Abstract
Background and aims
Agroforestry, whereby trees are associated with crops and/or livestock, is expected to mitigate nitrogen (N) losses from agriculture. However, little is known about how nitrification potential, an important process that drives N losses, is affected by agroforestry systems. This study aimed to investigate the effect of different silvopastoral agroforestry systems on soil nitrification potential.
Methods
Nitrification potential was evaluated in two agroforestry systems (hedgerow and alley cropping) associated with temporary grasslands in Brittany, France. In each system, soil was sampled along a transect spanning from the center of the tree row into the grass alley. Soil nitrification potential was determined ex situ and was explained by univariate and multivariate analysis of variables describing vegetation, physicochemical soil properties, and soil organisms.
Results
Nitrification potential differed between the two agroforestry systems and among the positions in relation to the trees. In the alley cropping system, nitrification potential was on average 1.5 times higher in the tree row than at 1.5 and 10 m into the grass alley, while in the hedgerow system, nitrification potential at 1.5 m into the grass alley was on average 40% lower than at 10 m into the grass alley. Nitrification potential was strongly correlated with soil pH, whereas no correlation was observed between nitrification potential and community size of soil nitrifiers.
Conclusion
Our results point out the diverse effects of agroforestry systems on nitrification, a key soil process that is involved in the regulation of N losses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agroforestry, i.e. the spatial combination of trees and crops and/or livestock on the same field, has gained attention as a sustainable agricultural practice that can contribute to developing multifunctional and resilient agroecosystems that promote ecosystem services (IPCC 2022, Veldkamp et al. 2023). Among these ecosystems services, the regulation of the nitrogen (N) cycle in agroecosystems is critical (Rockström et al. 2009). N is a key element for agricultural production in agroecosystems such as in agroforestry systems. Depending on the system, plants take up approximately half of the applied N (Lassaletta et al. 2014), while the remaining N poses a risk of being lost from the system, which greatly disrupts the global biogeochemical N cycle (Rockström et al. 2009). Thus, N losses threaten the integrity of ecosystems by negatively impacting soil, air and water quality, as well as contributing to climate change and the decline in biodiversity (e.g. Erisman et al. 2011; Galloway et al. 2003). In the context of climate change, N losses may increase due to the increasing frequency of extreme weather events (Bowles et al. 2018; Greaver et al. 2016; Jeppesen et al. 2011). Understanding the internal N cycle (i.e. the processes that drive the transformation and retention of N in the soil-plant-continuum) helps to identify the drivers that can mitigate such losses (Elrys et al. 2022).
Nitrification, which is the oxidation of ammonium (NH4+) to nitrate (NO3− ), is a key process that controls the fate of N in soils, especially regarding the formation of NO3− and the regulation of the denitrification pathway (Beeckman et al. 2018). Three types of nitrification pathways have been reported in agricultural soils (Gao et al. 2022; Norton 2008; Robertson and Groffman 2015): (i) autotrophic nitrification, which is performed in two steps under aerobic conditions by ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA) and nitrite oxidizing bacteria (NOB); (ii) heterotrophic nitrification, which is performed under aerobic conditions by certain groups of fungi and bacteria; and (iii) anammox, an anaerobic oxidation of NH4+ to N2 by nitrite-dependent bacteria. In agricultural soils, the size, composition and activity of nitrifying communities (nitrifiers) are governed by several biotic and abiotic factors (Norton 2008; Prosser and Nicol 2012; Robertson and Groffman 2015). For example, soil physical and chemical properties (e.g. NH4+-N content, NO3−-N content, soil pH) were described as the main drivers of niche differentiation for multiple nitrifiers (Clark et al. 2020; Jia et al. 2020; Prosser and Nicol 2012; Zhang et al. 2023). Soil structure, texture and water content also influence activity of nitrifiers since they drive soil oxygen availability (Clark et al. 2020; Robertson and Groffman 2015). Moreover, Abalos et al. (2019) reported direct and indirect effects of plant traits on N-cycling processes, including activity of nitrifiers, such as the inhibition of nitrification by root exudates.
In areas of high livestock density, controlling nitrification could help decrease losses of N (Di and Cameron 2016). In grazed grasslands especially, NH4+-N inputs due to organic and inorganic N inputs such as fertilizers and animal waste, stimulate nitrification (Bei et al. 2021; Clark et al. 2020; Di and Cameron 2016; Jia et al. 2020). Moreover, grasslands often contain multiple plant species, including legumes, which increase nitrification rates (Le Roux et al. 2013). High nitrification activity in grassland soils has often been attributed to the size and activity of AOB populations (Di and Cameron 2018; Le Roux et al. 2013); however, a recent meta-analysis highlighted that heterotrophic nitrification performed by bacteria and fungi represented a non-negligible part of nitrification in grasslands (Zhang et al. 2023).
In livestock production areas, agroforestry (i.e. trees combined with crops and/or livestock on the same field) is increasingly supported by local stakeholders and successfully adopted by farmers (den Herder et al. 2017). Accordingly, a variety of silvapastoral agroforestry systems that associate trees with livestock production have been established in these areas. For example, in northwestern France, silvopastoral agroforestry is associated with hedgerows (i.e. trees and shrubs planted on field edges) and alley cropping agroforestry (i.e. multiple tree rows planted in a field). One objectives for establishing these agroforestry systems is to decrease N leaching and thus improve water quality (Viaud and Thomas 2019). In a review, Kim and Isaac (2022) highlighted the influence of trees and their associated understory vegetation on the spatial variability in biological processes involved in regulating the internal N cycle. Thus, soil communities in agroforestry systems have gained increasing attention. For example, a recent study suggested niche differentiation of nitrifier communities in an alley cropping system, in which AOB populations were larger in crop rows than in tree rows (Beule et al. 2019). This niche differentiation was attributed to environmental modifications induced by the presence of trees. For example, tree traits such as crown architecture, leaf and root characteristics influence the microclimate by modifying solar radiation, water uptake and wind speed (Benhamou 2012; Dufour et al. 2012; Kanzler et al. 2019; van Ramshorst et al. 2022). Furthermore, trees and understory vegetation can modify nitrification (Laughlin 2011; Schimel and Hättenschwiler 2007) by increasing above- and belowground litter inputs and decomposition (Battie-Laclau et al. 2020; Cardinael et al. 2019) and through competition for nutrients (Robertson and Groffman 2015; Abalos et al. 2019). Moreover, trees and understory vegetation can improve soil structure (Mettauer et al. 2022; Rivest et al. 2013) and decrease soil pH (Litza 2022; Biffi et al. 2022), both of which drive nitrification in the soil. Lastly, a recent meta-analysis reported that hedgerows and alley cropping agroforestry influenced the carbon (C) cycle differently (Mayer et al. 2022); consequently, these two agroforestry systems may influence the internal N cycle differently.
