Abstract
Background and aims
Germination as a function of soil water potential (h) is modelled using polyethylene glycol (PEG). But, PEG would not consider soil properties. Our objective is to show the limitations of PEG to model germination in real soils. Using a new device, the tension germinator (TG), we show the interaction between soil type, h and seed characteristics on seed germination.
Methods
TG (A Mariotte reservoir that supplies water at constant h to a porous substrate on which seeds are deposited). Barley (Hordeum vulgare L.) and vetch (Vicia sativa L.) seeds were placed on TG with loam (TG-loam) and sand (TG-sand), and h of 0, -0.002, -0.006 MPa. Then, the imbibition curves were monitored. PEG experiments (0 to -2.5 MPa) were performed to estimate the critical h, hPEG, or h from which the imbibition curve decreases compared with that at 0 MPa. PEG curves for 0 > h > -0.01 MPa were compared with TG.
Results
PEG and TG curves were not different at 0 MPa. hPEG for barley and vetch was within [0, -0.01] MPa. While no differences were observed between PEG at [0, -0.01] MPa and TG-loam curves, TG-sand curves at -0.002 and -0.006 MPa were different to those of PEG. Conversely to PEG at -0.01 MPa, no imbibition was observed in TG-sand (-0.006 MPa). A negligible influence of h in TG-loam was observed.
Conclusions
PEG is not adequate to describe seed germination in soil. But the TG allows monitoring seed germination in real soils and controlled h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed germination acts as a key step for the establishment of plant species in ecosystems in natural habitats (Donohue et al. 2010; Huang et al. 2016), in weed populations in agricultural land and is also crucial to guarantee a homogeneous crop establishment. In ecology, the correct characterization of germination requirements is of paramount importance for understanding community assembly and, as a consequence, also for successful ecosystem restoration (Jimenez-Alfaro et al. 2016).
The process of germination can be separated into three phases (da Silva et al. 2018). Phase I (or seed imbibition): physical process in which soil water enters the seed due to a difference in water potential (h) between the seed and the surrounding medium (Hegarty 1978). During this phase there is an increase in seed weight. Phase II: seed weight reaches a threshold and then the rate of respiration and other metabolic processes increase. A successful Phase I does not necessarily imply the beginning of Phase II, since this latter also depends on the dormancy of the seed, oxygen availability in the soil and temperature. Phase III: there is an increase in water absorption, and respiratory activity and seed elongation and division leading to radicle grow. During this phase, that lasts until radicle emergence occurs, seed weight increases again. While in the first two phases the processes are reversible, seeds that reach the third phase cannot return to previous stages, and in the case of unfavorable environmental conditions for the progression of this phase, the seed dies (da Silva et al. 2018).
For decades, studies on germination as a function of soil moisture have been carried out in experiments in which seeds imbibe at different water potentials and temperatures (Sharma 1973; Liu et al. 2020). To study the influence of h on germination, polyethylene glycol (PEG) aqueous solutions are used to generate different levels of h. These solutions generate an osmotic potential, which is assumed to properly simulate the soil water potential (Sharma 1973). This technique has been employed, for instance, to study seed after-ripening (Christensen et al. 1998), drought tolerance of seed germination (Macar 2009; Jiang and Zhou 2022), to create hydrotime models (Patané and Tringali 2010) and in general studies related to seed germination ecology (Krichen et al. 2017).
Thus, PEG experiments allow determining the minimum h below which seeds can’t imbibe (Frischie et al. 2019). However, since seed imbibition is the result of the interaction between seed and the surrounding medium, it should depend not only on the seed characteristics that determine their ability to absorb water but also on the properties of the surrounding substrate, which regulates the supply of water to the seeds (Williams and Shaykewich 1971; Hadas and Russo 1974; Camacho et al. 2021). Indeed, it is hypothesized that a higher contact between seeds and soil particles even favors seed survival (Terpstra 1990; Reuss et al. 2001).
When PEG experiments show that seeds are able to germinate at h of -1.82 or even -2 MPa (i.e. Dürr et al. 2015; Frischie et al. 2019), it is usually interpreted that seeds may germinate at any h value between 0 MPa and these most negative h threshold, regardless of the medium where the seeds are placed. However, if we assume that, for most soils, the water content at the most negative h threshold for seed imbibition corresponds to the residual water content (van Genuchten 1980; Carsel and Parrish 1988), seed imbibition is not possible at these limits, simply because there is no biologically available water in the soil. This simple example demonstrates that PEG experiments report information about the most negative h threshold from which seeds stop imbibing, but not about the ability of soils to supply water to seeds. Thus, to study seed imbibition processes in field conditions, both seed characteristics and soil hydraulic properties must be considered. Ignoring the role of the soil hydraulic properties in the germination process can lead to erroneous conclusions. Among the very few studies carried out to quantify the influence of the surrounding medium on seed imbibition, Williams and Shaykewich (1971) demonstrated that the hydraulic conductivity of the soil was a limiting factor in the germination process, and Hadas and Russo (1974) observed that decreasing hydraulic conductivity reduced water absorption and germination rates. More recently, Camacho et al. (2021) showed that hydraulic conductivity, rather than h, was a more informative variable to predict seed germination, and advised that caution must be taken when considering results obtained using PEG solutions to infer germination behavior under field conditions. In another work, Moret-Fernández et al. (2023) presented a new methodology to determine the hydraulic properties of seeds and demonstrated that the hydraulic properties of the seeds varied among different species. Although all these studies provide valuable information on the importance of soil hydraulic conductivity on seed germination, they do not provide a clear picture about the relationship between seed germination and soil water. To our knowledge, to date there is no work that has experimentally demonstrated how soil properties combined with h can affect the seed imbibition process.
