Abstract
Background and aims
The Richmond District of Queensland (Australia) hosts seleniferous soils and is the habitat of selenium (Se) hyperaccumulator plants. These soils are also naturally enriched in vanadium (V) and molybdenum (Mo). This study aimed to survey soils and plants of the Richmond area with a focus on the Se hyperaccumulator Neptunia amplexicaulis.
Methods
Field collection of plant specimens and soils from the seleniferous outcrops of the Richmond District (including the Julia Creek area) was undertaken and the samples were analyzed for Se, V, Mo and other elements.
Results
The results show that three modes of Se accumulation can be observed with most plant species having from ~10 μg Se g−1 up to 70 μg Se g−1 in shoots, some having between 75 and 250 μg Se g−1 in shoots while both N. amplexicaulis and Acacia tephrina contained 450–700 μg Se g−1 in their shoots. There was high Se accumulation in the phyllode tips of A. tephrina. Previously unrecorded populations of N. amplexicaulis were discovered near Julia Creek. Selenium concentrations in soil are lower than historically reported from similar localities (up to 23.2 mg Se kg−1). Molybdenum concentrations between 213 and 329 μg Mo g−1 occurred in the two Se hyperaccumulators N. amplexicaulis and A. tephrina, whilst Salsola australis had the highest accumulation of V (31–66 μg V g−1).
Conclusions
The results suggest that the Toolebuc Formation is the source of Se in the region, but temporal variability alters how much Se is bioavailable to plants. The presence of high Mo in plant tissues is linked to Se hyperaccumulation, and V accumulation appears to have little impact on plant ecology.
Similar content being viewed by others
Introduction
Selenium (Se) is a metalloid element that is essential to animals at low concentrations, but highly toxic at higher exposure (Hartikainen 2005; Rotruck et al. 1973; Schwarz and Foltz 1957). Selenium poisoning in cattle (selenosis) often results in loss of hair and hooves, in turn stopping grazing and often leading to death; as such, selenosis has historically been of agricultural and scientific interest and surveys of affected areas often identify seleniferous soils (>2 μg Se g−1) and plants with unusually high Se concentrations (Beath et al. 1934; Draize and Beath 1935; O'Toole and Raisbeck 1995). These plants are known as hyperaccumulators, and they can accumulate metal(loid) elements in extremely high concentrations in their tissues without showing symptoms of toxicity (Jaffré et al. 1976; van der Ent et al. 2013). Selenium hyperaccumulation is referred to as plants having >1000 μg Se g−1 in their shoots where most ‘normal’ plants are incapable of tolerating over 25 μg Se g−1 in their leaves, and has only been recorded in 45 taxa, though there are also many ‘secondary accumulators’ which accumulate 100–1000 μg Se g−1 (Anderson 1993; Brown and Shrift 1982; Cappa and Pilon-Smits 2014; White 2016; White et al. 2004). Members of the genus Astragalus (Fabaceae, 25 taxa) and Stanleya (Brassicaceae), were discovered in Wyoming and Utah in the USA as part of selenosis investigations; hyperaccumulating species within these genera are able to accumulate up to 10,000 μg Se g−1 (Astragalus) and 4000 μg Se g−1 (Stanleya), where the soil only contains 2–10 μg Se g−1 (Beath et al. 1939; Schiavon and Pilon-Smits 2016). Several genera in the Asteraceae family also contain hyperaccumulating taxa, including Xylorhiza and Symphyotrichum which can accumulate up to 5390 μg Se g−1, with some members of the genus Oonopsis accumulated up to 9100 μg Se g−1 (El Mehdawi et al. 2014; Rosenfeld and Beath 1964; White 2016). In an assessment of Se hyperaccumulators, White (2016) developed an understanding of several other common factors, including higher Se:S ratios and decreased Mo uptake when exposed to higher soil Se or S (Harris et al. 2013; White et al. 2007).
Incidences of selenosis in Australia in relation to selenium hyperaccumulation
Some of the earliest reports of selenosis in Australia came from Silver Hills Station in the Richmond District of north-west Queensland (Knott and McCray 1959). The Richmond cases of selenosis were highly concentrated over a small area (0.3 km2 paddock irrigated from a bore hole) at Silver Hills Station. Selenium in the soil was measured at up to 69 μg Se g−1 within the ‘poison strip’ of the paddock, which is among some of the most seleniferous soils recorded globally. Several secondary Se accumulators were discovered but the highest recorded Se concentrations were found in Neptunia amplexicaulis Domin (Fabaceae/Mimosaceae), a native sensitive plant, which contained up to 4334 μg Se g−1 (Knott and McCray 1959). The average Se in tissues from N. amplexicaulis specimens collected from the most seleniferous soils contained an average of 3028 μg Se g−1 thereby placing this as a Se-hyperaccumulator comparable to the major Se-hyperaccumulators in the genera Astragalus and Stanleya. Specimens of N. amplexicaulis further from the main seleniferous paddock were recorded with an average 471 μg Se g−1 (Knott and McCray 1959). Other selenosis cases in Queensland have been linked to hyperaccumulator Coelospermum decipiens in Cape York (Knott and McCray 1959). No cases of naturally occurring selenosis in cattle in these regions has been reported since.
The nature of seleniferous soils and selenium status
The seleniferous soils of the Richmond area derive from the Late Cretaceous marine shales and limestones of the Toolebuc Formation outcropping across Richmond and further west to Julia Creek (Gray et al. 2002; Vine et al. 1963, 1970, 1974). Detailed mapping of the broader Wilgunya subgroup in this area is provided by the surface geology maps from the Bureau of Mineral Resources, Geology and Geophysics, now Geoscience Australia (Vine et al. 1963, 1970, 1974). The Toolebuc Formation contains mudstones, limestones, sandstones, coquinite and oil shale, with notable shell fossils and a high organic content at certain levels (Ozimic 1986; Senior et al. 1975). The organic content is primarily derived from planktonic/algal origins, which results in oxidation and sulphur incorporations (Boreham and Powell 1987). The landscape in this area often has outcropping sections of this surface geology, and large limonitic boulders locally known as ‘moon rocks’ (McCray and Hurwood 1963). This formation is overlain by the Allura mudstone (limestones, siltstones, sandstone and pyritic mudstones, outcropping south of Silver Hills) and underlain by the Wallumbilla Formation (mudstone, siltstone, sandstones, thin limestone and strandline accumulations) outcropping north of Silver Hills (Vine et al. 1970). Quaternary silts and sands overlay much of this area, deposited by the channels of the Flinders River. When investigating the trace elements from a core out of the Julia Creek area of Toolebuc Formation surface geology, Se was found in concentrations between 12 and 55 μg Se g−1 in the oil shale, up to 24 μg Se g−1 in the coquinite, and at 58 μg Se g−1 in a fish bed layer (Patterson et al. 1986). Mudstones contained less Se, with the Allura mudstone containing 5 μg Se g−1 and the Wallumbilla mudstone containing 10 μg Se g−1.
McCray and Hurwood (1963) reported the Se concentrations in soil and plants at Silver Hills at the time; several types of soil in the area contained normal to slightly elevated Se (up to 8.2 μg Se g−1) but the dark brown-grey clay soils from a flat area near a bore drain contained 32.2 μg Se g−1 and when recently agitated and overturned, were densely colonized by N. amplexicaulis. Surrounding areas contained plants with higher Se concentrations than usual, including buffel grass (Cenchrus ciliaris L.) (6.9–77.6 μg Se g−1), couch grass (Elymus repens (L.) Gould) (11.1–263 μg Se g−1) and variousFabaceae species (22.5–79.4 μg Se g−1). The Silver Boree tree (Acacia tephrina Pedley) was also accumulating between 902 and 1121 μg Se g−1, classifying it as another Se hyperaccumulator, though likely not responsible for the selenosis in the paddock. Shale ridges in the paddock and limestone shales from outside the paddock contained 24.6 μg Se g−1, likely making these the primary source of Se, as physical disturbance of uncapped ridges would transport Se-enriched sediments to soils. Historically the area was used for grazing, before the soil was disturbed to develop gravel pits, a pool and a Sorghum plantation in the late 1950s, and it had been noted by Knott and McCray (1959) in their original assessment that “any drastic change in management or in land use might be disastrous”, and it was often newly introduced cattle that were the most vulnerable.
Outside of the Silver Hills area, McCray and Hurwood (1963) additionally described Se in limonitic concretions (176–385 μg Se g−1) and gravel pits (1.0–96.2 μg Se g−1) with most other eroded outcrops containing rocks and soil with 1.0–6.4 μg Se g−1. They observed that N. amplexicaulis was only found in “the softer areas of the exposed formation” and specimens contained <0.5–227 μg Se g−1 across numerous bankside habitats of ‘undulating brown-soil downs’; these were the sites for minor Se accumulation. Other Neptunia species were present, containing <0.5–25.4 μg Se g−1. Along the highway running from Hughenden to Julia Creek, through Richmond, Se was measured in soils from 0.3–14 μg Se g−1, plants sampled in these locations included N. amplexicaulis (34.3–105 μg Se g−1), other Neptunia species (<0.5 μg Se g−1) and several shrubs (7.9–18.5 μg Se g−1).