Although temperate silvopastoral agroforestry systems are encouraged and adopted by local stakeholders to mitigate N losses, little is known on the impact of hedgerows and alley-cropping systems on the internal N cycle (Kim and Isaac 2022). In the present study, we explored for the first time the influence of hedgerow and alley cropping systems on potential soil nitrification, a process that can have implications for N losses from agricultural systems. Soil nitrification potential was assessed at three positions in relation to the trees in two young agroforestry systems in Brittany, France: one with a hedgerow planted as a windbreak and one with an alley cropping design. To follow a holistic approach, nitrification potential was related to other physicochemical soil properties, soil microbiota properties and vegetation properties. According to previous findings on soil organic C (SOC) (Mayer et al. 2022), we hypothesized (i) that nitrification potential would depend on the position in relation to the trees due to the latter’s influence on environmental factors that impact the size and activity of soil nitrifier community and (ii) that the hedgerow would have a higher effect on soil nitrification potential compared to the tree row in the alley cropping system.
Materials and methods
Study site
The study was performed in April-May 2022 on a farm in southwestern Brittany, France (47°45’36.3” N 1°54’54.0” W) (Fig. 1a). The study site had a temperate oceanic climate, with a mean annual temperature of 12.1 °C and mean annual precipitation of 721 mm (1991–2021, CLIMATE-DATA.ORG 2022). The soil was a Luvisol with a silty-loam texture, with a mean of 59% silt, 24% sand and 18% clay. The soil had developed on schist parental material and had a mean depth of 30 cm. The soil had mean pHKCl of 5.4 and mean cation exchange capacity of 9.7 cmol+ kg−1 (NF X 31–130). The study site had north-south slope of 6–10%.
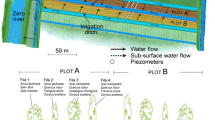
The study site contained two adjacent agroforestry systems (Fig. 1b) that were managed by the same farmer. Both systems represent the main types of silvopastoral agroforestry in the Brittany region. In one system, a hedgerow had been planted in 2012 on the western edge of a 1.8 ha field. The other system, alley cropping, was established in 2014 by planting two tree rows on a 2.1 ha field. In both systems, trees were planted in north-south orientation. The hedgerow consisted of trees planted 4 m apart. Between two adjacent trees, shrubs were planted at a distance of 1 m (Fig. 2). The hedgerow was planted with the following tree species: Viburnum opulus, Populus tremula, Fraxinus sp., Rhamnus frangula, Acer campestre, Prunus avium, Crataegus sp., Malus sylvestris and Sorbus domestica. Tree rows of the alley cropping system consisted of trees planted every 6 m and were separated by a 27 m wide grass alley (Fig. 2). The tree rows were planted with the following tree species: Juglans regia, Castanea crenata, Fraxinus sp., Tilia cordata, S. domestica, and P. avium. In both systems, trees were pruned once per year, in winter, except in 2021. The pruned branches were removed from the systems. The understory vegetation consisted of spontaneous vegetation and was mowed once per year.
In both agroforestry systems, cultivated areas were managed identically. The crop rotation was defined as four years of temporary grassland with ryegrass (Lolium spp.) and red clover (Trifolium pratense), followed by one year of rapeseed (Brassica napus) and one year of a cereal-legume intercrop. The grass was harvested in June and September and grazed by 45 cows and 10 heifers once or twice per year. The grass was not fertilized, and intercrops were fertilized using organic cow manure at the rate of ca. 110 kg N ha−1. Lime was applied every two years with 2 t ha−1 shell sand. During the study period, both systems were in the fourth year of grassland. Neither cattle grazing nor liming had been performed in the month before sampling.
Sampling design
In each agroforestry system, 30 trees were selected (Fig. 1b). In the hedgerow system, the following tree species were selected: Fraxinus sp. (n = 6), P. tremula (n = 5), V. opulus (n = 4), A. campestre (n = 4), P. avium (n = 3), Crataegus sp. (n = 3), R. frangula (n = 2), S. domestica (n = 2) and M. sylvestris (n = 1). In the alley cropping system, the following tree species were selected: Fraxinus sp. (n = 11), S. domestica (n = 10) and P. avium (n = 9). J. regia, C. crenata or T. cordata were not selected because they suffered from diseases and drought. See S.I.1 for the height and circumference of the selected trees. For each selected tree, the soil was sampled and the vegetation biomass was measured in an area of 1 m² at three positions in relation to the tree (Fig. 2): (i) in the tree row 1 m from the tree (position A); (ii) in the grass alley 1.5 m from the tree (position B) and (iii) in the grass alley 10 m from the tree (position C). Position A was always south of the tree, while positions B and C were always east of the tree.
Analysis of soil properties, earthworm abundance, vegetation biomass and soil microorganisms
The protocols used to measure soil properties, earthworm abundance, and vegetation biomass are presented in Table 1. All soil measurements were performed using soil samples collected from a depth of 0–30 cm. On the same sampling date, additional soil samples for the analysis of soil microorganisms by the means of real-time PCR were collected at 0–30 cm soil depth from the soil blocks used for earthworm extraction (see Table 1). Soil samples were stored at -20 °C until DNA extraction. Prior to DNA-extraction, soil samples were freeze-dried for 48 h and finely ground using a vortexer as described by previous study (Beule et al. 2019). Soil DNA was extracted from 50 mg finely ground soil following a cetyltrimethylammonium bromide (CTAB)-based protocol as described by Beule et al. (2019). Briefly, soil was suspended in 1 mL CTAB buffer containing 2 µL 2-mercaptoethanol and 1 µL Pronase E (30 mg/ml (w/v), followed by incubation at 42 °C (10 min) and subsequent incubation at 65 °C (10 min). After incubation, 800 µL phenol was added and the mixture was shaken and centrifuged. The DNA in the supernatant was extracted twice by adding chloroform/isoamylalcohol (24:1 (v/v), followed by incubation on ice (10 min) and centrifugation. Following the second extraction, the supernatant was transferred to a fresh 1.5-mL tube and DNA was precipitated by adding 200 µL PEG 6000 (30% (w/v) and 100 µL 5 M NaCl. Following 20 min of incubation at room temperature, DNA was pelleted by centrifugation, and washed twice with 500 µl 70% (v/v) EtOH to remove remaining salts. The obtained pellets were dried using vacuum centrifugation, resuspended in 50 µL 1× Tris-EDTA) (TE) buffer (10 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), adjusted to pH 8.0 with HCl) and incubated to allow the resuspension of the DNA (42 °C, 2 h). The quantity and quality of the extracted DNA were assessed by agarose gel electrophoresis using 1.0% (w/v) agarose gels stained with SYBR Green I (Thermo Fisher Scientific GmbH, Dreieich,Germany). Prior to real-time PCR, the DNA samples were diluted 1:50 (v/v) in double-distilled H2O to overcome PCR inhibition (Guerra et al. 2020). Soil Ascomycota, Basidiomycota, AOA and AOB community size were quantified based on the number of gene copies as described by Beule et al. (2020). Briefly, amplification was performed in 4 µL reaction volume in a Peqstar 96Q (PEQLAB, Erlangen, Germany) real-time PCR thermocycler. The reaction volume was composed of 3 µL mastermix and 1 µL sample DNA or double-distilled H2O for negative controls. The composition of the mastermix, the choice of primer, and the thermocycling conditions are listed in the Tables S.I.2, S.I.3, and S.I.4, respectively.
