Abstract
Background and aims
Plant Ni uptake in aboveground biomass exceeding concentrations of 1000 μg g−1 in dry weight is defined as Ni hyperaccumulation. Whether hyperaccumulators are capable of mobilizing larger Ni pools than non-accumulators is still debated and rhizosphere processes are still largely unknown. The aim of this study was to investigate rhizosphere processes and possible Ni mobilization by the Ni hyperaccumulator Odontarrhena chalcidica and to test Ni uptake in relation to a soil Ni gradient.
Methods
The Ni hyperaccumulator O. chalcidica was grown in a pot experiment on six soils showing a pseudo-total Ni and labile (DTPA-extractable) Ni gradient and on an additional soil showing high pseudo-total but low labile Ni. Soil pore water was sampled to monitor changes in soil solution ionome, pH, and dissolved organic carbon (DOC) along the experiment.
Results
Results showed that Ni and Fe concentrations, pH as well as DOC concentrations in pore water were significantly increased by O. chalcidica compared to unplanted soils. A positive correlation between Ni in shoots and pseudo-total concentrations and pH in soil was observed, although plant Ni concentrations did not clearly show the same linear pattern with soil available Ni.
Conclusions
This study shows a clear root-induced Ni and Fe mobilization in the rhizosphere of O. chalcidica and suggests a rhizosphere mechanism based on soil alkalinization and exudation of organic ligands. Furthermore, it was demonstrated that soil pH and pseudo-total Ni are better predictors of Ni plant uptake in O. chalcidica than labile soil Ni.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel hyperaccumulation in plants has primarily evolved as an adaptation to Ni-rich ultramafic soils in different world regions (Baker and Brooks 1989; Lange et al. 2017; van der Ent et al. 2016, 2018). The threshold level of nickel hyperaccumulation was set to 1000 μg g−1 in dry shoots (Baker and Brooks 1989; van der Ent et al. 2013). Such plants have received growing scientific attention due to their potential applications in the remediation of metal contaminated soils and more recently in nickel phytomining (Bani et al. 2015a, b; Chaney et al. 2007; Krämer 2005; Lombi et al. 2000; Nkrumah et al. 2016; Pardo et al. 2018; Rosenkranz et al. 2019; van der Ent 2015; Wenzel et al. 1999). To allow those exceptionally high metal concentrations in plant tissues, enhanced metal uptake and translocation mechanisms should have developed in hyperaccumulator plants (Baker 1981, 1987). Whether hyperaccumulators are able to modify metal availability in their soil and to access larger metal fractions which are unavailable to non-accumulator plants is still debated. Recent studies have contributed to clarify plant internal (Assunção et al. 2003; Brooks 1998; Krämer et al. 1996, 1997, 2000; Krämer 2010; Lombi et al. 2000) and rhizosphere processes (Álvarez-López et al. 2021; Dessureault-Rompré et al. 2010; Puschenreiter et al. 2005; Wenzel et al. 1999, 2003) associated with metal hyperaccumulation. Nevertheless, the mechanisms of metal acquisition and specific rhizosphere processes of hyperaccumulator plants are still largely unknown. Labile metal fractions assessed on soil often poorly correlate with metal uptake in hyperaccumulators and literature shows contradictory results. While some research provides evidence of increased Ni plant uptake with higher Ni soil availability (Centofanti et al. 2012; Massoura et al. 2004), other studies suggest the contrary (Bani et al. 2014; Noller 2017; Puschenreiter et al. 2019). Several studies support the hypothesis that hyperaccumulators rely on metal mobilization from less available metal fractions (Álvarez-López et al. 2021; Chardot-Jacques et al. 2013; Puschenreiter et al. 2005; Wenzel et al. 2003); however, literature also suggest that hyperaccumulators access the same metal pools as non-accumulators (Echevarria et al. 2006; Hammer et al. 2006; Hutchinson et al. 2000; Massoura et al. 2004; Salt et al. 2000). Non-linear relations would imply a role of active Ni mobilization processes at the roots level, which might not necessarily be targeting the hyperaccumulated metals (such as Ni) but could possibly occur to enhance plant uptake of other essential nutrients. As possible mechanism for high Ni plant accumulation, it was suggested that hyperaccumulator species may release root exudates containing Ni-chelators with the potential to enhance Ni uptake and translocation (Álvarez-López et al. 2021; Puschenreiter et al. 2005; Salt et al. 2000; Wenzel et al. 2003). Despite the extensive knowledge about rhizosphere process involved in plant nutrient acquisition, the role of root induced metal mobilization in hyperaccumulator plants has not been yet clarified. Higher dissolved organic carbon (DOC) measured in rhizosphere soil suggested an active release of roots exudates by the Ni hyperaccumulator Noccaea goesingensis (Wenzel et al. 2003) and Odontarrhena serpillyfolia (Álvarez-López et al. 2021) which was associated with higher Ni availability in rhizosphere soil. Puschenreiter et al. (2005) also found some indication for the release of citric acid in the rhizosphere of Noccaea goesingensis. Li et al. (2012) indicated higher organic acid levels in the rhizosphere of the hyperaccumulator Sedum alfredii compared with a non-hyperaccumulator ecotype, which was associated with higher Zn mineral dissolution. On the contrary, in other studies no or little indication of root-induced solubilization was observed (Salt et al. 2000; Whiting et al. 2001; Zhao et al. 2001). To the best of our knowledge, no studies have been specifically investigating rhizosphere processes of the Ni hyperaccumulator Odontarrhena chalcidica (formerly Alyssum murale).