The poison strip at Silver Hills was marked as an area of major Se enrichment (McCray and Hurwood 1963), as it contained an abundance of Se hyperaccumulators and several secondary Se accumulator grass species. It was established that disruption of the ridges during agricultural practices allowed for the release of Se, and disruption of the vegetation allowed for the establishment on N. amplexicaulis, increasing selenosis cases. McCray and Hurwood (1963) proposed that the entire Rolling Downs Formation could represent a potential distribution of seleniferous soils in Queensland, given the variable yet high Se in limestones present throughout the area. They deliberately noted the similarities between this location and the western-United States seleniferous geology, climate, and vegetation. However, further assessments of the extent of seleniferous soils in Queensland have not been published to follow up the authors’ hypothesis. The research that has been conducted around stratigraphic sequences and oil shales in the region, while not specifically focussing on Se, allow this hypothesis to be refined further (Coxhell and Fehlberg 2000; Lewis et al. 2010).
Significant concentrations of V have been found in the Toolebuc Formation outcrop just east of Julia Creek, which prompted geological explorations in relation to both the V and the potential economic benefits of the oil shales (Jiang et al. 2018; Mao et al. 2023; Riley and Saxby 1982). Molybdenum (Mo) was also noted as in high concentrations at this outcrop, and V and Mo have both been well investigated with regard to distribution and mineralogy (Coxhell and Fehlberg 2000; Lewis et al. 2010; Patterson et al. 1986). Geological and geochemical discussions of the Toolebuc Formation often do not investigate Se concentrations with the exception of Patterson et al. (1986). Similarities can be drawn with the geochemistry of the Yellow Cat area of Utah, a mining area for uranium (U) and V (Cannon 1964). A significant relationship was found between elevated Se, V and Mo and U in the soils and plants of the area, with a strong presence of Se indicator plants that had caused historical cases of selenosis in sheep and cattle. The orebody source of the elements at Yellow Cat had over 9500 mg V kg−1, 200 mg Se kg−1 and 100 mg Mo kg−1, with high Se still present in the overlying and surrounding sandstones. Selenium plants in this region were notable as their distribution could be a key indicator for not only Se, but also geologically favourable drilling results. Molybdenum was often found in high concentration in plant tissues (including Se-indicator plants) at Yellow Cat, with the increased accumulation of both Se and Mo in certain species resulting in increased lethality to sheep. This element is of particular interest as molybdate (MoO42−) chemically resembles sulphate (SO42−) and may increase in concentration in plants where root sulphate pathways are consistently upregulated, i.e., certain Se hyperaccumulators (Shinmachi et al. 2010). Tolerable V concentrations in plant tissues are generally species dependant, though concentrations exceeding 2 μg V g−1 tend to have a detrimental effect on most plants (Nawaz et al. 2018), whereas plant concentrations of V from Yellow Cat could exceed 100 μg V g−1, with the highest concentrations found in Astragalus flavus (another known Se indicator/accumulator) (Cannon 1964). While V toxicity has been observed in cattle, it is often linked with anthropogenic V pollution (Frank et al. 1996; McCrindle et al. 2001).
Neptunia amplexicaulis has been further investigated in controlled environments in relation to biochemical forms of Se and metabolic pathways (Burnell 1981; Ng and Anderson 1978; Peterson and Butler 1962, 1967; Peterson and Robinson 1972). Recently, it was discovered that Se is primarily concentrated in the reproductive tissues, youngest leaves and root tissues of N. amplexicaulis, where it is likely that phloem cycling of Se out of the older leaves and back into the young leaves is occurring, using the taproot as a storage organ (Harvey et al. 2020). As such, concentrations in the youngest leaves can reach 13,600 μg Se g−1 in controlled conditions, far above those previously reported in nature. It is likely that the Se elemental analysis used by McCray and Hurwood (1963) combined tissues that included a large portion of low-Se old leaves, giving a plant average that is lower than the maximum Se accumulating capacity in the young leaves. Furthermore, the studies failed to distinguish between the presence of glabrous leaves (N. amplexicaulis f. amplexicaulis) or villous leaves (N. amplexicaulis f. richmondii), which was later discussed as the distinguishing feature between the forms of this species (Windler 1966). Acacia tephrina by comparison was unassessed further than the description of Se accumulation by McCray and Hurwood (1963), though it is now understood that the extent of this species stretches from the Northern Territory to the east coast, through central Queensland (Pedley 1981).
Here, we aim to revisit the Silver Hills and larger Richmond-Julia Creek area to assess soil and plant Se status. Specifically, our objectives were to locate areas of N. amplexicaulis colonisation to assess (i) the Se concentrations and properties in the surface soil; (ii) the Se concentrations in the plant biomass from different species; and (iii) comparative Se concentrations in different organs of N. amplexicaulis and Se distribution in A. tephrina tissues. Additionally, we assessed the biogeochemical similarities of north-west Queensland and the Yellow Cat area, by examining the Mo and V concentrations of soils and plant biomass occurring on these geological features. Through these objectives, we aimed to add to the understanding of seleniferous areas of north-west Queensland, including changes in plant and soil Se concentrations since the last assessment in 1963.
Materials and methods
Field sample collection in the Richmond area
Plant and soil samples were collected from Richmond, Queensland in April 2019, after a disruptive monsoon flooding event (469 mm total rainfall in 10 days) in early February 2019 (Figs. 1 and 2; Bureau of Meteorology, 2022). Due to the large quantity of rainfall, plant biomass in the region was plentiful, and several areas of disrupted soil along roadsides had abundant early stages of plant colonisation. Plant sample collection occurred at the Silver Hills Station (here denoted as SH), in and around the locality known as the ‘poison strip’ and surrounding areas detailed in McCray and Hurwood (1963). The poison strip itself had been filled in and covered over with a gravel airplane landing strip, so areas adjacent to the air strip were sampled. More sampling occurred along roads on the eastern side of the Silver Hills Station (oSH). Outside of the Silver Hills property, sampling occurred at the Richmond Dinosaur Quarry (DQ), outside the Quarry (OQ) and on the roadside of the Flinders Highway in Richmond (HW) where populations of Neptunia amplexicaulis were visible. At Julia Creek, samples were taken in and around the St. Elmo Station (StE) and limestone quarry (Q) leading up from the Flinders Highway. Neptunia amplexicaulis and Neptunia heliophila (formerly N. gracilis in Harvey et al. 2020; Pinto Irish et al. 2021) were collected and separated into young leaves, old leaves, stems, and roots, with pods and flowers also collected if present. Soil was collected from the location of N. amplexicaulis root extraction at 5–20 cm depth alongside the taproot. Per locality, one to two specimens were selected randomly within a short geographic area, with more specimens sampled if the locality had a larger geographic area populated by N. amplexicaulis. Several N. amplexicaulis specimens from Julia Creek were collected for bulk leaf and stem tissue. At each locality, all other visible species were opportunistically sampled for leaves, stems and/or flowers and photographed for later identification. A portable handheld X-ray Fluorescence device (pXRF) was also utilised at these locations for preliminary results. Collected samples were put into envelopes and dried at 60 °C for 48 hrs.
Maps showing extent of Toolebuc Formation surface geology as adapted from Bureau of Mineral Resources, Geology and Geophysics (1970), clockwise from top left: Map of the Australia (ArcGIS World Topographic Map) with extent of the surface geology of the Toolebuc Formation (outlined in green) in Queensland, map of Richmond and Julia Creek area with Toolebuc Formation extent, collections at the outside Quarry (OQ) site, and St Elmo (StE) and Quarry (Q) sites
Environment and localities of Richmond. a SH site dominated by Salsola australis and (b) Neptunia amplexicaulis (blue green) with other plant species at SH site (c) environment along road at the oSH site (d) population of N. amplexicaulis at the oSH site (e) monoculture of N. amplexicaulis at the OQ site (f) examples of limonitic concretions (“moon rocks”) common in the Richmond District (g) and (h) outcropping of coquinite shales
Preparation of plant tissue samples for bulk chemical analysis
Plant tissue samples were ground to a fine powder in an impact mill at 19000 rpm (Tube Mill 100 control with disposable titanium blades) for 30–90 seconds and weighed to 100 ± 5 mg in 6 mL polypropylene tubes. These samples were pre-digested using 2 mL 70% HNO3 in tubes for at least one day before being digested in a Thermo Scientific™ digital dry bath block heater for 1 hr. at 70 °C followed by 1 hr. at 125 °C. Digests were then filtered using Whatman® Grade 41 filter paper and diluted to 10 mL with ultrapure water (Millipore 18.2 MΩ·cm at 25 °C) in 10 mL ICP tubes before analysis with Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) with a Thermo Scientific iCAP 7400 instrument for macro-elements (Na, Mg, Al, P, S, K, Ca), trace-elements (Mn, Fe, Co, Ni, Cu, Zn) and ultra-trace elements (Se, V, Mo) in radial mode. All elements were calibrated with a 4-point curve covering analyte ranges in the samples. In-line internal addition standardization using yttrium was used to compensate for matrix-effect interferences. Quality controls included matrix blanks, certified reference material (Sigma-Aldrich Periodic table mix 1 for ICP TraceCERT®), Standard Reference Material (NIST Apple leaves 1515 digested with HNO3), internal reference materials (homogenised powdered N. amplexicaulis tissues) and soil standards (SI Table 1–4).