To fill this gap, this paper experimentally evaluates the importance of the interaction between soil type, h and seed characteristics on seed imbibition. In order to achieve our goal, a new device, the tension germinator (TG), that allows monitoring weight gain due to imbibition of seeds placed on a substrate at controlled h, is presented.
Materials and methods
Tension germinator design
The tension germinator, TG, is based on the upward infiltration method at constant soil tension developed to estimate the soil hydraulic properties (Moret-Fernández et al. 2016) and the double disc method used to determine the soil hydraulic properties from drainage experiments with tension gradients (Moret-Fernández and Latorre 2022). These devices have so far been used to study the soil hydraulic properties but not yet for seed germination experiments. The TG consists of a 10 cm diameter aluminum receptacle containing the soil where tension is controlled and maintained (Fig. 1). The receptacle has a 5 cm diameter perforated base, the surface of which is covered with a dry nylon mesh of 10 μm pore size, that can hold suctions up to -0.008 MPa. The mesh is hermetically sealed against the aluminum receptacle with an aluminum ring. A 2.0 cm-high stainless-steel cylinder (5 cm internal diameter -i.d.-) is placed on the nylon mesh. This cylinder contains the soil in which the seeds will be placed. The bottom of the aluminum receptacle is connected to a Mariotte tube 30 cm-high and 2.5 cm i.d. that supplies water to the soil. An open/closed valve (Valve 1) is placed in the tube connecting the Mariotte tube and the aluminum receptacle. The Mariotte tube has a movable pipe, the lower end of which is at the same height as the nylon mesh. This implies that the same pressure is found at the lower end of the pipe and at the mesh surface. At the top of the Mariotte tube there is a syringe that allows generating vacuum, if necessary. The upper end of the movable tube of the Mariotte tube is connected to a bubbler tower and a water tank. The bubbling tower consists of a 70 cm-high and 2.5 cm i.d. tube filled with water and crossed longitudinally by a movable pipe. This bubbling tower is responsible for setting the desired tension below the nylon mesh, corresponding to the length of the pipe immersed in water (60 cm or 0.006 MPa in Fig. 1). The water tank consists of a 40 cm-high and 5 cm i.d. reservoir filled of water. This is a second Mariotte tube connected at the base to a 1 m long pipe, in which a flow regulating valve (Valve 2) is inserted. A third valve (Valve 3) to depressurize the system, if necessary, is placed at the top of the water tank. Finally, a water column manometer, to display the current tension of the system, is connected to the top of the water tank.
The water falling through the pipe inserted in the base of the water tank generates a suction inside the reservoir equal to the difference in height between the lower end of the pipe and the air inlet into the water tank (> 60 cm in Fig. 1). To allow for this water drop, the air inlet into the water tank comes from the bubbling tower. To set the desired tension, the length of the pipe submerged in the bubbling tower must be slightly shorter (60 cm in Fig. 1) that the distance between the bottom end of the pipe falling from the water tank and the air inlet height in the water tank. Finally, the suction generated inside the water tank is transmitted to the Mariotte tube and from there to the nylon mesh and, finally, to the soil.
Tension germinator setup
Before starting up the tension germinator, the entire air circuit must be empty of water droplets and the system must be depressurized. The latter is achieved by opening Valve 3, which must be kept open during the next two steps. Valve 2 is then closed and the water tank is filled with water. Next, the bubbling tower is also filled with water and the internal mobile pipe is immersed to the desired h (60 cm in Fig. 1). After closing Valve 1, the Mariotte tube is filled with water and the lower end of the immersed pipe is placed at the same level as the nylon mesh. Once all these steps are completed, Valve 3 is closed to allow vacuum in the system and Valve 1 is opened to allow water to flow into the aluminum receptacle. At this point, a mixture of water and air bubbles appears under the saturated nylon mesh. Air bubbles are removed from under the mesh by suctioning them out with a syringe. Once the nylon mesh is free of air bubbles, a vacuum is generated in the system by sucking air through the syringe located at the top of the Mariotte tube. The system will be at the desired tension when the bubbling tower begins to bubble. At this point, the difference in height at the water column manometer should be equal to the length of the pipe submerged in the bubbling tower (60 cm in Fig. 1). It must be ensured that no air enters the aluminum receptacle through the nylon mesh. If air inlets are observed, the nylon mesh should be replaced probably due to the existence of small unwanted holes. Once vacuum has been generated into the system, Valve 2 is opened and the difference in height between the lower ends of the external and internal pipes of the water tank is equaled to the length of pipe immersed in the bubbling tower. At this point, a slight drip through the outer tube of the water tank should be ensured by adjusting the opening of Valve 2.
