Abstract
Background and Aims
Beneficial plant–microbe interactions can improve plant performance under drought; however, we know less about how drought-induced shifts in microbial communities affect plant traits.
Methods
We cultivated Zea mays in fritted clay with soil microbiomes originating from contrasting environments (agriculture or forest) under two irrigation treatments (well-watered or water limited). Using this design, we investigated whether water conditioning was carried forward through the microbiome to affect a subsequent plant cohort that was subjected to either a well-watered or water limited treatment.
Results
Regardless of the microbiome-origin, plants inoculated with a microbiome from a water limited legacy had traits that allowed them to avoid stress but conserve water. They produced longer roots to explore soil, generated greater soil dissolved organic carbon, potentially stimulating the microbiome, and slower soil water content loss during drought. A well-watered legacy resulted in plants that delayed permanent stomatal closure and higher photosynthetic nitrogen use efficiency. In plants with a forest-originated microbiome, a well-watered legacy and water treatment also resulted in higher rates of photosynthesis and stomatal conductance.
Conclusion
These results demonstrate that soil microbiomes can be developed to influence plant drought performance, impacting crop resilience, using short-term microbial conditioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The frequency and duration of drought events is increasing across agricultural land area (Masson-Delmotte et al. 2021), driving growing concerns about global food supplies and bioenergy security. The effects of climate change are compounding and increasing the negative impacts of drought on world-wide crop yield (Lesk et al. 2016). These declines in crop yield not only threaten food security but cascade into severe economic costs (Boyer et al. 2013). Drought often leads to lower crop productivity by decreasing stomatal conductance, eventually limiting CO2 uptake and photosynthesis (Farquhar and Sharkey 1982). Lower CO2 uptake, or productivity, leads to declines in plant growth (Xu et al. 2009; Hussain et al. 2019), resulting in lowered crop yield (Ciais et al. 2005). Maintaining plant productivity and growth under limited water availability is key to maintaining crop yields as world population and food demand continues to rise (Ciais et al. 2005; Passioura 2007; Boyer et al. 2013).
There is growing interest in utilizing microbes to improve plant and ecosystem functions for specific functions, such as improved productivity (Backer et al. 2018; Cavicchioli et al. 2019; Compant et al. 2019; de Vries et al. 2020). Soil and phyto- microbiomes create close associations with their host plant and can play large roles in ecosystem functioning and plant productivity (van der Heijden et al. 2008), providing a promising solution to help alleviate plant stress. Microbial associations can increase a plant’s ability to deal with drought by, for example, directly altering root growth (Fan et al. 2011; Marasco et al. 2013; Henkes et al. 2018) and water and nutrient scavenging through the extension of the root system (Orfanoudakis et al. 2010). These responses improve the plant’s ability to maintain higher rates of stomatal conductance and continued CO2 uptake during moderate drought (Ulrich et al. 2019). Microbes can also directly influence the rhizosphere soil water available to the plant by excreting exopolysaccharides that create a gel-like substance that increases soil adhesion to roots and maintains soil structure (Sandhya et al. 2009). Beyond drought tolerance, many studies have shown improved plant structure and form through microbial inoculations (Timmusk et al. 2014; Dhawi et al. 2015). Trees and crops inoculated with beneficial microbes can have higher growth (Timmusk et al. 2014; Rolli et al. 2015) and produce higher yields (Chen et al. 2021). Most of these applications focus on individual plant growth promoting bacteria or mycorrhizal fungi; however, interactions between microbes can alter their effects on plants (Lau and Lennon 2011), and developing complex microbial communities may provide a more robust method of improving plant traits (Busby et al. 2017; Compant et al. 2019). Inoculations of microbial communities can provide advantages over a single microbial strain as they may have a competitive advantage and longer persistence in natural systems.
Plants can alter the soil chemistry and microbial communities creating plant-soil feedbacks, where the feedbacks have legacies that affect the growth, function, and interactions of future plant communities (Bever 2003; Van der Putten et al. 2013). Legacies of plant community-induced shifts in microbiomes can influence future generations of microbial-plant interactions (Heinen et al. 2020), and abiotic factors, such as extreme weather, warming, and nitrogen enrichment can shift the microbial community in a way that alters future plant-plant interactions (Meisner et al. 2013). These microbial legacies can be beneficial for plant drought avoidance and tolerance, and understanding these interactions could help identify robust microbiomes that can support plant growth under limited water availability. Some plant soil-feedback studies have investigated drought effects on plant growth and competition, where drought legacies on microbiomes can give some plant species a competitive advantage over others (Kaisermann et al. 2017). Further, bacteria isolated from a droughted environment can enhance plant drought tolerance traits (Marulanda et al. 2009), and natural microbial consortia sourced from water stressed conditions can allow maize to avoid drought stress by increasing root length compared to microbiomes from non-stressed plants (Zhang et al. 2022). Additionally, one study found that a microbiome intentionally developed under drought can improve the fitness of future plant generations experiencing drought conditions (Lau and Lennon 2012), suggesting a beneficial legacy. While these studies provide evidence that drought-induced rapid shifts in microbial communities can influence plant competition and fitness, we are still lacking information on how these stress-induced shifts in microbial communities can affect plant growth and function.
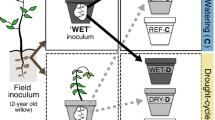
In this study we investigated microbiome legacy effects on Zea mays (maize) growth and function using two different source microbiomes, one originated from a forest (MForest) and one from an agricultural field (MAg), under well-watered (WW) and limited water (WL) conditions. We used a plant-soil feedback approach to condition the microbiomes under an initial microbial selection generation (G0) of maize growth with these two watering conditions. We then propagated these microbiomes into a second, experimental, generation (G1) of plants and tested for a legacy effect under the same and altered watering treatments (Fig. 1). This design allowed us to assess how a short-term drought inflicted on microbiomes originating from diverse sources affects plant growth and function. We hypothesized that the watering treatment would have a strong effect on plant growth; however, we also expected a growth-promoting legacy from microbiomes that received a WL treatment during the microbial conditioning phase. The legacy would manifest so that microbiomes that received a WL conditioning treatment would promote plant growth and function (photosynthesis and stomatal conductance) compared to microbiomes that received a WW conditioning treatment. We further hypothesized that the legacy of the WL conditioning would be stronger in plants inoculated with the microbiome originating from agricultural soil, as this microbiome was adapted to maize under low irrigation conditions.
Representation of experimental design. Generation 0 (G0) describes the water conditioning phase where the microbiomes were either well-watered (Ww; large water drop) or water limited (WL; small water drop). The parent microbiomes in the conditioning phase originated from either agricultural (MAg; yellow) or forest (MForest; green) soil. Soil was collected from each conditioning phase pot and the microbiome was transferred to a new pot and soil for Generation 1 (G1). Half of the pots containing WW-conditioned microbiome were selected at random to receive WW watering treatment again at G1, while the remaining pots containing WW-conditioned microbiome were instead given WL watering treatment at G1. Likewise, half of the pots containing WL-conditioned microbiome received WW watering treatment and half received WL watering treatment at G1. This obtains all eight combinations of microbial conditioning treatment, watering treatment, and microbiome parent source (shown in the bottom row of the figures) specified by the experimental design. Image courtesy of LANL. Don Montoya
Methods
Experimental design
We investigated the effects of microbial conditioning, microbiome parent, and watering treatments on plant growth and function. We were interested in studying two levels of microbial conditioning treatment (WL and WW); two levels of watering treatment (WL and WW); and two microbiome parent sources (MAg and MForest). The experiment was performed as a completely randomized 2 × 2 × 2 full factorial design, consisting of all possible combinations of these levels, giving eight combinations in total. The experiment was carried out in seven replicates per condition for a total of 56 plants.