Collection and chemical analysis of soil samples
Rhizosphere soils were air dried to constant weight and sieved through a 2 mm screen. Soil sub-samples were weighed to 100 ± 5 mg in quartz digestion vessels and 5 mL HNO3 (70%) and 2 mL HCl (37%) was added. The samples were then digested for 15 min at 80% power using a ColdBlock system (CB15S 15 channel system, ColdBlock Technologies Inc) which uses high-intensity infrared irradiation to aid rapid acid digestion (Wang et al. 2014). The digestates were quantitatively transferred to 50 mL tubes, brought to volume (40 mL), filtered (Whatman® Grade 1 filter paper) before analysis with ICP-AES. Plant available Se was extracted using the AB-DTPA method (Soltanpour and Schwab 1977; Sharmasarkar and Vance 1995) which consists of 1 M ammonium bicarbonate and 0.005 M DTPA (diethylenetriaminepentaacetic acid). The AB-DTPA solution was prepared by dissolving 1.97 g of DTPA in 800 mL distilled water to which 2 mL of NH4OH was added. Then 79.06 g NH4HCO3 was added and dissolved, and the pH adjusted to 7.6 using NH4OH or HCl and brought to volume (1.0 L). 10 g of soil was extracted with 20 mL AB-DTPA solution for 30 min on a reciprocating shaker, then centrifuged (Eppendorf Centrifuge 5804, 10 min. at 4000 rpm), filtered (Whatman® Grade 41 filter paper) and 10 mL was transferred into 10 mL ICP tubes. Finally, 0.5 mL of 70% HNO3 was added to drive off CO2 before analysis with ICP-AES as described above. In addition, Sr(NO3)2-extraction (0.01 M) was performed using a method adapted from Kukier & Chaney to determine weakly exchangeable metal(loid) concentrations in the soil (solid/liquid ratio, m:v, of 1:4 for 2 hr.) (Kukier and Chaney 2001).
Micro-X-ray fluorescence elemental mapping
The UQ microXRF facility contains a modified IXRF ATLAS X system, mounting two 50 W X-ray sources fitted with polycapillary focussing optics: XOS microfocus Mo-target tube producing 17.4 keV X-rays (flux of 2.2 × 108 ph s−1) focussing to 25 μm FWM and a Rh-target tube producing 20.2 keV (flux of 1.0 × 107 ph s−1) focussing to 5 μm FWM. The system is fitted with two silicon drift detectors (SDD) of 150 mm2 coupled to a XIA Mercury X4 signal processing unit. Measurements were conducted at room temperature (~20 °C), using the Mo 25 μm X-ray source at a 40 kV and 1000 uA, with a per-pixel dwell of 100 ms. The hydrated A. tephrina samples were mounted between two sheets of 4 μm Ultralene thin film in a tight sandwich to limit evaporation and analysed within 10 minutes after excision. The mounted samples between Ultralene thin film were stretched over a Perspex frame magnetically attached to the x-y motion stage.
Synchrotron X-ray absorption spectroscopy of soils
Compacted hydrated soil samples from the field were inserted in plastic cells, sealed in Kapton tape, and cooled to ~5 K in a He expansion cryostat. XAS spectra of the samples were recorded in fluorescence mode using a 100-element solid-state Ge fluorescence detector array at 90° to the incident beam. The energy ranges to be utilised for XANES Se K-edge data collection are pre-edge region 12,430–12,635 eV (10 eV steps); near edge region 12,635–12,685 eV (0.25 eV steps); post-edge region 12,685–12,875 eV. A spectrum of a hexagonal Se standard, recorded simultaneously in transmission downstream of the sample, was used to calibrate the energy scale to the first peak of the first derivative of the elemental selenium edge (12,658.0 eV). Spectra of model Se compounds, as previously reported, for target transformation analysis and XANES linear combination fitting were (abbreviations used in tables and figures in parentheses): aqueous selenate at pH 7 (selenate pH 7), aqueous selenite at pH 5 and 7 (selenite pH 5, selenite pH 7), seleno-L-cysteine (CysSeSeCys), sulfoseleno-L-cysteine (CysSSeCys), seleno-L-methionine (SeMet), Se-methylselenocysteine (MeSeCys), selenodiglutathione (GSSeSG), protonated and deprotonated seleno-L-cysteine (CysSeH and CysSe−), dimethyl selenoxide (DMSeO) and dimethyl selenone (DMSeO2) (Weekley et al. 2013, 2014). The red allotrope of elemental selenium was synthesised by reduction in a solution of Na2SeO3 with excess ascorbic acid.
Statistical analyses
Statistical analyses were performed using R version 4.0.2 and RStudio (Integrated Development for R. RStudio, PBC, Boston, MA, http://www.rstudio.com) and Microsoft Excel 2016 (Redmond). Measured elemental levels <LOD were replaced with LOD/√2 before conversion to μg g−1. The Se, Mo and V concentrations of wild plant shoot biomass are presented as histograms and boxplots (R package ggplot2) and in tables. Key to symbols of boxplots: open squares are the interquartile range, whiskers are the maximum and minimum range and circles are outliers. The mean ± standard deviation and statistical tests were determined using R package rstatix. Normality was tested using Shapiro-wilks test and QQ plots on model residuals and transformed with log transformation and re-tested if necessary. Variance was checked using Levene’s test. Outliers were not removed unless the coincided with a sample that may have affected analytical output (small volume of sample, volatilisation during acid digestion etc.). Significant difference was tested using one-way analysis of variance (one-way ANOVA) performed on data with Se, Mo and V concentration after log transformation to satisfy normality and variance with Tukey’s HSD post-hoc test (for the soil data), Welch one way ANOVA in the cases where variances were not normal (vanadium data), or Kruskal Wallis tests with Wilcoxon pairwise post-hoc on non-normal data (for field plant data, where values <LOD removed instead of replaced by LOD/√2 as raw data). The XAS data were interpreted with standard approaches using EXAFSPAK. All data were energy calibrated, background corrected and normalized. The XRF spectra on the UQ microXRF facility were acquired in mapping mode using the instrument control package, Iridium (IXRF systems), and then imported into the GeoPIXE software package version 7.5 s (beta). The XRF spectra were subsequently fitted using the Dynamic Analysis method (Ryan et al. 1990, 2005; Ryan 2000; Ryan and Jamieson 1993). This method generates elemental images, which are line overlap-resolved, and in with the Mo-tube Bremsstrahlung background has been subtracted.
Herbarium XRF measurement of selenium in Neptunia amplexicaulis specimens
The use of handheld X-ray fluorescence (XRF) instruments is a non-destructive and effective method for the systematic quantitative assessment of hyperaccumulation in vast numbers of herbarium specimens (van der Ent et al. 2019). The XRF analyser uses a miniaturised X-ray tube (Ag-target, 25–50 kV, 200 μA) as its main excitation source. Each measurement was taken from a specimen attached to standard herbarium cardboard sheet placed on top of a 100 cm2 99.995% pure titanium plate (to block transmitted X-rays and to provide a uniform background). Soil dust contamination adhering to leaves can confound measurements, but may be gauged from unusually high concomitant Cr, Fe, and Ti concentrations (Cary and Kubota 1990) and suspect specimens were omitted from the dataset. The XRF analysis was undertaken at the Queensland Herbarium (BRI) in Brisbane on all available specimens of N. amplexicaulis. Where distinguishable, specimens were scanned on both older and younger leaves.
Results
Site descriptions and extent of seleniferous soils
Roads and creek crossings had been recently disturbed by severe flooding and road repairs, and the rains had aided with vegetative regeneration in this arid region. Neptunia amplexicaulis was observed growing in localities either on, or adjacent to, the layers of Toolebuc Formation at Silver Hills Station (SH) and St. Elmo Station (StE) near Julia Creek (Figs. 1, 2 and 3). Both localities contained seleniferous rhizosphere soils of statistically similar ranges (SH total Se mean is 10.7 mg Se kg−1, StE mean is 11.1 mg Se kg−1), where the maximum value recorded was 23.2 mg Se kg−1 total at locality SH (Table 1). The former ‘poison strip’ described in McCray and Hurwood’s investigation, now a gravel airstrip, had N. amplexicaulis growing along the sides and ridges. Neptunia amplexicaulis and N. heliophila A.R.Bean were found growing further on the SH property, where the soils had up to 15.4 mg Se kg−1 (Fig. 3; the mean is 8.59 mg Se kg−1). Other sampled localities include two localities outside the Dinosaur Quarry at Richmond (OQ, DQ). The OQ locality hosted N. amplexicaulis in mono-dominant populations in freshly disturbed soils, and only mildly seleniferous rhizosphere soils (Fig. 2; the mean is 7.35 mg Se kg−1 total). Acacia tephrina specimens were sampled at the DQ site (Fig. 3). The Flinders Highway (HW) site had a similar range of total soil Se (Fig. 2; mean is 8.65 mg Se kg−1 total). There were no significant differences between the ranges of total Se concentrations across the localities (p 0.25). It should be noted that ranges of total Se can include mineralised or elemental Se, and concentrations of bioavailable Se were much lower – the highest average bioavailable soil Se was found at the SH site (0.252 mg Se kg−1), which was significantly higher than the range found at the oSH sites (p 0.033) but not significantly different from the bioavailable Se concentrations from the other localities (from AB-DTPA extractions) (Table 1).