The installation of a continuous vacuum generation system is needed because small misalignments in the system can cause undesirable vacuum leaks in the piping circuits and tanks. A similar process is applied, for instance, in the pressure plates used in soil physics, where a flow of air is constantly introduced inside the plates. Thus, if we want to guarantee a constant tension under the nylon mesh, a constant vacuum must be generated inside the circuit.
Once the system is at the desired tension, a layer of dry substrate is placed on the nylon mesh. At this point, bubbling will appear in the Mariotte tube, until the tension of the wet soil is in equilibrium with that inside the germinator circuit. Finally, the seeds can be placed on the soil layer. More details about seeds placement are described in “TG experiments” section.
Soil hydraulic model
The water retention, \(\theta \left(h\right)\) (Eq. 1) and unsaturated hydraulic conductivity, \(K\left(h\right)\) (Eq. 2) functions used to characterize the substrates were those described by van Genuchten (1980) and Mualem (1976)
where h is the matric potential [L] and θ is the volumetric water content [L3 L−3], θs and θr are the saturated and residual volumetric water content, Ks is the saturated hydraulic conductivity [L T−1], α [L−1] and n [−] are a scale and shape parameter, respectively, and m = 1 − 1/n. According to van Genuchten (1980), θr is defined as the water content for which the gradient 𝑑𝜃/dℎ becomes zero.
The one-dimensional water flux, Q [L T-1], between two points inside a porous material is defined by the Darcy’s law for unsaturated media (Hillel 2012), which can be expressed as
where l [L] and Δh are the distance and the water tension gradient between two points inside a porous media, respectively.
Seed imbibition experiments with TG and PEG
Two different germination experiments were performed, one using polyethylene glycol (PEG) solutions and the other using the TG device. For both experiments, we used seeds of two species: barley (Hordeum vulgare L.) and vetch (Vicia sativa L.). The selection of these species was based on several criteria: (1) their ready commercial availability, (2) a high germination rate with rapid germination and absence of dormancy, (3) distinct morphological characteristics, specifically being a monocotyledonous (barley) and a dicotyledonous (vetch). Moreover, vetch is a non-endospermic seed with a well-developed embryo, characterized by a larger size compared to barley. Conversely, barley exhibits a less developed embryo, possesses an endosperm, and is smaller in size compared to vetch. (4) These species were previously used in experiments conducted by the research team; hence we are familiar with them. (5) Additionally, their imbibition curves differ, with a gradual slope for barley and a more pronounced, steep slope for vetch (Moret-Fernández et al. 2023). Volume, bulk densities and dry weight of the two seed species are shown in Table 1. More details about calculation of these seed parameters can be found in Moret-Fernández et al. (2023).
PEG experiments
PEG experiments consisted of measuring the cumulative seed imbibition curve, calculated as the average weight (W) gain of a set of seeds at seven different levels of h (0, -0.001, -0.01, -0.1, -0.5, -1.5 and -2.5 MPa). These values correspond to the standard h used by Moret-Fernández et al. (2023) to estimate the hydraulic properties of seeds. In addition, these same PEG experiments were used to estimate the water potential threshold, hPEG, or h from which the imbibition curve decreases compared with that at h = 0 MPa. In a second step, and in order to simplify further analysis, 0, -0.001, -0.01, -1.5 and -2.5 MPa water potentials were used to compare PEG imbibition curves with those obtained with TG.
The h values were simulated using polyethylene glycol solutions (PEG 6000, Polyethylene glycol 6,000, Sigma-Aldrich; MDL Number: MFCD01779614), where the concentration was calculated using standard equations (Michel and Kaufmann 1973). Distilled water was used as control. Ten seeds of each species were used at each PEG concentration. Three replicates were considered per water potential and species. Seed viability range was 95–98% and 95% for barley and vetch, respectively. Air-dried seed lots were first weighted to obtain the initial weight of seeds. These seeds were then placed in a 9 cm diameter Petri dish containing a filter paper on which a volume of 8 ml distilled water or PEG solution was applied. Petri dishes were placed in a controlled temperature chamber, in the dark, at 18 ºC during 5–7 days. All seeds were weighted three and two times per day, for the first and remaining days, respectively. To this purpose, the seeds were extracted from Petri dishes, dried with a dry filter paper, weighted and placed back into the Petri dishes, which were returned to the chamber. At the end of each day, the Petri dishes plus the wet seeds were weighted and the loss of water, due to the extraction of the seeds to be weighted, was replaced with distilled water or the corresponding PEG solution. The volume of water or PEG solution to be added corresponded to the difference between the first weighing of Petri dishes plus seeds and the successive measurements. Water loss by evaporation was considered negligible due to the short duration of the experiments and the fact the Petri dishes were at saturated atmospheric conditions. The experiment lasted until a constant seed weight or the emergence of the radicle (Bewley et al. 2013) was observed in 80% of the seeds placed in the control treatment. At the end of the experiment, the percentage of germinated seeds (defined by seeds with a 0.5 mm emerged radicle) was also assessed. In summary, a set of three average cumulative seed imbibition curves was obtained for each species.
