3 and TC-vigour lines) produced significantly (P < 0.05) more veins than their commercial parents Wyalkatchem and Yitpi. Condo, included as a vigorous cultivar control produced significantly more leaf veins than cv. Mace, included as a low vigour control. However, the number of cell files between two leaf veins (10.9 ± 0.2, SE) was not significantly different across genotypes. Consequently, the total number of cell files was strongly correlated with leaf width (r = 0.95, P < 0.001) (Supplementary data Fig. 1B).
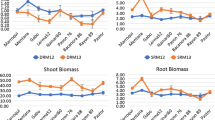
Biomass accumulation in these early growth stages was greater in the TC-vigour lines compared with commercial cultivars (Supplementary data Fig. 2). TC-vigour lines accumulated ~19% more root biomass and ~ 20% shoot biomass than commercial cultivars. Thus, while TC-vigour lines produced more root and shoot biomass than the commercial cultivar parents, root-to-shoot ratios were not significantly different between wheat genotypes. Only the triticale cultivar, Chopper, included for its known high vigour and high weed competitiveness, had a higher root-to-shoot ratio than the tested wheat entries. Triticale also had a significant larger root biomass than all the wheat entries.
Early root growth- root length and root angles
Total root length of plants at second leaf stage were significantly different between the different genotypes (Fig. 3A) The triticale cultivar Chopper had the longest total root system of all entries. Among the wheat entries the more vigorous cultivar Condo had 17% longer root system than the less vigorous cultivar Mace. The heritage cultivar Federation also produced longer primary and secondary roots than all the wheat cultivars. The length of the root systems of the TC-vigour lines did not differ from the C3 vigour parent. Compared with the commercial parent, the Yitpi derived vigour lines had ~50% longer roots. The Wyalkatchem derived TC-vigour lines produced ~40% longer roots compared with its commercial parent.
Early root growth of different wheat and triticale genotypes. Root length (A) length of the main seminal root length (B) and length of the secondary roots (C) were measured for the high vigour W lines, their respective commercial cultivar parents (W010709 and W670704 generated from Wyalkatchem, and W400203 and W470201 generated from Yitpi), the recurrent selection for shoot vigour parent (C3) and the triticale Chopper. The error bars represent standard errors (n = 24). Significant differences were determined with a one-way ANOVA. Letters identify significant differences between means (P ≤ 0.05) as determined by pairwise comparisons with the Benjamini-Hochberg (BH) procedure
Total root system length in the wheat genotypes was closely associated with length of the main seminal roots (Fig. 3B). All TC-vigour lines had longer seminal roots than their respective commercial cultivar parents and some were as long as the C3 parent and the triticale control. The total length of secondary or branching roots was not significantly different between wheat genotypes (Fig. 3C). Only the triticale had significantly longer branch roots. Root angles of the second pair of seminal roots were not significantly different (Supplementary data Fig. 3).
Rhizosheath size
Rhizosheath sizes were carefully measured among all vigour lines and compared them to those of the respective commercial parent. Generally, the TC-vigour lines had larger rhizosheaths than their respective commercial parents but rhizosheaths were smaller than the C3 vigour parent (Fig. 4). Rhizosheaths on the TC-vigour lines derived from Yitpi were ~ 14% larger than Yitpi but 27% smaller than C3. Similarly, the rhizosheaths on the vigour lines derived from Wyalkatchem were 38% larger than Wyalkatchem but 21% smaller than C3. Differences between the vigour lines and triticale were not significant for rhizosheath size.
Rhizosheath sizes of the vigour lines compared to their parental genotypes and triticale. The rhizosheath, or amount of soil material attached to given root length, was measured for high vigour lines, their respective commercial cultivar parents (W010709 and W670704 generated from Wyalkatchem, and W400203 and W470201 generated from Yitpi), C3 parents and triticale Chopper. The error bars represent standard errors (n = 24). Significant differences were determined with a one-way ANOVA. Letters identify significant differences between means (P ≤ 0.05) as determined by pairwise comparisons with the Benjamini-Hochberg (BH) procedure
Competitive ability
Competitive ability of wheat genotypes during early growth stages (GS21) was first examined in growth pouches by assessing how well these lines suppress the growth of perennial ryegrass (Fig. 5A, B, C) and how their own growth was affected by different numbers of ryegrass plants growing nearby (tolerance) (Fig. 5D, E, F). The TC-vigour lines suppressed up to 50% more weed biomass than their respective commercial cultivar parents (Fig. 5A) while the more vigorous and competitive commercial cultivar Condo was more suppressive than low vigour cultivar Mace. At the highest level of ryegrass pressure (six ryegrass plants) (Fig. 5C) the TC-vigour lines supressed weed growth significantly more than the recurrent selection parent, Condo and the commercial cultivars and this suppression was not significantly different from the triticale control, Chopper.