Experimental set-up
The experiment was conducted in a greenhouse with air temperature averaging 21–22 °C during both day and nighttime hours. Fifty-six Zea mays plants (B73 line: USDA National Plant Germplasm System) were grown from seed under 14 h/10 h day/night cycles. To equalize the daylight hours over the season, natural light was supplemented with growth lights (ROI-E720, Grower’s Choice, Ontario, CA) set to 50% of maximum natural light intensity (PPFD 2000 µmol m−2 s−1) to produce PPFD 1000 µmol m−2 s−1 both in the morning and evening. All plants were grown in 9.8L pots in 6L 20–30 mesh size artificial, fritted clay (GreensGradeTM, PROFILE(R) Products LLC, Buffalo Grove, IL, USA) to control soil chemistry and sterility prior to inoculation. We did not autoclave the clay due to leaching issues; however, the fritted clay manufacturing process produced an extremely low microbial load. Fritted clay was rinsed multiple times prior to planting to reduce the potential for solute toxicity, especially manganese (Adams et al. 2014).
Following the experimental design, half of the 56 plants were selected at random to be inoculated with microbiome source MAg and half were inoculated with source MForest. The agricultural microbiome (MAg) was collected from a maize agricultural field from Akron, CO (40.1605° N, 103.2144° W) that has historically not been irrigated. The forest microbiome (MForest) was collected from a mid-elevation mixed conifer forest in Los Alamos, NM (35.8800° N, 106.3031° W). The two source microbiomes started with different community compositions that remained different throughout the water conditioning phase of the experiment (Moore et al., 2023). Plant inoculation consisted of inoculating both the fritted clay and the seed. The fritted clay was inoculated using a 1:20 soil–water slurry. Z. mays seeds were soaked in a 10% bleach solution for 10 min, rinsed three times with sterile water, and soaked in the natural soil–water slurry for 20 min. Two seeds were then planted per pot and 500 mL of the water slurry was used to water each pot.
Generation 0 (G0) was used as the conditioning phase. Half of the plants inoculated with MAg and half of the plants inoculated with the MForest were chosen at random to be grown under well-watered (WW) conditions at G0; all remaining plants were grown under water limited (WL) conditions at G0. Plants were maintained at 64% and 45% volumetric water content, respectively. To allow for sufficient germination, all plants were maintained at 64% volumetric water content for two weeks after planting. Moisture contents were selected based on fritted clay measured saturation and the moisture characteristic curve (Steinberg et al. 2005). The 45% moisture condition was selected because it was high enough to allow plant growth, but low enough to impose a water limited condition. Soil moisture in each pot was monitored using a soil moisture probe (TEROS 10, METER Group, Inc, WA, USA), and each pot was watered to the target volumetric water content three times per week using filtered tap water enhanced with FloraGro, FloraMicro, and FloraBloom (General Hydroponics, Santa Rosa, CA, USA) fertilizers with doses of 200, 200, and 100 μL L−1 of water, respectively. When both seeds germinated, the smaller seedling was pruned from the pot. When neither planted seed germinated, both seeds were carefully dug up with a sterilized spatula and removed, and the pot re-planted. Plants were grown until the emergence of the 11th leaf. Because the G0 watering treatment affected plant growth rate, WW plants were harvested at 8 weeks and WL plants at 10 weeks. 10 cm soil cores were then collected to sample the soil and rhizosphere microbiome of each pot and stored to inoculate a new seed. Microbial communities remained distinct after Gen0 and differences between Gen0 and Gen1 show that microbial communities continued to shift throughout the experimental Gen1 (Fig. S1a).
After the completion of G0, we used serial propagation of the microbiome (i.e., the microbiome from each pot in G0 was used to inoculate one pot in G1) to transfer the conditioned microbiome to a microbial selection generation with a fresh seed from the same seed batch as used in G0 (G1). G1 seeds were inoculated with G0 microbiome using the same method described above. The G0 soil cores were collected surrounding the intact plants. Therefore, soil cores included fine roots, which were also incorporated in the inoculum slurry. All seeds were planted in fresh, rinsed fritted clay so that the conditioned microbiome, and not differences in soil chemistry, provided the legacy factor. While, we do acknowledge that our soil slurry likely had nutrient carry-over from G0, this would be very minimal due to high dilution at planting. Seedlings were allowed to germinate and grow under WW treatment for two weeks, after which half were given the WW watering treatment and half were given the WL watering treatment (Fig. 1), as specified by the experimental design. After emergence of the 11th leaf, plant height, stem diameter, and maximum photosynthesis rates were measured as described below. After measurement, the plants were subjected to a terminal drought phase where irrigation was discontinued. During this phase, stomatal conductance (gs) and soil volumetric water content (VWC) were measured daily during the hours of 08:00–10:00 using a porometer (SC-1; Decagon Devices Inc., WA, USA). Once the newest fully-expanded leaf reached gs = 0, we proceeded with the post terminal drought measurements described below.
Plant and soil traits pre-terminal drought
We measured plant growth response characteristics non-destructively starting one day before initiating the terminal drought. Plant height (cm) was measured from the base of the stem to the tip of the longest leaf using a ruler. Stem diameter (mm) was measured in two orthogonal directions approximately 2 cm from the base of the plant using calipers. Stem diameter was calculated as the averaged of two orthogonal measurements per plant.
We evaluated plant function from light saturated photosynthesis (Asat) and gs measured on the second fully expanded leaf using an open-system infrared gas exchange system (LI6400XT, LI-COR Inc, Lincoln, NE, USA). Light was controlled at saturating light (measured from light response curves; data not shown) set to 2,000 μmol m−2 s−1. Reference CO2 concentrations and flow rate were controlled at 400 ppm and 400 μmol m−2 s−1, respectively. Relative humidity inside the chamber was controlled at 20%, similar to the ambient humidity inside the greenhouse. Intrinsic water use efficiency (WUEi) was calculated as Asat/gs.
Leaf traits, such as leaf carbon and nitrogen content, are often used as proxies to describe plant function and were measured on the same leaf as plant gas exchange, from 2–3 cm excised from the apex of the leaf. Leaf area (cm2) was measured on the apex cutting of the leaf using a scanner (Canon Image Runner C5560i) and analyzed using ImageJ v1.53 image analysis software. The leaf was placed in a 60 °C drying oven for 48 h for dry mass (g) and then ground using a ball mill (12621–1664, VWR International LLC, PA, USA) to analyze for % nitrogen (N) and % carbon using an elemental analyzer (Eurovector, Pavia, Italy). Nitrogen per unit area (Narea; g m−2) was calculated as nitrogen per unit mass (Nmass; mg g−1) multiplied by leaf mass/ leaf area. Photosynthetic nitrogen use efficiency (PNUE) was calculated as Asat divided by Narea.