TG experiments
Within the TG experiments, a calibrated sand (TG-sand), and 1-mm sieved loam soil (TG-loam), were used as substrates. The grain size of the sand was 80–160 μm. Van Genuchten (1980) parameters of the water retention curves and saturated hydraulic conductivity of the two substrates were measured according to the Moret-Fernández et al. (2021) procedure. Hydraulic parameters, textural characteristics and organic carbon content of the two substrates are shown in Table 2. To setup the TG experiments, the stainless-steel cylinders were filled with the corresponding substrates, and the system was left unaltered until the matric potential of the substrate was balanced with that on the nylon mesh (see “Tension germinator setup” section). Three different h were applied: 0, -0.002 and -0.006 MPa. Based on previous experiments, the h used in the TG were selected because, (i) they are within the range of h values allowed by the TG nylon membrane, and (ii) it is a range of h within which the sand exhibits the highest and practically zero hydraulic conductivity. Once the tension in the substrate was equilibrated, a set of 10 air-dried seeds was weighted and placed on the substrate. The seeds were buried until the top of the seeds was level with the surface of the substrate. Next, a small amount of soil was sprinkled over the remaining surface of the seed, to create a thin layer of substrate over the seed. This procedure allowed the seed to remain covered and in contact with the substrate, while allowing the seed to be easily located for subsequent weighing. This process was repeated each time the seed was removed for weighting and placed back in the TG. The experiment lasted until a constant seed weight or the emergence of the radicle was observed in 80% of the seeds placed in the control treatment. At the end of the experiment, the percentage of germinated seeds was calculated. Three replications per species, substrate and soil tension were done. This means a total of 36 measurements with the tension germinator. At the end of the experiment, three average cumulative seed imbibition curves were obtained per species and substrate.
Statistical analysis
Within each seed species, hPEG was calculated using the Se variable, which was defined as the slope of the linear regressions between the PEG imbibition curve measured at 0 MPa and the corresponding curves measured at -0.001, -0.01, -0.1, -0.5, -1.5 and -2.5 MPa, respectively. The relationship between the PEG water potentials and the corresponding Se values was fitted to the dimensionless form of Eq. (1), which is obtained for θs equal to one and θr equal to zero. The optimized function, S, allowed obtaining α and n parameters, from which \({h}_{PEG}= \frac{1}{\alpha }\). This optimization process was performed with the R version 4.3.1 software (Project for Statistical Computing), using a nonlinear (weighted) least-square method that incorporates the Levenberg-Marquardt optimization algorithm.
To compare the imbibition curves of vetch and barley at different tensions in the PEG and TG experiments we compared the values of seed weight at each sampling point using ANOVA analysis.
To this end, the R version 4.3.1 (Project for Statistical Computing) was used.
Results
Different shapes of \(K\left(h\right)\) and \(\theta \left(h\right)\) functions calculated according to Eqs. 1 and 2 and input data of Table 2 were obtained for the sand and loam soil (Fig. 2). While the water content of sand at -60 cm (-0.006 MPa) of matric potential was close to the residual water content, θr (Eq. 1), the corresponding water content in the loam soil was much higher (Fig. 2a). In contrast, while Ks of sand was seven times larger than that for a loam soil, the unsaturated hydraulic conductivity, K(h), of sand at -60 cm of soil tension was two orders of magnitude smaller than the corresponding K(h) for the loam soil (Fig. 2b). These figures show that the hydraulic behavior of sand, with an extremely small K(h) at -60 cm, is different from that of a loam soil.
Figure 3 shows the average imbibition curves measured in barley and vetch seeds wetted in the PEG experiments at 0, -0.001, -0.01, -0.5 and -2.5 MPa water potentials and in TG-loam and TG-sand at 0, -0.002 and -0.006 MPa soil tensions. Overall, different shapes of cumulative imbibition curves were observed between barley and vetch in both types of experiment (PEG and TG). While the long-time cumulative imbibition curve measured in barley increased at an almost constant rate, the corresponding trend in vetch showed a steep increase during the first 24 h of seed imbibition followed by a general flattening.
Average cumulative imbibition curves measured in barley and vetch seeds immersed in PEG solutions at 0, -0.001, -0.01, -0.5 and -2.5 MPa, and curves measured in the tension germinator, TG, with loam soil and sand at 0, -0.002 and -0.006 MPa water potential. * denotes significant differences (p < 0.05) among seed weights within each sampling time
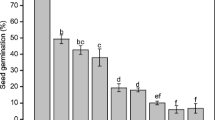
Comparison between experimental Se and the corresponding S function measured from the PEG imbibition curves (Fig. 4) showed that in both species the hPEG value, which represents the water potential at which the imbibition curve starts to be significantly different from that at 0 MPa, was -0.097 and -0.156 MPa for barley and vetch, respectively. It means that within both seed species, the Se values remained constant for 0 > h > -0.01 MPa (Fig. 4). These results were supported by the absence of differences within each sampling time between the PEG imbibition curves measured at 0, -0.001 and -0.01 MPa, and by a similar percentage of germination within the range [0, -0.01] MPa (Table 3). Thus, the results showed that between 0 and -0.01 MPa, the imbibition curves measured in PEG experiments were not affected by the water potential. In contrast, for h < -0.01 MPa, a significant influence of PEG solution on seed imbibition was observed, with decreasing slopes of cumulative imbibition curves (Fig. 3) and germination rates (Table 3) as water potential decreases. In both seed species, the time needed for germination tended to increase with lower osmotic potentials in PEG solutions (Table 3), e.g. for vetch, potentials lower than -0.5 MPa prevented germination in the PEG experiments.