The aim of this study was to investigate if: i) Ni accumulation in hyperaccumulator plants is following a gradient of total and available Ni in soil; ii) evidence of root exudation and Ni mobilization can be observed in the rhizosphere of a Ni hyperaccumulator; iii) co-mobilization of Ni with other nutrients such as Fe and P occurs; iv) root-induced changes in soil pH can be linked to enhanced Ni soil availability. We hypothesise that Ni plant uptake rather correlates with total soil Ni than plant available Ni fractions and that Ni (and possibly Fe and P) might be co-mobilized in the rhizosphere of hyperaccumulators due to plant root activity. To test our hypotheses, a pot experiment was conducted with the Ni hyperaccumulator O. chalcidica on seven soils ranging from moderate to pronounced ultramafic characteristics and showing a gradient in total and available Ni.
Material and methods
Experimental soil characterisation
Experimental soils were collected from an ultramafic forest area near Redlschlag (eastern Austria) previously described by Puschenreiter et al. (2005), Wenzel and Jockwer (1999) and Wenzel et al. (2003). Soils were sieved < 4 mm in the field and left to air-drying for several weeks. To investigate effects of increasing Ni concentrations, a linear Ni gradient was artificially created by mixing two soils in different percentages, resulting in four mixed soils. Therefore, a soil with lower Ni concentrations (S1) was mixed, based on the dry weight, with a higher Ni soil (S6) as shown in Table 1. An additional soil, representing a subsoil exposed to the surface by a landslide (LS), was included in the experiment because it was characterised by high pseudo-total Ni but low labile Ni content. All soil analyses were performed in triplicates on < 2 mm sieved, air-dried subsamples and batch extractions were corrected for the total water content determined at 105 °C. Pseudo-total element concentrations were determined by microwave (CEM Mars 6) assisted digestion of 0.5 g milled subsamples in HNO3 65% and HCl 37% at 1:3 ratio (aqua regia) for 40 min at 200 °C according to Austrian standards (ÖNORM L 10852009). Readily-available metal fraction was assessed by 0.01 M Sr(NO3)2 extraction (Everhart et al. 2006) as adapted by Madden (1988) using 1:4 w/v ratio and 2 h shaking. Complexable metals were determined through DTPA (diethylene triamine pentaacetic acid) extraction after Lindsay and Norvell (1978) with 1:2 w/v and 2 h shaking. Plant available P was extracted according to Olsen (1954) as described in Schoenau and O'Halloran (2008) and P determined by molybdenum blue colometry (Murphy and Riley 1962). Cation exchange capacity (CEC) was estimated as the sum of the exchangeable cations Ca, Mg and K using 0.1 M BaCl2 extraction according to Austrian standards (ÖNORM L1086-891989). Soil pH was measured in ultrapure H2O (conductivity: 0.055 < 0.080 mS) at 1:2.5 w/v with a pH meter (SCHOTT ProLab 4000). Trace elements in soil digests, Sr(NO3)2 and DTPA extraction were determined with an ICP-MS (NexION 2000, Perkin Elmer). Macronutrients for aqua regia and CEC assays with an ICP-OES (Optima 8300, Perkin Elmer).
Pot experiment and pore water sampling
In order to observe changes in Ni hyperaccumulation and rhizosphere processes of Odontarrhena chalcidica at increasing soil Ni concentrations, a pot experiment with a soil Ni gradient was set up and plants were grown under controlled conditions. For S1 to S6, 450 g soil and for LS 550 g soil, because of higher density, was added to PVC pots in four experimental replicates. Experimental control consisted of unplanted pots, which were also setup for each soil in four replicates. Rhizon pore water (PW) samplers (Rhizosphere Research Products, Wageningen, NL) were installed in each pot to allow soil pore water sampling. Odontarrhena chalcidica seeds were germinated in washed vermiculite and transplanted after seven days into the pot experiment. After seven more days, when the plants showed 2–3 pair of leaves, one plant was kept per pot. Plants were grown in a growth cabinet (CLF Plant Climatics, SE59-AR2cLED), with the following settings: 16 h photoperiod with 400 μmol photons m−2 s−1, temperatures of 24/18 °C during the light/dark period and humidity maintained at 60%. The pots were arranged randomly and shuffled every two days, soil moisture was kept gravimetrically at 60% water holding capacity (WHC) by daily watering with ultrapure H2O. Soil pore water was sampled four times during the experiment: seven days after transplantation (DAT) of seedlings into the pots (T0), 21 DAT (T1), 49 DAT (T2), and 71 DAT (T3), when the experiment ended. Each time 10 mL of filtered (mean pore size of rhizon sampler = 0.15 μm) PW was sampled from water saturated pots and analysed for pH (SCHOTT ProLab 4000 pH meter), P content (Murphy and Riley 1962), dissolved organic carbon (DOC) (Vario TOC Cube, Elementar) and trace metals (ICP-MS, NexION 2000, Perkin Elmer).
Plant biomass analyses
Plant shoots were harvested after 71 DAT, carefully rinsed with deionised water, oven dried at 60 °C for 38 h, weighed and milled. Shoots were digested in a 4:1 mixture of HNO3 65% and H2O2 30% in a microwave oven (CEM Mars 6) at 200 °C for 25 min. Elemental concentrations in plant biomass were analysed for trace elements with ICP-MS (NexION 2000, Perkin Elmer) and for macronutrients with ICP-OES (Optima 8300, Perkin Elmer).
Soil analyses after the pot experiment
To investigate changes to soil parameters resulting from the pot experiment, Sr(NO3)2, DTPA, pH and Olsen-P analyses were performed on planted and control pots as previously described. The seven initial soils were also included in all analyses for comparing with the initial conditions without operational biases.