Plants can directly encourage microbial growth during drought by altering their root exudation chemistry (Karst et al. 2017; Preece et al. 2018; de Vries et al. 2019); therefore, we also investigated soil pore water chemistry. We analyzed the concentration of dissolved organic carbon (DOC; mg L−1) and nitrate (NO3−; mg L−1) in the pore water, which was collected using Rhizon filter pore water collectors (Rhizosphere Research Products, Wageningen, Netherlands). We placed two collectors per pot, one at the surface (1-11 cm) and one in the deeper profile (6-17 cm). To get a representative sample covering the entire pot depth, surface and deep profile samples were combined to collect at least 20 mL pore water. Samples were stored at -20 °C until thawed and analyzed for DOC using OI Analytical Aurora 1030 TOC analyzer. NO3− and NH4+ were analyzed using a Dionex ICS-2100 (Thermo Fisher Scientific Inc., USA) and Varian Cary 100Bio UV–VIS Spectrometer, respectively. 10 cm soil cores and biocrust samples were then collected for microbiome analysis (Supplemental Methods S1).
Post terminal drought traits
During the terminal drought, VWC declined. The rate of decline was obtained by fitting a linear decay function:
where t indicates time passed since beginning the terminal drought in days, a is the starting VWC before beginning the terminal drought and b is the decay factor. Parameters a and b were estimated for each pot separately using non-linear least squares to determine the best fit. The WW water treatment plants had higher starting VWC; therefore, we normalized each VWC and terminal drought day count measurement to a proportion of the highest VWC and total drought days for each plant. VWC at permanent stomatal closure was measured as the VWC on the morning when the plant reached zero gs.
After the first fully expanded leaf reached gs = 0, plants were harvested and measured for root biomass. To harvest roots, stems were cut 2 cm from the base of the plant. Root systems were carefully pulled from the pot and gently shaken to release soil. Root bulbs were washed in 500 mL sterilized filtered water. Remaining soil in the pot was hand searched for remaining fine roots. Roots were immediately frozen in -20 °C for root morphology analysis (total root length (cm); root length of < 1 mm diameter, 1–2 mm diameter, and > 2 mm diameter (cm), and root projected area (cm2)). For root morphology analysis, roots were placed in a refrigerator to thaw for no more than 72 h. Root systems were imaged (4800 Perfection, Epson America Inc., Epson, CA, USA.) at 600 dpi and analyzed using WinRhizo pro software (Regent Instruments Inc., QC, Canada). Samples were dried at 60 °C for at least 48 h. Specific root length (SRL, m g−1) of the entire root system was calculated as root length per dry root mass.
Statistical analyses
We employed the analysis of variance (ANOVA) to investigate the simultaneous effects of microbiome source, conditioning treatment, and watering treatment on each above- and belowground trait of interest, using a significance threshold of \(\alpha =0.05\). We carried out separate statistical analyses for each trait. All analyses were conducted using R statistical software (R Core Team 2020). When no statistically significant interaction effects were identified, we estimated main effects. For statistically significant main effects, we estimated and reported the difference in factor level means (WW—WL (G0); WW—WL (G1); MForest—MAg) and constructed a 95% confidence interval for the true difference. When statistically significant interaction effects were identified, we carried out multiple comparisons of all treatment means using the Tukey procedure to examine factor effects jointly, as it is not appropriate to combine factor levels to investigate main effects when interaction effects are present. When assumptions of a linear model were not met, we performed a Box-Cox transformation of the response Y, given by
where \(\lambda\) is a parameter that is determined from the data (Box and Cox 1964; Sakia 1992).
Differences in microbial community composition were statistically compared with a PERMANOVA analysis using the adonis function in the R package vegan (Oksanen et al. 2022; see Supplemental Methods S1).
Results
Conditioning microbial legacy effect
A single generation of water-limited microbial conditioning enhanced root and stem growth in the subsequent generation regardless of the microbiome parent and water treatment. Microbiome conditioning did not affect plant height (Table 1; Fig. 2a), but plants grown under a microbiome with WL conditioning had thicker stems than plants with a WW conditioned microbiome (11.95 ± 0.40 mm vs 10.95 ± 0.45 mm, respectively, Fig. 2b). Interestingly, dry root mass was unaffected (Fig. 2c); however, there was a microbiome conditioning effect on root surface area and length of mid and coarse diameter roots. Plants showed marginal evidence for longer fine roots (p = 0.069), and had longer mid (440.93 ± 31.86 cm vs 330.25 ± 27.08 cm) and coarse (1341.88 ± 90.10 cm vs 1045.32 ± 101.12 cm) roots when grown with microbes having a WL than WW conditioning (Table 1; Fig. 2d–f). WL conditioned microbes produced plants with greater root surface area than WW conditioned microbiomes (1407.88 ± 96.17 cm2 vs 1132.45 ± 102.54 cm2; Fig. 2g). Plants grown with WL conditioned microbes had marginally longer SRL (47.47 ± 2.62 m g−1 vs 40.74 ± 2.88 m g−1) than those with WW conditioned microbes (Table 1; Fig. 2h).
Plant growth trait response differences between the two factor level means for each of the three experimental factors: microbiome conditioning (G0), watering treatment (G1), and microbiome parent. Effects on plant height (a), stem diameter (b), dry root mass (c), fine root length (d), mid-diameter root length (e), coarse root length (f), root surface area (g), and specific root length (h). Bars in figures show the differences in mean response between plants treated with well-watered (WW) and water-limited (WL) conditioning (light blue); WW and WL watering treatment (dark blue); and microbiomes originating from forest (MForest) and agricultural (MAg) soil (brown). Factor level mean differences are not shown for characteristics with hypothesis test results for main effects with p > 0.05 (Table 2)
For Asat, the conditioning legacy effect depended on both the watering treatment and the parent microbiome, characterized by a statistically significant three-factor interaction involving conditioning treatment, watering treatment, and microbiome treatment (p = 0.006, Table 2). Tukey multiple comparisons showed that MForest plants treated with Ww at G1 obtained statistically significantly higher mean Asat (17.71 ± 0.85 μmol m−2 s−1; Fig. 3a) when given the Ww conditioning treatment than when conditioned with the WL treatment (12.06 ± 0.67 μmol m−2 s−1; p = 0.011). Additionally, MForest plants treated with Ww at G1 also obtained statistically significantly higher mean Asat than WL MAg plants treated with the WL conditioning treatment (11.36 ± 1.28 μmol m−2 s−1; mean ± se; p = 0.003).
Three-factor interaction plots for response of interest (a) saturated photosynthesis (Asat) and (b) stomatal conductance (gs) (b). The left panels contain watering treatment-by-microbiome two-factor interaction plots when the third factor, conditioning, is WL. The right panels contain watering treatment-by-microbiome two-factor interaction plots when the third factor, conditioning, is WW. The x-axis indicates the two water treatment levels, WL or WW. Letters indicate significant Tukey posthoc analysis differences between means. Interaction plots were based on the significant three-way interaction between microbiome, watering treatment, and conditioning shown in Table 2 (Asat p = 0.006, gs p = 0.031)
There was a similar three-factor interaction for gs (p = 0.031). Under the WL conditioning, varying the levels of microbiome or watering treatment had no discernable effect on mean gs (Fig. 3b; left panel). Under the WW conditioning, increasing the watering treatment from WL to WW resulted in an increase in mean gs for MForest but not for MAg. Tukey multiple comparisons showed that MForest plants treated with WW at G1 obtained statistically significantly higher mean gs (0.17 ± 0.03 mol m−2 s−1; Fig. 3b) when given the Ww conditioning treatment than plants given three of the four treatment combination involving WL conditioning: (1) WL conditioning with MAg and WL watering treatment (0.09 ± 0.03 mol m−2 s−1; p = 0.001); (2) WL conditioning with MForest and WW watering treatment (0.11 ± 0.03 mol m−2 s−1; p = 0.029); (3) WL conditioning with MForest and WL watering treatment (0.11 ± 0.03 mol m−2 s−1; p = 0.025). Additionally, Ww-conditioned MForest plants treated with WW at G1 obtained statistically significantly higher mean gs than Ww-conditioned MForest plants treated with WL at G1 (0.11 ± 0.03 mol m−2 s−1; p = 0.025).