Examples of hyperaccumulators found in the Richmond regions (a) a stand of Acacia tephrina at the DQ site (b) large Neptunia amplexicaulis at the SH site with Sesbania cannabina (Retz.) Pers. (c) Neptunia amplexicaulis with flowers (d) Neptunia heliophila with flowers (e) Neptunia amplexicaulis (upper) and N. heliophila (lower) growing directly next to each other
Molybdenum and vanadium in site soils
Total molybdenum in the rhizosphere soils was below the limit of detection (Table 1). Vanadium, however, occurred in concentrations of up to 1090 mg V kg−1 total (at the StE site) and with a mean value of 353 mg V kg−1 across all of the sites. The only sites with significant difference in soil V concentrations were the SH site (mean is 558 mg V kg−1), which was significantly higher in V than the Flinders Highway (HW) site (mean is 92.1 mg V kg−1). Strontium nitrate extractable concentrations of V in the soil ranged between 0.0141–4.31 mg V kg−1, where the SH site had higher average V concentration (1.54 mg V kg−1) than the soil at the HW or StE. The AB-DTPA extractable V ranged between 1.61–50.5 mg V kg−1 where, similarly, the SH site (mean is 30.4 mg V kg−1) was significantly higher in V than the HW (2.79 mg V kg−1) and oSH (12.7 mg V kg−1) and StE (6.98 mg V kg−1) sites.
Chemical form of selenium found Silver Hills station soil
X-ray absorption spectroscopy at the Se K absorption edge of the sample of Silver Hills soil was conducted to provide an overall picture of the Se speciation that was present (Weekley et al. 2013; Weekley et al. 2014; Weekley and Harris 2013). The low concentration of Se (~10 mg Se kg−1) led to a moderate level of noise being observed in the near edge spectrum and precluded the collection of useful EXAFS data. A fit of the edge spectrum with a set of model compound spectra indicated that solid elemental selenium was the primary chemical form of selenium in the soil, in addition to other minor components (see Table 2; SI Fig. 1) including oxyanion forms such as selenite. This finding was unexpected but can be explained by bacterial reduction of Se (Lindblom et al. 2013). The lack of detectable selenate in the soil may be explained by leaching during the major flooding event that had just occurred. The residual from the fitting procedure was high indicating that important models may have been absent, and as such the identification of the exact chemical nature of the minor components should not be considered reliable. However, the good fit to the main Se peak at lower energy provides confidence that the identification of elemental Se is valid because the peak energy for the element is distinct from other known forms (SI Fig. 1). We note that the model compound library did not include spectra of metal selenides or other likely mineral forms of Se which are potentially present in the soil sample (Weekley et al. 2013, 2014; Weekley and Harris 2013).
Concentrations of selenium, molybdenum, and vanadium in plants
In all localities, N. amplexicaulis was observed growing sympatrically with a variety of other species, which were measured for their Se concentrations (Fig. 4, SI Table 5). Three modes of Se accumulation were observed: non-accumulators were in the first mode with Se concentrations ranging from 0 to 70 μg Se g−1, the highest frequency of Se concentration occurred around 10 μg Se g−1, with lowering frequencies towards 70 μg Se g−1 (Fig. 4). This first mode was represented by most species before the mode peak and Fabaceae species N. amplexicaulis, A. tephrina, Crassula sp. (Crassulaceae), Cucumis melo L. (Cucurbitaceae), Cryptostegia grandiflora R.Br. (Apocynaceae), Eucalyptus sp. (Myrtaceae) and Flaveria australasica Hook. (Asteraceae) in the tail of the mode. Secondary accumulators occurred in another mode between 75 and 250 μg Se g−1, with the highest frequency of this mode occurring at 100–150 μg g−1 Se. This mode was represented by specimens of N. amplexicaulis, A. tephrina and a Crassula sp. The highest recorded values of Se in leaf tissues occurred in three species, N. amplexicaulis, A. tephrina, and one specimen of F. australasica, between 450 and 700 μg Se g−1. Neptunia amplexicaulis and most other plants were observed with evidence of herbivory, such as damage to leaves and boring into seedpods. Bioconcentration of the elements Mo and V were observed in some plants from all localities (SI Table 6). Vanadium was present in concentrations of up to 66.4 μg V g−1 across plant samples. Plants at the StE locality had significantly lower V concentrations (max is 1.48 μg V g−1) compared to the OQ, Q and SH localities, and plants at the SH locality had significantly higher V concentrations (mean is 4.70 μg V g−1) compared to the OQ, Q and oSH localities. Salsola australis (Chenopodiaceae) had the highest range of V (31–66 μg V g−1), whereas the mode between 14 and 22 μg V g−1 contained S. australis, Crassula, Hibiscus (Malvaceae) and Scaevola (Goodeniaceae) species and Aerva javanica (Amaranthaceae). All concentrations of Mo observed in plant tissues were within the expected tolerable limits for plants (Marschner 2011); however, there was higher than commonly recorded field accumulation (between 213 and 329 μg Mo g−1) occurring in A. tephrina and N. amplexicaulis leaf tissues.
Stacked histogram showing frequency of Se concentration occurrence in all species with Se recorded >LOD. Neptunia amplexicaulis leaf/young leaf/biomass Se concentrations in green and Acacia tephrina leaf Se concentrations in pink, with all other species shown in orange. X axis is Se (in μg g−1 Se, transformation log10), y axis is frequency
Selenium concentrations in Neptunia plant tissues
Neptunia amplexicaulis specimens collected in the field were separated into young leaves (youngest set of opened leaves and all unopened leaves per limb) and old leaves (all other leaves), stem and root for 20 unique specimens from Richmond and four from Julia Creek (Fig. 5, SI Table 7). Seeds (mean is 182, n = 23) and flowers (mean is 319, n = 7) were significantly higher in Se than all other tissues (p < 0.025). The only samples above 1000 μg Se g−1 were one flower sample (1540 μg Se g−1) and one seedpod sample (1319 μg g−1 Se), and only four leaf tissue specimens were > 100 μg Se g−1 (100–682 μg Se g−1). Selenium concentrations were invariant across young leaf, old leaf and stem tissues (p > 0.05), and the taproot was significantly more concentrated in Se than the stems and old leaves (p < 0.0015). Twenty-six samples from young leaves, old leaves, stems, and roots were below the limit of detection. There was no significant difference in organ Se concentrations between localities (p > 0.06), and tissue Se concentration was not correlated with the total or bioavailable Se in the soils for either N. amplexicaulis or N. heliophila (p > 0.095). All N. amplexicaulis collected (bulk tissues and separated tissues), in total 27 specimens from Richmond and 37 specimens from Julia Creek, were compared to determine forma ratios (relative proportions of N. f. amplexicaulis and f. richmondii). Neptunia amplexicaulis f. richmondii accounted for 63% of collected specimens from Richmond, and only 13.5% of Julia Creek specimens.
Boxplot showing Se concentrations (in μg g−1) all N. amplexicaulis tissues from field-collected specimens. Tissues measure include seeds (+ seed pod material), flowers, young leaf, old leaf, stem, and root (primarily taproot) material, Se concentration is on a log scale. Boxplot open squares are the ± mean, whiskers are ± standard deviation (SD) and circles are outliers. Values <LOD were replaced with LOD/√2 before conversion to μg g−1
Comparatively, samples of N. heliophila were targeted in the same sampling localities as N. amplexicaulis (Table 3). The flowers were more concentrated in Se than other tissues (the maximum is 63.3 μg Se g−1, p < 0.024). The concentrations in the young leaves (mean is 9.52 μg Se g−1), old leaves (mean is 6.4 μg Se g−1) and stems (mean is 8.93 μg Se g−1) shared no significant difference in Se concentration between them (p > 0.175). These tissues were not significantly different in Se concentrations from their N. amplexicaulis tissue counterparts (p > 0.08). The root (mean is 6.88 μg Se g−1) and fruit tissues, while not being significantly different from the leaves and stems (p > 0.175), were significantly lower in Se concentration than the N. amplexicaulis root and stem tissues (p < 0.014).
Concentrations of selenium and other elements in Acacia tissues
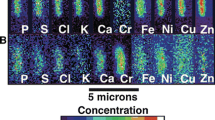
Acacia tephrina biomass from phyllodes, wood and bulk tissue (phyllodes and stems) were collected from a stand outside of the Dinosaur Quarry (DQ) and at the SH site (Table 4). The woody twigs from the Dinosaur Quarry (DQ) site had the highest average Se concentration (247 μg Se g−1, highest value 353 μg Se g−1) followed by the phyllodes from the same site (mean is 114 μg Se g−1, highest value is 377 μg Se g−1). The Silver Hills (SH) site bulk tissues of A. tephrina had the lowest average Se concentration (56 μg Se g−1), however one sample contained the highest concentrations analysed with this method (510 μg Se g−1). Additionally, Mo values in this plant are elevated, particularly in the DQ leaves (mean is 180 μg Se g−1, maximum value 329 μg Se g−1). Micro-X-ray fluorescence elemental mapping of branches and phyllodes shows that Se is concentrated towards the apex of each phyllode, around the marginal areas, with higher concentrations in the younger phyllodes on the branch (Fig. 6). The stem of this branch also has high Se. GeoPIXE was used to determine in situ measurements of the whole phyllode, which recorded Se concentrations of 132 μg Se g−1, however the leaf tips (apex) of this plant contained 1730 μg Se g−1 (Fig. 7). Potassium (described as a structural indicator) is also more concentrated towards the apex of the phyllodes, though covering a larger region of the leaf, and the distribution of Ca is the opposite, more enriched towards the phyllode bases.
