Abstract
Background and aims
Redox potential is a promising soil health indicator, which integrates the combined effect of chemical oxidation–reduction reactions into a single measurement. However, this method has been tested only on a few soils. The aim of this study was to test redox potential as a soil health indicator, to see if it responds to management practices and to compare it with existing soil health metrics for microbial activity (“CO2burst”) and soil structure.
Methods
We collected 35 soil samples in agricultural fields from a carbon farming trial, where contrasting management practices have been applied to increase carbon stock. The soil samples were dried, rewetted and analyzed for redox and microbial respiration during rewetting. In addition, soil structure, texture and organic matter content was measured. The data was analyzed for correlations between the indicators and for the differences between management and control fields.
Results
Redox was well correlated with microbial activity, structure, and texture. A low redox state was connected to high microbial activity, indicating bioavailable organic matter availability. Soils with good structure had an oxidized redox status, possibly reflecting high gas-transport. The carbon farming practices resulted in lower oxidation, possibly due to build-up of plant residues.
Conclusions
The findings supported the use of redox as a soil health indicator, but highlighted further research needs for identifying the shared mechanisms linking structure, redox and microbial activity. As such, redox can be a low-cost additional measurement to map changes in soil health, but it cannot replace existing structure or microbial activity measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The capability of soil to provide ecosystem services through four soil functions: carbon cycle, nutrient cycle, pest regulation, and soil structure maintenance (Kibblewhite et al. 2008) is defined as “soil health.” It is closely related to soil quality and productivity but is more integrative and focused on soil biological functions (Lal 2016). These can be quantified through process indicators (e.g. carbon metabolism: basal respiration, CO2burst test, aerobic and anaerobic incubations) (Weil and Brady 2016). One of the most commonly used functional indicators is the 24 h CO2 respiration test for microbial activity (Franzluebbers et al. 1996). In this method, a soil sample is dried and rewetted, which results in a flush of CO2 (“CO2burst”). Physical, chemical, and biological soil processes control the flush of CO2 (Barnard et al. 2020); therefore, it is an integrative indicator for many soil properties, but the exact mechanisms have puzzled research for decades (Birch 1958; Hicks et al. 2022). The CO2burst has two valuable properties: i) it responds to management and ii) it reflects nitrogen mineralization, making it important for fertilizer recommendations. Despite the unclear mechanisms, CO2burst has become an important tool for planning fertilizer recommendations (Haney et al. 2018).
While the CO2burst took 50 years from the description of the process to its application in management, soil redox potential had a faster development cycle. A review by Husson (2013) brought redox from the use in submerged and paddy soils to aerated mineral soils and highlighted its importance for plant and soil health. Subsequent development has focused on improving measurement (Husson et al. 2016), tracking management-induced changes (Husson et al. 2018), and evaluating the role of redox potential for plant health (Husson et al. 2021). Most of the work has been done by a single research group and only on a few soils and locations, but there is increasing interest among agriculturalists in using redox as an integrative measurement of soil and plant condition (Husson et al. 2021). The redox is a relatively easy measurement, a sensor is pressed to a dried and rewetted sample and results are achieved in 1–2 min.
The redox potential of soil measures the general availability of electrons, or the relative difference between oxidation (loss of electrons) and reduction (gain of electrons) (Zhang and Furman 2021). In soils, one of the main reactions is the oxidation of organic matter ((CH2O)n), which supplies electrons. In fully aerobic soils, oxygen serves as the electron acceptor, resulting in the production of CO2 and H2O. For example, in rewetted agricultural soil, redox is a strong predictor of CO2 flux (Bartolucci et al. 2021). When oxygen availability is low (inside aggregates) or when organic matter supply is high, redox may decrease and other electron acceptors can also be used simultaneously with O2, resulting in manganese, iron, and nitrogen reduction (Marschner 2021) and improved soil nutrient availability for plants (Husson 2013). Based on this, redox could be a promising indicator for the soil carbon cycle (labile carbon pools) and structure (oxygen availability), two key components of soil health (Kibblewhite et al. 2008).