There were no significant pairwise differences among any other treatment combinations for Asat or gs responses. There was no conditioning effect on WUEi (Fig. 4a; data shown as differences between treatment means) or leaf chemistry (Table S1; Fig. S2), but WW conditioning increased PNUE (31.14 ± 14.21 μmol CO2 g−1N s−1) over WL conditioning (25.31 ± 10.06 μmol CO2 g−1N s−1; Table 2; Fig. 4b).
Plant function response differences between the two-factor level means for each of the three experimental factors: microbiome conditioning (G0), watering treatment (G1), and microbiome parent. Effects on intrinsic water use efficiency (WUEi; a), photosynthetic nitrogen use efficiency (PNUE; b), time to stomatal closure (c), pore water dissolved organic carbon (DOC; d), and nitrate (NO3−; e). Bars in figures show the differences in mean response between plants treated with well-watered (WW) and water-limited (WL) conditioning (light blue); WW and WL watering treatment (dark blue); and microbiomes originating from forest (MForest) and agricultural (MAg) soil (brown). Factor level mean differences are not shown for characteristics with hypothesis test results for main effects with p > 0.05 (Table 1)
WW conditioned microbiomes also allowed plants to delay permanent stomatal closure longer during the terminal drought, even when the rate of VWC decline was elevated for WW conditioning. Plants grown with WL conditioned microbiomes reached permanent stomatal closure almost two days earlier than plants grown with WW conditioned microbiome (13.29 ± 0.66 days vs 15.07 ± 0.71 days, respectively; Table 2, Fig. 4c). This occurred despite an increase in the decay factor describing VWC decline over time in plants with WW conditioned microbiomes (0.60 ± 0.02% vs 0.54 ± 0.02%; Table 2; Fig. 5) relative to plants with WL conditioned microbiomes. VWC at stomatal closure did not respond to microbial water conditioning. Microbial WL conditioning increased pore DOC over WW conditioning (11.11 ± 0.145 vs 8.25 ± 0.98 mg L−1; Table 1; Fig. 4d). However, pore water NO3− and NH4+ did not respond to microbial water conditioning (Table 2; Fig. 4e). Soil microbial community composition varied and showed distinct clustering by conditioning treatment (Fig. S1b; p = 0.001).
Volumetric water content (VWC) decline during the terminal drought. Normalized to highest content per pot, the rate that VWC declined until the plant reached the wilting point where stomatal conductance = 0 for plants that received the well-water conditioning (WW; black) or water limited conditioning (WL; gray) microbiomes. Symbols represent the current watering treatment: WL (circle) or WW (triangle)
Watering treatment effect
The G1 watering treatment affected some plant traits without interacting with the G0 conditioning treatment, showing that our watering treatment imposed stress while still allowing plants to continue functioning. Aboveground growth reduced and root length increased under WL watering treatment, even when root biomass decreased. Plants grown under the WW treatment were taller (115.89 ± 2.32 vs 103.42 ± 2.28 cm; Table 1, Fig. 2a) with increased stem diameter (12.97 ± 0.35 vs 9.94 ± 0.29 mm; Fig. 2b) compared to those grown under WL. Plants from WW had greater root biomass than WL treatment plants (3.66 ± 0.19 vs 2.94 ± 0.12 g; Table 1; Fig. 2c). However, when separated into root diameter classes, there was only an overall watering treatment effect for mid diameter root length with WW treatment producing plants with longer mid root length than WL treatment (445.99 ± 33.85 vs 325.19 ± 23.62 cm; Fig. 2e). SRL of WW plants was shorter than for WL plants (40.11 ± 2.48 vs 48.11 ± 2.94 m g−1; Fig. 2h).
The only treatment that affected WUEi was the watering treatment, where plants grown under the WW treatment had lower WUEi (0.12 ± 0.02 μmol CO2 mmol H2O−1 vs 0.13 ± 0.02 μmol CO2 mmol H2O−1) than under WL treatment (Table 2; Fig. 4a). In contrast, WW had higher PNUE (36.73 ± 11.35 μmol CO2 g−1N s−1 vs 19.71 ± 6.44 μmol CO2 g−1N s−1; Fig. 4b) than WL watering treatment. This, combined with the microbiome conditioning results, strongly suggest that WW conditions are beneficial for PNUE. Leaf carbon to nitrogen ratio (C:N) was higher in WW (33.05 ± 5.13 unitless vs 26.75 ± 0.94 unitless; p < 0.001; Table S1; Fig. S2a) compared to WL. Nmass was lower in WW (12.53 ± 2.02 mg g−1 vs 15.95 ± 2.85 mg g−1; p < 0.001; Table S1; Fig. S2b) than WL. Similarly, Narea was lower in the WW (0.43 ± 0.09 g m−2 vs 0.71 ± 0.14 g m−2) than WL watering treatment (p < 0.001; Table S1; Fig. S2c).
Plants grown under WL watering treatment experienced stomatal closure ~ 2.5 days more quickly (12.93 ± 0.54 days vs 15.43 ± 0.77 days; Fig. 4c) than plants grown under a WW treatment. Similarly, the VWC decay factor was higher in plants given the WW treatment (0.60 ± 0.01% vs 0.54 ± 0.02%VWC day−1) than in plants given WL. VWC at stomatal closure was lower for the WL watering treatment (21.62 ± 0.35%VWC) than WW (23.06 ± 0.34%VWC; Table 2). WL watering treatment pots also had higher DOC (14.98 ± 1.28 mg L−1 vs 5.38 ± 0.26 mg L−1; Fig. 4d) than WW treatment pots. Pore water NO3− was almost three times higher in WL compared to WW pots (33.21 ± 3.64 mg L−1 vs 11.36 ± 2.21 mg L−1; Fig. 4e). NH4+ did not respond to water treatment (Table 1). Soil microbial community composition varied and showed distinct clustering by watering treatment (Fig. S1b; p = 0.001).
Parent microbiome effect
Plant growth was strongly influenced by the parent microbiome, where above- and belowground growth were all higher in MForest compared to MAg. Plants with a microbiome parent originating from MForest were taller (116.04 ± 1.88 cm vs 103.27 ± 2.34 cm; Table 1, Fig. 2a) than MAg. Plants with MForest parent had thicker stems (12.34 ± 0.40 mm vs 10.56 ± 0.41 mm; Fig. 2b) than MAg. Plants with a microbiome originating from MForest had higher root biomass (3.71 ± 0.67 g vs 2.89 ± 0.0.18 g) than plants with MAg. Plants that had a parent microbiome originating from MForest had longer fine (14,879.50 ± 653.78 cm; Table 1; Fig. 2d) mid (449.31 ± 23.63 cm; Fig. 2e), and coarse (1443.38 ± 67.60 cm; Fig. 2f) roots than MAg fine (8879.64 ± 1132.76 cm), mid (321.87 ± 33.40 cm), and coarse (943.82 ± 103.83 cm) roots. MForest produced higher root surface area (1550.81 ± 59.88 cm2; Fig. 2g) than MAg (989.52 ± 108.39 cm2). MForest microbiome plants had a higher SRL (50.37 ± 2.10 m g−1; Fig. 2h) than MAg plants (37.84 ± 2.94 m g−1).