Determination of nitrification potential
The potential nitrification rate (PNR) was determined in the laboratory according to ISO 15.685. First, soil samples were stored at ambient temperatures for 2 months to air dry. Next, the samples were sieved at < 1 mm and gently rewetted for two weeks to slowly reach 60% of mean water holding capacity, which was determined from a composite soil sample (Richards and Fireman 1943). Once rewetted, the samples were kept at 20 °C for four weeks at 60% water holding capacity. Next, PNR was determined by adding 10 mL (NH4)2SO4 solution (1.5 mmol L−1) containing sodium chlorate to 2.5 g of soil. Samples were then incubated at 20 °C for 6 h and shaken vertically at 40 rpm to ensure aerobic conditions. Since sodium chlorate restricts oxidation of NO2− to NO3−, NO2−-N production was measured after 2 and 6 h of incubation by colorimetry using a microplate reader (TECAN Infinite® 200 PRO). PNR was expressed as ng NO2-N g dry soil−1 h−1.
Statistical analysis
To increase data quality, the data were pre-treated to exclude statistical outliers (i.e. > 3 times as large as the standard deviation for more than one variable). After removal of 18 outliers, the final dataset contained 162 observations: 80 for the hedgerow system and 82 for the alley cropping system.
Linear models were used to analyze (i) effects of the agroforestry system (hedgerow vs. alley cropping), (ii) the position in relation to the tree (A, B and C) and (iii) interactions between them. We performed analysis of variance (ANOVA) of each model when residual normality and homoscedasticity were met according the Shapiro-Wilk and Levene tests (car package; Fox and Weisberg 2018). If at least one of these conditions was not met, a Kruskal-Wallis test with Bonferroni adjusted p-values was performed. Post-hoc analyses with Tukey’s honestly significant difference test or with Bonferroni adjustment were performed after the ANOVA and Kruskal-Wallis test, respectively. The contribution of each factor to the overall R2 of each linear model was determined.
Redundancy analysis (RDA) was performed (rda() function; vegan package, Legendre and Legendre 2012) to assess effects of soil and vegetation properties on the nitrification potential and microbial community size. Before the RDA, replicates with missing variables were removed, and data were Z-standardized (n = 105). To identify soil and vegetation variables that significantly affected nitrification potential and microbial community size, we used the step() function (stats package), which added soil and vegetation variables individually to the RDA model. Variables were selected if they decreased the Akaike information criterion (AIC) of the RDA model. Relations among nitrification potential, microbial community size and soil and vegetation properties, as well as the effect of the agroforestry system and the position in relation to the tree on these relations, were analyzed using the developed model. Additionally, Spearman’s rank correlation analysis (cor () function; stats package) was performed to complement the RDA analysis on the strength of the relations among all studied variables. Statistical significance of the correlations was tested using the cor.mtest() function (corrplot package). We considered p-values ≤ 0.05 as statistically significant. All statistical analyses were performed using R statistical software v.4.2.0 (R Core Team 2022).
Results
Influence of the agroforestry system and position in relation to the tree on nitrification potential and microbial community size
The agroforestry system, the position in relation to the tree and the interaction between these two factors significantly influenced PNR (Table 2). The position and the interaction between the position and the agroforestry system explained the most variability in PNR (46% and 42%, respectively). Thus, the spatial patterns of PNR differed between the agroforestry systems (Fig. 3). In the alley cropping system, PNR was higher in the tree row (position A, 199.2 ± 56.7 ng NO2-N g dry soil−1 h−1) than in the grass alley in positions B (136.9 ± 32.8 ng NO2-N g dry soil−1 h−1) (p = 0.005) and in position C (127.2 ± 57.5 ng NO2-N g dry soil−1 h−1) (p = 0.001). In the hedgerow system, PNR was lower at position B (96.6 ± 72.2 ng NO2-N g dry soil−1 h−1) than at C (157.1 ± 77.1 ng NO2-N g dry soil−1 h−1) (p = 0.006).
AOA were not detected in the soil samples. The abundances of AOB and Basidiomycota were not influenced by the agroforestry system or the position in relation to the tree (Table 2, S.I. 5). Only the abundance of Ascomycota differed significantly between the agroforestry systems (Table 2, S.I. 5). Despite high variability, Ascomycota abundance was higher in the alley cropping system (25.5 ± 38.3 × 106 gene copies g dry soil−1) than in the hedgerow system (14.1 ± 39.5 × 106 gene copies g dry soil−1) (p = 0.044).
Influence of the agroforestry system and the position in relation to the tree on soil and vegetation properties
Among the soil variables, only NH4+-N and SOC were influenced by the agroforestry system: both were higher in the hedgerow (3.58 ± 1.36 mg NH4+-N kg dry soil−1 and 2.88 ± 0.33%, respectively) than in the alley cropping system (2.67 ± 0.75 mg NH4+-N kg dry soil−1 and 2.60 ± 0.28%, respectively) (Table 2, S.I. 6, p = 1 × 10−5 for NH4+-N and p = 7 × 10−9 for SOC). Six of the eight soil properties were influenced by the position in relation to the tree (p < 0.01): SOC, the C:N ratio, bulk density, root biomass, vegetation biomass and soil pH (Table 2, S.I. 6). SOC was significantly higher in the tree row (position A: 2.87 ± 0.34%) than in the positions B (p = 0.015) and C (p = 0.006) (2.70 ± 0.32% and 2.67 ± 0.33%, respectively), as were the C:N ratio (10.78 ± 0.42 vs. 10.46 ± 0.45 and 10.51 ± 0.40, respectively) (A vs. B: p = 5 × 10−4; A vs. C: p = 0.002) and vegetation biomass (217.4 ± 83.0 vs. 100.2 ± 35.8 and 112.3 ± 35.8 g.m−2, respectively) (A vs. B and A vs. C: p < 2 × 10−16). Soil bulk density was higher in the grass alleys (positions B and C: 1.30 ± 0.10 and 1.29 ± 0.10 g.cm−3, respectively) than in the tree row (position A: 1.16 ± 0.12 g.cm−3) (A vs. B: p = 7 × 10−10; A vs. C: p = 8 × 10−12). Root biomass was higher at positions A (3.05 ± 1.82 g.cm−3) and B (2.60 ± 1.27 g.cm−3) than at position C (1.97 ± 1.09 g.cm−3) (A vs. C: p = 3 × 10−4; B vs. C: p = 0.037).