Experimental data (Se, represented by dots) and best fits (S functions represented by solid lines) for the relationship between water potentials and the slopes of the linear regressions between PEG imbibition curves measured at 0 MPa and the corresponding imbibition curves measured for -0.001, 0.01, -1, -0.5, -1.5 and -2.5 MPa, calculated for barley and vetch seeds. hPEG (represented by dotted lines) is the water potential threshold at which the imbibition curve starts to be significantly different from that at 0 MPa
Overall, within TG-loam experiments, the water potentials between 0 and -0.006 MPa did not have any significant effect on the imbibition curves (except for vetch on TG loam 5 h) (Fig. 3), where all curves measured in barley and vetch almost overlapped each other. In contrast, a very different behavior was observed in TG-sand, where the average seed weight measured in barley and vetch at the different sampling times and the three different water potentials were significantly different from each other. Thus, while a null imbibition curve was observed in barley and vetch at -0.006 MPa, the imbibition curve at -0.002 MPa was midway between 0 and -0.006 MPa. In both species, the highest percentage of germination corresponded with h = -0.002 MPa (Table 3).
The significant linear relationship, with a slope value close to one, for the comparison between seed weights measured in both species and sampling times with PEG at 0 MPa and the corresponding TG-loam and TG-sand also at 0 MPa, indicates quasi-equal seed imbibition curves was obtained in both PEG and TG at 0 MPa (Fig. 5).
Discussion
Since seed imbibition is a passive process that depends on the relationship between internal water potential of the seed and that of the surrounding medium (Hegarty 1978), the imbibition rate of a dry seed decreases with the increase of the concentration of PEG solutions (Bewley et al. 2013; Vertucci 1989). However, according to Fig. 4, under the absence of water supply restrictions between the seed and the surrounding medium, the imbibition process in PEG between 0 and -0.01 MPa was not affected by the h of the germination medium. In addition, the significant relationship, with a slope value equal to one, between the barley and vetch seed weights measured with PEG at 0 MPa and the corresponding values measured with TG-loam and TG-sand at 0 MPa (Fig. 5) indicates that seeds exhibit similar imbibition curves in the absence of water supply restrictions, and hence the imbibition process at 0 MPa is independent of the media where the seeds are placed.
Although PEG imbibition curves at -0.002 and -0.006 MPa are not available, these curves can be extrapolated from the PEG imbibition curves measured between 0 and -2.5 MPa. Thus, according to Fig. 4, the PEG imbibition curves at -0.002 and -0.006 MPa should correspond to that measured at 0 MPa. On the other hand, if we assume that PEG and TG curves at 0 MPa are equal to each other (Fig. 5), then the PEG curves at 0, -0.002 and -0.006 MPa are equivalent to the TG curve at 0 MPa. Taking these assumptions into account, results showed that although no differences are observed between TG-loam imbibition curves and PEG curves ranging between 0 and -0.01 MPa, the imbibition curves in TG-sand at h ≤ -0.002 MPa are significantly different to the PEG curves within the [0, -0.01] MPa interval. Since seed imbibition curves in PEG depend only on h, the different behavior between sand and loam soil indicates that the imbibition process should depend not only of h but also on the medium where the seed is placed. As reported by Williams and Shaykewich (1971) and Hadas and Russo (1974) these differences should be caused by the hydraulic properties of the porous substrate that surrounds the seeds, e.g. soil. Thus, while in PEG experiments the imbibition of completely wet seeds is only limited by the Δh between seed and the external solution, the seed imbibition process within porous substrates is controlled by both Δh (Eq. 3) and the K(h) (Eq. 2) of the substrate. All this information is summarized by the Darcy’s law for unsaturated media (Eq. 3), which can be applied to the seed-soil system. According to Eq. 3 the flow of water from the soil to a seed depends on the soil K(h) and the relationship between the distance and the Δh between a point of soil and the seed surface. In conclusion, unlike to PEG experiments, where there is no hydraulic restriction between the seed and the water solution, the imbibition curve of a seed buried in a soil depends on both Δh and the K(h) of the soil. This theoretical description agrees with Camacho et al. (2021), who observed that K(h) rather than h, was a more informative variable to predict seed germination in soil.
Based on these general theoretical considerations, we can then discuss in detail the different behaviors observed between the seed imbibition curves obtained in the PEG and TG experiments:
-
i.
The significant relationship with slope close to one obtained in the two species for the comparison between PEG and TG-loam and TG-sand at 0 MPa (Fig. 5) is explained by the fact that Ks (Eq. 2) of the sand and loam soils was not a limiting factor for the seed imbibition process. At h = 0 MPa, the high imbibition rate during the first steps of seed hydration is explained by the large Δh between the seed (around -50 to -350 MPa, Bewley et al. 2013) and the surrounding medium. However, as water is absorbed by seeds, the h of the seed increases reducing Δh and thus the rate of water absorption.
-
ii.