The parent microbiome did not affect WUEi, PNUE, leaf chemistry traits, pore water DOC, or traits describing time to stomatal closure or soil VWC at wilting (Table 2). NO3− (28.75 ± 4.01 mg L−1) was almost double in MAg compared to MForest (15.82 ± 2.79 mg L−1; Fig. 4e). NH4+ did not respond to parent microbiome (Table 1).
MForest demonstrated a higher abundance of nitrogen-fixing bacteria during the conditioning phase (G0). This trend continued and was more pronounced in G1, where MForest showed a higher abundance (hits) of nitrogen-fixing bacteria than MAg (Fig. S2).
Discussion
Our experiment showed that just a single generation of water treatment conditioning generated a microbial community that could carry forward its water treatment legacy to influence the structure and function of a new maize plant. Root and belowground traits were strongly influenced by a WL legacy with root surface area, specific root length, lengths of mid- to coarse-roots, and soil pore water dissolved organic carbon increasing in plants inoculated with a WL conditioned microbiome. A WW conditioned microbiome also positively affected some plant functional traits. Plants with a WW conditioning legacy kept stomata open longer before reaching their point of permanent stomatal closure, had higher photosynthetic nitrogen use efficiency, and had higher rates of photosynthesis and stomatal conductance under MForest and WW treatment. These results provide important evidence of how quickly microbiomes can shift to affect the function of future plant growth. Our findings provide a promising experimental design approach toward the development of microbiomes to secure agriculture under future climate stressors.
Water conditioning legacy on plant structure
Many studies have shown that microbiomes influence plant belowground growth, where plant growth promoting bacteria increases root dry or fresh biomass (Marulanda et al. 2009; Marasco et al. 2013; Schwartz et al. 2013) by, e.g., altering plant signal cascades to increase lateral root growth or promoting production of root hairs that increase plant surface area for water uptake (Creus et al. 2005; Molina-Favero et al. 2008). Our watering conditioning induced a legacy effect, such that depending on the trait, the WL conditioning either benefitted or disadvantaged plant structure in relation to drought independent from the original microbiome source. Plants with a microbiome originating from WL conditioning had greater root length due to shift in the morphology of their root system such that they produced longer roots per biomass investment (e.g., longer SRL) than plants growing with a microbiome that originated from WW conditioning. Greater root length (Fig. 2, S5; Table 1), theoretically assists plants growing under limited water availability in foraging for water (Comas et al. 2013; Marasco et al. 2013). Similarly, plants that originated from the WL conditioning had thicker stems, even though height did not increase. Plant-associated microbiomes, particularly plant growth promoting bacteria, can either increase (Bashan et al. 2012; Molina-Romero et al. 2017) or decrease stem diameter (Molina-Romero et al. 2017). Greater stem thickness could lead to higher rates of water transport or, overall, higher biomass. The longer root length resulting from WL conditioning could have increased plant water uptake; therefore, thicker stems, if associated with larger conduits and stem cross sections, could have developed to better facilitate water movement through the plant (Abd El-Rahim et al. 1998). Although we did not specifically measure hydraulic conductance in this study, greater water transport through the stem often leads to greater primary productivity (Brodribb and Feild 2000; Mencuccini 2003; Liu et al. 2020). However, during times of water scarcity, greater water transport can cause more rapid water uptake from the soil and eventually lead to more rapid water deficits (Leakey et al. 2006). In our experiment, WL conditioning still had a soil water retention advantage over WW conditioning in that WL-conditioned plants lost VWC more slowly during the terminal drought (Table 2; Fig. 5); therefore, this may not apply to our system. Overall, these results suggest that microbiomes quickly respond to drought in a way that affects plant growth traits toward water uptake and that shift is conserved in the next plant cohort.
Water conditioning legacy on plant functioning
While WL legacy resulted in beneficial plant structural shifts, whether WW or WL legacy was beneficial differed between plant functional traits. The WL conditioning resulted in greater DOC (Fig. 2d), showing the important control that the microbial legacy had on DOC. Pre-experimental growth substrate (fritted clay + fertilized water) pore water DOC was quite low (~ 5 mg l−1); however, it is likely that the fertilized water caused some accumulation of DOC over time in our pots. Even so, we saw a conditioning treatment effect on DOC regardless of the water treatment; therefore, the conditioning treatment effects are due to alterations of the microbiome during the conditioning phase. DOC treatment differences could be an indicator of either increased microbial metabolism of soil organic carbon, cells sloughing off during growth, or direct exudation by roots (Calvo et al. 2019). Root exudates play a direct role in signaling and providing a carbon source for the microbial community that can help alleviate drought stress (Kiers et al. 2011). Although severe droughts may limit root exudation (Preece and Peñuelas 2016; Karst et al. 2017), greater carbon exudation or DOC is commonly found in systems exposed to moderate drought compared to a control treatment (Sanaullah et al. 2012; Preece et al. 2018). The amount of DOC has been shown to be controlled by microbial taxa (Sanaullah et al. 2012; Albright et al. 2020). 14C labeling studies have shown that drought-induced increases in DOC do not necessarily lead to increases in recently exuded C in microbial biomass (Sanaullah et al. 2012), suggesting DOC is directly coming from the plant instead of increased microbial biomass. The legacy of WL conditioning promoted higher DOC in the soil, suggesting that a drought adapted microbiome stimulates C exudation or root sloughing from the plant that, in principle, could promote microbiome activity that may also benefit the plant under drought stress.
A WW conditioning treatment benefitted MForest, which produced higher rates of photosynthesis and stomatal conductance under well-watered conditions. However, due to higher rates of photosynthesis and stomatal conductance in the forest soil when microbiomes experienced WW during both the conditioning and water treatment phase, WL during the conditioning or water treatment phases seemed to prohibited gas exchange rate increases (Fig. 3). It is possible that the MAg treatment did not experience an observable WW conditioning treatment legacy because this microbiome was already adapted to maize drought conditions. Less responsive stomata are more commonly found in more drought tolerant species (Skelton et al. 2015; Henry et al. 2019) and, while often associated with lower stomatal conductance, lower stomatal sensitivity and less efficient xylem can help plants maintain hydraulic safety (Gleason et al. 2017). Further, we hypothesize that plants grown under MForest combined with WW water treatment and WW conditioning may have experienced upregulation of plant gas exchange compared to the WL treatment because MForest was enriched with potential nitrogen fixing bacteria (Figs. S3, S4). N fixation could positively influence photosynthetic capacity and CO2 uptake via impacts of increased N on photochemistry (Henneron et al. 2020). We note that further experiments are needed to definitely attribute function to these microbial taxa. These are taxa that could explain treatment differences, and nitrogen fixation is one potential mechanism, but we acknowledge that these taxa and other taxa in our systems could be driving different functions and, thus, the different plant responses observed between our treatments. Even so, rhizospheric nitrogen fixing bacteria play a key role in plant nutrient availability as they are able to directly reduce atmospheric nitrogen, creating higher nitrogen availability to plants while also producing phytohormones and solubilizing phosphorus (Swarnalakshmi et al. 2020). This could lead to higher nitrogen availability for the photosynthetic apparatus (Cleveland et al. 1999; Reed et al. 2011) and, therefore, increased rates of photosynthesis.