Soil pH was the only property that was influenced by the interaction between the agroforestry system and the position in relation to the tree (Table 2, S.I. 6). For the alley cropping system, soil pH was higher at position A (5.6 ± 0.2) than at position C (5.3 ± 0.2) (p = 0.009), and did not differ from the other two positions at position B (5.4 ± 0.2). In contrast, for the hedgerow system, soil pH was lower at position B (5.1 ± 0.3) than at positions A (5.7 ± 0.3) (p = 2 × 10−5) and C (5.6 ± 0.3) (p = 2 × 10−8). Earthworm abundance was not influenced by any of the factors studied (Table 2, S.I. 6).
Relations among nitrification potential, microbial community size and soil and vegetation properties
Four soil and vegetation variables were selected to explain the variability in PNR: soil pH, which decreased the AIC of the RDA the most (from 146.6 to 127.2), followed by the C:N ratio, root biomass and soil NH4+-N (Table 3). The final AIC when considering all four variables equaled 123.7 (Table 3). These variables explained 22.5% of the variability in nitrification potential and microbial community size. The first and second dimensions of the RDA explained 76.8% and 16.2% of the constrained variability, respectively. On the first dimension, PNR and soil pH were strongly correlated and positively represented, followed by Ascomycota abundance (Fig. 4a). Complementary, the Spearman’s rank correlation analysis revealed that only pH was significantly correlated with nitrification potential (Fig. 5, S.I. 7). The second dimension was negatively related to Ascomycota abundance and root biomass, and positively related to C:N ratio, NH4+-N and AOB gene abundance. A significant negative correlation between Ascomycota gene abundance and NH4+-N was revealed by the correlation analysis (Fig. 5).
RDA results revealed that the position in relation to the tree significantly influenced the relations among PNR, microbial community size and soil properties (Fig. 4b). Observations at position A differed from those at position B (p = 0.039) in having higher PNR, pH and, to a lesser extent, Ascomycota community size. In contrast, position B was associated with lower PNR, pH and Ascomycota community size. Observations at position C did not differ significantly from those at the two other positions. Lastly, the type of agroforestry system did not significantly influence the RDA results.
Boxplots with jittered datapoints (n = 162) of nitrification potential as a function of the agroforestry system and position in relation to the tree: A (within the tree row 1 m from the tree), B (in the grassland 1.5 m from the tree) and C (in the grassland 10 m from the tree). Whiskers represent 1.5 times the interquartile range. Black dots represent outliers. Boxplots concerning nitrification potential in the alley cropping agroforestry system are colored in orange. Boxplots concerning nitrification potential in the hedgerow system are colored in blue. Boxplots with different letters above them are significantly different (p < 0.05) according to a linear model
Results of the redundancy analysis tested on the following model: (Nitrification potential (PNR) + ammonia-oxidizing (AOB) gene copies + Basidiomycota gene copies + Ascomycota gene copies) ~ (pH + C:N ratio + Root biomass + NH4+-N). Biplot a shows the replicates (black dots) and the correlations between the variables (black arrows). Biplot b shows the replicates colored as a function of the position in relation to the tree: A in red (in the tree row 1 m from the tree), B in green (in the grassland 1.5 m from the tree) and C in blue (in the grassland 10 m from the tree)
Discussion
Spatial patterns of nitrification potential in young agroforestry systems have little relation to the size of microbial communities
Despite the young age of the two agroforestry systems studied, the study revealed spatial differences in nitrification potential between them and among positions in relation to the tree. These observations may have been exacerbated by the shallow soil at the study site. Thus, the study sheds light on the structural and functional complexity of the N cycle in different agroforestry systems. In the alley cropping system, nitrification potential was on average 1.5 times as high in the tree rows as in the grass alleys, while in the hedgerow system, it was on average 40% lower in the grass alley next to the trees (position B) than at 10 m in the grass alley (position C). These results indicate that the agroforestry system drives not only C-cycle processes (Mayer et al. 2022) but also N-cycle processes, which has been studied little to date.
Neither the RDA analysis nor the correlation analysis revealed a relation between the community size of soil nitrifiers and the PNR, which disagrees with our first hypothesis. Autotrophic nitrification at the study site was driven by bacteria rather than archaea, since AOA were not detectable. This result differs from those of other studies, which observed higher abundance of AOA than AOB in acidic and organically fertilized grassland soils (Clark et al. 2020; Jia et al. 2020; Prosser and Nicol 2012). Inorganic fertilization is known to favor AOB over AOA (Jia et al. 2020; Prosser et al. 2020), but the site in the present study had not received any inorganic fertilizer for the past 15 years. Thus, AOB may have dominated at the study site due to repeated ammonia inputs from cow urine and manure, which has been shown to favor AOB in grazed grasslands (Di et al. 2009). However, AOB community size was not influenced by the agroforestry system or the position in relation to the tree, which is consistent with results of Beule et al. (2019). Moreover, AOB community size was weakly correlated with the soil properties measured. Overall, AOB population size did not drive the spatial patterns of nitrification potential in the two agroforestry systems studied, which is surprising since agricultural practices and the presence of cows drive AOB population size (Di and Cameron 2018; Verhamme et al. 2011). Although previous studies revealed that AOA and AOB gene abundances are proxies for variations in nitrification in grasslands systems (Di and Cameron 2018), we argue that further investigations are required in order to broaden our understanding of nitrification processes in agroforestry systems.