The similarity observed when comparing the TG-loam curves between 0 and -0.006 MPa and the PEG curves within the [0, -0.01] MPa interval, indicates that the K(h) of the loam soil for 0 > h > -0.006 MPa (Fig. 2) was not a limiting factor for seed imbibition either.
-
iii.
The completely different seed imbibition behavior observed between the null seed imbibition measured in TG-sand at -0.006 MPa (Fig. 3) and the PEG curves measured at more negative water potential (i.e. -0.01 MPa), indicates that this porous media has a significant effect on seed imbibition. By allusions, there would also be a contradiction between this null imbibition curve in TG-sand and the PEG curve at -2.5 MPa, since this latter shows an increasing trend despite the more negative h value. As suggested by Hadas and Russo (1974) and Camacho et al. (2021), the null imbibition curves found in sand should be related to the low unsaturated hydraulic conductivity of this material at -0.006 MPa (Fig. 2), which drastically restricted the water flow between the sand and the seeds. Note that this hydraulic limitation does not exist in the PEG experiments, where the seeds are in constant contact with a film of water. Thus, these results, which demonstrate that seed imbibition in soil depends on Δh and the soil K(h), clearly indicate that PEG experiments are not adequate to describe seed germination under soil conditions.
-
iv.
The intermediate imbibition curve behavior observed in the TG-sand experiment at -0.002 MPa for both species, indicates that there was a moderate restriction of water flow between the sand and the seeds at this soil tension. Although this behavior could be explained by the low unsaturated hydraulic conductivity of the sand at -0.002 MPa (Fig. 2) (Hadas and Russo 1974; Camacho et al. 2021), there seems to be a sort of contradiction between these results and those observed in TG-loam at -0.006 MPa, which, with lower unsaturated hydraulic conductivity (Fig. 2), the corresponding imbibition curve was less affected by h (Fig. 3). Thus, the higher water flow restriction between the seeds and sand at -0.002 MPa could be also explained by low water content of sand at that soil tension (Fig. 2) or a poor contact between seeds and the surrounding soil particles. Thus, these results suggest that seed imbibition could depend not only on the surrounding h and K(h), but also on other factors such as: (1) soil water content; while PEG experiments show that vetch can germinate at -2.5 MPa, in most soils, germination is not possible at this water potential because its soil moisture corresponds to the residual water content, or (2) the surface contact between seeds and soil, which determines the capacity of the soil to provide water to the seed independently of the soil K(h) and Δh between seed and soil. Thus, these results open the door to new applications of TG, which could be used, for instance, to study the role of seed shape or the mucilage as a strategy to increase the surface contact with the soil (Tsai et al. 2021).
All these results indicate, as reported by Camacho et al. (2021), that PEG experiments are not appropriate to infer germination behavior under field conditions. This conclusion, however, does not mean that PEG experiments are not useful in other kind of experiments about seed germination in controlled conditions, e.g. they provide information about the most negative h below which the seed stops absorbing water. And they are an essential tool to characterize the hydraulic properties of the seeds (Moret-Fernández et al. 2023).
The different shapes of imbibition curves obtained in TG for barley and vetch and 0 and -0.002 MPa (Fig. 3) suggest that the imbibition process does not only depend on the soil hydraulic properties but also the intrinsic hydraulic properties of the seeds (Vertucci 1989): the water retention curve and the hydraulic conductivity function (Moret-Fernández et al. 2023). Finally, compared to -0.002 MPa, the lower germination percentage obtained in TG-sand and TG-loam at 0 MPa (Table 3), may be due to the lack of soil aeration (Bewley et al. 2013). Previous articles have found that, at saturation, lower oxygen conductivity reduces seed germination, but this effect depends on soil texture (Dasberg and Mendel 1971), in our case the soil pore space at 0 MPa is completely filled with water, which can be producing a limitation in oxygen diffusion. However, it needs to be studied yet why this does not occur in PEG.
Usually, wet thermal accumulation models to simulate seed germination are built using PEG to simulate soil water potential or to test osmotic stress on germination of certain weed species. Based on our results, this technique is not accurate enough. But these models are used with some success in predicting seed germination in field (e.g. 60% of successful predictions in Rawlins et al. 2012). These good results could be explained by the fact that over some potentials interval, the PEG experiments have similar imbibition curves than soil (i.e. loam soil at [0, -0.01] MPa). Our findings have the potential to enhance the precision of wet thermal accumulation models. These models traditionally rely on the assumption of a soil water potential threshold below which germination is deemed impossible. This threshold is sometimes arbitrarily set, as using values such as the wilting point or others (Bullied et al. 2012 or Rawlins et al. 2012), or it is derived as the minimum potential required for germination in PEG germination experiments (Boddy et al. 2012; Watt et al. 2010; Patané and Tringali 2010). Our results challenge both of these assumptions. Firstly, we illustrate that the seed imbibition process depends on both seed and soil properties. Consequently, assuming a uniform threshold potential for all soil and seed combinations is not realistic. Secondly, we establish that the thresholds provided by PEG germination experiments are unrealistic because they do not account for soil properties, resulting in excessively negative thresholds that may not correspond to real soil water availability.