Statistical analyses
Differences in physiochemical parameters of pore water, plant and soil samples between planted replicates were assessed using two-way ANOVA with Tukey’s HSD post-hoc test. If normal distribution was not met data was either log-transformed or non-parametric Kruskal–Wallis H test was applied, followed by Wilcoxon rank sum test. Differences between planted and unplanted replicates within each soil group were assessed using Student’s t-test. If normal distribution was not met, data were either log-transformed or a non-parametric Mann–Whitney-U test applied. To test for correlation between two variables Pearson’s product-momentum correlation was performed after checking for normality and homoscedasticity of the data. All statistical analyses were carried out using R version 4.2.2 (2022–10-31). Sample size was n = 4 for pore water, plant, and soil samples after the pot experiment and n = 3 for initial soil samples and a significance level of p < 0.05 was used.
Results
Experimental soil characterisation
An overview of the main characteristics of the experimental soils, such as pseudo-total elemental concentrations, DTPA- and Sr(NO3)2-extractable metals, plant available P, CEC and pH is shown in Table 2. Aqua regia digestion as well as DTPA and Sr(NO3)2 extraction confirmed that soil mixing successfully created a Ni gradient from S1 to S6 in both labile and pseudo-total concentrations. Pseudo-total Ni concentrations ranged from 552 mg kg−1 (S1) to 1465 mg kg−1 (S6) and was even higher in LS (1613 mg kg−1). DTPA-extractable Ni accounted for about 10% of pseudo-total concentrations and increased threefold from S1 (41.6 mg kg−1) to S6 (158 mg kg−1) as well, whereas LS showed low DTPA-extractable Ni (53.1 mg kg−1, ~ 3% of pseudo-total Ni). Sr(NO3)2-extractable Ni was distinctly low in LS (376 µg kg−1) and increased twofold from S1 (641 µg kg−1) to S6 (1247 µg kg−1). Regarding nutrient contents, pseudo-total K (2.23 g kg−1) concentrations decreased to 1.03 g kg−1 K from S1 to S6, while P, S and Fe showed the opposite trend with lowest concentrations in S1 and highest in S6. LS showed comparable concentration regimes regarding Fe, Mg, K and Na within the S1 to S6 range but was considerably higher in S (732 mg kg−1) and lower in pseudo-total P (131 mg kg−1). All soils of the experiment showed very low concentrations of plant-available P, especially low in LS (1.17 mg kg−1) and marginally increasing from S1 to S6 (2.94 to 6.51 mg kg−1). Soil pH ranged from slightly acidic in S1 (5.93) towards nearly neutral (6.46) in S6 and was alkaline in LS (8.08).
Shoot biomass and elemental concentrations
Average values of plant dry weight (DW) are presented in Fig. 1 and Table 2. Odontarrhena chalcidica grown on S1 showed the lowest biomass (477 mg DW), whereas S6 plants grew nearly twice as much (946 mg DW), significantly higher than S1 to S5 plants and in the same range as LS plants (773 mg DW). S1 to S5 did not show any differences in DW of the shoots and only S1 plants had significantly lower biomass than LS plants.
Elemental concentrations in shoots are shown in Table 3. The lowest dry shoots Ni concentrations were measured in S1 and S2 plants (2.44 and 3.87 g kg−1) and the highest in S6 and LS plants (7.67 and 7.51 g kg−1, Fig. 2 a). S4 and S5 showed plant Ni concentrations comparable with S1, S2 and S3 without significant differences. No significant differences were observed among Fe concentrations in plant shoots (41 to 62 mg kg−1) from all soils (Fig. 2 b), while O. chalcidica showed significantly elevated shoot P concentrations (900 mg kg−1) when growing on LS soil compared to the other soils (Fig. 2 c).
Pore water sampling
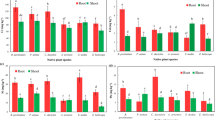
Pore water samples were collected to detect root-induced changes to soil solution in the rhizosphere and results are shown in Fig. 3. At the beginning of the experiment (T0) DOC concentrations in PW were highest in planted S1 (54.7 µg L−1) and lowest in LS control pots (25.3 µg L−1), but no major differences between the pots were evident. Control pots remained comparable for DOC concentrations from T0 to T3, whereas increasing DOC concentrations were measured in planted pots after 49 days (T2), significant for S2, S3, S4 and especially S6 (144 µg L−1, S6 control: 41 µg L−1). At T3, planted S5 and LS pots also showed significantly elevated DOC concentrations in PW and planted S4 contained highest DOC contents in PW (134 µg L−1). The pH as well as Fe and Ni concentrations in PW between planted and control pots likewise showed an increase starting at T2 and continuing for T3. In this regard alkalinization of the pH of the PW at T3 was clearly visible for S6 (pH increase of 0.71) and least pronounced but still significant for LS (pH increase of 0.11). Nickel concentrations in PW resulted highest for S6 at T2 (265 µg L−1) with more than two-fold increase compared to controls. At T3 Ni concentrations significantly increased for S3, S4 and S5, with 76, 191 and 174 µg L−1, respectively, corresponding to a 44%, 90% and 73% increase compared to the unplanted controls. In contrast, for LS there was a small reduction of Ni concentrations, which started at T2 and became significant at T3 (33 µg L−1). Iron concentrations in soil PW showed the most pronounced increase along the experiment with elevated concentrations at T2 significant for S3 and strongly significant for S4 and S6. At T3 dissolved Fe concentrations in planted pots were highest in S4 (1420 µg L−1), S6 increased more than 8 times (828 µg L−1) compared to controls, whereas LS was the only soil without a significant increase with very low values (29 µg L−1) in general. Phosphorus concentrations in PW were very low, so that the majority of the samples were below the quantification limit of 20 µg L−1 and thus could not be reliably determined.