The effect of competition between ryegrass and crop species on plant growth. The relative decrease in total biomass of A) two ryegrass plants, B four ryegrass plants, or C) six ryegrass plants when they were grown in the presence or absence of a single wheat or triticale plant (Chopper). The relative decrease in the crop biomass when a single plant is grown in the presence of D) two ryegrass plants, E four ryegrass plants or F) six ryegrass plants compared with no ryegrass plants. One factor ANOVAs were performed for data from each density of ryegrass. Letters identify significant differences between means (P ≤ 0.05) as determined by pairwise comparisons with the Benjamini-Hochberg (BH) procedure
Tolerance to weed presence was measured as the reduction in wheat growth in the presence of an increasing number of ryegrass plants relative to growth in the absence of weeds (2 ryegrass plants Fig. 5D, 4 ryegrass plants Fig. 5E, 6 ryegrass plants Fig. 5F). Under all regimes of weed pressure, growth of the TC-vigour lines was less affected than the parental cultivars at all ryegrass densities. Interestingly, growth of the TC-vigour lines was reduced less by the presence of ryegrass than the cultivar Condo at the different levels of weed pressure. The cultivar Mace (low vigour and uncompetitive) showed the greatest reductions in growth in the presence of ryegrass plants. At the highest levels of weed pressure, growth reduction of the TC-vigour lines was only a third that shown by their parental cultivars and Condo, and only a quarter the reduction in Mace. Tolerance of the high vigour lines was similar to the tolerance of triticale.
Following from the pouch experiments, we selected lines to grow and validate ryegrass competition responses in PVC tubes filled with field soil. The different regimes of competition from ryegrass were imposed and these included: no competition, four ryegrass seedlings and eight ryegrass seedlings (respectively equivalent to 0, 625 and 1250 weed seedlings per m2). To determine the importance of below-ground competition during early stages of growth wheat plants were managed to compete with ryegrass below-ground only, or both above- and below-ground. The results suggest that the TC-vigour lines reduced growth of ryegrass significantly more than their commercial cultivar parents at all the levels of weed presence (Fig. 6). Indeed, the suppression of weed growth by the Wyalkatchem-derived vigour lines was three-fold greater than for Wyalkatchem. Similarly, Yitpi-derived vigour lines were up to twice more suppressive than Yitpi. There were no significant differences in the suppression of ryegrass between plants competing below-ground only and plants that competed both above- and below-ground.
Competitive ability of wheat and triticale genotypes in field soil at GS25. The reduction of ryegrass biomass was measured for the high vigour W lines, their respective commercial cultivar parents (W010709 and W670704 generated from Wyalkatchem, and W400203 and W470201 generated from Yitpi), the recurrent selection for shoot vigour parent (C3). The error bars represent standard errors (n = 12). Significant differences were determined with a one-way ANOVA. Letters identify significant differences between means (P ≤ 0.05) as determined by pairwise comparisons with the Benjamini-Hochberg (BH) procedure
Metabolomics
Seven benzoxazinoids and four aminophenoxazinones were detected in both the wheat root dips and root extracts (Supplem Table 1), and their abundance varied significantly among samples (Figs. 7 and 8). Abundance of benzoxazinoid glycosides were greatest in triticale Chopper, followed by Mace, W010709, W400203 and Yitpi. The presence of soil extractives in the culturing water generally resulted in uniformly higher levels of all benzoxazinoids and aminophenoxazinoids detected, but particularly the benzoxazinoid glycosides DIBOA-glc and DIM2BOA-glc. Due to differences in methods used to extract these compounds from the root dips and root extracts, it was not possible to directly compare the abundance of metabolites between these sample types. Therefore,comparisons were made only within a sample source (e.g., either root dips or root extracts).
Heatmap showing relative abundance of benzoxazinones and aminophenoxazinones in root dips of wheat seedlings cultured in root pouches with A) distilled water and B) soil water. Colouration of cells indicates normalised abundance (dark red to dark blue, from highest to lowest abundance). Similarity in patterns of abundance by genotype and by metabolite are indicated by the dendrograms along the top and left side, respectively, of the table
Heatmap showing relative abundance of benzoxazinones and aminophenoxazinones in root extracts of wheat seedlings cultured in root pouches with A) distilled water and B) soil water. Colouration of cells indicates normalised abundance (dark red to dark blue, from highest to lowest abundance). Similarity in patterns of abundance by genotype and by metabolite are indicated by the dendrograms along the top and left side, respectively, of the table
Statistical analysis of the root dip samples showed that presence of soil extractives in the culturing water significantly affected the abundance of the benzoxazinoids DIBOA-glc, DIM2BOA-glc and HDMBOA-glc (Table 2). There were no significant differences in the levels of benzoxazinoids among the wheat cultivars nor were there any significant interactions between culturing water and genotype (Supplem Table 3). Among the aminophenoxazinones, only AAMPO levels were significantly impacted by the presence of soil extractives, but in this case the soil extractives caused a decrease in AAMPO levels (Supplem Table 3). The levels of APO and AMPO were significantly different among genotypes, and this effect for AMPO differed with culturing water (the interaction between water and genotype was significant for AMPO at P < 0.05) (Supplem Table 3). The levels of APO were greatest for Yitpi, the recurrent selection, high vigour germplasm C6 and Chopper, and smallest for Wyalkatchem and Mace (Supplem Table 4). Levels of AMPO in root dips of the 11 genotypes varied with culturing water. Specifically, when plants were grown in distilled water, the root dips of Yitpi and the high-vigour W670709 line derived from Wyalkatchem contained the highest levels of AMPO while the high-vigour line W470201 derived from Yitpi had the lowest levels. By contrast, when plants were grown in soil-infused water, Condo had the highest levels of AMPO and Wyalkatchem had the lowest levels (Supplem Table 5).
Heat maps showing the abundance of metabolites and similarities in the patterns of abundance are shown in Figs. 7 and 8. The groupings of genotypes do not closely follow what might be expected by the relatedness of the genotypes, but there is some consistency in patterns of abundance of metabolites. For example, the benzoxazinoid glycosides are well-clustered in the root dips from seedlings cultured in soil water (Fig. 7B) and in the root extracts from seedlings cultured in distilled water (Fig. 8A).