Selenium concentrations in Neptunia amplexicaulis from the Queensland herbarium
Selenium in young leaves ranged from levels below the limit of detection to 8120 μg Se g−1 (SI Table 8). The three specimens with Se >1000 μg Se g−1 in the young leaves were all collected at Silver Hills station between the years 1957 to 1960. Levels of Se in the older leaves ranged from below the limit of detection to 239 μg Se g−1. The 12 specimens occurring outside Richmond (in Hughenden and Maxwelton, primarily) had a maximum of 120 μg Se g−1 found in the young leaves.
Discussion
Given that the poison strip had been covered over with an airplane landing strip since previous investigations, any chance to directly compare results was lost, and previously selenosis-inducing levels of Se were not as present in the soil. Nevertheless, N. amplexicaulis was still locally common, and the recorded Se in this area suggests that plants are still exposed to Se and accumulating Se in higher concentrations than most other species found in the area, including closely related non-accumulator N. heliophila. Similarity in accumulation capacity was only seriously linked to other known hyperaccumulator A. tephrina, which localised Se in the twigs and phyllode tips. Concentrations of Se observed here matched the ranges of Se in N. amplexicaulis from areas further away from the poison strip from previous field investigations (McCray and Hurwood 1963). The St. Elmo Station collection site, with lightly seleniferous soils and concentrations of up to 200 μg Se g−1 in N. amplexicaulis young leaves, was a previously unknown locality for this species – record collections in the Atlas of Living Australia show N. amplexicaulis distributions around the Richmond-Hughenden area, with one record at Kilterry, north-west of Richmond and 58 km north-east of the St Elmo Site (with negligible concentrations of Se within its tissues).
Nature of seleniferous soils near Richmond and Julia Creek in relation to hyperaccumulation
In the original field studies, McCray and Hurwood observed highly variable Se in soil, from 0.15–32.2 mg Se kg−1 from across the Silver Hills soils, comparable to our results of up to 23.2 mg Se kg−1 total from around the same areas (McCray and Hurwood 1963). Historically exceptional concentrations of Se (67.0–385 mg Se kg−1) were only found in the clay bands and limonitic concretions from gravel pits and excavation sites of the Toolebuc Formation outcrop, or in cores of Toolebuc Formation (up to 58 mg Se kg−1), arguably the source material for Se in the region (McCray and Hurwood 1963; Patterson et al. 1986). The Toolebuc Formation also outcrops at the St. Elmo site, potentially linking the outcropping of this formation with the distribution of N. amplexicaulis, though only a broad scale vegetation survey of the north-west Queensland region could confirm this hypothesis. Here, elemental concentrations are reported in rhizosphere soils, which will not demonstrate a direct temporal comparison with the bulk soil and rock samples analysed previously. Here it was observed that there was very little link between Se in the rhizosphere soil and Se uptake in N. amplexicaulis, as evidenced by low correlation between soil and plant Se. In our case, such a high proportionality of elemental Se as seen here would indicate that microbial activity would be a factor in soil Se conditions and control over bioavailability, introducing a third variable to the relationship (Li et al. 2017; Lindblom et al. 2013; White et al. 2004). There may be several other contributing variables, such as the size and age of the plants, the timing of seed development and a natural heterogeneity and generally low concentration range of soil Se in the region that could explain the lack of a statistically viable link.
In certain productive Se hyperaccumulators, the bioconcentration factor of Se is so extreme that small differences in seleniferous soils and their fluctuating bioavailability make correlation difficult, though ideally Se accumulation ought to be proportional to bioavailable soil Se (Statwick and Sher 2017). Dosing experiments performed under controlled conditions have shown that the presence of environmental Se can increase the concentrations of Se in the plant but small changes in Se concentrations for already seleniferous soils often do not influence the concentrations of Se in the plant to significant differences (Statwick et al. 2016). Only in the case of exceptionally toxic environments (such as 75 or 100 mg Se kg−1 as selenate, conditions not currently seen in Richmond) is there a serious noticeable difference in Se accumulation for N. amplexicaulis when compared to lower soil Se concentrations (Harvey et al. unpublished). Previous studies of the Richmond environment were performed soon after agricultural land disturbance that eroded the ridges of outcropping Toolebuc Formation, which may have played a role in higher Se availability in a dynamic system (McCray and Hurwood 1963). Indeed, the late 1950’s was the only time Se was recorded in excess of 1000 μg Se g−1 in N. amplexicaulis leaves, as evidenced by herbarium specimens.
Regardless of seleniferous heterogeneity in soils, it is expected that natural collected hyperaccumulator plants will have a strong natural variability of Se, given previous evidence; sites that gave rise to record accumulation in A. bisulcatus were later revisited and samples contained considerably less Se, with the highest bulk tissue results only reaching ‘secondary accumulator’ status (Shrift and Ulrich 1969; Sors et al. 2009). Similarly, sampling of S. pinnata from the same general field site demonstrated considerable difference in measured leaf Se range in less than a decade (Cappa et al. 2014; Lima et al. 2022). It is important to consider temporal variability when it comes to the seleniferous ecologies – not just seasonal variability, but also the effect of long-term land use, geological disturbance, and climate.
The overlapping growth of N. amplexicaulis and N. heliophila was at first surprising, before the lower soil Se concentrations were revealed. McCray and Hurwood (1963) had previously recorded N. heliophila outside of the poison strip with elevated but generally ‘non-accumulator concentrations’ of Se, a result that has been validated in this study. In a dosing experiment, N. heliophila growing in spiked soils could tolerate up to 10 mg Se kg−1 as sodium selenate before noticeable mortality, which may be comparable to the Se in Richmond soils accounting for bioavailable factions (Harvey et al. unpublished). Supposedly, higher concentrations of Se in the immediate vicinity of hyperaccumulators due to leaf litter may have an allelopathic effect on non-tolerant species (El Mehdawi et al. 2011a). In the case here, leaf litter from N. amplexicaulis may have a negligible effect on soil Se, due to the low presence of Se in the older leaves experiencing senescence and phloem-based Se-remobilisation (Harvey et al. 2020). Comparatively, low concentrations of soil Se can have a beneficial effect on non-accumulator species (Pilon-Smits et al. 2009) and growing adjacent to hyperaccumulators can be noticeably beneficial to more Se tolerant non-accumulators through increases in biomass and protection from herbivory through the cycling and remobilisation of Se (El Mehdawi et al. 2011a, b). Neptunia amplexicaulis may be providing this nurse plant effect to the hardier plants sharing its habitat, especially given the overall lower Se concentrations present in the soil and older leaves.
Other elements from the Toolebuc formation and their relationship to plants
The presence of Mo in the soil, while observations could only be limited to soluble and bioavailable fractions, was low. Arguably, total soil concentrations of Mo may be comparable to concentrations found in Yellow Cat, (<7–13 mg Mo kg−1) based on the concentrations of bioavailable Mo at Silver Hills (Cannon 1964). It was interesting to find strong accumulation of Mo in A. tephrina and N. amplexicaulis from soils with such low Mo; similarly, higher concentrations of Mo were recorded in Se accumulator species in Yellow Cat, between 100 and 1000 μg Mo g−1 on soils with 1–3 mg Mo kg−1 (Cannon 1964). The highest Mo accumulation at Yellow Cat occurred in the shoots of an Astragalus species - there have been similar results regarding the higher accumulation of Mo in Se hyperaccumulators in the field more recently (DeTar et al. 2015). Under controlled conditions this effect has yet to be replicated: in a dosing experiment, significant accumulation of Mo (>1000 μg Mo g−1) was measured in Astragalus species regardless of Se hyperaccumulator or non-accumulator status, though it should be noted that enhanced Mo accumulation did not reduce Se concentrations within the plants studied (DeTar et al. 2015). For N. amplexicaulis and A. tephrina, despite Mo accumulation, Mo in soils would not have been concentrated enough to inhibit Se accumulation. It has been assumed that enhanced accumulation of Mo in Se plants is due to the similarity between the molybdate and sulphate ions – if upregulation of sulphate transporters is responsible for the increased accumulation of selenate, then molybdate would also be accumulated (Schiavon et al. 2015; Shinmachi et al. 2010). However, without the capacity to replicate this effect in controlled conditions, there may be some transport mechanism with a preference of Se over Mo (DeTar et al. 2015). Similarly, it was noted that S. pinnata transporters likely prefer Se over Mo, as increasing soil Se lowered supplied Mo concentrations in the plant, when compared to a non-accumulator (Harris et al. 2014).