Pore water concentrations of Ni (a), Fe (b), DOC (c) and pH (d) of pots with Odontarrhena chalcidica and controls for the samplings T0 to T3, presented as mean ± standard deviation (n = 4). Letters indicate significance between planted soils (p < 0.05), asterisks show significance between planted and control pots (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***)
Changes in soil characteristics after the pot experiment
Soil pH, Olsen-P and extractable Ni (DTPA and Sr(NO3)2 extraction) are shown in Table 4 and Fig. 4. Planted pots for both assessments showed a significant reduction of labile Ni compared to control pots for almost all soils. The reduction was higher in Sr(NO3)2-extractable Ni ranging from 38 µg kg−1 (11.3%) in LS up to 422 µg kg−1 (30.4%) in S6 and less pronounced in DTPA-extractable Ni with lowest values in S1 and LS, 6.8 and 5.2 mg kg−1 (14.2% and 8.5%) and highest in S6, 30.5 mg kg−1 (16.2%). No significant differences between planted and control pots were detected for DTPA- or Sr(NO3)2-extractable Fe. A significant alkalinization of soil pH of soils S1 to S6 after the pot experiment was measured with averaged values ranging from 0.19 (S4 and S5) to 0.34 (S6). In contrast, no significant differences in soil pH between planted and control pots were evident for LS. Plant-available P did not significantly change before and after the pot experiment and also no differences between planted and control pots were visible.
Sr(NO3)2-extractable Ni (a), DTPA-extractable Ni (b) and pH (c) as well as pH (d) of the different soils after the pot experiment, presented as mean ± standard deviation (n = 4). Letters indicate significance between planted soils (p < 0.05), asterisks show significance between planted and control pots (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***)
Discussion
Plant biomass and shoot analyses
The study of plant growth and Ni accumulation in typical to weakly ultramafic soils provided interesting insights into plant growth and the acquisition of Ni and other nutrients by O. chalcidica. Remarkably, the highest plant shoots biomass was obtained from the two soils with the more pronounced ultramafic characteristics (soils S6 and LS, Fig. 1), despite the very different plant availability of essential nutrients such as P, N (Fig. S2, supplementary information) and Fe (Table 2). Similarly, the highest Ni shoot uptake in O. chalcidica was obtained from the more distinctly ultramafic soils S6 and LS (Fig. 2), suggesting that specific ultramafic soil properties might promote Ni shoot uptake more than Ni availability itself. Ni accumulation in shoots from all soils was above the hyperaccumulation threshold of 1000 μg g−1 in dry shoots (Baker and Brooks 1989; van der Ent et al. 2013) and comparable with previous experimental work on O. chalcidica (Bani et al. 2015a, b; Rosenkranz et al. 2019; Tognacchini et al. 2020). Of particular note is the significantly higher P uptake by plants in LS (Fig. 2c), which has the lowest P availability (Table 2). This might indicate a very efficient P uptake strategy or favourable soil conditions for P uptake in LS soil, such as higher pH or very low concentrations of soluble Fe in pore water.
Soil pore water analyses
The significant increase in Ni pore water (PW) concentrations in planted pots from T0 to T3 in soils S3, S4, S5 and S6, and marginally S1 and S2 (Fig. 3) is a clear evidence of plant-induced Ni solubilization from insoluble Ni pools to soil water solution. Conversely, the decrease in PW Ni concentrations in soil LS over the course of the experiment indicates either that no Ni mobilization occurred, or that plant uptake had a stronger effect than solubilization. The strong Fe solubilization observed in the planted pots during the experiment in all soils except LS (Fig. 3) further confirms rhizosphere processes involved in metal mobilization; the high correlation (r = 0.81) between Ni and Fe solubilization also suggests a co-mobilization of those metals from the same soil fractions, possibly from Ni associated with soil Fe oxides, which has been shown to be one of the main sources of labile Ni in ultramafic soils (Álvarez-López et al. 2021; Chardot et al. 2005; Massoura et al. 2004). As for Ni, also the limited Fe solubilization in LS is reflecting the different geochemistry and rhizosphere processes for this soil. Furthermore, the significant increase in DOC in planted pots along the experiment (Fig. 3) is likely due to root-related increase of soluble organic compounds and a possible indication of root exudation by O. chalcidica in all soils. The increased DOC in pore water also seems to promote Ni and Fe soil solubilization in planted pots, as suggested by the high positive correlation between ΔDOC /ΔNi and ΔDOC/ΔFe in PW at T3 (r = 0.96 and r = 0.86 respectively, Fig. S1 supplementary information). As further indication that plant-derived DOC is involved in Ni and Fe solubilization, no Ni and Fe PW mobilization was observed in soil LS, where DOC increase was negligible. Similarly to our results, an increased Ni solubility in rhizosphere of ultramafic soils compared to bulk soil was observed in Wenzel et al. (2003) and Álvarez-López et al. (2021) on, respectively, the Ni hyperaccumulator species Noccaea goesingensis and Odontarrhena serpyllifolia. In both studies, increased Ni solubility was associated with higher DOC and pH in soil and a positive correlation between soil Ni availability and DOC in the rhizosphere was also observed. Puschenreiter et al. (2005) reported higher concentrations of oxalic and citric acid in the rhizosphere compared to bulk soil of Thlaspi goesingense (syn. Noccaea goesingensis) from a natural ultramafic site. Based on chemical speciation analysis (MINTEQA2), Wenzel et al. (2003) suggested the formation of Ni-organic complexes in the rhizosphere of N. goesingensis and that organic ligands excreted by roots form stronger complexes with Ni than organic compounds from bulk soil. This might explain the enhanced Ni present in PW, which seems to be in complexed form with organic ligands from root exudation.