Photosynthetic nitrogen use efficiency (PNUE) also experienced a watering treatment legacy effect; however, this was not dependent on parent microbiome (Fig. 4b). WW conditioning led to plants with higher PNUE than plants with WL legacy, independent of the parent microbiome. This is aligned with the Least-cost Hypothesis that states that plants optimize their traits to efficiently use and acquire the more limiting resource (either water or nitrogen) (Field et al. 1983; Wright et al. 2003). In our study, WL microbiome conditioning tended to produce lower rates of photosynthesis, (significant interaction effect, Table 2); however, Narea did not differ between plants with different microbiomes (Table 2). This suggests that the plants experiencing the WW treatments were better able to utilize similar concentrations of leaf nitrogen. Water and nitrogen are the factors that most limit global crop growth (Mueller et al. 2012), and importantly, these results show that the microbiome plays a role in this relationship, where WW conditioning of the microbiome allowed the plant to maximize plant nitrogen utilization per the rate of photosynthesis.
Time to stomatal closure strongly controlled by microbial community
Contrary to our hypothesis, corn plants that were inoculated with a WW conditioned microbiome were able to last longer during the terminal drought before reaching the point of permanent stomatal closure (Fig. 4c). This suggests the microbiomes retained a feature from WW conditioning that allowed them to prolong plant activity even under drought. The microbial community can have direct and indirect effects on how long plants keep stomata open during drought and have specifically been shown to delay wilting in rice (Redman et al. 2011). Microbes can exude exopolysaccharides that directly retain water and nutrients within the rhizosphere (De Caire et al. 1997; Alami et al. 2000; Mehboob et al. 2009). In addition, microbes can control plant hormonal signals and can reduce abscisic acid induced stomatal closure (Perrig et al. 2007; Kang et al. 2014). In our system, we also found that pots with WW conditioning lost soil water more quickly than pots with a WL legacy, yet these plants also took longer to close their stomata (Fig. 5). This extended time to stomatal closure under faster soil drying might be accomplished by altering plant hormonal signals or by allowing the plants to better access the more limited water available in these pots.
Implications and conclusions
We found that just a single generation of plant growth under different watering conditions induced rapid shifts in microbial function to influence a subsequent cohort of plant structure and function under varying watering treatments. These results present novel microbiome selection methods to improve plant performance relative to other studies that have used mycorrhizal fungi (Bowles et al. 2016), plant growth promoting bacteria (Rolli et al. 2015), and arid region microbiomes (Marulanda et al. 2009) in that they show that, regardless of the microbiome origin, we can very quickly modify the entire microbiome to produce plant traits of interest using droughting techniques. Similarly, we know from previous studies that drought alters the legacy of microbial communities (de Vries et al. 2019); however, our work further shows that shifting the microbial community alone can influence plant structure and function.
We hypothesized a priori that, of our two microbiomes, the microbiome originating from non-irrigated agricultural soil would show a stronger microbial legacy response to drought than the forest originated microbiome. Surprisingly, our results do not support this hypothesis. Except for Asat and gs grown under MForest and WW treatment (Fig. 3), there were no clear differences between the conditioning legacies of the two microbiomes even though had different starting compositions (Tables 1 and 2; Moore et al. 2023). What was clear, however, was that the MForest promoted greater plant growth than MAg (Figs. 2e–g, 5), a trend that occurred regardless of watering treatment and further supports results discussed in Moore et al. (2023). The shorter plants and lower root mass seen in MAg plants might suggests that, similar to our WL legacy effects, plants exposed to MAg would use less water and might be able to withstand drought for longer periods of time independently of the microbiome conditioning treatment. However, the decrease in aboveground growth was not accompanied by increased root length or area that would improve the plant’s ability to access sources of water (Fig. 2; Comas et al. 2013). There is a trade-off associated with drought tolerance and plant size where a smaller plant might have a greater ability to survive but the smaller, drought adaptive traits often lead to lower productivity. Our results suggest that MAg, when compared to MForest, might actually be limited in its ability to shift in response to drought in a way that is beneficial for the crops growing under water limitation. Further, depressed growth in MAg plants could have been influenced by the yield drag phenomenon, where years of agricultural plant growth in the same field decreases yield through an altered microbiome (Johnson et al. 1992; Benitez et al. 2021). While the difference in growth suggests important functional differences between plants grown in the presence of different microbiomes, it is important to note that height alone might not indicate improved plant yield, especially under drought where light interception is key (Comas et al. 2019). The impacts of microbiomes from a historically droughted environment on plant drought tolerance traits are currently under investigated. However, the differences we found between the two original parent microbiomes are opposite to a recent study by Zhang et al. (2022) who showed that a microbiome sourced from a droughted grass conferred higher maize drought tolerance than a microbiome sourced from irrigated agricultural conditions. Our results, however, agreed with Zhang et al. (2022) in that the microbiome able to best improve plant traits came from a natural soil rather than controlled, agricultural conditions. These results have important implications for agricultural applications trying to improve plant growth or yield through modification of the microbiome, where it is important to perform rigorous investigations of the community being amended. Further, sourcing that a microbiome from agricultural soil that has already gone through generations of selection under plant species and nutrient amendments might not produce the most effective microbial community to improve plant performance.
Our study shows that it is possible to rapidly induce a shift in complex microbial communities to alter plant performance. Examples showing that microbial communities can be pressured to modify composition in a way that alters plant function, rather than plant growth alone, are rare; however, our results are aligned with a study where a microbiome from a three-generation drought experiment improved aspects of plant fitness, such as fruiting and flowering time (Lau and Lennon 2012). In addition, Panke-Buisse et al., (2015) also adjusted plant flowering time through microbiome directed evolution. These results suggest that, while directly trying to evolve plants in a timely manner in response to global change may be an unlikely or impractical solution for improving plant structure and function in future environments (Lau et al. 2007; Lau and Terhorst 2020), achieving improved plant performance by imposing short-term treatments on microbial communities may be a promising avenue to ameliorate plant drought stress.
Data availability
Data are available as supplemental dataset.