In contrast, V accumulation, discussed here due to the oil-shale geochemistry of the Toolebuc Formation, was found most prominently in Salsola australis, a common saltbush in the area. The highest V specimens came from the Silver Hills Station site, though V was more concentrated in the soils at St. Elmo Station. In most instances, plants with reported concentrations of V seem to accumulate more V in the roots than aerial tissues (Aihemaiti et al. 2020; Tian et al. 2015; Vachirapatama et al. 2011). According to a recent review, plant V concentrations from naturally V-enriched sites can exceed concentrations of 100 μg V g−1 in the roots, but rarely exceed 40 μg V g−1 in the shoots, with the exception of Settaria viridis (Poaceae), which recorded up to 157 μg V g−1 in the shoots in Hubei Province, China (Hanus-Fajerska et al. 2021). In other cases, bulk shoot tissues from species found growing near a V mine with comparable total V soil concentrations only accumulated up to 11 μg V g−1, with notable toxicity effects (Panichev et al. 2006). Fellow Chenopodiaceae species Beta vulgaris, however, did accumulate comparable concentrations of V to S. australis, though not approaching the 66 μg V g−1 maximum found in S. australis (Khan et al. 2011). In most other cases, exceptionally high V tissue concentrations are induced by growing crop plants in controlled hydroponic conditions, circumstances which are difficult to compare to field conditions (van der Ent et al. 2015). In any case, keeping track of V concentrations (as well as Se and Mo) in plants grown on Toolebuc Formation-derived soils will be important, particularly with the risk of changing land use disturbing potential trace element enriched sources (Multicom 2021). Considering the V enriched geology of Yellow Cat, Utah, plants were often seen as not indicators for V due to their actual V concentrations, but due to their distribution over V enriched deposits (Cannon 1964). This was influenced by the overlying surface geology, soils, water table presence and species i.e., S. pinnata was not indicative of V enrichment due to its intolerance of growing on metalliferous soils. Perhaps N. amplexicaulis may be an indicator for V-enriched soils, and thus V-enriched orebodies, due to distribution by association, and not by quantifiable within-tissue V concentrations.
Ecological implications of selenium hyperaccumulation
It has been a long-held suggestion that Se uptake in hyperaccumulators is an herbivory defence (Boyd 2007; El Mehdawi and Pilon-Smits 2012; Quinn et al. 2008). Various species of insects, fungi and even small mammals have been reported to be deterred by Se hyperaccumulation in S. pinnata and A. bisulcatus or enhanced Se in non-accumulators (Freeman et al. 2010; Hanson et al. 2003; Quinn et al. 2010). However, field studies have shown that increases of tissue Se concentration in S. pinnata may not significantly deter predators, as Se-resistant insects can be found in seleniferous areas (Freeman et al. 2012; Lima et al. 2022). There is also evidence that insect-plant interactions in the form of floral visitors such as honeybees may result in no effect on the basis of enhanced floral Se (Quinn et al. 2011), or may suffer from Se toxicity, especially if flowers contain Se as selenate (Hladun et al. 2012). The role that herbivores and insects in the cycling of Se throughout a seleniferous ecosystem is largely understudied – in Richmond, this effect is almost entirely unknown. Evidence of widespread insect herbivory of N. amplexicaulis was observed in the field, though whether this was generalists unaffected by the lower Se concentrations (like in S. pinnata) or specifically adapted herbivores remains to be seen (Quinn et al. 2011). Floral visitors, regardless of the selfing nature of this species, would be affected by the highly enriched Se in the ovaries, anthers, and pollen of the flowers (Harvey et al. 2020).
The continued presence of Se in the ecosystem has important implications for the survival of N. amplexicaulis. This species is seemingly restricted to allegedly seleniferous soils; this could be because it outcompetes species in a seleniferous niche that it would not be able to compete against elsewhere, but there may be a stronger physiological role that Se plays in this plant. Indeed, it has been suggested that Se may hold a degree of essentiality for plants, although this is still far from proven conclusively (Pilon-Smits and Quinn 2010). We did find that growing N. amplexicaulis in soil or hydroponics without Se results in obvious malformation to the leaves and inability to flower (Harvey et al. unpublished) and that young N. amplexicaulis exhibit root seeking behaviour and fine root proliferation in Se-spiked soils (Goodson et al. 2003; Pinto Irish et al. 2021). This is a strong clue that N. amplexicaulis may have a physiological need for Se, but this requires identification of the nature of the dependence on Se in further research.
What is observed in Richmond is a unique type of ecosystem in Australia. All observed sightings of N. amplexicaulis, few as they are, may be a key indicator for the extent of this ecosystem in Queensland. However, these sightings only roughly match the range of where the Toolebuc Formation coincides with the Flinders Highway and publicly accessible land, and much of the range of N. amplexicaulis beyond this is largely unknown. Thus, the landscape changes over time, and the ongoing impact of changing land use, may also remain unknown. Disruption to seleniferous ecosystems in central Queensland have occurred over the last two centuries given the bovine grazing agricultural land use and introduction of pasture grass species, bringing about changes to the functioning of this ecosystem. The Toolebuc Formation is increasingly being developed as a mineable source of V, in particular with new mining projects starting in the next few years, due to the current global focus on the need for clean energy resources. Neptunia amplexicaulis is a localised endemic species, with a limited and vulnerable habitat due to its Se-specialisation which may be sensitive to the changes in Se geology, seleniferous soils and ecosystems. This plant and its region should be more thoroughly and systematically studied and monitored in the coming decades in better understand the environmental responses in Australia’s unique seleniferous areas.
Data availability
The datasets used and generated during the formulation of this research can be accessed upon request to the corresponding author.
References
Aihemaiti A, Gao Y, Meng Y, Chen X, Liu J, Xiang H, Xu Y, Jiang J (2020) Review of plant-vanadium physiological interactions, bioaccumulation, and bioremediation of vanadium-contaminated sites. Sci Total Environ 712:135637
Anderson JW (1993) Selenium interactions in sulfur metabolism. Sulfur nutrition and assimilation in higher plants: regulatory, agricultural and environmental aspects. SPB Academic Publishing, The Hague
Beath OA, Draize JH, Eppson HF, Gilbert CS, McCreary OC (1934) Certain poisonous plants of Wyoming activated by selenium, and their association with respect to soil types. J Am Pharm Assoc 23:94–97
Beath OA, Gilbert CS, Eppson HF (1939) The use of indicator plants in locating seleniferous areas in western United States. I General Am J Bot 26:257–269. https://doi.org/10.1002/j.1537-2197.1939.tb12900.x
Boreham CJ, Powell TG (1987) Sources and preservation of organic matter in the cretaceous Toolebuc formation, eastern Australia. Org Geochem 11:433–449. https://doi.org/10.1016/0146-6380(87)90001-5
Boyd RS (2007) The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant Soil 293:153–176. https://doi.org/10.1007/s11104-007-9240-6
Brown T, Shrift A (1982) Selenium: toxicity and tolerance in higher plants. Biol Rev Camb Philos Soc 57:59–84
Bureau of Meteorology (2022) Daily Rainfall, Richmond Airport. Commonwealth of Australia. http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=136&p_display_type=dailyDataFile&p_startYear=2019&p_c=-181983072&p_stn_num=030161 Accessed 12/06/2022
Burnell JN (1981) Selenium metabolism in Neptunia amplexicaulis. Plant Physiol 67:316–324. https://doi.org/10.1104/pp.67.2.316
Cannon HL (1964) Geochemistry of rocks and related soils and vegetation in the Yellow Cat area, Grand County, Utah. US Geological Survey Bulletin 1176. https://doi.org/10.3133/b1176
Cappa J, Pilon-Smits E (2014) Evolutionary aspects of elemental hyperaccumulation. Int J Plant Biol 239:267–275. https://doi.org/10.1007/s00425-013-1983-0
Cappa JJ, Cappa PJ, El Mehdawi AF, McAleer JM, Simmons MP, Pilon-Smits EAH (2014) Characterization of selenium and sulfur accumulation across the genus Stanleya (Brassicaceae): a field survey and common-garden experiment. Am J Bot 101:830–839. https://doi.org/10.3732/ajb.1400041
Cary EE, Kubota J (1990) Chromium concentration plants: effects of soil chromium concentration and tissue contamination by soil. J Agric Food Chem 38:108–114
Coxhell S, Fehlberg B (2000) Julia Creek vanadium and oil shale deposit. Paper 2000-11 AIG Journal