Redox can also serve as an indicator of nutrient availability and pest regulation (Husson 2013). Redox can be thought of as a parallel to pH which measures proton availability whereas redox (Eh) measures electron availability. Like pH, also redox can influence nutrient availability considerably: redox is managed to avoid toxic As and Cd buildup in rice paddies (Evans et al. 2021) and to improve Mn supply (Husson 2013; Zhang and Furman 2021). However, a key challenge for using redox as an indicator is that it changes rapidly with soil water and oxygen conditions (Zhang and Furman 2021). To standardize the evaluation of a soil, Husson et al. (2016) proposed the use of redox potential as a soil test from dried soil samples. In the proposed test, dried soil was rewetted to field capacity and the redox was measured for 2 min. In theory, the test is similar to the CO2burst test, but takes only 2 min instead of 24 h. It therefore has great potential as a high-throughput indicator for soil health, but it should be tested in different soils and management and also compared with established soil tests.
To evaluate redox as a soil biological health indicator, we compared it with commonly used indicators, focusing on carbon cycling (CO2burst) and structure (visual evaluation of soil structure VESS, Ball and Munkholm 2015). It was also compared to soil organic matter, which is a key driver for both biological activity and structure maintenance (Weil and Brady 2016). We used 20 sites from an ongoing carbon sequestration experiment (Carbon Action experiment 2019–2023, described in Mattila et al. 2022), where each site had a carbon farming trial plot and a control plot. The aim was to see if i) redox could be explained by microbial activity and soil structure or if it is a new complementary measure of soil health and ii) if three years of management had an effect on the measured soil health indicators.
Materials and methods
The materials for the study were soils sampled from the Carbon Action experiment (Mattila et al. 2022) intensive observation set of farms (Mattila 2020). The intensive observation farms are located in a 200 × 500 km area, which covers the main agricultural areas of Finland (ranging from 63.18 N to 60.36 N in latitude). The farms tested five carbon sequestration practices (cover crops, compost, grazing practices, leyfarming, and subsoiling). The experiment started in the spring of 2019 and the samples were collected in July 2021, the third growing season of the experiment. Each farm had a split field, where one side had carbon farming practices implemented and the other was held as a continued normal-practice control. Both sides of each field were sampled using a composite sample of pooled soil cores. Samples for each field side were collected around 3 GPS reference points from a 10 m radius, resulting in 30 cores (16 mm diameter, 0–17 cm sample depth), which were pooled to a composite sample.
The composite samples were used in the experiment monitoring for routine soil analysis (organic matter, clay, pH, nutrients; Mattila and Girz 2021; presented in Table 1), but for this study we also took a 100 ml part of the sample for redox and CO2burst assessment. This allowed comparing redox with existing indicators as well as with soil properties and management.
The collected samples were dried at room temperature and gently sieved through a 5 mm sieve following the established guidelines for CO2burst (Franzluebbers and Haney 2018; Woods End Laboratories 2019). (Due to an unfortunate laboratory accident, 5 samples were lost during processing, resulting in an overall sample amount of n = 35. The lost samples resulted in the loss of two farm replicates in both treatment and control and one treatment sample from one farm. The farm with only one sample was ignored when calculating the statistical treatment effect, but was included in the correlations with other metrics.) The sampled soils covered a large range of soil texture and organic matter: the median clay content was 35% (4–63%) and the OM 6.8% (2.6–15.5%). The farming systems covered annual cropping, grass in rotation, and perennial pastures.
For CO2burst analysis, the dry samples were rewetted to approximately 50% pore space (i.e. 30 ml of soil and 9 ml of water; Woods End Laboratories 2019). The sample was placed in a 475 ml container and sealed with a CO2 respirometer cap (Woodsend IRTH). The CO2 concentration was measured for 24 h at 5 min intervals with the integrated infrared cell-datalogger and the increase in CO2 level (ppm) over 24 h was converted to mg CO2-C/kg3 soil by multiplying with container air space and dividing by sample mass (26–39 g/30 ml sample, average 31 g).