Thus, the TG is a solution that allows a more realistic study of seed imbibition processes, which vary for each combination of soil and seed. This device, which is inexpensive and easy to implement, present great stability and precision and does not require external electrical vacuum pumps, has proven to be effective in maintaining a constant tension on the soil layer. However, the main limitation of this new device is that the 10 μm pore size nylon mesh used in the design limits the minimum water potential to -0.008 MPa. Above this suction, the rupture of the water film contained within the pores of the mesh breaks the vacuum inside the germinator. Although more negative suctions could be obtained by using meshes with smaller pore size, this would imply lower permeabilities, which could restrict the replenishment of water lost by evaporation. Thus, although the nylon mesh used allowed demonstrating that the hydraulic characteristics of the soils have a significant influence on seed germination, further studies would be needed to test alternative meshes of smaller pore size that could achieve more negative stresses without significantly affecting mesh permeability. However, although this device is limited to relatively coarse textured soils, its applications in seed ecology are very broad. Since the unsaturated hydraulic conductivity of sand is very sensitive to h, small variation in h will result in larger changes of K(h) (Fig. 2). This large elasticity would allow, for instance, studying the influence of K(h) on the seed imbibition time or quantifying a hypothetical critical θ for seed germination. In addition, TG could be also used, for instance, to study the strategies of some seed species to improve the seed imbibition under soil hydraulic conductivity restrictions.
One could argue that these results are unreliable due to the low number of seeds per replicate. In fact, if this were an experiment aimed at studying the germination characteristics of vetch and barley seeds (e.g., germination speed), this low number would make it impossible to draw reliable conclusions. However, since the goal of the article is not to investigate germination but rather to test the TG and compare PEG with real soils, this seed quantity is sufficient. There is another necessary caveat, regarding the methodology, maybe the measures of weight we did to calculate the imbibition curves are not enough to capture the shape of the imbibition curve at the very beginning of the imbibition, when imbibition rate is high. In the current study, this is not relevant, since the objective of the experiment is to demonstrate the use of the TG and compare it with PEG. However, in future experiments, in which the objective is to compare germination curves between species or varieties, it will be necessary to measure the imbibition curves with a higher frequency at the beginning of the experiments. In fact, tests should be done for different species, since this intensive weight sampling period may be shorter or longer depending on the species studied.
References
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: physiology of development, germination 133 and dormancy, 3rd edn. https://doi.org/10.1007/978-1-4614-4693-4_4
Boddy LG, Bradford KJ, Fischer AJ (2012) Population-based threshold models describe weed germination and emergence patterns across varying temperature, moisture and oxygen conditions. J Appl Ecol 49:1225–1236. https://doi.org/10.1111/j.1365-2664.2012.02206.x
Bullied WJ, Van Acker RC, Bulloc PR (2012) Hydrothermal modeling of seedling emergence timing across topography and Soil Depth. Agron J 104:423
Camacho ME, Heitman JL, Gannon TW, Amoozegar A, Leon RG (2021) Seed germination responses to soil hydraulic conductivity and polyethylene glycol (PEG) osmotic solutions. Plant Soil 462:175–188. https://doi.org/10.1007/s11104-021-04857-5
Carsel RF, Parrish RS (1988) Developing joint probability distributions of soil water retention characteristics. Water Resour Res 24:755–769. https://doi.org/10.1029/WR024i005p00755
Christensen M, Meyer SE, Allen PS (1998) A simulation model to predict seed dormancy loss in the field for Bromus tectorum L. J Exp Bot 49:1235–1244. https://doi.org/10.1093/jxb/49.324.1235
da Silva AR, Leão-Araújo EF, Rezende BR, dos Santos WV, Santana HA, Silva SCM, Fernandes NA, Costa DS, Mesquita JCP (2018) Modeling the three phases of the Soaking kinetics of seeds. Agron J 110:164–170
Dasberg S, Mendel K (1971) The effect of soil water and aeration on seed germination. J Exp Bot 22:992–998. https://doi.org/10.1093/jxb/22.4.992
Donohue K, Rubio de Casas R, Burghardt L et al (2010) Germination, postgermination adaptation, and species ecological ranges. Ann Rev Ecol Evol Syst 41:293–319. https://doi.org/10.1146/annurev-ecolsys-102209-144715
Dürr C, Dickie JBB, Yang XY, Pritchard HWHW (2015) Ranges of critical temperature and water potential values for the germination of species worldwide: contribution to a seed trait database. Agric for Meteor 200:222–232. https://doi.org/10.1016/j.agrformet.2014.09.024
Frischie S, Fernández-Pascual E, Ramirez CG, Toorop P, González MH, Jiménez-Alfaro B (2019) Hydrothermal thresholds for seed germination in winter annual forbs from old-field Mediterranean landscapes. Plant Biol 21:449–457. https://doi.org/10.1111/plb.12848
Hadas A, Russo D (1974) Water uptake by seeds as affected by water stress, capillary conductivity, and seed-soil water contact. I. Experimental study 1. Agron J 66:643–647
Hegarty TW (1978) The physiology of seed hydration and dehydration, and the relation between water stress and the control of germination: a review. Plant Cell Environ 1:101–119