DeTar RA, Alford ÉR, Pilon-Smits EAH (2015) Molybdenum accumulation, tolerance and molybdenum–selenium–sulfur interactions in Astragalus selenium hyperaccumulator and nonaccumulator species. J Plant Physiol 183:32–40. https://doi.org/10.1016/j.jplph.2015.05.009
Draize JH, Beath OA (1935) Observation on the pathology of ‘blind staggers’ and ‘alkali disease’. J Am Vet Med Assoc 86:753–763
El Mehdawi AF, Pilon-Smits EAH (2012) Ecological aspects of plant selenium hyperaccumulation. Plant Biol 14:1–10. https://doi.org/10.1111/j.1438-8677.2011.00535.x
El Mehdawi AF, Quinn CF, Pilon-Smits EAH (2011a) Effects of selenium hyperaccumulation on plant–plant interactions: evidence for elemental allelopathy? New Phytol 191:120–131. https://doi.org/10.1111/j.1469-8137.2011.03670.x
El Mehdawi AF, Quinn Colin F, Pilon-Smits Elizabeth AH (2011b) Selenium hyperaccumulators facilitate selenium-tolerant neighbors via phytoenrichment and reduced herbivory. Curr Biol 21:1440–1449. https://doi.org/10.1016/j.cub.2011.07.033
El Mehdawi AF, Reynolds RJB, Prins CN, Lindblom SD, Cappa JJ, Fakra SC, Pilon-Smits EAH (2014) Analysis of selenium accumulation, speciation and tolerance of potential selenium hyperaccumulator Symphyotrichum ericoides. Physiol Plant 152:70–83. https://doi.org/10.1111/ppl.12149
Frank A, Madej A, Galgan V, Petersson LR (1996) Vanadium poisoning of cattle with basic slag. Concentrations in tissues from poisoned animals and from a reference, slaughter-house material. Sci Total Environ 181(1):73–92. https://doi.org/10.1016/0048-9697(95)04962-2
Freeman J, Tamaoki M, Stushnoff C, Quinn CF, Cappa JJ, Devonshire J, Fakra S, Marcus M, McGrath SP, Van Hoewyk D, Pilon-Smits EAH (2010) Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol 153:1630–1652. https://doi.org/10.1104/pp.110.156570
Freeman JL, Marcus MA, Fakra SC, Devonshire J, McGrath SP, Quinn CF, Pilon-Smits EAH (2012) Selenium hyperaccumulator plants Stanleya pinnata and Astragalus bisulcatus are colonized by Se-resistant, Se-excluding wasp and beetle seed herbivores (seed herbivores – selenium hyperaccumulator plants). 7:e50516. https://doi.org/10.1371/journal.pone.0050516
Goodson CC, Parker DR, Amrhein C, Zhang Y (2003) Soil selenium uptake and root system development in plant taxa differing in Se-accumulating capability. New Phytol 159:391–401. https://doi.org/10.1046/j.1469-8137.2003.00781.x
Gray ARG, McKillop M, McKellar JL (2002) Eromanga basin stratigraphy. In: JJ Draper (ed) Geology of the Cooper and Eromanga Basins, Queensland. Queensland Department of Natural Resources and Mines, Queensland Minerals and Energy Review Series, Brisbane, Australia pp 30–56
Hanson B, Garifullina GF, Lindblom SD, Wangeline A, Ackley A, Kramer K, Norton AP, Pilon-Smits CBLEAH (2003) Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol 159:461–469. https://doi.org/10.1046/j.1469-8137.2003.00786.x
Hanus-Fajerska E, Wiszniewska A, Kamińska I (2021) A dual role of vanadium in environmental systems—beneficial and detrimental effects on terrestrial plants and humans. Plants 10:1110
Harris J, Schneberg KA, Pilon-Smits EAH (2013) Sulfur–selenium–molybdenum interactions distinguish selenium hyperaccumulator Stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 239:479–491. https://doi.org/10.1007/s00425-013-1996-8
Harris J, Schneberg KA, Pilon-Smits EA (2014) Sulfur–selenium–molybdenum interactions distinguish selenium hyperaccumulator Stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 239:479–491
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318. https://doi.org/10.1016/j.jtemb.2005.02.009
Harvey M-A, Erskine PD, Harris HH, Brown GK, Pilon-Smits EAH, Casey LW, Echevarria G, Van Der Ent A (2020) Distribution and chemical form of selenium in Neptunia amplexicaulis from Central Queensland, Australia. Metallomics 12:514–527. https://doi.org/10.1039/c9mt00244h
Hladun KR, Smith BH, Mustard JA, Morton RR, Trumble JT (2012) Selenium toxicity to honey bee (Apis mellifera l.) pollinators: effects on behaviors and survival. PLoS One 7:e34137–e34137. https://doi.org/10.1371/journal.pone.0034137
Jaffré T, Brooks RR, Lee J, Reeves RD (1976) Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193:579–580. https://doi.org/10.1126/science.193.4253.579
Jiang K, Lin C, Zhang X, He W, Xiao F (2018) Geochemical characteristics and possible origin of shale gas in the Toolebuc formation in the northeastern part of the Eromanga Basin, Australia. J Nat Gas Sci Eng 57:68–76. https://doi.org/10.1016/j.jngse.2018.06.031
Khan S, Kazi TG, Kolachi NF, Baig JA, Afridi HI, Shah AQ, Kumar S, Shah F (2011) Hazardous impact and translocation of vanadium (V) species from soil to different vegetables and grasses grown in the vicinity of thermal power plant. J Hazard Mater 190:738–743
Knott SG, McCray CWR (1959) Two naturally occurring outbreaks of selenosis in Queensland. Aust Vet J 35:332–334. https://doi.org/10.1111/j.1751-0813.1959.tb08505.x
Kukier U, Chaney RL (2001) Amelioration of nickel phytotoxicity in muck and mineral soils. J Environ Qual 30(6):1949–1960
Lewis S, Henderson R, Dickens G, Shields G, Coxhell S (2010) The geochemistry of primary and weathered oil shale and coquina across the Julia Creek vanadium deposit (Queensland, Australia). Int J Geol Mineral Geochem Min Deposits 45:599–620. https://doi.org/10.1007/s00126-010-0287-6
Li Z, Liang D, Peng Q, Cui Z, Huang J, Lin Z (2017) Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: a review. Geoderma 295:69–79. https://doi.org/10.1016/j.geoderma.2017.02.019
Lima LW, Castleberry M, Wangeline AL, Aguirre B, Dall’Acqua S, Pilon-Smits EA, Schiavon M (2022) Hyperaccumulator Stanleya pinnata: in situ fitness in relation to tissue selenium concentration. Plants 11:690
Lindblom SD, Valdez-Barillas JR, Fakra SC, Marcus MA, Wangeline AL, Pilon-Smits EAH (2013) Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environ Exp Bot 88:33–42. https://doi.org/10.1016/j.envexpbot.2011.12.011
Mao X, Rodrigues S, Esterle JS, Golding SD (2023) Reservoir lithofacies study of the Toolebuc formation, Eromanga Basin, Australia as a potential unconventional target. Int J Coal Geol 265:104163. https://doi.org/10.1016/j.coal.2022.104163
Marschner H (2011) Marschner's mineral nutrition of higher plants. Academic press
McCray CWR, Hurwood IS (1963) Selenosis in north west Queensland associated with marine cretaceous formation. Queensland J Agric Sci 20:475–498
McCrindle CME, Mokantla E, Duncan N (2001) Peracute vanadium toxicity in cattle grazing near a vanadium mine. J Environ Monit 3:580–582. https://doi.org/10.1039/B107244G
Multicom (2021) Environmental Impact Statement, Saint Elmo Vanadium project. In: MR Limited (ed) https://saintelmoproject.com.au, Brisbane. Accessed 06/05/2022
Nawaz MA, Chen C, Shireen F, Zheng Z, Jiao Y, Sohail H, Afzal M, Imtiaz M, Ali MA, Huang Y (2018) Improving vanadium stress tolerance of watermelon by grafting onto bottle gourd and pumpkin rootstock. Plant Growth Regul 85:41–56
Ng BH, Anderson JW (1978) Synthesis of selenocysteine by cysteine synthases from selenium accumulator and non-accumulator plants. Phytochemistry 17:2069–2074. https://doi.org/10.1016/S0031-9422(00)89282-1
O'Toole D, Raisbeck MF (1995) Pathology of experimentally induced chronic selenosis (alkali disease) in yearling cattle. J Vet Diagn Invest 7:364–373. https://doi.org/10.1177/104063879500700312
Ozimic S (1986) The geology and petrophysics of the Toolebuc formation and its time equivalents, Eromanga and Carpentaria basins. Spec Publ Geol Soc Aust 12:119–137
Panichev N, Mandiwana K, Moema D, Molatlhegi R, Ngobeni P (2006) Distribution of vanadium(V) species between soil and plants in the vicinity of vanadium mine. J Hazard Mater 137:649–653. https://doi.org/10.1016/j.jhazmat.2006.03.006
Patterson JH, Ramsden AR, Dale LS, Fardy JJ (1986) Geochemistry and mineralogical residences of trace elements in oil shales from Julia Creek, Queensland, Australia. Chem Geol 55:1–16. https://doi.org/10.1016/0009-2541(86)90123-3
Pedley L (1981) Further notes on Acacia in Queensland. Austrobaileya 1:339–345
Peterson PJ, Butler GW (1962) The uptake and assimilation of selenite by higher plants. Aust J Biol Sci 5:126–146
Peterson PJ, Butler GW (1967) Significance of selenocystathionine in an Australian selenium-accumulating plant, Neptunia amplexicaulis. Nature 213:599. https://doi.org/10.1038/213599a0
Peterson PJ, Robinson PJ (1972) L-cystathionine and its selenium analogue in Neptunia amplexicaulis. Phytochemistry 11:1837–1839. https://doi.org/10.1016/0031-9422(72)85047-7
Pilon-Smits EA, Quinn CF (2010) Selenium metabolism in plants. In: Hell, R., Mendel, RR. (eds) Cell Biology of Metals and Nutrients. Plant Cell Monographs, vol 17. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-10613-2_10
Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12:267–274. https://doi.org/10.1016/j.pbi.2009.04.009
Pinto Irish K, Harvey M-A, Erskine PD, van der Ent A (2021) Root foraging and selenium uptake in the Australian hyperaccumulator Neptunia amplexicaulis and non-accumulator Neptunia gracilis. Plant Soil 462:219–233. https://doi.org/10.1007/s11104-021-04843-x