For the redox analysis, 30 ml of soil was also rewetted to 50% pore space by adding 9 ml water. This followed the 30% humidity recommendation for measuring redox from rewetted soils in Husson et al. (2016) and used in Husson et al. (2018). The redox was measured using an Extech RE300 Exstik Platinum oxidation–reduction-potential (ORP) sensor (platinum electrode, silver/chloride reference electrode). The flat end ORP sensor was pressed to the moist soil sample and allowed to stabilize 1–3 min, until the ORP reading changed only slowly. Three repeated measurements were made of the same sample and the average value was used. The sample pH was measured with a Horiba LAqua Twin pH meter using a 1:1 ratio of distilled water:soil. The ORP reading was converted to Eh (mV) by adding the reference electrode voltage (200 mV) and pH corrected to a relative hydrogen score by the equation rH2 = 33.83 Eh + 2 pH (where Eh is in V; Husson et al. 2016). For interpretation, the readings were compared to suggested norms for”healthy” soils (Husson 2013). The Eh value was used for soil classification in the Eh/pH parameter space (Husson 2013). Otherwise the analysis was based on the rH2 metric which includes both pH and Eh (i.e. a pH corrected redox).
For additional interpretation, the soil samples were classified according to organic matter (OM) content, soil clay content, soil structure (VESS (Ball and Munkholm 2015)), and type of crop (perennial, annual). These were collected and published as ongoing monitoring in the Carbon Action experiment (Mattila and Girz 2021). Soil OM and clay content were determined in a commercial lab (Eurofins) by loss-on-ignition and dry sieving, respectively. The VESS analysis was performed in the field, it is a visual soil assessment (VSA) method developed in 2007 from existing methods and used subsequently in hundreds of studies (Franco et al. 2019). Although the method is visual, it gives numerical scores that are repeatable and correlate well with traditional measurements such as bulk density (Johannes et al. 2017). In the VESS, the soil is divided into layers and each layer is scored in a range of 1–5 based on the severity of structural damage. The layer scores are then multiplied by the layer depth and divided by the total depth of the sample to give a depth-weighted average score.
The statistical analysis was done in R programming language (R Core Team 2022). In order to compare redox measurements with other soil health metrics, Spearman’s correlation coefficients were calculated between redox, CO2burst, OM, soil structure, and clay content. These soil indicators range from highly labile (structure) to permanently stabile (clay content). CO2burst is known to respond to OM quality more than to OM amount (Haney et al. 2012). To test if redox could represent organic matter quality in forecasting CO2burst, a least squares linear regression model was used to explain CO2burst based on redox (Eh) and OM. If this provided a strong fit, the 24-h CO2burst could be estimated with a 2-min redox test. To test the effect of carbon farming practices on redox and CO2burst, a Wilcoxon test was used to test if the difference between control and treatment was larger (CO2burst) or smaller (redox) than zero.
Results
The redox potential (Eh) of the 35 studied soils ranged from reduced (+ 340 mV) to oxidized (+ 560 mV) and the pH ranged from acidic (pH 5.8) to slightly alkaline (pH 7.6) (Fig. 1). Most of the soils were in an Eh/pH space considered favorable for plant growth (pH 5.5–8; Eh + 350–500 mV; Husson 2013). One soil was more reduced than the favorable area, it was a seasonally waterlogged silty clay loam with horse manure applied previously. Ten soils were classified as more oxidized than the plant-favorable region: the alkaline and oxidized soil was a low OM loamy sand, but the acidic and oxidized soils were a diverse group with high OM (> 12%) or high clay (> 60%).