In contrast to the correlation analysis, the RDA highlighted a relation between Ascomycota abundance and nitrification potential. Recent literature revealed that both bacteria and fungi can perform heterotrophic nitrification (Martikainen 2022), especially in grassland soils (Zhang et al. 2023). Primers that can detect ammonia monooxygenase genes of bacteria have not been developed yet due to lack of knowledge about the underlying processes (Li et al. 2018). Thus, we may have underestimated the contribution of NH4+-oxidizing heterotrophic bacteria. However, we studied Ascomycota and Basidiomycota abundance and observed higher Ascomycota gene abundance in the alley cropping system than in the hedgerow system (p = 0.044). Since some Ascomycota can nitrify organic matter and NH4+ (Li et al. 2018), and in line with reviews of heterotrophic nitrification (Martikainen 2022; Song et al. 2021), heterotrophic nitrification driven by fungi may have contributed greatly to nitrification in the agroforestry systems studied. Future in field and laboratory studies using NH4+-15N tracers (Gao et al. 2022) may help disentangle the relative contributions of autotrophic and heterotrophic nitrification in the soils of different agroforestry systems.
Soil properties explain nitrification potential the most in young agroforestry systems
Soil properties such as SOC, NH4+-N availability, soil structure and pH are considered as drivers of autotrophic and heterotrophic nitrification (Gao et al. 2022). The RDA indicated no relation between nitrification potential and SOC, NH4+-N availability or soil bulk density, but it did highlight a strong correlation with soil pH (Fig. 4, S.I. 7).
Soil pH was strongly influenced by the position in relation to the tree and its interaction with the agroforestry system. In particular, pH and nitrification potential decreased strongly at the interface between tree row and grass alley (position B) in the hedgerow system, while smaller variations were observed in the alley cropping system. Biffi et al. (2022) and Litza and Diekmann (2017) reported similar decreases in soil pH near hedgerows in the United Kingdom and Germany that were associated mainly with grazed grasslands, while the comprehensive study of Pardon et al. (2017) reported no increased soil acidity in alley cropping systems. In line with these studies, our results underline that hedgerow systems might be associated with increased soil acidity. In our study, the increase of soil acidity may have been related to higher input of tree litter, since the hedgerow had 33% higher tree density than the tree rows of the alley cropping system. An unbalanced proton budget due to litter input and plant uptake between proton-generating processes (e.g. dissociation of carbonic and organic acids, nitrification, and cation uptake by plants) and proton-consuming processes (e.g. mineralization of soil organic matter) is known to drive soil acidification in forest ecosystems (Fujii et al. 2022). Moreover, high cow density near the hedgerow during grazing (the farmer’s observation, which agrees with the scientific literature, e.g. Giro et al. (2019) in the present study likely resulted in high input of animal waste at position B and thus may have stimulated proton-generating processes.
The correlation observed between nitrification potential and soil pH agrees with results of previous studies (Martikainen 2022; Prosser 1990). Soil pH is thus one factor that alters the resistance and resilience (i.e. insensitivity and rate of recovery of communities, respectively, when experiencing a disturbance (Shade et al. 2012) of soil-N-cycling microorganisms to stresses that will occur in the future due to a changing climate (Mod et al. 2021; Shu et al. 2023). In the long term, Litza and Diekmann (2017) observed a decrease in pH of 0.5 units over 50 years near hedgerows. This highlights that pH and thus soil nitrification potential might be influenced even more by the presence of trees once the systems in the present study mature.
Although soil bulk density was not significantly influenced by the type of agroforestry system, both NH4+-N and SOC were altered by presence of trees in the grassland. Like Mayer et al. (2022), we observed higher SOC and soil NH4+-N in the hedgerow system than in the alley cropping system. Mayer et al. (2022) explained this result by higher root biomass and tree density, which increased litter input. In the present study, however, the agroforestry system did not explain differences in root biomass. Like for soil pH, higher tree density and high cow density next to the hedgerow could explain the higher SOC and NH4+-N in the hedgerow system. Moreover, shrubs were planted between the trees, which may increase litter input next to the hedgerow. Because we did not study litter quality or quantity, we can hypothesize about their influence on the spatial patterns of SOC and NH4+-N observed only by referring to other studies (Cardinael et al. 2017; Guillot et al. 2021; Laughlin 2011; Schimel and Hättenschwiler 2007).
We expected that understory vegetation biomass and earthworm abundance would influence the nitrification potential (Le Roux et al. 2013; Patoine et al. 2020), but we found no relations between them. Unfortunately, practical limitations caused by the large dataset (n = 180) prevented acquiring other relevant proxies, such as specific root length (Cantarel et al. 2015; Freschet et al. 2021) or ecological categories of earthworms (Bottinelli et al. 2020; Xue et al. 2022). Thus, we cannot draw any conclusions from our data about effects of understory vegetation or earthworms on nitrification potential in the two agroforestry systems.
Since the studied agroforestry systems were young, the observed trends will need to be confirmed in the future. In particular, the trends in soil pH call for spatially explicit estimates of proton budgets (Helyar and Porter 1989) in temperate agroforestry systems. Since soil pH is one of the main drivers of soil chemical reactions and processes (Hartemink and Barrow 2023), this approach would help consider the spatial complexity of agroforestry systems, especially that of biological activities or the bioavailability of nutrients (Hinsinger 2001; Neina 2019), when managing them. In particular, lower soil pH can decrease ammonia volatilization and nitrate leaching in grazed pastures (Le Cadre et al. 2009) but increases phosphorus availability and leaching (Devau et al. 2011), as well as aluminum toxicity.
Conclusion
We explored the influence of hedgerow and alley cropping agroforestry systems on soil nitrification in temperate pastures. Spatial patterns of nitrification potential at the study site differed between the two systems and were related mainly to spatial differences in soil pH. In contrast, the size of the nitrifier community did not drive nitrification potential. By studying two young agroforestry systems, the study revealed early regulation of nitrification potential by agroforestry systems. Nitrification potential was particularly stimulated in the tree row of the alley cropping system and was lower near the hedgerow. These results suggest that the influence of the type of agroforestry system on the N cycle should be considered, especially regarding the provision of ecosystem services.
Data Availability
The dataset generated during and/or analysed during the current study is available at : https://doi.org/10.57745/ZV5TJ8.