Quinn CF, Freeman J, Galeas ML, Klamper EM, Pilon-Smits EAH (2008) The role of selenium in protecting plants against prairie dog herbivory: implications for the evolution of selenium hyperaccumulation. Oecologia 155:267–275. https://doi.org/10.1007/s00442-007-0907-8
Quinn CF, Freeman JL, Reynolds RJB, Cappa JJ, Fakra SC, Marcus MA, Lindblom SD, Quinn EK, Bennett LE, Pilon-Smits EAH (2010) Selenium hyperaccumulation offers protection from cell disruptor herbivores. BMC Ecol 10:19. https://doi.org/10.1186/1472-6785-10-19
Quinn CF, Prins CN, Freeman JL, Gross AM, Hantzis LJ, Reynolds RJB, In Yang S, Covey PA, Bañuelos GS, Pickering IJ, Fakra SC, Marcus MA, Arathi HS, Pilon-Smits EAH (2011) Selenium accumulation in flowers and its effects on pollination. New Phytol 192:727–737. https://doi.org/10.1111/j.1469-8137.2011.03832.x
Riley KW, Saxby JD (1982) Association of organic matter and vanadium in oil shale from the Toolebuc formation of the Eromanga Basin, Australia. Chem Geol 37:265–275. https://doi.org/10.1016/0009-2541(82)90082-1
Rosenfeld I, Beath OA (1964) Selenium: Geobotany, biochemistry, toxicity and nutrition. Academic Press, New York
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588. https://doi.org/10.1126/science.179.4073.588
Ryan CG (2000) Quantitative trace element imaging using PIXE and the nuclear microprobe. Int J Imaging Syst Technol 11:219–230
Ryan CG, Jamieson DN (1993) Dynamic analysis: on-line quantitative PIXE microanalysis and its use in overlap-resolved elemental mapping. Nucl Instrum Methods Phys Res, Sect B 77:203–214
Ryan CG, Cousens DR, Sie SH, Griffin WL (1990) Quantitative analysis of PIXE spectra in geoscience applications. Nucl Instrum Methods Phys Res, Sect B 49:271–276
Ryan C, Etschmann B, Vogt S, Maser J, Harland C, Van Achterbergh E, Legnini D (2005) Nuclear microprobe–synchrotron synergy: towards integrated quantitative real-time elemental imaging using PIXE and SXRF. Nucl Instrum Methods Phys Res, Sect B 231:183–188
Schiavon M, Pilon-Smits EAH (2016) The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytol 213:1582–1596. https://doi.org/10.1111/nph.14378
Schiavon M, Pilon M, Malagoli M, Pilon-Smits EAH (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation—a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00002
Schwarz K, Foltz CM (1957) Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc 79:3292–3293. https://doi.org/10.1021/ja01569a087
Senior BR, Exon NF, Burger D (1975) The Cadna-owie and Toolebuc formations in the Eromanga Basin, Queensland. Queensland Government Mining Journal 76:445–455
Sharmasarkar S, Vance GF (1995) Fractional partitioning for assessing solid-phase speciation andgeochemical transformations of soil selenium. Soil Sci 160(1):43–55
Shinmachi F, Buchner P, Stroud JL, Parmar S, Zhao F-J, McGrath SP, Hawkesford MJ (2010) Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol 153:327–336
Shrift A, Ulrich JM (1969) Transport of selenate and selenite into Astragalus roots. Plant Physiol 44:893–896. https://doi.org/10.1104/pp.44.6.893
Soltanpour PN, Schwab AP (1977) A new soil test for simultaneous extraction of macro- and micro-nutrients in alkaline soils. Commun Soil Sci Plant Anal 8:195–207. https://doi.org/10.1080/00103627709366714
Sors TG, Martin CP, Salt DE (2009) Characterization of selenocysteine methyltransferases from Astragalus species with contrasting selenium accumulation capacity. Plant J 59:110–122. https://doi.org/10.1111/j.1365-313X.2009.03855.x
Statwick J, Sher AA (2017) Selenium in soils of western Colorado. J Arid Environ 137:1–6. https://doi.org/10.1016/j.jaridenv.2016.10.006
Statwick J, Majestic BJ, Sher AA (2016) Characterization and benefits of selenium uptake by an Astragalus hyperaccumulator and a non-accumulator. Plant Soil 404:345–359. https://doi.org/10.1007/s11104-016-2842-0
Tian L-Y, Yang J-Y, Huang J-H (2015) Uptake and speciation of vanadium in the rhizosphere soils of rape (Brassica juncea L.). Environ Sci Pollut Res 22:9215–9223
Vachirapatama N, Jirakiattikul Y, Dicinoski G, Townsend AT, Haddad PR (2011) Effect of vanadium on plant growth and its accumulation in plant tissues. Sonklanakarin J Sci Technol 33:255
van der Ent A, Baker A, Reeves RD, Pollard A, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Int J Plant-Soil Relationsh 362:319–334. https://doi.org/10.1007/s11104-012-1287-3
van der Ent A, Baker A, Reeves R, Pollard A, Schat H (2015) Commentary: toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00554
van der Ent A, Ocenar A, Tisserand R, Sugau JB, Echevarria G, Erskine PD (2019) Herbarium X-ray fluorescence screening for nickel, cobalt and manganese hyperaccumulator plants in the flora of Sabah (Malaysia, Borneo Island). J Geochem Explor 202:49–58
Vine RR, Jauncey W, Chertok I (1963) Julia Creek, sheet SF 54–03 1st edn. 1:250 000 Geological Series. Australia: Bureau of Mineral Resources, Geology and Geophysics
Vine RR, Bastian LV, Casey DJ, Chertok I (1970) Richmond, sheet SF 54–04 1st edn. 1:250 000 Geological Series. Australia: Bureau of Mineral Resources, Geology and Geophysics
Vine RR, Bastian LV, Paine AGL, Harding RR, De Keyser F, De Ferranti RZ, Reynolds MA, Casey DJ, Clarke DE, Day RW, Chertok I (1974) Hughenden, sheet SF 55–01 1st edn. 1:250 000 Geological Series. Australia: Bureau of Mineral Resources, Geology and Geophysics
Wang Y, Kanipayor R, Brindle ID (2014) Rapid high-performance sample digestion for ICP determination by ColdBlock™ digestion: part 1 environmental samples. J Anal At Spectrom 29:162–168. https://doi.org/10.1039/C3JA50333J
Weekley CM, Harris HH (2013) Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev 42:8870–8894. https://doi.org/10.1039/c3cs60272a
Weekley CM, Aitken JB, Finney L, Vogt S, Witting PK, Harris HH (2013) Selenium metabolism in cancer cells: the combined application of XAS and XFM techniques to the problem of selenium speciation in biological systems. Nutrients 5:1734. https://doi.org/10.3390/nu5051734
Weekley CM, Aitken JB, Witting PK, Harris HH (2014) XAS studies of Se speciation in selenite-fed rats. Metallomics 6:2193–2203
White PJ (2016) Selenium accumulation by plants. Ann Bot 117:217–235. https://doi.org/10.1093/aob/mcv180
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937. https://doi.org/10.1093/jxb/erh192
White PJ, Bowen HC, Marshall B, Broadley MR (2007) Extraordinarily high leaf selenium to sulfur ratios define 'Se-accumulator' plants. Ann Bot 100:111–118. https://doi.org/10.1093/aob/mcm084
Windler DR (1966) A revision of the genus Neptunia (Leguminosae). Aust J Bot 14:379–420. https://doi.org/10.1071/BT9660379
Acknowledgements
The authors would like to acknowledge the Tritton family from the Silver Hills Station and the Hick Family from St. Elmo Station for allowing access to their property for sample collection. We would also like to acknowledge the Richmond Shire council for approving the collection of samples from roadside areas. We thank Philip Nkrumah for operating the ICP-AES for the sample analysis. Thanks to Raphael Colin for help preparing field plant samples for analysis and performing the pXRF scanning. This research was undertaken on the XAS beamline at the Australian Synchrotron, part of ANSTO. We thank Bernt Johannessen (ANSTO) for support during the XAS experiment. M-A. Harvey was the recipient of a UQ Graduate School RTP Scholarship. This research forms part of the PhD thesis of M-A. Harvey.
Funding
This publication is part of the project “Living on the edge: unravelling the secrets of selenium hyperaccumulator plants” (with project number VI.Vidi.213.037) of the research programme ENW-VIDI which is (partly) financed by the Dutch Research Council (NWO). A. van der Ent acknowledges The Australian Academy of Science for the 2021 Thomas Davies Research Grant for Marine, Soil and Plant Biology which funded part of this project.
Author information
Authors and Affiliations
Contributions
Maggie-Anne Harvey and Antony van der Ent conceptualized and designed the study. Maggie-Anne Harvey, Antony van der Ent and Peter D. Erskine conducted the fieldwork. Maggie-Anne Harvey undertook the chemical analysis of soil and plant samples and conducted the statistical analysis and interpretation. Hugh H. Harris, Maggie-Anne Harvey and Antony van der Ent conducted the XAS experiment, and Hugh H. Harris and Jemma I. Virtue performed the XAS data analysis and interpretation. Maggie-Anne Harvey, Antony van der Ent, Hugh H. Harris and Peter D. Erskine wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest relevant to the content of this manuscript.
Additional information
Responsible Editor: Fangjie Zhao.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 88 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harvey, MA., Erskine, P.D., Harris, H.H. et al. Plant-soil relations of selenium, molybdenum and vanadium in the Richmond District of Central Queensland, Australia. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06633-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06633-7