The pH increase of pore water samples from rooted pots along the experiment (Fig. 3) suggests a significant root-induced soil alkalinization, as observed in previous studies on hyperaccumulator plants (Álvarez-López et al. 2021; Kukier et al. 2004; Luo et al. 2000; Puschenreiter et al. 2005; Singer et al. 2007; Wenzel et al. 2003, 2004). Wenzel et al. (2003) hypothesised that the pH increase in the rhizosphere of N. goesingensis could be related with the release of hydroxyl ions during mineral dissolution of Mg and Ni-bearing orthosilicates. From a hydroponic test with O. chalcidica (Tognacchini et al. 2022, unpublished) a substantial pH increase in a sampling solution was also observed within two hours of root exposure, which implies other mechanisms of pH increase besides mineral dissolution. As previously proposed in literature, possible beneficial effect of pH increase in the rhizosphere might be the stabilization of metal–organic ligands (Li et al. 2003; van der Ent et al. 2016), thus keeping in solution metals as Ni and Fe associated with soluble organic compounds. Because of the very low P concentrations in PW (results not shown) and partially unclear results, a thorough discussion about P geochemistry and mobilization is unfortunately limited. Despite this, a general trend in PPW increase in planted pots was observed, which would suggest a plant-promoted solubilization. Exception, again, is made for soil LS, where a significant P depletion occurred, which might be reflected in the enhanced plant uptake observed in plant shoots of O. chalcidica.
Soil Ni availability and plant uptake
Another main research question we wanted to investigate was whether a linear relation between a soil Ni gradient and plant uptake could be observed. Previous studies conducted on ultramafic soils from the same location show a weak correlation of Ni concentration in Noccaea goesingensis shoots with soil available Ni assessed by Sr(NO3)2 extraction, DTPA extraction and DGT (diffusive gradients in thin films) assessment (Noller 2017; Puschenreiter et al. 2019). Bani et al. (2014) observed that Ni accumulation in Odontarrhena chalcidica was independent from soil DTPA-extractable Ni. On the contrary, Centofanti et al. (2012) showed that Ni accumulation in Alyssum corsicum was dependent upon the solubility of the Ni mineral present in the growth substrate. From our results, the increasing Ni availability from soil S1 to soil S6 (NiPW, NiDTPA and NiSr(NO3)2) was not entirely reflected in shoot Ni uptake in O. chalcidica and seems to be limited to a specific range of Ni availability (e.g. from soil S1 to S3). This shows limitations in predicting shoot Ni uptake based on soil availability assessments such as NiPW, NiDTPA, NiSr(NO3)2 and could partially explain contradictory literature results. Surprisingly, our experiment revealed that Ni uptake by shoots of O. chalcidica is highly predictable from the soil pseudo-total Ni and pH of soil and PW, (respectively: r = 0.87, r = 0.72 and r = 0.75; see Fig. S1 in supplementary information), suggesting that soil total Ni pools and pH might be better predictors of shoot Ni concentrations than the Ni plant-available fractions. This might indicate that: i) Ni mobilization processes from non-available Ni pools might play a central role in hyperaccumulation, or/and that ii) Ni uptake might be regulated by soil pH. As already underlined in literature, soil pH seems to have a central role in plant Ni uptake (Everhart et al. 2006; Ghafoori et al. 2022; Li et al. 2003; Kukier et al. 2004). In several studies a higher Ni accumulation by Alyssum (synonymous Odontarrhena) species was observed as soil pH was raised and thus soil available Ni (NiDTPA, NiSr(NO3)2 and Ni biosensor) declined (Everhart et al. 2006; Kukier et al. 2004; Li et al. 2003). Since increasing soil ultramafic properties (and total Ni) was associated with increasing pH (Table 2), the apparent linear relation of shoot uptake with total soil Ni may actually be a bias related to the effect of soil pH. Another relevant aspect is the opposite response to soil alkalinization of extraction-based Ni availability assessments (DTPA and Sr(NO3)2) compared to Ni in PW. The reduction in DTPA and Sr(NO3)2 extractable Ni after plant growth (Fig. 4) cannot be justified by plant uptake or PW removal alone, which accounts for approximately half of the Ni loss and it is seemingly a combined effect of immobilization due to pH increase. Considering the soil extractable Ni fractions only (NiDTPA and NiSr(NO3)2; Fig. 4) we would have concluded that rhizosphere processes have caused Ni immobilization, while PW analyses resulted to be crucial in observing Ni solubilization. In contrast with our results, Álvarez-López et al. (2021) measured a significantly higher available Ni (Sr(NO3)2 and DGT) in rhizosphere soil of O. serpillifoilia compared to bulk soil, showing that rhizosphere processes in ultramafic soil induced Ni mobilization. Being a field study, in Álvarez-López et al. (2021) the long-term rhizosphere effect might justify the different results obtained compared to our pot experiment. Contrasting literature results can be found regarding the capability of hyperaccumulator plants to access larger Ni pools than non-accumulators. For example, it was shown that hyperaccumulators within the genus Odontarrhena accesses the same Ni pool as non-hyperaccumulators (Massoura et al. 2004; Shallari et al. 2001). In contrast, Chardot-Jacques et al. (2013) observed enhanced dissolution of a Ni-bearing mineral in the rhizosphere of the Ni hyperaccumulator Bornmuellera emarginata (syn. Leptoplax emarginata). Although evidence of active Ni solubilization was observed in the rhizosphere of O. chalcidica in the present experiment, it cannot be stated whether this indicates mobilization from larger Ni pools that are not available to non-hyperaccumulators. The linearity observed between soil Ni total pools and plant uptake, would suggest that O. chalcidica could access non-available pools, while Ni uptake in O. chalcidica seems to derive from Ni solubilized from adsorbed and surface complexed soil Ni fractions which are also potentially available to non-accumulators. A clear link between Ni solubilization and Ni shoot uptake was not observed and high Ni accumulation in O. chalcidica occurred in soil LS without signs of solubilization. This suggests that even when root mobilization occurs, it is not a strategy to enhance Ni plant uptake. As already suggested by Álvarez-López et al. (2021), Ni mobilization appears to be a consequence of rhizosphere processes targeting other nutrients.