References
Abalos D, van Groenigen JW, Philippot L, Lubbers IM, De Deyn GB (2019) Plant trait-based approaches to improve nitrogen cycling in agroecosystems. J Appl Ecol 56:2454–2466. https://doi.org/10.1111/1365-2664.13489
Battie-Laclau P, Taschen E, Plassard C, Dezette D, Abadie J, Arnal D, Benezech P, Battie-Laclau P, Taschen E, Plassard C, Dezette D, Abadie J, Arnal D, Benezech P, Duthoit M, Pablo A-L, Jourdan C, Laclau J-P, Bertrand I, Taudière A, Hinsinger P (2020) Role of trees and herbaceous vegetation beneath trees in maintaining arbuscular mycorrhizal communities in temperate alley cropping systems. Plant Soil 453:153–171. https://doi.org/10.1007/s11104-019-04181-z
Beeckman F, Motte H, Beeckman T (2018) Nitrification in agricultural soils: impact, actors and mitigation. Curr Opin Biotechnol 50:166–173. https://doi.org/10.1016/j.copbio.2018.01.014
Bei S, Tian Y, Zhao J, Zhang H, Christie P, Li X, Jia Z, Zhang J (2021) Temperature-dependent changes in active nitrifying communities in response to field fertilization legacy. Biol Fertil Soils 57:1–14. https://doi.org/10.1007/s00374-020-01500-w
Benhamou C (2012) Modélisation de l’effet des interactions haies-cultures sur les transferts d’eau et d’azote à l’échelle d’un petit bassin versant agricole. Agrocampus Ouest, PhD Thesis, France, p 214
Beule L, Corre MD, Schmidt M, Göbel L, Veldkamp E, Karlovsky P (2019) Conversion of monoculture cropland and open grassland to agroforestry alters the abundance of soil bacteria, fungi and soil-N-cycling genes. PLoS ONE 14:e0218779. https://doi.org/10.1371/journal.pone.0218779
Beule L, Lehtsaar E, Corre MD, Schmidt M, Veldkamp E, Karlovsky P (2020) Poplar rows in temperate agroforestry croplands promote bacteria, fungi, and denitrification genes in soils. Front Microbiol 10:3108. https://doi.org/10.3389/fmicb.2019.03108
Biffi S, Chapman PJ, Grayson RP, Ziv G (2022) Soil carbon sequestration potential of planting hedgerows in agricultural landscapes. J Environ Manage 307:114484. https://doi.org/10.1016/j.jenvman.2022.114484
Blake GR, Hartge KH (1986) Bulk density. In: Methods of soil analysis. John Wiley & Sons, Ltd, pp 363–375. https://doi.org/10.2136/sssabookser5.1.2ed.c13
Bottinelli N, Hedde M, Jouquet P, Capowiez Y (2020) An explicit definition of earthworm ecological categories – Marcel Bouché’s triangle revisited. Geoderma 372:114361. https://doi.org/10.1016/j.geoderma.2020.114361
Bowles TM, Atallah SS, Campbell EE, Gaudin ACM, Wieder WR, Stuart Grandy A (2018) Addressing agricultural nitrogen losses in a changing climate. Nat Sustain 1:399–408. https://doi.org/10.1038/s41893-018-0106-0
Cantarel AAM, Pommier T, Desclos-Theveniau M, Diquélou S, Dumont M, Grassein F, Kastl EM, Cantarel AAM, Pommier T, Desclos-Theveniau M, Diquélou S, Dumont M, Grassein F, Kastl E-M, Grigulis K, Laîné P, Lavorel S, Lemauviel-Lavenant S, Personeni E, Schloter M, Poly F (2015) Using plant traits to explain plant–microbe relationships involved in nitrogen acquisition. Ecology 96:788–799. https://doi.org/10.1890/13-2107.1
Cardinael R, Chevallier T, Cambou A, Béral C, Barthès B, Dupras C, Durand C, Kouakoua E, Chenu C (2017) Increased soil organic carbon stocks under agroforestry: a survey of six different sites in France. Agric Ecosyst Environ 236:243–255. https://doi.org/10.1016/j.agee.2016.12.011
Cardinael R, Hoeffner K, Chenu C, Chevallier T, Béral C, Dewisme A, Cluzeau D (2019) Spatial variation of earthworm communities and soil organic carbon in temperate agroforestry. Biol Fertil Soils 55:171–183. https://doi.org/10.1007/s00374-018-1332-3
Clark DR, McKew BA, Dong LF, Leung G, Dumbrell AJ, Stott A, Grant H, Nedwell DB, Trimmer M, Whitby C (2020) Mineralization and nitrification: Archaea dominate ammonia-oxidising communities in grassland soils. Soil Biol Biochem 143:107725. https://doi.org/10.1016/j.soilbio.2020.107725
CLIMATE-DATA.ORG (2022) Climat Saint-Just: Pluviométrie et Température moyenne Saint-Just, diagramme ombrothermique pour Saint-Just - Climate-Data.org. https://fr.climate-data.org/europe/france/bretagne/saint-just-720919/. Accessed 19 Dec 2022
den Herder M, Moreno G, Mosquera-Losada RM, Palma JHN, Sidiropoulou A, Santiago Freijanes JJ, Crous-Duran J, den Herder M, Moreno G, Mosquera-Losada RM, Palma JHN, Sidiropoulou A, Santiago Freijanes JJ, Crous-Duran J, Paulo JA, Tomé M, Pantera A, Papanastasis VP, Mantzanas K, Pachana P, Papadopoulos A, Plieninger T, Burgess PJ (2017) Current extent and stratification of Agroforestry in the European Union. Agric Ecosyst Environ 241:121–132. https://doi.org/10.1016/j.agee.2017.03.005
Devau N, Hinsinger P, Le Cadre E, Colomb B, Gérard F (2011) Fertilization and pH effects on processes and mechanisms controlling dissolved inorganic phosphorus in soils. Geochim Cosmochim Acta 75:2980–2996. https://doi.org/10.1016/j.gca.2011.02.034
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sediments 16:1401–1420. https://doi.org/10.1007/s11368-016-1403-8
Di HJ, Cameron KC (2018) Ammonia oxidisers and their inhibition to reduce nitrogen losses in grazed grassland: a review. J R Soc N Z 48:127–142. https://doi.org/10.1080/03036758.2017.1354894
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624. https://doi.org/10.1038/ngeo613
Dufour L, Metay A, Talbot G, Dupraz C (2012) Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J Agron Crop Sci 199:217–227. https://doi.org/10.1111/jac.12008
Elrys AS, Uwiragiye Y, Zhang Y, Abdel-Fattah MK, Chen Z, Zhang H, Meng L et al (2022) Expanding agroforestry can increase nitrate retention and mitigate the global impact of a leaky nitrogen cycle in croplands. Nat Food 4:109–121. https://doi.org/10.1038/s43016-022-00657-x
Erisman JW, Galloway J, Seitzinger S, Bleecker A, Butterbach-Bahl K (2011) Reactive nitrogen in the environment and its effect on climate change. Curr Opin Environ Sustain 3:281–290. https://doi.org/10.1016/j.cosust.2011.08.012
Fox J, Weisberg S (2018) An R companion to Applied Regression. Third Edition (Eds) Sage Publication Inc, p 608
Freschet GT, Roumet C, Comas LH, Weemstra M, Bengough G, Rewald B, Bardgett RD et al (2021) Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol 232:1123–1158. https://doi.org/10.1111/nph.17072
