Abstract
Background and Aims
To sustainably manage N in oil palm systems quantities of N fixed by cover legumes need to be understood. Current values are scarce, based on shoot N measures and do not include litter which releases nitrate as it decomposes. We aimed to quantify N2 fixed by legumes under oil palm systems in PNG and to determine if soil nitrate influenced dependence on N2 fixation (Ndfa).
Methods
The ureide technique for estimating tropical legume Ndfa was calibrated for Calapogonium mucunoides and Pueraria phaseoloides using 15N isotope dilution, and then used to assess Ndfa for legume cover under oil palms (2 to 25 years old) in Papua New Guinea. Amounts of fixed N in above-ground legume biomass (shoot plus litter) were calculated incorporating % groundcover. Soil nitrate under the legume litter was also measured.
Results
Legume Ndfa was highly negatively correlated with soil nitrate concentration but independent of palm age. Legume groundcover, shoot and litter dry matter, and quantity of fixed N were greater under oil palms less than 5 years old, decreasing under older plantations where solely C. caeruleum was present. DM and N content of litter were similar to shoots for legumes in plantations less than 6 years old.
Conclusion
The calibrated ureide technique can be used, together with estimates of annual legume N accumulation, to quantify N input from legume groundcover during the life cycle of oil palm plantations and other tropical ecosystems, in order to support more sustainable management of N.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil palm (Elaeis guineensis) has become one of the world’s major agricultural crops, driven by unprecedented increase in consumer demand (Corley 2009) coupled with rapid commercial development, with production occurring in lowland moist humid tropical zones in Central Africa, South America and Southeast Asia (Byerlee et al. 2016). Although 85% of global production currently comes from Indonesia and Malaysia, the oil palm industry is a highly important component of the export economy for many other countries, including Papua New Guinea (PNG), where it is the largest non-government employer and underpins rural livelihoods (Allen et al. 2009; Cramb and Curry 2012). There is increasing pressure from consumers and governments for agriculture and food production systems to be more sustainable, with oil palm under particular public scrutiny to improve productivity per current area rather than encroach further on important natural environments (Teoh 2010; Sayer et al. 2012). Many palm oil producing countries, including PNG, are committed to increase the sustainability of the oil palm industry via activities undertaken as members of the Roundtable on Sustainable Palm Oil (RSPO, https://rspo.org/as-an-organisation/).
A key factor for sustainability of any agricultural system is the management of nitrogen (N) in order to maximise productivity and minimise off-site effects, particularly eutrophication of waterways caused by run off and nitrate (NO3−) leaching, and greenhouse gas (GHG) emissions from denitrification and volatilisation (Galloway et al. 2013). Nitrogen fertilizers, a significant component of the cultivation costs in oil palm systems (Corley and Tinker 2016), are considered a major potential source for such N losses (Choo et al. 2011; Comte et al. 2012). Amounts of mineral fertiliser N applied to oil palm plantations vary widely, ranging from 48 to 90 kg N ha/year for immature palms to 56–206 kg N/ha/yr for mature palms (see references cited in Pardon et al. 2016). Whilst there is a dearth of comprehensive data to describe the complete N cycle of an oil palm system from planting to harvest, modelling using the limited data available suggests potential for N losses is greatest during the immature phase (first 2–3 years) when N uptake by palms is low, and then later during the mature phase when groundcover under palms tends to be sparse and N fertiliser rates higher (Pardon et al. 2016). The most influential parameters for N loss from an oil palm system were identified as mineral N fertiliser rate, drainage and fraction of legume in groundcover (Pardon et al. 2017).
Perennial legume species such as Pueraria phaseoloides, Calapogonium mucunoides Calopogonium caeruleum, and Mucuna pruriens have long been utilised as groundcover for control of soil erosion and suppression of weeds under oil palm plantations (Fairhurst and Hardter 2003; Samedani et al. 2015), as well as for N input via biological N2 fixation or BNF (Giller and Fairhurst 2003). Amounts of N fixed by cover legumes, including P. phaseoloides under 2–3 year old oil palm in Malaysia, were estimated at 150 kg N/ha/yr (Agamuthu and Broughton 1985) based on the mean difference in N content between the vegetation of the N2 fixing legume and non-fixing “natural” covers (N difference method). Another Malaysian oil palm study using 15N isotope-dilution methodology reported similar amounts of N2 fixation by P. phaseoloides (Zaharah et al. 1986). However, published measures of BNF for cover legumes in oil palm systems in situ remain scant (Giller and Fairhurst 2003; Pardon et al. 2016), despite a number of methodologies available for field use (Unkovich et al. 2008).
One approach for assessing the dependence of a legume cover on BNF that does not require the presence of a non-fixing cover is the ureide technique (Herridge 1982). This method is based on the premise that many tropical legume plants, from the tribes Phaseoleae and Desmoideae within the subfamily Papilionoideae, transport identifiable N2 fixation products in the xylem sap as ureides in contrast to N from mineral N assimilated into amides (Peoples et al. 2009). While the method itself is inexpensive and relatively simple, it must be calibrated with another technique such as the natural abundance δ15N (%o) or 15N isotope dilution technique (Unkovich et al. 2008). Ureide calibrations have been carried out for a number of legume species, including Desmodium ovalifolium and Centrosema spp. that are commonly used as oil palm covers. Stem relative ureide N (RUN) for these species has been shown to be highly correlated with the proportion of N derived from N2 fixation (pNdfa) assessed using 15N isotope dilution, proving the reliability of the calibrated ureide technique (Unkovich et al. 2008). Although there is mention of an unpublished ureide calibration for C. caeruleum (Wilson et al. 1995), there appear to be no published calibrations for this species, or for C. mucunoides, P. phaseoloides and M. pruriens (Unkovich et al. 2008) which are widely used as cover legumes in oil palm systems.