Tested soils in a pH/Eh Pourbaix plot. Triangles = carbon farming plots, rounds = control plots. Color = OM content. Favorable and optimum regions for plant growth drawn based on Husson 2013
The carbon farming plots had lower Eh than the corresponding control plots (Figs. 1 and 2): the management-induced reduction was on average -20 mV (Wilcoxon p = 0.039; 95% confidence interval -90.. + 40 mV) and the largest reductions (-90 mV) were on fields where compost had been applied.
The effect of five carbon farming practices on soil redox potential. (Light shade = control plot; dark shade = carbon farming plot. Dot color = OM content (range 3–16%, legend in Fig. 1). Samples taken in July 2021, on the third growing season of the Carbon Action experiment.)
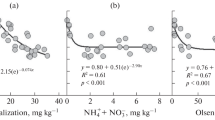
Soil redox potential correlated with soil properties as well as with two other soil health metrics (soil structure VESS and microbial activity CO2burst) (Table 2). The correlation with CO2burst was statistically significant and strong (p < 0.01; r = 0.44). Eh explained variability of CO2burst better than pH (p = 0.17; r = 0.23), soil texture (p = 0.04; r = 0.46) or OM content (p > 0.10; r = 0.43). A linear model of Eh and OM could explain 46% of the variability in CO2burst, with both input variables being significant (p = 0.01, CO2burst = 211·OM-220·Eh). The highest microbial activity was found in reduced soil conditions, but some soils with reduced soil conditions had only moderateCO2burst. Oxidized soils did not have high CO2burst values, but the microbial activity ranged between moderate and low, suggesting other controls than redox. The carbon farming treatment resulted in slightly increased CO2burst (8 mg CO2-C/kg, 95% confidence interval -16..68 mg CO2-C/kg, Wilcoxon test p-value 0.044). The highest increases were in farms that tested improved grazing practices (average 17 mg CO2-C/kg).
Soil structure had a noticeable effect on the redox potential (Fig. 4) (VESS vs. rH2, = r = 0.43, p = 0.04*), but almost no effect on CO2burst (r = 0.007). In soils with good structure (VESS < 2.75), the redox status was more oxidized (rH2 30.5) compared to soils with poor structure (VESS > 3.25; rH2 28.6) (Fig. 4). The redox in poorly structured soils was highly variable, with some soils having increased oxidation status. The structure score also correlated with soil texture (r = 0.44, p = 0.008) and OM (r = -0.47, p = 0.005) with poorer structure in higher clay and better structure with higher OM levels.
Discussion
Redox potential describes soil conditions and responds to soil management
In this study, redox could be used to evaluate plant growing conditions (Fig. 1), and it also followed management-induced changes (Fig. 2). Redox correlated with microbial activity (CO2burst, Fig. 3) and soil structure (Fig. 4), making it a promising indicator of soil health. Redox potential is rapid to measure (c.a. 2 min per measurement) and integrates many biological and physical processes. It can serve as an additional measure for quick screening or used in parallel with existing measures to help interpretation.
Soil microbial activity vs. redox status (rH2). The colors represent OM concentration (range 3–16%, legend in Fig. 1) and the shape is the treatment (triangle) vs. control group (round)
The redox status (rH2) of soils with a good structure (VESS 1–2.75) was more oxidized than soils with a poor structure (VESS 3.25–5). The colors on the points describe the soil OM concentration (Fig. 1 legend)
Finding a large variation in redox (Fig. 1) in an experiment where dried and sieved soil was rewetted was somewhat unintuitive. One would assume that in an artificial setting, the gas transport would be sufficient to keep the conditions oxidized, especially as the rewetting aimed only to saturate 50% of the pore space (Franzluebbers and Haney 2018). However, the strong correlation with microbial activity (CO2burst) and the response to management, which added carbon compounds to soil (manures and compost), suggested that the intense microbial activity and high availability of bioavailable carbon compounds can also reduce redox conditions in a well-aerated experimental setting. This confirmed earlier findings, where plant environments could be classified on the basis of redox and pH (Husson 2013). In our study, those soils that fell clearly outside the recommended “favourable” range (pH 5.5–8.0, Eh + 350–500 mV) had distinct soil health problems (waterlogging or low OM, weak structure sands). This supported the use of redox as a universal soil health indicator, although the oxidized state of high OM or clay soils in the experiment (upper left corner in Fig. 1) warrants further investigation on the sample pre-treatment and rewetting conditions. Currently, the redox potential is an interesting indicator, but it is still under development, especially when applied to dried and rewetted soil samples. With the currently widely used CO2burst method, standardizing sample pretreatment has been critical to reduce variability (Franzluebbers and Haney 2018). Similar work needs to be done for redox to ensure application to different textures and OM categories.