Fujii K, Funakawa S, Kosaki T (2022) Effects of forest management on soil acidification in cedar plantation. Geoderma 424:115967. https://doi.org/10.1016/j.geoderma.2022.115967
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling AB, Cosby BJ (2003) The Nitrogen Cascade. Bioscience 53:341–356. https://doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
Gao W, Chen M, Xu X (2022) Tracing controls of autotrophic and heterotrophic nitrification in terrestrial soils. Eur J Soil Biol 110:103409. https://doi.org/10.1016/j.ejsobi.2022.103409
Giro A, Pezzopane JRM, Barioni Junior W, de Faria Pedroso A, Prudencio Lemes A, Botta D, Romanello N, do, Nascimento Barreto A, Garcia AR (2019) Behavior and body surface temperature of beef cattle in integrated crop-livestock systems with or without tree shading. Sci Total Environ 684:587–596. https://doi.org/10.1016/j.scitotenv.2019.05.377
Greaver TL, Clark CM, Compton JE, Vallano D, Tlhelm AF, Weaver CP, Band LE et al (2016) Key ecological responses to nitrogen are altered by climate change. Nat Clim Change 6:836–843. https://doi.org/10.1038/nclimate3088
Guerra V, Beule L, Lehtsaar E, Liao H, Karlovski P (2020) Improved protocol for DNA extraction from Subsoils using phosphate lysis buffer. Microorganisms 8:532. https://doi.org/10.3390/microorganisms8040532
Guillot E, Bertrand I, Rumpel C, Gomez C, Arnal D, Abadie J, Hinsinger P (2021) Spatial heterogeneity of soil quality within a Mediterranean alley cropping agroforestry system: comparison with a monocropping system. Eur J Soil Biol 105:103330. https://doi.org/10.1016/j.ejsobi.2021.103330
Hartemink AE, Barrow NJ (2023) Soil pH - nutrient relationships: the diagram. Plant Soil 486:209–215. https://doi.org/10.1007/s11104-022-05861-z
Helyar KR, Porter WM (1989) Soil acidification, its measurements and the processes involved. Soil acidity and plant growth, Robson. Academic Press, London, UK, pp 61–101
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195. https://doi.org/10.1023/A:1013351617532
IPCC (2022) Climate Change 2022: impacts, adaptation and vulnerability. In: Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (eds) Contribution of Working Group II to the sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambrigde University Press, Cambridge, p 3056
Jeppesen E, Kronvang B, Olesen JE, Audet J, Søndergaard M, Hoffmann CC, Andersen HE, Jeppesen E, Kronvang B, Olesen JE, Audet J, Søndergaard M, Hoffmann CC, Andersen HE, Lauridsen TL, Liboriussen L, Larsen SE, Beklioglu M, Meerhoff M, Özen A, Özkan K (2011) Climate change effects on nitrogen loading from cultivated catchments in Europe: implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 663:1–21. https://doi.org/10.1007/s10750-010-0547-6
Jia Z, Zhou X, Xia W, Fornara D, Wang B, Wasson EA, Christie P, Polz MF, Myrold DD (2020) Evidence for niche differentiation of nitrifying communities in grassland soils after 44 years of different field fertilization scenarios. Pedosphere 30:87–97. https://doi.org/10.1016/S1002-0160(19)60803-9
Kanzler M, Böhm C, Mirck J, Schmitt D, Veste M (2019) Microclimate effects on evaporation and winter wheat (Triticum aestivum L.) yield within a temperate agroforestry system. Agroforest Syst 93:1821–1841. https://doi.org/10.1007/s10457-018-0289-4
Kim D-G, Isaac ME (2022) Nitrogen dynamics in agroforestry systems. A review. Agron Sustain Dev 42:60. https://doi.org/10.1007/s13593-022-00791-7
Lassaletta L, Billen G, Grizzetti B, Anglade J, Garnier J (2014) 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ Res Lett 9:105011. https://doi.org/10.1088/1748-9326/9/10/105011
Laughlin DC (2011) Nitrification is linked to dominant leaf traits rather than functional diversity. J Ecol 99:1091–1099. https://doi.org/10.1111/j.1365-2745.2011.01856.x
Legendre P, Legendre L (2012) Numerical ecology. Elsevier Science Ltd Eds, p 1007
Le Cadre E, Gérard F, Génermont S, Morvan T, Recous S (2009) Which formalism to Model the pH and temperature dependence of the microbiological processes in soils? Emphasis on Nitrification. Environ Model Assess 14:539–544. https://doi.org/10.1007/s10666-008-9155-5
Li Y, Chapman SJ, Nicol GW, Yao H (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301. https://doi.org/10.1016/j.soilbio.2017.10.023
Litza K (2002) The vegetation of hedgerows in changing landscapes - past and present patterns. Universität Bremen, PhD thesis, Germany, p 145
Litza K, Diekmann M (2017) Resurveying hedgerows in Northern Germany: Plant community shifts over the past 50years. Biol Conserv 206:226–235. https://doi.org/10.1016/j.biocon.2016.12.003
Martikainen PJ (2022) Heterotrophic nitrification – an eternal mystery in the nitrogen cycle. Soil Biol Biochem 168:108611. https://doi.org/10.1016/j.soilbio.2022.108611
Mayer S, Wiesmeier M, Sakamoto E, Hübner R, Cardinael R, Kühnel A, Kögel-KnabnerI (2022) Soil organic carbon sequestration in temperate agroforestry systems – A meta-analysis. Agric Ecosyst Environ 323:107689. https://doi.org/10.1016/j.agee.2021.107689
Mettauer R, Thoumazeau A, Le Gall S, Soiron A, Rakotondrazafy N, Bérard A, Brauman A, Mézière D (2022) Soil health in temperate agroforestry: influence of tree species and position in the field. Arch Agron Soil Sci 0:1–20. https://doi.org/10.1080/03650340.2022.2116013
Mod HK, Buri A, Yashiro E, Guex N, Malard L, Pinto-Figueroa E, Pagni M, Niculita-Hirzel H, van der Meer JR, Guisan A (2021) Predicting spatial patterns of soil bacteria under current and future environmental conditions. ISME J 15:2547–2560. https://doi.org/10.1038/s41396-021-00947-5
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci 2019:e5794869. https://doi.org/10.1155/2019/5794869
Norton JM (2008) Nitrification in agricultural soils. In: Scheppers JS, Raun WR (Eds) Nitrogen in agricultural systems. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Wiley, New York, pp 173–199
Pardon P, Reubens B, Reheul D, Mertens J, De Frenne P, Coussement T, Janssens P, Verheyen K (2017) Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agric Ecosyst Environ 247:98–111. https://doi.org/10.1016/j.agee.2017.06.018