Conclusions
This study presents new insights into rhizosphere processes involved in Ni biogeochemistry of the Ni hyperaccumulator species O. chalcidica. We demonstrated that Ni uptake by plants was favoured by ultramafic soil characteristics and correlated well with soil characteristics like pseudo-total Ni content or pH, whereas Ni bioavailability assessments (NiDPTA and NiSr(NO3)2) resulted to be poor predictors of plant Ni uptake. A strong increase in pore water Ni and Fe concentrations combined with elevated DOC content and alkalinization in most soils suggested solubilization of Ni and Fe mediated by exudation of organic ligands. This led us to conclude that root-related activities clearly influence the fate of Ni in the rhizosphere. However, it remains unclear whether O. chalcidica has access to a larger Ni pool that is unavailable to non-hyperaccumulator plants. Further studies should investigate in more detail the composition of root exudates from O. chalcidica and include comparisons with non-accumulator plant species adapted to ultramafic soils.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Álvarez-López V, Puschenreiter M, Santner J, Lehto N, Prieto-Fernández Á, Wenzel WW, Monterroso C, Kidd PS (2021) Evidence for nickel mobilisation in rhizosphere soils of Ni hyperaccumulator Odontarrhena serpyllifolia. Plant Soil 464(1–2):89–107. https://doi.org/10.1007/s11104-021-04944-7
Assunção AG, Schat H, Aarts MG (2003) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159:351–360. https://doi.org/10.1046/j.1469-8137.2003.00820.x
Baker AJM (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654. https://doi.org/10.1080/01904168109362867
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Baker AJM, Brooks R (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Bani A, Echevarria G, Montargès-Pelletier E, Gjoka F, Sulçe S, Morel JL (2014) Pedogenesis and nickel biogeochemistry in a typical Albanian ultramafic toposequence. Environ Monit Assess 186:4431–4442. https://doi.org/10.1007/s10661-014-3709-6
Bani A, Echevarria G, Sulçe S, Morel JL (2015a) Improving the agronomy of Alyssum murale for extensive phytomining: a five-year field study. Int J Phytoremediation 17:117–127. https://doi.org/10.1080/15226514.2013.862204
Bani A, Echevarria G, Zhang X, Benizri E, Laubie B, Morel JL, Simonnot M-O (2015b) The effect of plant density in nickel-phytomining field experiments with Alyssum murale in Albania. Aust J Bot 63:72–77. https://doi.org/10.1071/BT14285
Brooks RR (1998) Phytochemistry of hyperaccumulation. In: Brooks RR (ed) Plants that Hyperaccumulate metals. CAB International, Wallingford, pp 261–287
Centofanti T, Siebecker MG, Chaney RL, Davis AP (2012) Sparks DL (2012) Hyperaccumulation of nickel by Alyssum corsicum is related to solubility of Ni mineral species. Plant Soil 359:71–83. https://doi.org/10.1007/s11104-012-1176-9
Chaney RL, Angle JS, Broadhurst CL, Peters CA, Tappero RV, Sparks DL (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36:1429–1443. https://doi.org/10.2134/jeq2006.0514
Chardot-Jacques V, Calvaruso C, Simon B, Turpault MP, Echevarria G, Morel JL (2013) Chrysotile dissolution in the rhizosphere of the nickel hyperaccumulator Leptoplax emarginata. Environ Sci Technol 47:2612–2620. https://doi.org/10.1021/es301229m
Chardot V, Massoura ST, Echevarria G, Reeves RD, Morel JL (2005) Phytoextraction potential of the nickel hyperaccumulators Leptoplax emarginata and Bornmuellera tymphaea. Int J Phytoremediation 7:323–335. https://doi.org/10.1080/16226510500327186
Dessureault-Rompré J, Luster J, Schulin R, Tercier-Weiber M-L, Nowack B (2010) Decrease of labile Zn and Cd in the rhizosphere of hyperaccumulating Thlaspi caerulescens with time. Environ Pollut 158:1955–1962. https://doi.org/10.1016/j.envpol.2009.10.032
Echevarria G, Massoura ST, Sterckeman T, Becquer T, Schwartz C, Morel JL (2006) Assessment and control of the bioavailability of nickel in soils. Environ Toxicol Chem 25:643–651. https://doi.org/10.1897/05-051R.1
Everhart JL, McNear D, Peltier E, van der Lelie D, Chaney RL, Sparks DL (2006) Assessing nickel bioavailability in smelter-contaminated soils. Sci Total Environ 367:732–744. https://doi.org/10.1016/j.scitotenv.2005.12.029
Ghafoori M., Shariati M., van der Ent A, Baker AJM (2022). Nickel hyperaccumulation, elemental profiles and agromining potential of three species of Odontarrhena from the ultramafics of Western Iran. International journal of phytoremediation, 1–12. https://doi.org/10.1080/15226514.2022.2086213
Hammer D, Keller C, McLaughlin MJ, Hamon RE (2006) Fixation of metals in soil constituents and potential remobilization by hyperaccumulating and non-hyperaccumulating plants: results from an isotopic dilution study. Environ Pollut 143:407–415. https://doi.org/10.1016/j.envpol.2005.12.008