Hillel D (2012) Introduction to environmental soil physics. Academic, San Diego. ISBN-10: 012395455X
Huang Z, Liu S, Bradford KJ, Huxman TE, Venable DL (2016) The contribution of germination functional traits to population dynamics of a desert plant community. Ecology 97:250–261. https://doi.org/10.1890/15-0744.1
Jiang T, Zhou L (2022) Effects of PEG simulated drought on the germination of Chimonanthus praecox seeds and the physiological response of seedlings to drought. Seed Sci Techn 50:227–233
Jiménez-Alfaro B, Silveira FAO, Fidelis A et al (2016) Seed germination traits can contribute better to plant community ecology. J Veg Sci 27:637–645. https://doi.org/10.1111/jvs.12375
Krichen K, Vilagrosa A, Chaieb M (2017) Environmental factors that limit Stipa tenacissima L. germination and establishment in Mediterranean arid ecosystems in a climate variability context. Acta Physiol Plant 39:1–14
Liu S, Bradford KJ, Huang Z, Venable DL (2020) Hydrothermal sensitivities of seed populations underlie fluctuations of dormancy states in an annual plant community. Ecology 101:e02958. https://doi.org/10.1002/ecy.2958
Macar K (2009) Effects of water deficit induced by PEG and NaCl on Chickpea (Cicer arietinum L.) cultivars and lines at Early Seedling stages. G. U J Sci 22:5–14
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Phy 51:914–916
Moret-Fernández D, Latorre B (2022) A novel double disc method to determine soil hydraulic properties from drainage experiments with tension gradients. J Hydrol 615:128625. https://doi.org/10.1016/j.jhydrol.2022.128625
Moret-Fernández D, Latorre B, Peña-Sancho C, Ghezzehei TA (2016) A modified multiple tension upward infiltration method to estimate the soil hydraulic properties. Hydrol Process. https://doi.org/10.1002/hyp.10827
Moret-Fernández D, Latorre B, Lòpez MV, Pueyo Y, Tormo J, Nicolau JM (2021) Hydraulic properties characterization of undisturbed cores under different soil managements. Catena. https://doi.org/10.1016/j.catena.2020.104816
Moret-Fernández D, Tormo J, Latorre B (2023) A new methodology to characterize the kinetics of a seed during the imbibition process. Plant Soil. https://doi.org/10.1007/s11104-023-06427-3
Mualem Y (1976) New model for predicting hydraulic conductivity of unsaturated porous-media. Water Resour Res 12:513–522. https://doi.org/10.1029/WR012i003p00513
Patané C, Tringali S (2010) Hydrotime analysis of Ethiopian Mustard (Brassica carinata A. Braun) seed germination under different temperatures. J Agron Crop Sci. https://doi.org/10.1111/j.1439-037X.2010.00448.x
Rawlins JK, Roundy BA, Egget D, Cline N (2012) Predicting germination in semi-arid wildland seedbeds II. Field validation of wet thermal- time models. Environ Exp Bot 76:68–73
Reuss SA, Buhler DD, Gunsolus J (2001) Effects of soil depth and aggregate size on weed seed distribution and viability in a silt loam soil. Appl Soil Ecol 16:209–217
Sharma ML (1973) Simulation of drought and its effect on germination of five pasture species 1. Agron J 65:982–987. https://doi.org/10.2134/agronj1973.00021962006500060041x
Terpstra R (1990) Formation of new aggregated and weed seed behaviour in a coarse - and fine-textured loam soil. A laboratory experiment. Soil till Res 15:285–296
Tsai AYL, McGee R, Dean GH, Haughn GW, Sawa S (2021) Seed mucilage: biological functions and potential applications in Biotechnology. Plant Cell Phy 62:1847–1857. https://doi.org/10.1093/pcp/pcab099
van Genuchten MT (1980) A closed form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci Soc Am J 44:892–898
Vertucci CW (1989) The kinetics of seed imbibition: controlling factors and relevance to seedling vigor. In: Stanwood PC, McDonald MB (eds) Seed moisture, vol 14. CSSA Special publications, pp 93–115. https://doi.org/10.2135/CSSASPECPUB14.C6
Watt MS, Xu V, Bloomberg M (2010) Development of a hydrothermal time seed germination model which uses the Weibull distribution to describe base water potential. Ecol Mod 221:1267–1272
Williams J, Shaykewich CF (1971) Influence of soil water matric potential and hydraulic conductivity on the germination of rape (Brassica napus L). J Exp Bot 22:586–597. https://doi.org/10.1093/jxb/22.3.586
Acknowledgements
The authors thank Pepa Salvador for her support in the laboratory work. The discussions that led to this article took place while the authors were working on the project LIFE TECMINE (LIFE 16 ENV/ES/000159).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
D. Moret-Fernández has participated in the design and fabrication of the tension germinator, the experimental tests and in the writing of the article.
J. Tormo has contributed in the general concept of the paper, discussion about the soil-seed relationship, the agronomic and ecological context of the article and the revision of the article.
M.V. López has participated in the experimental design and the revision of the article.
A. Cirujeda and E. Bochet provided key insights during discussions for the design of the TG and the agronomic and ecological context of the article and have actively participated in the revision of the article.
Corresponding author
Additional information
Responsible Editor: Ricardo Aroca.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moret-Fernández, D., Tormo, J., López, M.V. et al. A new experimental device for germinating seeds under controlled soil water potentials, a step beyond PEG. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06642-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06642-6