Correlation heat maps showed that abundance of MBOA in root dips of seedlings cultured in soil water was positively correlated only with the abundance of AMPO (Supplem. Fig. 4B), the first breakdown product of MBOA by soil microbes. However, in root extracts, MBOA was most strongly correlated with the abundance of HMBOA-glc and DIM2BOA-glc (Supplem. Fig. 4D) which are the primary metabolites produced by the plant metabolism.
Discussion
This study demonstrated that the incorporation of shoot vigour into commercial wheat cultivars significantly modified root growth. Compared with their commercial parents, the TC-vigour lines had significantly longer roots and larger rhizosheaths which positively correlated with root hair length. Larger roots would enable the vigour lines to grow and rapidly occupy a larger volume of soil contributing to their weed competitiveness (Craine and Dybzinski 2013). The vigour lines had significantly larger root systems compared with their commercial cultivar parents. Therefore our results are in agreement with the “functional equilibrium” model of Brouwer (1963) suggesting a coordination between shoot and root growth parents and similar root-to-shoot ratios. Similarly, Palta and Watt (2009) found that wheat genotypes varying for above-ground vigour had similar ratios of root carbon to total plant carbon. These findings are consistent with other studies showing wheat selected for increased leaf area also exhibited more vigorous root growth (Richards et al. 2007). The competition experiments performed in pouches and in field soil indicate that both tolerance to weeds and the ability to suppress weed growth was significantly greater in the TC-vigour lines compared with the parental lines and other commercial genotypes.
The competitive advantage of high vigour wheat lines was observed during early growth in the field (Hendriks et al. 2022a). This work shows that the competitiveness of wheat based on the suppression of weed growth occurred as early as the second leaf stage, a developmental stage in which wheat seedlings are less likely to be competing with weeds for light or space. This study also showed that the more competitive wheat lines and cultivars all had longer seminal roots and larger rhizosheaths than the less competitive cultivars. The increase in rhizosheath size and total root length indicates that breeding greater above-ground vigour also modifies root traits at early seedling stages. Moreover, increased vigorous early root growth was associated with greater nitrogen uptake (Liao et al. 2006) potentially providing a competitive advantage against weeds (Bingham 1995). Similarly, crop-weed competition modelling concluded that intensity of soil foraging (plant density and branch spacing) and the rate with which root systems are established are key factors enabling crops to out-compete weeds because they allow for the rapid occupation of a greater soil volume from which resources can be extracted (Dunbabin 2007).
In contrast to the findings above, it has been argued by others that cereal root systems can be unnecessarily large and wasteful (Lynch 2018; Passioura 1983). Researchers have expressed concerns that early vigour might be associated with excessive water use during pre-anthesis growth (Figueroa-Bustos et al. 2020; Lemerle et al. 2001a) without impacting on weed suppression (Lemerle et al. 2001b) and perhaps even having negative effects on harvest index (Palta et al. 2011). However, these outcomes are likely to depend on the other weed management practices being undertaken and on environmental conditions. In dry seasons, vigorous wheats produced higher grain yields and a greater water-use efficiency (Botwright et al. 2002). Under terminal drought, the better early competitor remained the superior competitor throughout the season up to maturity (Cousens et al. 2003a, b). On the other hand, semi-dwarf wheat cultivars grown in a controlled environment could grow their root systems deeper into the soil profile in case of drought stress (Friedli et al. 2019). Smaller root systems may well be sufficient to support reasonable yields if the supply of water and nutrients remains high (Van Noordwijk and de Willigen 1987) but larger root systems can reduce risk when the environmental conditions are uncertain, especially at the end of season (Van Noordwijk and de Willigen 1987) and when weeds are present (Craine 2006).
The root dip experiments performed in this study showed there were abundant levels of the benzoxazinoids on the surface of living roots and our results found no significant effect of shoot vigour on the exudation of secondary metabolites. Interestingly, some vigorous wheat genotypes showed greater abundance of both benzoxazinoids metabolites and aminophenoxazinones compared with cultivar parents. In fact, root extracts of Wyalkatchem and Yitpi seedlings cultured in distilled water contained no detectable levels of DIMBOA-glc. However, when challenged with soil extractives, root exudates showed abundant levels of DIMBOA-glc which is readily converted by soil microbes to bioactive and herbicidal metabolites such as aminophenoxazinones. Moreover, the observed correlation between MBOA and AMPO levels in root dips is entirely expected as MBOA is first degraded to the bioherbicidal compound AMPO by soil microbes (Reiss et al. 2018). Because aminophenoxazinones are generated by soil processes (Belz 2007), their presence in the root samples is likely to be incidental only. Reduced levels of aminophenoxazinones and parent BX metabolites were noted in aqueous extracts containing soil extractives in where microbial activity was likely maintained. This confirms that soil microbes actively degrade both BX and aminophenoxazinone compounds. Benzoxazinoids have not yet been targeted for selection in plant breeding programs (Niculaes et al. 2018) with the exception of the development of a wheat variety with higher allelopathic ability in Sweden (Bertholdsson 2007). Our findings show that, in controlled environment, the Australian heritage wheat cultivar Federation released more BX metabolites than modern cultivars. These results align with previous studies (Bertholdsson et al. 2012; Vandeleur and Gill 2004) suggesting that wheat’s historic allelopathic ability may have reduced with ancient cereal cultivars or landraces found to be more competitive with weeds than modern cultivars. For example, Federation was found to have higher abundance of MBOA than recent wheat cultivars tested in multiple sites and years (Mwendwa et al. 2021). However, the impact of climatic factors on the regulation of biosynthesis of BX and their transformation is further needed (Hussain et al. 2022).