Hutchinson JJ, Young SD, McGrath SP, West HM, Black CR, Baker AJM (2000) Determining uptake of ‘non-labile’ soil cadmium by Thlaspi caerulescens using isotopic dilution techniques. New Phytol 146:453–460. https://doi.org/10.1046/j.1469-8137.2000.00657.x
Krämer U (2005) Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotechnol 16:133–141. https://doi.org/10.1016/j.copbio.2005.02.006
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534. https://doi.org/10.1146/annurev-arplant-042809-112156
Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that hyperaccumulate nickel. Nature 379:635–638. https://doi.org/10.1038/379635a0
Krämer U, Smith RD, Wenzel WW, Raskin I, Salt DE (1997) The role of metal transport and tolerance in nickel hyperaccumulation by Thlaspi goesingense Hálácsy. Plant Physiol 115:1641–1650. https://doi.org/10.1104/pp.115.4.1641
Krämer U, Pickering IJ, Prince RC, Raskin I, Salt DE (2000) Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species. Plant Physiol 122:1343–1353. https://doi.org/10.1104/pp.122.4.1343
Kukier U, Peters CA, Chaney RL, Angle JS, Roseberg RJ (2004) The effect of pH on metal accumulation in two Alyssum species. J Environ Qual 33(6):2090–2102. https://doi.org/10.2134/jeq2004.2090
Lange B, van der Ent A, Baker AJM, Mahy G, Malaisse F, Meerts P, Echevarria G, Pourré O, Verbruggen N, Faucon MP (2017) Copper and cobalt accumulation in plants: a critical assessment of the current status of knowledge. New Phytol 213(2):537–551. https://doi.org/10.1111/nph.14175
Li T, Xu Z, Han X et al (2012) Characterization of dissolved organic matter in the rhizosphere of hyperaccumulator Sedum alfredii and its effect on the mobility of zinc. Chemosphere 88:570–576. https://doi.org/10.1016/j.chemosphere.2012.03.031
Li YM, Chaney RL, Brewer EP, Angle JS, Nelkin J (2003). Phytoextraction of nickel and cobalt by hyperaccumulator Alyssum species grown on nickel-contaminated soils. Environ Sci Technol 37(7):1463–1468. https://doi.org/10.1021/es0208963
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Lombi E, Wenzel WW, Gobran GR, Adriano DC (2000) Dependency of phytoavailability of metals on indigenous and induced rhizosphere processes: a review. In: Gobran GR, Wenzel WW, Lombi E (eds) Trace elements in the Rhizosphere. CRC Press LLC, Boca Raton, pp 3–24
Luo Y, Christie P, Baker A (2000) Soil solution Zn and pH dynamics in non-rhizosphere soil and in the rhizosphere of Thlaspi caerulescens grown in a Zn/Cd-contaminated soil. Chemosphere 41(1):161–164. https://doi.org/10.1016/S0045-6535(99)00405-1
Madden MS (1988) Adapting the Sr(NO3)2 method for determining available cations to a routine soil testing procedure. University of Wisconsin--Madison
Massoura ST, Echevarria G, Leclerc-Cessac E, Morel JL (2004) Response of excluder, indicator, and hyperaccumulator plants to nickel availability in soils. Aust J Soil Res 42:933–938. https://doi.org/10.1071/SR03157
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nkrumah PN, Baker AJM, Chaney RL, Erskine PD, Echevarria G, Morel JL, van der Ent A (2016) Current status and challenges in developing nickel phytomining: an agronomic perspective. Plant Soil 406:55–69. https://doi.org/10.1007/s11104-016-2859-4
Noller C (2017) Evaluating relationships between soil characteristics and Ni accumulation by the hyperaccumulator Noccaea goesingensis. Master Thesis, University of Hohenheim
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate, Vol 939. US Department of Agriculture
ÖNORM L 1085 (2009) Chemische Bodenuntersuchungen: Extraktion von Elementen mit Königswasser oder Salpetersäure-Perchlorsäure-Gemisch. Österreichisches Normungsinstitut, Vienna, Austria
ÖNORM L1086–89 (1989) Chemische Analyse von Böden: Bestimmung von austauschbaren Kationen und Kationenaustauschkapazität. Österreichisches Normungsinstitut, Vienna, Austria
Pardo T, Rodríguez-Garrido B, Saad RF, Soto-Vázquez JL, Loureiro-Viñas M, Prieto-Fernández A, Echevarria G, Benizri E, Kidd PS (2018) Assessing the agromining potential of Mediterranean nickel-hyperaccumulating plant species at field-scale in ultramafic soils under humid-temperate climate. Sci Total Environ 630:275–286. https://doi.org/10.1016/j.scitotenv.2018.02.229
Puschenreiter M, Schnepf A, Millán IM, Fitz WJ, Horak O, Klepp J, Schrefl T, Lombi E, Wenzel WW (2005) Changes of Ni biogeochemistry in the rhizosphere of the hyperaccumulator Thlaspi goesingense. Plant Soil 271(1–2):205–218. https://doi.org/10.1007/s11104-004-2387-5
Puschenreiter M, Kidd P, Rosenkranz T, Noller C (2019) Influence of soil characteristics on Ni hyperaccumulation by Noccaea goesingensis. Book of Abstracts; International Conference of Trace Element Biogeochemistry, May 5–9 2019, Nanjing, China