Patoine G, Bruelheide H, Haase J, Nock C, Ohlmann N, Schwarz B, Scherer-Lorenzen M, Eisenhauer N (2020) Tree litter functional diversity and nitrogen concentration enhance litter decomposition via changes in earthworm communities. Ecol Evol 10:6752–6768. https://doi.org/10.1002/ece3.6474
Prosser JI (1990) Autotrophic Nitrification in Bacteria. In: Rose AH, Tempest DW (eds) Advances in Microbial Physiology. Academic Press, pp 125–181
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531. https://doi.org/10.1016/j.tim.2012.08.001
Prosser JI, Hink L, Gubry-Rangin C, Nicol GW (2020) Nitrous oxide production by ammonia oxidizers: physiological diversity, niche differentiation and potential mitigation strategies. Glob Chang Biol 26:103–118. https://doi.org/10.1111/gcb.14877
R Core Team (2022) R: a language and environnement for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Richards LA, Fireman M (1943) Pressure-plate apparatus for measuring moisture sorption and transmission by soils. Soil Sci 56:395. https://doi.org/10.1097/00010694-194312000-00001
Rivest D, Lorente M, Olivier A, Messier C (2013) Soil biochemical properties and microbial resilience in agroforestry systems: Effects on wheat growth under controlled drought and flooding conditions - ScienceDirect. Sci Total Environ 463–464:51–60. https://doi.org/10.1016/j.scitotenv.2013.05.071
Robertson GP, Groffman PM (2015) 14- Nitrogen transformations. Soil Microbiology, Ecology and Biochemistry (Fourth Edition). Academic Press, San Diego, pp 421–446
Rockström J, Steffen W, Noone K, Persson A, Chapin FS, Lambin EF, Lenton TM et al (2009) A safe operating space for humanity. Nature 461:472–475. https://doi.org/10.1038/461472a
Le Roux X, Schmid B, Poly F, Barnard RL, Niklaus PA, Guillaumaud N, Habekost M, Le Roux X, Schmid B, Poly F, Barnard RL, Niklaus PA, Guillaumaud N, Habekost M, Oelmann Y, Philippot L, Salles JF, Schloter M, Steinbeiss S, Weigelt A (2013) Soil environmental conditions and microbial build-up mediate the effect of plant diversity on soil nitrifying and denitrifying enzyme activities in temperate grasslands. PLoS ONE 8:e61069. https://doi.org/10.1371/journal.pone.0061069
Schimel JP, Hättenschwiler S (2007) Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39:1428–1436. https://doi.org/10.1016/j.soilbio.2006.12.037
Shade A, Peter H, Allison S, Baho D, Berga M, Buergmann H, Huber D, Langenheder S, Lennon J, Martiny J, Matulich K, Schmidt T, Handelsman J (2012) Fundamentals of microbial community resistance and resilience. Front Microbiol 417:1-19. https://doi.org/10.3389/fmicb.2012.00417
Shu X, Daniell TJ, Hallett PD, Baggs EM, Griffiths BS (2023) Soil pH moderates the resistance and resilience of C and N cycling to transient and persistent stress. Appl Soil Ecol 182:104690. https://doi.org/10.1016/j.apsoil.2022.104690
Song T, Zhang X, Li J, Wu X, Feng H, Dong W (2021) A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci Total Environ 801:149319. https://doi.org/10.1016/j.scitotenv.2021.149319
van Ramshorst JGV, Siebicke L, Baumeister M, Moyano FE, Kohl A, Markwitz C (2022) Reducing wind erosion through agroforestry: a case study using large Eddy simulations. Sustainability 14:13372. https://doi.org/10.3390/su142013372
Veldkamp E, Schmidt M, Markwitz C, Veldkamp E, Schmidt M, Markwitz C, Beule L, Beuschel R, Biertümpfel A, Bischel X, Duan X, Gerjets R, Göbel L, Graß R, Guerra V, Heinlein F, Komainda M, Langhof M, Luo J, Potthoff M, van Ramshorst JGV, Rudolf C, Seserman D-M, Shao G, Siebicke L, Svoboda N, Swieter A, Carminati A, Freese D, Graf T, Greef JM, Isselstein J, Jansen M, Karlovsky P, Knohl A, Lamersdorf N, Priesack E, Wachendorf C, Wachendorf M, Corre MD (2023) Multifunctionality of temperate alley-cropping agroforestry outperforms open cropland and grassland. Commun Earth Environ 4:20. https://doi.org/10.1038/s43247-023-00680-1
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5:1067–1071. https://doi.org/10.1038/ismej.2010.191
Viaud V, Thomas Z (2019) Une réflexion sur l’état des connaissances des fonctions du bocage pour l’eau dans une perspective de mobilisation pour l’action. Sci Eaux Territoires Numéro 30:32–37. https://doi.org/10.3917/set.030.0032
Xue R, Wang C, Liu X, Liu M (2022) Earthworm regulation of nitrogen pools and dynamics and marker genes of nitrogen cycling: a meta-analysis. Pedosphere 32:131–139. https://doi.org/10.1016/S1002-0160(21)60063-2
Zhang Y, Cai Z, Zhang J, Müller C (2023) The controlling factors and the role of soil heterotrophic nitrification from a global review. Appl Soil Ecol 182:104698. https://doi.org/10.1016/j.apsoil.2022.104698
Acknowledgements
The authors thank their colleagues from the SAS mixed research unit, as well as Elodie Joubrel (Agrobio 35), Isabelle Senegas (Chambre d’Agriculture de Bretagne) and Brendan Godoc (Idele), who helped with field preparation and sampling. We are grateful to Esther Guillot (INRAE) for her advice on statistical analysis, and to Michael S. Corson for English proofreading. We also especially thank the farmer who warmly welcomed us to his farm for field sampling.
Funding
This study was financially supported by La Fondation de France (grant no. 00117721/WB-2021-35937).
Author information
Authors and Affiliations
Contributions
RM, OG and ELC contributed to the conception and design of the study. RM and MM performed the field work and determined soil and vegetation properties with the help of OG and ELC. LB and ZB performed laboratory work to determine microbial community size, and RM determined PNR. RM performed the statistical analysis. RM and ELC wrote the first draft of the manuscript, which was critically revised by LB, ZB, OG and MM. All authors read and approved the final manuscript.
Corresponding author
Additional information
Responsible Editor: Zucong Cai.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mettauer, R., Beule, L., Bednar, Z. et al. Influence of two agroforestry systems on the nitrification potential in temperate pastures in Brittany, France. Plant Soil 494, 651–667 (2024). https://doi.org/10.1007/s11104-023-06309-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06309-8