Interestingly, two of the benzoxazinoids detected in our study (DIM2BOA-glc and HDMBOA-glc) have previously been reported from maize but not wheat (Kumar et al. 1993). We could not confirm the identity of these compounds with authentic standards but mass spectral data for these analytes were consistent with the nominal molecular formulae for these metabolites. In addition, formate adducts of these putative compounds were evident, as was observed for the other benzoxazinoids monitored in this study (i.e. the formate ion arises from the formic acid used in the HPLC mobile phase). Detailed MS analysis and comparison with authentic standards of these compounds, if available, would be required to confirm their presence in these samples.
The competitive growth experiments indicated that below-ground competition between wheat and ryegrass commenced earlier and was greater than the above-ground competition. No significant differences in weed suppression or tolerance were detected up to the beginning of tillering between plants that were competing above and below-ground and the plants competing below-ground only. These observations agree with meta-analyses in wheat concluding below-ground competition occurred earlier and was more intense than above-ground competition (Kiær et al. 2013; Wilson 1988). One possible explanation for this is that during the early stages of growth canopies typically do not overlap, so competition between crops and weeds for water and nutrients is greater than the competition between canopies for light and space (Weaver et al. 1992). The early establishment of a large and robust root system would affect the subsequent development of shoots (Zimdahl 2004) thus enhancing a plants overall competitiveness (Hodge et al. 1998).
There is ongoing discussion whether vigorous root genotypes release more carbon into the rhizosphere (e.g. Palta and Watt 2009). Root and shoot growth are linked as root system growth depends on the assimilates supplied by shoots while the size and activity of the root system determine the rate at which the shoot is supplied with water and nutrients to fix carbon (Manschadi et al. 2013). Vigorous wheat genotypes assimilated up to 60% more carbon than less vigorous genotypes and accumulated more carbon in their root systems (Palta and Watt 2009). The amount of carbon deposed in the soil fluctuates between 10 and 40% depending on plant (Grayston et al. 1996). More recently it was suggested that approximately 20% of total plant carbon allocated below-ground (Iannucci et al. 2021). Exudates have been reported to only represent a small part of the total carbon rhizodeposition (Lambers 1987). Plants with vigorous root growth have previously been suggested to exude less organic carbon to the rhizosphere per root length (Watt et al. 2006). However, others speculated that more vigorous wheat lines containing larger root systems with longer and denser root hairs, may also exude a greater amount of carbon into the rhizosphere, which may include allelochemicals useful for the suppression of competing species (Venturelli et al. 2015; Niculaes et al. 2018; Mwendwa et al. 2021). Previously Wu et al., (2000) suggested that allelopathic interference was not well correlated with other traits related to competitiveness. In this study we show that an increased investment of carbon in longer root systems, we note that the TC-vigour lines appear to maintain the expression and production of key allelopathic metabolites. Therefore, it is unlikely that the introgression of early shoot vigour wheat has adversely affected the allelopathic potential of the HV lines. Our study reported exudation per root weight. While our results suggest no significant differences in allelopathic metabolites, we also showed increased root weight in the TC-vigour lines; also, one might suggest that the overall release of allelopathic molecules in the surrounding of vigorous lines could be greater than in the less vigorous lines.
Conclusions
This study demonstrated that competition between plants grown under controlled environments commences below-ground. Introgression of increased shoot vigour had strong pleiotropic effects on key root traits including total root length and rhizosheath size without negatively affecting exudation of secondary metabolites. The TC-vigour lines displayed up to 50% increased weed suppression and weed tolerance compared with their commercial parents. Genetic improvement of the competitive ability of wheat is potentially a cost-effective tool in integrated weed management as it does not require growers to invest in new equipment or adopt drastically new management practices (Lowry and Smith 2018).
Watt et al. (2013) reported that root traits measured in controlled environments and the field correlate well during the early stages of growth but not at crop maturity. Others found poor correlations between seedling root architecture in glasshouse and field-grown plants (Rich et al. 2020) and that root traits vary even with different types of growth media in controlled environment conditions (Wojciechowski et al. 2009). This highlights the uncertainty associated with genotype-environmental interactions and the need to extend this work to the field. Field trials will avoid some of the pitfalls inherent in small-scale pot and plot experiments (Colbach et al. 2020) and provide a more realistic view of the economic gains that are possible using competitive wheats. Therefore, although challenging to perform, it would be useful to assess wheat growth from seedling stages to maturity under field conditions to evaluate root architectural traits and determine whether the observed differences between early TC-vigour lines and commercial cultivars are maintained under diverse environments.
Data availability
The data supporting the findings of this study are available from the corresponding author, P.-W. Hendriks on reasonable request.