Rosenkranz T, Hipfinger C, Ridard C, Puschenreiter M (2019) A nickel phytomining trial using Odontarrhena chalcidica and Noccaea goesingensis on an Austrianserpentine soil. J Environ Manag 242:522–528. https://doi.org/10.1016/j.envman.2019.04.073
Salt DE, Kato N, Krämer U, Smith R, Raskin I (2000) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and non-accumulator. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton, p 189
Schoenau JJ, O’Halloran DD (2008) Sodium bicarbonate-extractable phosphorus. In: Carter MR, Gregoric EG (eds) Soil sampling and methods of analysis, vol 2. Taylor & Francis
Shallari S, Echevarria G, Schwartz C and Morel JL (2001) Availability of nickel in soils for the hyperaccumulator Alyssum murale Waldst. & Kit. S Afr J Sci 97:11:568-570. https://doi.org/10.10520/EJC97243
Singer AC, Bell T, Heywood CA, Smith JAC, Thompson IP (2007) Phytoremediation of mixed-contaminated soil using the hyperaccumulator plant Alyssum lesbiacum: Evidence of histidine as a measure of phytoextractable nickel. Environmental Pollution 147(1):74–82. https://doi.org/10.1016/j.envpol.2006.08.029
Tognacchini A, Rosenkranz T, van der Ent A, Machinet GE, Echevarria G, Puschenreiter M (2020) Nickel phytomining from industrial wastes: growing nickel hyperaccumulator plants on galvanic sludges. J Environ Manage 254:109798. https://doi.org/10.1016/j.jenvman.2019.109798
Tognacchini A, Puschenreiter M (2022). Sampling root exudates of Odontarrhena chalcidica from a hydroponic experiment. Unpublished results
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334. https://doi.org/10.1007/s11104-012-1287-3
van der Ent A, Baker AJM, Reeves RD, Chaney R, Anderson CW, Meech JA, Erskine PD, Simonnot MO, Vaughan J, Morel JL, Echevarria G, Fogliani B, Rongliang Q, Mulligan DR (2015) Agromining: farming for metals in the future? Environ Sci Technol 49(8):4773–4780. https://doi.org/10.1021/es506031u
van der Ent A, Echevarria G, Tibbett M (2016) Delimiting soil chemistry thresholds for nickel hyperaccumulator plants in Sabah (Malaysia). Chemoecol 26:67–82. https://doi.org/10.1007/s00049-016-0209-x
van der Ent A, Cardace D, Tibbett M, Echevarria G (2018) Ecological implications of pedogenesis and geochemistry of ultramafic soils in Kinabalu Park (Malaysia). Catena 160:154–169. https://doi.org/10.1016/j.catena.2017.08.015
Wenzel WW, Jockwer F (1999) Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ Pollut 104:145–155. https://doi.org/10.1016/S0269-7491(98)00139-0
Wenzel WW, Lombi, E, Adriano DC (2004) Root and rhizosphere processes in metal hyperaccumulation and phytoremediation technology, In: Prasad, M.N.V. (Ed.) Heavy metal stress in plants: from biomolecules to ecosystems. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 313–344. https://doi.org/10.1007/978-3-662-07743-6_13
Wenzel WW, Lombi E, Adriano DC (1999) Biogeochemical processes in the rhizosphere: role in phytoremediation of metalpolluted soils. In: Prasad NMV, Hagemeyer J (eds) Heavy metal stress in plants- from molecules to ecosystems. Springer Verlag, Heidelberg, pp 273–303
Wenzel WW, Bunkowsky M, Puschenreiter M, Horak O (2003) Rhizosphere characteristics of indigenously growing nickel hyperaccumulator and excluder plants on serpentine soil. Environ Pollut 123:131–138. https://doi.org/10.1016/S0269-7491(02)00341-X
Whiting SN, Leake JR, McGrath SP, Baker AJM (2001) Hyperaccumulation of Zn by Thlaspi caerulescens can ameliorate Zn toxicity in the rhizosphere of cocropped Thlaspi arvense. Environ Sci Technol 35:3237–3241. https://doi.org/10.1021/es010644m
Zhao FJ, Hamon RE, McLaughlin MJ (2001) Root exudates of the hyperaccumulator Thlaspi caerulescens do not enhance metal mobilization. New Phytol 151:613–620. https://doi.org/10.1046/j.0028-646x.2001.00213.x
Acknowledgements
We wish to thank Monika Laux and Alireza Golestanifard for the help and company with soil sampling as well as Christian Benold for providing a very helpful mobile XRF device in the field. Aida Bani is sincerely thanked for the supply of Odontarrhena chalcidica seeds. We would also like to thank Michael Santangeli for reviewing the manuscript. We acknowledge the Austrian Science Fund FWF for financially supporting this study (Project P 34719).
Funding
Open access funding provided by Austrian Science Fund (FWF). The work was supported by the Austrian Science Fund FWF (Project P 34719).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Hugh Harris.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Risse, S.B.L., Puschenreiter, M. & Tognacchini, A. Rhizosphere processes by the nickel hyperaccumulator Odontarrhena chalcidica suggest Ni mobilization. Plant Soil 495, 43–56 (2024). https://doi.org/10.1007/s11104-023-06161-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06161-w