The management-induced changes were very similar in our study compared with earlier findings (Husson et al. 2016). Compared to the four soils evaluated in Husson et al. (2016), the soils in this study were more reduced (average + 530 vs. + 470 mV), which reflects differences in climate (warm vs. cool temperate) and organic matter level (2% vs. 8% average). However, carbon farming management practices resulted in similar redox potential reductions to those found in Husson et al. (2016) for conservation agriculture. In our study, the reduction was on average -20 mV (p < 0.05; 95% range -90.. + 40 mV). The largest reductions (-90 mV) were on sites that had compost soil amendments applied. This supports the findings of Husson et al. (2016) where conservation agriculture (residue retention, cover crops and minimum tillage) reduced Eh by -10–25 mV. This suggests that soil redox potential reacts rapidly to the addition of readily decomposable OM. It may take decades before the total soil OM changes enough to detect management effects; therefore, rapid indicators are valuable in guiding management. Microbial activity (CO2burst) has been used as a rapid indicator that shows change even after a few seasons of different management (Haney et al. 2018; Weil and Brady 2016). In this study, redox showed similar changes, making it a promising indicator for soil carbon cycling.
Interactions between redox, structure, and microbial activity
The significant correlations of redox with microbial activity, structure and texture (Table 2) make interpretation challenging. Further research into the reaction mechanisms of redox decrease during rewetting and the effect of sample pretreatment may be necessary. The group of oxidized and acidic soils in Fig. 1 (upper left corner) also had high clay or OM concentrations. This oxidized reading may therefore be a result of high water holding capacity, where the water applied for rewetting (9 ml water per 30 ml soil) did not sufficiently saturate the pores. On the other hand, the structure has a strong effect on redox (Table 2 and Fig. 4). Some poor-structure soils had high oxidation status, perhaps due to sharp and angular aggregates, which had air gaps during the experiment. Refining the sample pretreatment (milling and sieving) may reduce the variability in the redox of poor structured soils, but it may result in more artificial settings by exposing protected organic matter, as has been found for CO2burst (Franzluebbers and Haney 2018).
Although structure was reflected in the redox, it can not be used to replace the field assessment of soil structure. Good structure scores were generally found together with oxidized soil redox, but most poor structure soils were classified as oxidized. Only strong oxidation or reduction could be used as an indicator for good or poor soil structure as these extreme values were only found in those structural classes (Fig. 4).
Redox explained variability in microbial CO2burst better than OM. When redox was combined in a linear model with OM, the two variables could explain almost half of the variability in CO2burst. Redox was not significantly correlated with OM as such, suggesting that it may measure a separate pool of bioavailable OM, as has been found for water-soluble OM (Haney et al. 2012). This supports earlier findings that CO2burst is controlled more by the availability and quality of OM than by the quantity (Haney et al. 2012). Redox has been found to be an important indicator for soil respiration in field rewetting studies (Bartolucci et al. 2021). However, compared to the laboratory, the effect of redox in the field is the opposite: highest respiration is found with oxidized redox status (Bartolucci et al. 2021). In the field with saturated soils, bioavailable OM accumulates and subsequent drying results in both increased respiration and redox. The rewetting study starts from the opposite end of the moisture spectrum, with highly oxygenated and dry soils, where respiration is limited by water. In that setting, oxygen availability is high and the redox is more likely controlled by the availability of readily degradable organic compounds and microbial biomass (Barnard et al. 2020). The CO2burst test has been able to predict nitrogen mineralization in field conditions (Haney et al. 2018) but scaling the redox potential to field conditions may be challenging because it depends on texture and structure. Introducing textural classes to the interpretation of “favorable” and “optimum” ranges for plant growth (Husson 2013) would make the indicator more broadly applicable.