Abbreviations
- BX:
-
Benzoxazinoids
- DIBOA:
-
2,4-dihydroxy-(2H)-1,4-benzoxazin-3(4H)-one
- DIMBOA:
-
2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one
- BOA:
-
benzoxazolin-2-one
- MBOA:
-
6-methoxy-benzoxazolin-2-one)
- DIBOA-glc:
-
2,4-dihydroxy-1,4-benzoxazin-3-one-glucoside
- HMBOA-glc:
-
2-O-Glucosyl-7-methoxy-1,4(2H)-benzoxazin-3-one
- DIMBOA-glc:
-
2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one-glucoside
- DIM2BOA-glc:
-
2,4-dihydroxy-7,8-dimethoxy-1,4-benzoxazin-3-one-glucoside
- HDMBOA-glc:
-
2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one
- APO:
-
2-amino-3H-phenoxazin-3-one
- AAPO:
-
2-acetylamino-3-H-phenoxazin-3- one
- AMPO:
-
2-amino-7-methoxy-3H-phenoxazin-3-one
- AAMPO:
-
2- acetylamino-7-methoxy-3H-phenoxazin-3-one
References
Ahmed MA, Passioura J, Carminati A (2018) Hydraulic processes in roots and the rhizosphere pertinent to increasing yield of water-limited grain crops: a critical review. J Exp Bot 69:3255–3265
Belz RG (2007) Allelopathy in crop/weed interactions — an update, vol 63. Wiley, Chichester, pp 308–326
Bertholdsson NO (2005) Early vigour and allelopathy – two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Res 45:94–102
Bertholdsson NO (2007) Varietal variation in allelopathic activity in wheat and barley and possibilities for use in plant breeding. Allelopathy J 19:193–202
Bertholdsson NO, Andersson SC, Merker A (2012) Allelopathic potential of Triticum spp., Secale spp. and Triticosecale spp. and use of chromosome substitutions and translocations to improve weed suppression ability in winter wheat. Plant Breed 131(1):75–80
Bhaskar AV, Weedon OD, Finckh MR (2019) Exploring the differences between organic and conventional breeding in early vigour traits of winter wheat. Eur J Agron 105:86–95
Bingham IJ (1995) A comparison of the dynamics of root growth and biomass partitioning in wild oat (Avena fatua L.) and spring wheat. Weed Res 35:57–66
Botwright TL, Condon AG, Rebetzke GJ, Richards RA (2002) Field evaluation of early vigour for genetic improvement of grain yield in wheat. Aust J Agric Res 53:1137–1145
Botwright TL, Rebetzke GJ, Condon AG, Richards RA (2005) Influence of the gibberellin-sensitive Rht8 dwarfing gene on leaf epidermal cell dimensions and early vigour in wheat (Triticum aestivum L.). Ann Bot 95:631–639
Brouwer R (1963) Some aspects of the equilibrium between overground and underground plant parts. Mededelingen Intituut voor Biologish en Scheikundig Onderzoek van Landbouwgewassen Wageningen 213:31–39
Colas F, Cordeau S, Granger S, Jeuffroy MH, Pointurier O, Queyrel W, Rodriguez A, Villerd J, Colbach N (2020) Co-development of a decision support system for integrated weed management: contribution from future users. Eur J Agron 114:126010
Colbach N, Munier-Jolain N, Dugué F, Gardarin A, Strbik F, Moreau D (2020) The response of weed and crop species to shading. How to predict their morphology and plasticity from species traits and ecological indexes? Eur J Agron 121:126158
Coleman RK, Gill GS, Rebetzke GJ (2001) Identification of quantitative trait loci for traits conferring weed competitiveness in wheat (Triticum aestivum L.). Aust J Agric Res 52:1235–1246
Cousens R, Barnett A, Barry G (2003a) Dynamics of competition between wheat and oat: I. Effects of changing the timing of phenological events. Agron J 95:1295–1304
Cousens R, Rebetzke G, Barnett A (2003b) Dynamics of competition between wheat and oat: II. Effects of dwarfing genes. Agron J 95:1305–1313
Craine J (2006) Competition for nutrients and optimal root allocation. Int J Plant-Soil Relatsh 285:171–185
Craine JM, Dybzinski R (2013) Mechanisms of plant competition for nutrients, water and light. Funct Ecol 27:833–840
Debaeke P, Perronne R, Colbach N, Moreau D, Barre P, Lecouviour F, Durand-Tardif M (2024) Non-chemical weed management: which crop functions and traits to improve through breeding? Crop Prot 22:106631
Delhaize E, James RA, Ryan PR (2012) Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytol 195:609–619
Délye C, Jasieniuk M, Le Corre V (2013) Deciphering the evolution of herbicide resistance in weeds. Trends Genet 29:649–658
Donald CT (1968) The breeding of crop ideotypes. Euphytica 17:385–403
Duggan BL, Richards RA, Van Herwaarden AF (2005) Agronomic evaluation of a tiller inhibition gene (tin) in wheat. II. Growth and partitioning of assimilate. Aust J Agric Res 56:179–186
Dunbabin V (2007) Simulating the role of rooting traits in crop- weed competition. Field Crop Res 104:44–51
Einhellig FA (1996) Interactions involving allelopathy in cropping systems. Agron J 88(6):886–893
Evers J, Bastiaans L (2016) Quantifying the effect of crop spatial arrangement on weed suppression using functional- structural plant modelling. J Plant Res 129:339–351
Figueroa-Bustos V, Palta JA, Chen Y, Stefanova K, Siddique KHM (2020) Wheat cultivars with contrasting root system size responded differently to terminal drought. Front Plant Sci 11:1285–1285
Finch J, Guillaume G, French S, Davies J, Swarbreck S (2017) Wheat root length and not branching is altered in the presence of neighbours, including blackgrass. PLoS One 12:e0178176
Friedli CN, Abiven S, Fossati D, Hund A (2019) Modern wheat semi-dwarfs root deep on demand: response of rooting depth to drought in a set of Swiss era wheats covering 100 years of breeding. Euphytica 215:85