References
Abd El-Rahim MF, Fahmy GM, Fahmy ZM (1998) Alterations in transpiration and stem vascular tissues of two maize cultivars under conditions of water stress and late wilt disease. Plant Pathol 47:216–223. https://doi.org/10.1046/j.1365-3059.1998.00211.x
Adams C, Jacobson A, Bugbee B (2014) Ceramic aggregate sorption and desorption chemistry: implications for use as a component of soilless media. J Plant Nutr 37:1345–1357. https://doi.org/10.1080/01904167.2013.837921
Alami Y, Achouak W, Marol C, Heulin T (2000) Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl Environ Microbiol 66:3393–3398. https://doi.org/10.1128/AEM.66.8.3393-3398.2000
Albright MBN, Thompson J, Kroeger ME et al (2020) Differences in substrate use linked to divergent carbon flow during litter decomposition. FEMS Microbiol Ecol 96:1–15. https://doi.org/10.1093/femsec/fiaa135
Backer R, Rokem JS, Ilangumaran G et al (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 871:1–17. https://doi.org/10.3389/fpls.2018.01473
Bashan Y, Salazar BG, Moreno M et al (2012) Restoration of eroded soil in the Sonoran Desert with native leguminous trees using plant growth-promoting microorganisms and limited amounts of compost and water. J Environ Manage 102:26–36. https://doi.org/10.1016/j.jenvman.2011.12.032
Benitez MS, Ewing PM, Osborne SL, Lehman RM (2021) Rhizosphere microbial communities explain positive effects of diverse crop rotations on maize and soybean performance. Soil Biol Biochem 159:108309. https://doi.org/10.1016/j.soilbio.2021.108309
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol 157:465–473. https://doi.org/10.1046/j.1469-8137.2003.00714.x
Bowles TM, Barrios-Masias FH, Carlisle EA et al (2016) Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci Total Environ 566–567:1223–1234. https://doi.org/10.1016/j.scitotenv.2016.05.178
Box GEP, Cox DR (1964) An analysis of transformations. Journal, Source Stat R Ser Soc 26:211–252
Boyer JS, Byrne P, Cassman KG et al (2013) The U.S. drought of 2012 in perspective: a call to action. Glob Food Sec 2:139–143. https://doi.org/10.1016/j.gfs.2013.08.002
Brodribb TJ, Feild TS (2000) Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant Cell Environ 23:1381–1388. https://doi.org/10.1046/j.1365-3040.2000.00647.x
Busby PE, Soman C, Wagner MR et al (2017) Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol 15:1–14. https://doi.org/10.1371/journal.pbio.2001793
Calvo OC, Franzaring J, Schmid I, Fangmeier A (2019) Root exudation of carbohydrates and cations from barley in response to drought and elevated CO2. Plant Soil 438:127–142. https://doi.org/10.1007/s11104-019-03998-y
Cavicchioli R, Ripple WJ, Timmis KN et al (2019) Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol 17:569–586. https://doi.org/10.1038/s41579-019-0222-5
Chen L, Li K, Shang J et al (2021) Plant growth–promoting bacteria improve maize growth through reshaping the rhizobacterial community in low-nitrogen and low-phosphorus soil. Biol Fertil Soils. https://doi.org/10.1007/s00374-021-01598-6
Ciais P, Reichstein M, Viovy N et al (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533. https://doi.org/10.1038/nature03972
Cleveland CC, Townsend AR, Schimel DS et al (1999) Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem Cycles 13:623–645. https://doi.org/10.1002/(ISSN)1944-9224
Comas LH, Becker SR, Cruz VMV et al (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4:1–16. https://doi.org/10.3389/fpls.2013.00442
Comas LH, Trout TJ, DeJonge KC et al (2019) Water productivity under strategic growth stage-based deficit irrigation in maize. Agric Water Manag 212:433–440. https://doi.org/10.1016/j.agwat.2018.07.015
Compant S, Samad A, Faist H, Sessitsch A (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res 19:29–37. https://doi.org/10.1016/j.jare.2019.03.004
Creus CM, Graziano M, Casanovas EM et al (2005) Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 221:297–303. https://doi.org/10.1007/s00425-005-1523-7
De Caire GZ, De Cano MS, De Mulé MCZ et al (1997) Aggregation of soil particles by Nostoc muscorum Ag. (Cyanobacteria). J Appl Phycol 9:249–253
de Vries FT, Williams A, Stringer F et al (2019) Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol 224:132–145. https://doi.org/10.1111/nph.16001
de Vries FT, Griffiths RI, Knight CG et al (2020) Harnessing rhizosphere microbiomes for drought-resilient crop production. Science (80- ) 368:270–2714
Dhawi F, Datta R, Ramakrishna W (2015) Mycorrhiza and PGPB modulate maize biomass, nutrient uptake and metabolic pathways in maize grown in mining-impacted soil. Plant Physiol Biochem 97:390–399. https://doi.org/10.1016/j.plaphy.2015.10.028
Fan L, Dalpé Y, Fang C et al (2011) Influence of arbuscular mycorrhizae on biomass and root morphology of selected strawberry cultivars under salt stress. Botany 89:397–403. https://doi.org/10.1139/b11-028
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Field C, Merino J, Mooney HA (1983) Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384–389. https://doi.org/10.1007/BF00376856
Gleason SM, Wiggans DR, Bliss CA et al (2017) Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora Morphol Distrib Funct Ecol Plants 227:1–9. https://doi.org/10.1016/j.flora.2016.11.017
Heinen R, Hannula SE, De Long JR et al (2020) Plant community composition steers grassland vegetation via soil legacy effects. Ecol Lett 23:973–982. https://doi.org/10.1111/ele.13497
Henkes GJ, Kandeler E, Marhan S et al (2018) Interactions of mycorrhiza and protists in the rhizosphere systemically alter microbial community composition, plant shoot-to-root ratio and within-root system nitrogen allocation. Front Environ Sci 6:. https://doi.org/10.3389/fenvs.2018.00117
Henneron L, Kardol P, Wardle DA et al (2020) Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol 228:1269–1282. https://doi.org/10.1111/nph.16760
Henry C, John GP, Pan R et al (2019) A stomatal safety-efficiency trade-off constrains responses to leaf dehydration. Nat Commun 10:1–9. https://doi.org/10.1038/s41467-019-11006-1
Hussain HA, Men S, Hussain S et al (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-40362-7
Johnson NC, Copeland PJ, Crookston RK, Pfleger FL (1992) Mycorrhizae: possible explanation for yield decline with continuous corn and soybean. Agron J 84:387–390. https://doi.org/10.2134/agronj1992.00021962008400030007x
Kaisermann A, de Vries FT, Griffiths RI, Bardgett RD (2017) Legacy effects of drought on plant–soil feedbacks and plant–plant interactions. New Phytol 215:1413–1424. https://doi.org/10.1111/nph.14661
Kang SM, Khan AL, Waqas M et al (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9:673–682. https://doi.org/10.1080/17429145.2014.894587
Karst J, Gaster J, Wiley E, Landhäusser SM (2017) Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiol 37:154–164. https://doi.org/10.1093/treephys/tpw090
Kiers ET, Duhamel M, Beesetty Y et al (2011) Reciprocal rewards stabilize cooperation in the Mycorrhizal symbiosis. Science 333:880–883. https://doi.org/10.1126/science.1208473
Lau JA, Lennon JT (2011) Evolutionary ecology of plant-microbe interactions: Soil microbial structure alters selection on plant traits. New Phytol 192:215–224. https://doi.org/10.1111/j.1469-8137.2011.03790.x
Lau JA, Lennon JT (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci U S A 109:14058–14062. https://doi.org/10.1073/pnas.1202319109
Lau JA, Terhorst CP (2020) Evolutionary responses to global change in species-rich communities. Ann N Y Acad Sci 1476:43–58. https://doi.org/10.1111/nyas.14221
Lau JA, Shaw RG, Reich PB et al (2007) Strong ecological but weak evolutionary effects of elevated CO2 on a recombinant inbred population of Arabidopsis thaliana. New Phytol 175:351–362. https://doi.org/10.1111/j.1469-8137.2007.02108.x
Leakey ADB, Uribelarreà M, Ainsworth EA et al (2006) Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol 140:779–790. https://doi.org/10.1104/pp.105.073957