Overall, these results confirm the earlier results on the use of soil redox potential to measure soil health and track management-induced changes (Husson 2013; Husson et al. 2016). Previous work on redox and soil management was done in a very different climate and soil texture (warm vs. cool temperate climate, low vs. high OM, low clay content). The replication of those findings in cool, high clay and OM environment supports further investigation into the use of redox as a soil health metric. Strong correlations with microbial CO2burst and soil structure suggest common mechanisms. Further studies into the chemical reactions causing redox reduction and CO2burst during rewetting could help interpret the results across structural and textural soil categories. At the same time, redox measurement with an oxidation–reduction potential meter can be a low-cost and simple integrative measurement of both structure and microbial decomposition, which has practical implications for crop advisory and soil management zone mapping.
Conclusions
In conclusion, soil redox potential was found to correlate with soil texture, structure, and biological activity but not with soil organic matter. It also responded to management practice changes with carbon additions lowering the redox. These findings support the use of redox potential as an integrative measurement of soil function. In general, oxidized soil conditions were found in soils with good structure, and low redox corresponded to a high biological activity. Further research on the measurement methods (sample treatment) and the shared mechanisms between redox and microbial activity is necessary to facilitate a deeper interpretation in different soil textures. In spite of these limitations, redox can serve as a low-cost complementary indicator of carbon cycling and soil structure.
Data availability
The datasets generated during the current study are available in the Zenodo repository “Carbon Action” community.
Abbreviations
- OM:
-
Organic matter
- rH2 :
-
Relative hydrogen score
- VESS:
-
Visual evaluation of soil structure
- Eh:
-
Redox potential
References
Ball BC, Munkholm LJ (eds) (2015) Visual soil evaluation: realizing potential crop production with minimum environmental impact. CABI, Wallingford, 168 p
Barnard RL, Blazewicz SJ, Firestone MK (2020) Rewetting of soil: revisiting the origin of soil CO2 emissions. Soil Biol Biochem: 107819. https://doi.org/10.1016/j.soilbio.2020.107819
Bartolucci NN, Anderson TR, Ballantine KA (2021) Restoration of retired agricultural land to wetland mitigates greenhouse gas emissions. Restor Ecol 29(3):e13314. https://doi.org/10.1111/rec.13314
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10(1):9–31. https://doi.org/10.1007/BF01343734
Evans AE, Limmer MA, Seyfferth AL (2021) Indicator of redox in soil (IRIS) films as a water management tool for rice farmers. J Environ Manage 294:112920. https://doi.org/10.1016/j.jenvman.2021.112920
Franco HHS, Guimarães RML, Tormena CA, Cherubin MR, Favilla HS (2019) Global applications of the visual evaluation of soil structure method: a systematic review and meta-analysis. Soil Tillage Res 190:61–69. https://doi.org/10.1016/j.still.2019.01.002
Franzluebbers AJ, Haney RL (2018) Evaluation of soil processing conditions on mineralizable C and N across a textural gradient. Soil Sci Soc Am J 82(2):354–361. https://doi.org/10.2136/sssaj2017.08.0275
Franzluebbers AJ, Haney RL, Hons FM, Zuberer DA (1996) Determination of microbial biomass and nitrogen mineralization following rewetting of dried soil. Soil Sci Soc Am J 60(4):1133–1139