Gaba S, Perronne R, Fried G, Gardarin A, Bretagnolle F, Biju-Duval L, Colbach N, Cordeau S, Fernández-Aparicio M, Gauvrit C, Gibot-Leclerc S, Guillemin J-P, Moreau D, Munier-Jolain N, Strbik F, Reboud X (2017) Response and effect traits of arable weeds in agro-ecosystems: a review of current knowledge. Weed Res 57:123–147
Gaba S, Caneill J, Nicolardot B, Perronne R, Bretagnolle V (2018) Crop competition in winter wheat has a higher potential than farming practices to regulate weeds. Ecosphere 9:e02413
Gahoonia TS, Care D, Nielsen NE (1997) Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 191:181–188
Gallandt ER, Weiner J (2015) Crop-weed competition. ELs, pp 1–9
Giehl RFH, Gruber BD, von Wirén N (2014) It’s time to make changes: modulation of root system architecture by nutrient signals. J Exp Bot 65:769–778
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudations and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Haynes W (2013) Benjamini–Hochberg method. In: Encyclopedia of systems biology, p 78
Heap I (2014) Herbicide resistant weeds. In: Integrated pest management. Springer, Netherlands, Dordrecht, pp 281–301
Heap I (2018) The international survey of herbicide resistant weeds, vol 2019. www.weedscience.org. (Accessed 14/07/2022)
Hendriks P-W, Gurusinghe S, Ryan PR, Rebetzke GJ, Weston LA (2022a) Competitiveness of early vigour wheat (Triticum aestivum L.) genotypes is established at early growth stages. Agronomy (Basel) 12:377
Hendriks P-W, Ryan PR, Hands P, Rolland V, Gurusinghe S, Weston LA, Rebetzke GJ, Delhaize E (2022b) Selection for early shoot vigour in wheat increases root hair length but reduces epidermal cell size of roots and leaves. J Exp Bot 73(8):2499–2510
Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH (1998) Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytologist 139:479–494
Hussain MI, Araniti F, Schulz M, Baerson S, Vieites-Álvarez Y, Rempelos L, Bilsborrow P, Durán AG, Salcedo NC, Macías FA, Weston LA, Reigosa MJ, Sánchez-Moreiras AM (2022) Benzoxazinoids in wheat allelopathy – from discovery to application for sustainable weed management. Environ Exp Bot 204:105096
Iannucci A, Canfora L, Nigro F, De Vita P, Beleggia R (2021) Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. In: Applied soil ecology: a section of agriculture, ecosystems & environment, p 158
Inderjit R, Moral R (1997) Is separating resource competition from allelopathy realistic? Bot Rev 63:221–230
James RA, Weligama C, Verbyla K, Ryan PR, Rebetzke GJ, Rattey A, Richardson AE, Delhaize E (2016) Rhizosheaths on wheat grown in acid soils: phosphorus acquisition efficiency and genetic control. J Exp Bot 67:3709–3718
Jordan N (1993) Prospects for weed control through crop interference. Ecol Appl 3:84–91
Jungk A (2001) Root hairs and the acquisition of plant nutrients from soil. J Plant Nutr Soil Sci 164:121–129
Kaur S, Kaur R, Chauhan BS (2018) Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop Prot 103:65–72
Kiær LP, Weisbach AN, Weiner J (2013) Root and shoot competition: a meta-analysis. J Ecol 101:1298–1312
Kumar P, Gagliardo RW, Chilton WS (1993) Soil transformation of wheat and corn metabolites MBOA and DIM2BOA into aminophenoxazinones. J Chem Ecol 19:2453–2461
Lambers H (1987) Growth, respiration, exudation and symbiotic associations: the fate of carbon translocated to the roots. In: Gregory PJ, Lake JV, Rose DA (eds) Root development and function. Cambridge University Press, Cambridge, pp 125–145
Lemerle D, Verbeek B, Cousens RD, Coombes NE (1996) The potential for selecting wheat varieties strongly competitive against weeds. Weed Res 36:505–513
Lemerle D, Gill G, Murphy CE, Walker R, Cousens S, Mokhtari S, Peltzer D (2001a) Genetic improvement and agronomy for enhanced wheat competitiveness with weeds. Aust J Agric Res 52:527–527
Lemerle D, Verbeek B, Orchard B (2001b) Ranking the ability of wheat varieties to compete with Lolium rigidum. Weed Res 41:197–209
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2021) emmeans: Estimated marginal means, aka least-squares means [Computer software]. The Comprehensive R Archive Network. Available online at: https://CRAN.R-project.org/package=emmeans
Liao MT, Fillery IRP, Palta JA (2004) Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Funct Plant Biol 31:121–129
Liao M, Palta JA, Fillery IRP (2006) Root characteristics of vigorous wheat improve early nitrogen uptake. Aust J Agric Res 57:1097–1107
Lilley JM, Kirkegaard JA (2011) Benefits of increased soil exploration by wheat roots. Field Crop Res 122:118–130
Liu JG, Mahoney KJ, Sikkema PH, Swanton CJ (2009) The importance of light quality in crop-weed competition. Weed Res 49:217–224
Lowry CJ, Smith RG (2018) Weed control through crop plant manipulation. In: Jabran K, Chauhan BS (eds) Non chemical weed control. Elsevier Science, Amsterdam, pp 73–96
Lynch JP (2018) Rightsizing root phenotypes for drought resistance. J Exp Bot 69:3279–3292
Lynch JP, Strock CF, Schneider HM, Sidhu JS, Ajmera I, Galindo-Castañeda T, Klein SP, Hanlon MT (2021) Root anatomy and soil resource capture. Plant Soil 466:21–63
Mahé I, Chauvel B, Colbach N, Cordeau S, Gfeller A, Reiss A, Moreau D (2022) Deciphering field-based evidences for crop allelopathy in weed regulation. A review. Agron Sustain Dev 42(3):50