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529:84–87. https://doi.org/10.1038/nature16467
Liu J, Kang S, Davies WJ, Ding R (2020) Elevated [CO2] alleviates the impacts of water deficit on xylem anatomy and hydraulic properties of maize stems. Plant Cell Environ 43:563–578. https://doi.org/10.1111/pce.13677
Marasco R, Rolli E, Vigani G et al (2013) Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal Behav 8:. https://doi.org/10.4161/psb.26741
Marulanda A, Barea JM, Azcón R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM Fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J Plant Growth Regul 28:115–124. https://doi.org/10.1007/s00344-009-9079-6
Masson-Delmotte V, Zhai P, Priani A et al (2021) IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contrib Work Gr I to Sixth Assess Rep Intergov Panel Clim Change. Cambridge University Press, Cambridge, United Kingdom and New York, p 2391. https://doi.org/10.1017/9781009157896
Mehboob I, Naveed M, Zahir ZA (2009) Rhizobial association with non-legumes: mechanisms and applications. CRC Crit Rev Plant Sci 28:432–456. https://doi.org/10.1080/07352680903187753
Meisner A, De Deyn GB, De Boer W, Van Der Putten WH (2013) Soil biotic legacy effects of extreme weather events influence plant invasiveness. Proc Natl Acad Sci U S A 110:9835–9838. https://doi.org/10.1073/pnas.1300922110
Mencuccini M (2003) The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ 26:163–182. https://doi.org/10.1046/j.1365-3040.2003.00991.x
Molina-Favero C, Creus CM, Simontacchi M et al (2008) Aerobic nitric oxide production by Azospirillum brasilense Sp245 and its influence on root architecture in tomato. Mol Plant-Microbe Interact 21:1001–1009. https://doi.org/10.1094/MPMI-21-7-1001
Molina-Romero D, Baez A, Quintero-Hernández V et al (2017) Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 12:1–21. https://doi.org/10.1371/journal.pone.0187913
Moore E, Carter K, Heneghan J et al (2023) Microbial drivers of plant performance during drought depend upon community composition and the greater soil environment. Spectrum 11:e01476–e1522. https://doi.org/10.1128/spectrum.01476-22
Mueller ND, Gerber JS, Johnston M et al (2012) Closing yield gaps through nutrient and water management. Nature 490:254–257. https://doi.org/10.1038/nature11420
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) _vegan: Community Ecology Package_. R package version 2.6-4
Orfanoudakis M, Wheeler CT, Hooker JE (2010) Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. Mycorrhiza 20:117–126. https://doi.org/10.1007/s00572-009-0271-0
Panke-Buisse K, Poole AC, Goodrich JK et al (2015) Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J 9:980–989. https://doi.org/10.1038/ismej.2014.196
Passioura J (2007) The drought environment: Physical, biological and agricultural perspectives. J Exp Bot 58:113–117. https://doi.org/10.1093/jxb/erl212
Perrig D, Boiero ML, Masciarelli OA et al (2007) Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol 75:1143–1150. https://doi.org/10.1007/s00253-007-0909-9
Preece C, Peñuelas J (2016) Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 409:1–17. https://doi.org/10.1007/s11104-016-3090-z
Preece C, Farré-Armengol G, Llusià J, Peñuelas J (2018) Thirsty tree roots exude more carbon. Tree Physiol 38:690–695. https://doi.org/10.1093/treephys/tpx163
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statical Computing. In: R Found. Statical Comput. Vienna, Austria
Redman RS, Kim YO, Woodward CJDA et al (2011) Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 6:1–10. https://doi.org/10.1371/journal.pone.0014823
Reed SC, Cleveland CC, Townsend AR (2011) Functional biology of heterotrophic nitrogen-fixing bacteria. Annu Rev Ecol Evol Syst 42:489–512. https://doi.org/10.1146/annurev-ecolsys-102710-145034
Rolli E, Marasco R, Vigani G et al (2015) Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol 17:316–331. https://doi.org/10.1111/1462-2920.12439
Sakia R (1992) The Box-Cox transformation technique: a review. Wiley R Stat Soc 41:169–178
Sanaullah M, Chabbi A, Rumpel C, Kuzyakov Y (2012) Carbon allocation in grassland communities under drought stress followed by 14C pulse labeling. Soil Biol Biochem 55:132–139. https://doi.org/10.1016/j.soilbio.2012.06.004
Sandhya V, Z AS, Grover M et al (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol Fertil Soils 46:17–26. https://doi.org/10.1007/s00374-009-0401-z
Schwartz AR, Ortiz I, Maymon M et al (2013) Bacillus simplex—a little known pgpb with anti-fungal activity—alters pea legume root architecture and nodule morphology when coinoculated with rhizobium leguminosarum bv. Viciae. Agronomy 3:595–620. https://doi.org/10.3390/agronomy3040595
Skelton RP, West AG, Dawson TE (2015) Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc Natl Acad Sci U S A 112:5744–5749. https://doi.org/10.1073/pnas.1503376112
Steinberg SL, Kluitenberg GJ, Jones SB et al (2005) Physical and hydraulic properties of baked ceramic aggregates used for plant growth medium. J Am Soc Hortic Sci 130:767–774. https://doi.org/10.21273/jashs.130.5.767
Swarnalakshmi K, Yadav V, Tyagi D et al (2020) Significance of plant growth promoting rhizobacteria in grain legumes: Growth promotion and crop production. Plants 9:1–25. https://doi.org/10.3390/plants9111596
Timmusk S, Abd El-Daim IA, Copolovici L et al (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9:. https://doi.org/10.1371/journal.pone.0096086
Ulrich DEM, Sevanto S, Ryan M et al (2019) Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-018-36971-3
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Van der Putten WH, Bardgett RD, Bever JD et al (2013) Plant-soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276. https://doi.org/10.1111/1365-2745.12054
Wright IJ, Reich PB, Westoby M (2003) Least-cost input mixtures of water and nitrogen for photosynthesis. Am Nat 161:98–111. https://doi.org/10.1086/344920
Xu Z, Zhou G, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60:3737–3749. https://doi.org/10.1093/jxb/erp216
Zhang Z, Jatana BS, Campbell BJ et al (2022) Cross‐inoculation of rhizobiome from a congeneric ruderal plant imparts drought tolerance in maize (Zea mays ) through changes in root morphology and proteome . Plant J 1–18. https://doi.org/10.1111/tpj.15775
Acknowledgements
We are very grateful to the United States Department of Agriculture and the US National Plant Germplasm System, who provided seeds for this project. We thank Dr. John Dunbar for his contribution to the design of this project.
Funding
This work was funded by Los Alamos National Laboratory Directed Research and Development grant #20200109DR.
Author information
Authors and Affiliations
Contributions
Kelsey R. Carter, L. Turin Dickman, Eric R. Moore, Sangeeta Negi, John P. Heneghan, Anthony J. Sabella, Rose J. Harris, Oana C. Marina, Dea Musa, George B. Perkins, Sanna Sevanto performed experiments Kelsey R. Carter, Abigael C. Nachtsheim, Sanna Sevanto, L. Turin Dickman, Eric R. Moore, Sangeeta Negi, Christina R. Steadman, Christine M. Anderson-Cook, Louise H. Comas, Rose J. Harris, John P. Heneghan, Nicholas Lubbers, Dea Musa, Brent D. Newman, Jeffrey M. Heikoop wrote the manuscript. SS, Christina R. Steadman, Michaeline B. N. Albright, Abigael C. Nachtsheim, Christine M. Anderson-Cook, Louise H. Comas, John P. Heneghan, Nicholas Lubbers, Brent D. Newman, Scott Twary, Chris M. Yeager, John M. Dunbar, Sanna Sevanto designed the experiment. Kelsey R. Carter and Abigael C. Nachtsheim analyzed the data. All authors contributed to interpretation of data.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Anna Maria Pirttila.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carter, K.R., Nachtsheim, A.C., Dickman, L.T. et al. Drought conditioning of rhizosphere microbiome influences maize water use traits. Plant Soil 492, 587–604 (2023). https://doi.org/10.1007/s11104-023-06204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06204-2