Haney RL, Franzluebbers AJ, Jin VL, Johnson M-V, Haney EB et al (2012) Soil organic C:N vs. water-extractable organic C:N. Open J Soil Sci 02(03): 269–274. https://doi.org/10.4236/ojss.2012.23032
Haney RL, Haney EB, Smith DR, Harmel RD, White MJ (2018) The soil health tool—theory and initial broad-scale application. Appl Soil Ecol 125:162–168. https://doi.org/10.1016/j.apsoil.2017.07.035
Hicks LC, Lin S, Rousk J (2022) Microbial resilience to drying-rewetting is partly driven by selection for quick colonizers. Soil Biol Biochem 167:108581
Husson O (2013) Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362(1):389–417. https://doi.org/10.1007/s11104-012-1429-7
Husson O, Husson B, Brunet A, Babre D, Alary K et al (2016) Practical improvements in soil redox potential (Eh) measurement for characterisation of soil properties. Application for comparison of conventional and conservation agriculture cropping systems. Anal Chim Acta 906:98–109. https://doi.org/10.1016/j.aca.2015.11.052
Husson O, Brunet A, Babre D, Charpentier H, Durand M et al (2018) Conservation agriculture systems alter the electrical characteristics (Eh, pH and EC) of four soil types in France. Soil Tillage Res 176:57–68. https://doi.org/10.1016/j.still.2017.11.005
Husson O, Sarthou J-P, Bousset L, Ratnadass A, Schmidt H-P et al (2021) Soil and plant health in relation to dynamic sustainment of Eh and pH homeostasis: a review. Plant Soil 466(1):391–447. https://doi.org/10.1007/s11104-021-05047-z
Johannes A, Weisskopf P, Schulin R, Boivin P (2017) To what extent do physical measurements match with visual evaluation of soil structure? Soil Tillage Res 173:24–32. https://doi.org/10.1016/j.still.2016.06.001
Kibblewhite MG, Ritz K, Swift MJ (2008) Soil health in agricultural systems. Philos Trans R Soc Lond B Biol Sci 363(1492): 685–701. https://doi.org/10.1098/rstb.2007.2178
Lal R (2016) Soil health and carbon management. Food Energy Secur 5(4):212–222. https://doi.org/10.1002/fes3.96
Marschner P (2021) Processes in submerged soils – linking redox potential, soil organic matter turnover and plants to nutrient cycling. Plant Soil 464(1):1–12. https://doi.org/10.1007/s11104-021-05040-6
Mattila TJ (2020) Carbon action MULTA Finnish carbon sequestration experimental field dataset. https://doi.org/10.5281/zenodo.3670654
Mattila T, Girz A (2021) Carbon action MULTA Finnish carbon sequestration experimental field dataset 2021.https://doi.org/10.5281/zenodo.7142931
Mattila TJ, Hagelberg E, Söderlund S, Joona J (2022) How farmers approach soil carbon sequestration? Lessons learned from 105 carbon-farming plans. Soil Tillage Res 215:105204. https://doi.org/10.1016/j.still.2021.105204
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Weil RR, Brady NC (2016) The Nature and properties of soils, 15th edn. Pearson, Columbus
Woods End Laboratories (2019) Soil CO2 respiration official Solvita instructions. Woods End Laboratories, Mt Vernon
Zhang Z, Furman A (2021) Soil redox dynamics under dynamic hydrologic regimes - a review. Sci Total Environ 763:143026. https://doi.org/10.1016/j.scitotenv.2020.143026
Funding
Open access funding provided by Finnish Environment Institute (SYKE). The work has been funded by the Strategic Research Council of the Academy of Finland (grant number: 352437).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Iain Paul Hartley.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mattila, T.J. Redox potential as a soil health indicator – how does it compare to microbial activity and soil structure?. Plant Soil 494, 617–625 (2024). https://doi.org/10.1007/s11104-023-06305-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06305-y