Manschadi A, Christopher J, Devoil P, Hammer G (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33:823–837
Manschadi AM, Manske GGB, Vlek PLG (2013) Root architecture and resource acquisition: wheat as a model plant. In: Plant roots: the hidden half, pp 1–22
Maydup ML, Graciano C, Guiamet JJ, Tambussi EA (2012) Analysis of early vigour in twenty modern cultivars of bread wheat (Triticum aestivum L.). Crop Pasture Sci 63:987–996
Morris NL, Miller PCH, Jhorson F-WRJ (2010) The adoption of non-inversion tillage systems in the United Kingdom and the agronomic impact on soil, crops and the environment—a review. Soil Tillage Res 108:1–15
Mwendwa JM, Brown WB, Haque S, Heath G, Wu H, Quinn JC, Weidenhamer JD, Weston LA (2016) Field evaluation of Australian wheat genotypes for competitive traits and weed suppression. In: Randall R, Lloyd S, Borger C (eds) . Weeds Society of Western Australia, Perth, pp 48–53
Mwendwa JM, Weston PA, Weidenhamer JD, Fomsgaard IS, Wu H, Gurusinghe S, Weston LA (2021) Metabolic profiling of benzoxazinoids in the roots and rhizosphere of commercial winter wheat genotypes. Plant Soil 466:467–489
Niculaes C, Abramov A, Hannemann L, Frey M (2018) Plant protection by benzoxazinoids—recent insights into biosynthesis and function. Agronomy 8:143
Niemeyer HM (1988) Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the gramineae. Phytochemistry 27:3349–3358
Oerke EC (2006) Crop losses to pests. J Agric Sci 144:31–43
Page ER, Tollenaar M, Lee EA, Lukens L, Swanton CJ (2010) Shade avoidance: an integral component of crop-weed competition. Weed Res 50:281–288
Palta J, Watt M (2009) Vigorous crop root systems: form and function for improving the capture of water and nutrients. In: Applied crop physiology: boundaries between genetic improvement and agronomy. Academic, San Diego, pp 309–325
Palta J, Yang J (2014) Crop root system behaviour and yield preface. Field Crop Research 165:1–4
Palta JA, Fillery IRP, Rebetzke GJ (2007) Restricted-tillering wheat does not lead to greater investment in roots and early nitrogen uptake. Field Crop Res 104:52–59
Palta J, Chen X, Milroy S, Rebetzke G, Dreccer M, Watt M (2011) Large root systems: are they useful in adapting wheat to dry environments? Funct Plant Biol 38:347–354
Passioura JB (1983) Roots and drought resistance. Agric Water Manag 7:265–280
Petit S, Cordeau S, Chauvel B, Bohan D, Guillemin J-P, Steinberg C (2018) Biodiversity-based options for arable weed management. A review Agronomy for Sustainable Development 38:1–21
Pratley JE (1996) Allelopathy in annual grasses. Plant Protection Quarterly 11:213–214
Qin X, Niklas KJ, Qi L, Xiong Y, Li F (2012) The effects of domestication on the scaling of below- vs. aboveground biomass in four selected wheat (Triticum; Poaceae) genotypes. Am J Bot 99:1112–1117
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rebetzke GJ, Richards RA (1999) Genetic improvement of early vigour in wheat. Aust J Agric Res 50:291–301
Rebetzke GJ, Botwright TL, Moore CS, Richards RA, Condon AG (2004) Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat. Field Crop Res 88:179–189
Rebetzke G, Condon A, Richards R (2008) Inheritance of coleoptile tiller appearance and size in wheat. Aust J Agric Res 59:863–873
Rebetzke G, Ingvordsen C, Newman P, Weston LA, French B, Gill G (2018) Delivering weed-competitive wheat breeding lines to growers. GRDC grains research update. GRDC, Wagga Wagga, pp 35–40
Reiss A, Fomsgaard I, Mathiassen S, Stuart R, Kudsk P (2018) Weed suppression by winter cereals: relative contribution of competition for resources and allelopathy. Evolution and Mechanisms of the Chemical Base of Ecological Interactions 28:109–121
Reynolds MP, Acevedo E, Sayre KD, Fischer RA (1994) Yield potential in modern wheat varieties: its association with a less competitive ideotype. Field Crop Res 37:149–160
Rich SM, Christopher J, Richards R, Watt M (2020) Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. J Exp Bot 71(16):4751–4762
Richard C, Christopher J, Chenu K, Borrell A, Christopher M, Hickey L (2018) Selection in early generations to shift allele frequency for seminal root angle in wheat. Plant Genome 11
Richards MC (1989) Crop competitiveness as an aid to weed control, Crop Protection Conf Weeds, Brighton, The British Crop Protection Council, Farnham, pp. 573—578
Richards R, Watt M, Rebetzke G (2007) Physiological traits and cereal germplasm for sustainable agricultural systems. International Journal of Plant Breeding 154:409–425
Rogers ED, Benfey PN (2015) Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol 32:93–98
Ryan PR, Liao M, Delhaize E, Rebetzke GJ, Weligama C, Spielmeyer W, James RA (2015) Early vigour improves phosphate uptake in wheat. J Exp Bot 66:7089–7100
Schandry N, Becker C (2019) Allelopathic plants: models for studying plant–Interkingdom interactions. Trends Plant Sci 25(2):176–185
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Soane BD, Ball BC, Arvidsson J, Basch G, Moreno F, Roger-Estrade J (2012) No-till in northern, western and South-Western Europe: a review of problems and opportunities for crop production and the environment. Soil Tillage Res 118:66–87
Stoate C, Báldi A, Beja P, Boatman ND, Herzon I, van Doorn A, de Snoo GR, Rakosy L, Ramwell C (2009) Ecological impacts of early 21st century agricultural change in Europe – a review. J Environ Manag 91:22–46
Sue M, Nakamura C, Nomura T (2011) Dispersed benzoxazinone gene cluster: molecular characterization and chromosomal localization of glucosyltransferase and glucosidase genes in wheat and rye. Plant Physiol 157:985–997
Thijs H, Shann JR, Weidenhamer JD (1994) The Effect of phytotoxins on competitive outcome in a model system. Ecology 75:1959–1964
Van Den Boogaard R, Goubitz S, Veneklaas EJ, Lambers H (1996) Carbon and nitrogen economy of four Triticum aestivum cultivars differing in relative growth rate and water use efficiency. Plant, Cell and Environment 19:998–1004
Van Noordwijk M, de Willigen P (1987) Agricultural concepts of roots : from morphogentic to functional equilibrium between root and shoot growth. Neth J Agric Sci 35:487–496
Vandeleur RK, Gill GS (2004) The impact of plant breeding on the grain yield and competitive ability of wheat in Australia. Crop and Pasture Science 55(8):855–861
Venturelli S, Belz RG, Kämper A, Berger A, von Horn K, Wegner A, Böcker A, Zabulon G, Langenecker T, Kohlbacher O, Barneche F, Weigel D, Lauer UM, Bitzer M, Becker C (2015) Plants release precursors of histone deacetylase inhibitors to suppress growth of competitors. Plant Cell 27:3175–3189
Wasson A, Richards R, Chatrath R, Misra S, Prasad S, Saxena DC, Rebetzke G, Kirkegaard J, Christopher JT, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Oxford University Press, Oxford
Watt M, Kirkegaard JA, Rebetzke GJ (2005) A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Funct Plant Biol 32:695–706
Watt M, Silk WK, Passioura JB (2006) Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Ann Bot 97:839–855
Watt M, Moosavi S, Cunningham SC, Kirkegaard JA, Rebetzke GJ, Richards RA (2013) A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann Bot 112(2):447–455
Weaver SE, Kropff MJ, Groeneveld RMW (1992) Use of ecophysiological models for crop-weed interference: the critical period of weed interference. Weed Sci 40:302–307
Wezel A, Casagrande M, Celette F, Vian J-F, Ferrer A, Peigné J (2013) Agroecological practices for sustainable agriculture. A review. Agron Sustain Dev 34:1–20
White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot 112:207–222
Wilson J (1988) Shoot competition and root competition. J Appl Ecol 25:279–296
Wilson PB, Rebetzke GJ, Condon AG (2015) Of growing importance: combining greater early vigour and transpiration efficiency for wheat in Vari- able rainfed environments. Funct Plant Biol 42:1107–1115
Wojciechowski T, Gooding MJ, Ramsay L, Gregory P (2009) The effects of dwarfing genes on seedling root growth of wheat. J Exp Bot 60(9):2565–2573
Wu H, Pratley J, Lemerle D, Haig T (2000) Laboratory screening for allelopathic potential of wheat (Triticum aestivum) accessions against annual ryegrass (Lolium rigidum). Aust J Agric Res 51:259–266
Youssefian S, Kirby EJM, Gale MD (1992) Pleiotropic effects of the GA-insensitive Rht dwarfing genes in wheat. 2. Effects on leaf, stem, ear and floret growth. Field Crop Res 28:191–210
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zerner M, Gill G, Vandeleur R (2008) Effect of height on the competitive ability of wheat with oats. Agron J 100:1729–1734
Zerner MC, Rebetzke GJ, Gill GS (2016) Genotypic stability of weed competitive ability for bread wheat () genotypes in multiple environments. Crop and Pasture Science 67:695–702
Zhang L, Richards RA, Condon AG, Liu DC, Rebetzke GJ (2015) Recurrent selection for wider seedling leaves increases early biomass and leaf area in wheat (Triticum aestivum L.). J Exp Bot 66:1215–1226
Zimdahl RL (2004) Weed-crop competition a review. Blackwell Pub, Oxford
Zimdahl RL (2018) Fundamentals of weed science. Academic Press, an imprint of Elsevier, London
Acknowledgements
We thank the APPF Postgraduate Internship Awards Scholarship allowed access to the growth capsules and Dr. Richard Poiré for his assistance. P-WH thanks Charles Sturt University for his postgraduate research scholarship, and the Tim Healey Memorial Scholarship through the AE Howard Memorial Trust.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research is supported in part through the GRDC project, CSP00182, ‘Breeding weed competitive wheat’, and US00084, ‘New technologies for weed management in the Northern Region’. P-WH was supported by a CSU and CSIRO postgraduate research scholarship, the AE Howard Memorial Trust through the Tim Healey Memorial Scholarship and an APPF Postgraduate Internship Awards Scholarship.
Author information
Authors and Affiliations
Contributions
P-WH, MD, VR, PH, SG, and PR: design and coordination; P-WH: conducting experiments; P-WH, PW and PR: data collection; P-WH and PW data analysis; LAW and GJR: supervision; P-WH, PR, PW and MD: writing with reviewing and/or comments by all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Additional information
Responsible Editor: Gwendolyn Kristin Kirschner.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 482 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hendriks, PW., Gurusinghe, S., Weston, P.A. et al. Introgression of early shoot vigour in wheat modifies root systems, increases competitiveness and provides options for integrated weed management. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06653-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06653-3