As plantations age and the palm canopy closes, the legume cover often declines due to shading, although some species thrive under older plantations (Giller and Wilson 1991). Reduced legume cover growth means lower amounts of N2 fixed (Zaharah et al. 1986), but it is unclear how plantation age and accompanying increase in shade, affects the proportional dependence on N2 fixation of the legumes. Also, since pNdfa tends to be reduced if nitrate is present in the legume root zone (Streeter and Wong 1988), it might be expected that accumulation of nitrate in soil, resulting from increased accumulation and decomposition of cover legume litter as plantations age, would reduce legume dependence on N2 fixation. However, a glasshouse study to determine the effect of litter quantity on pNdfa for P. phaseoloides suggested that effects were small as it was only reduced from 87 to 84% when litter input was increased from zero to the equivalent of 8 Mg DM/ha (Vesterager et al. 1995). Legume covers in tropical environments accrue litter rapidly within 6–9 months of establishment (Broughton 1977; Chiu 2004; Samedani et al. 2015), but reports of the rate at which this litter decomposes vary widely from a few months up to a year or more (Vesterager et al. 1995; Turner and Gillbanks 2003; Chiu 2004; Philip and Abraham 2009). Furthermore, although incubation experiments with 15N labelled residues of the cover legumes P. phaseoloides and M. bracteata demonstrate rapid net N mineralisation (Mendham et al. 2004), it has been suggested the flux may be reduced in the plantation situation by N immobilisation due to the presence of other residues from living and previously felled trees in the plantation (Pardon et al. 2016). It has been reported that the presence of cover legumes actually reduced leaching of N in plantations (Agamuthu and Broughton 1985; Lehmann et al. 1999) but there appears to be no direct investigation of soil nitrate under cover legumes in the field specifically related to BNF inputs in oil palm systems.
Therefore, this study aimed to quantify N2 fixation by legume cover crops under oil palm systems in PNG and to determine possible relationships between pNdfa and amount of nitrate in the soil under the cover legumes. The ureide technique was calibrated for legume cover species in the glasshouse, subsequently used to estimate pNdfa of legume covers in the field under different ages of palm, and then amounts of fixed N in total above-ground biomass (shoot plus litter) of legume covers were calculated.
Materials and methods
-
1.
Glasshouse experiment to calibrate the xylem ureide technique using 15N isotope dilution
Plant culture and growing conditions
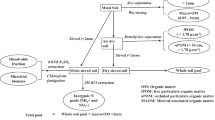
A glasshouse experiment was conducted to measure changes in the relative ureide abundance in stems of nodulated legumes grown at varying nitrate supply, with N2 fixation measured using 15N isotope dilution. The experiment took place under natural daylight over 9 months (May-January) at the Waite campus of the University of Adelaide, South Australia (34.9688o S, 138.6339o E). Average day/night temperature in the glasshouse for the growth period was 25/17oC. During winter (June - August) supplementary glasshouse lighting was used to ensure a daylight period of 12 h. Seeds of C. mucunoides, P. phaseoloides and M. pruriens were sourced from the Australian Tropical Grains Germplasm Centre (Queensland). Prior to sowing C. mucunoides seeds were soaked in hot (75oC ) water for three minutes to break dormancy (Giller and Fairhurst 2003) and P. phaseoloides seeds were soaked for one hour in glycerol at 50oC. M. pruriens seeds required no pre-treatment. Single seeds of each species were sown in free-draining 4-L pots containing fine acid-washed river sand in May (C. mucunoides and M. pruriens) and July 2011 (P. phaseoloides). All pots were inoculated with Group M Bradyrhizobium strain (CB756, Becker Underwood, Australia), by mixing peat inoculum in water and pouring a small amount of slurry into each seeding hole before the seeds were sown.
Nutrient solution and nitrate treatments
The glasshouse experiment had five nitrate (NO3−) concentration treatments including zero, four replicates and two harvests for each species, giving a total of 120 pots, arranged in a randomized complete block design. Pots were watered as needed with tap water until the first true leaves were observed on the plants, and until 10 weeks of age fed with a basal minus N nutrient solution for tropical legume culture (Herridge and Peoples 2002b) containing (as g/L) 0.0625 MgSO4, 0.0368 CaCl2, 0.02188 KH2PO4, 0.0188 KCl, and 0.0259 FeEDTA and as (mg/L) 0.715 H3BO4, 0.4525 MnCl2, 0.0275 ZnCl2, 0.0125 CuCl2 and 0.0063 NaMoO4, initially diluted 1/8 or ¼ according to the growth rate of the seedlings. Nitrate feeding of plants commenced ten weeks after sowing using 15N labelled potassium nitrate (5.0 atom% 15N) mixed with unlabelled Ca(NO3)2.4H2O and unlabelled KNO3 to make 1, 3, 5 and 10 mM NO3− solutions. Resulting enrichments of these nitrate solutions were 1 (1mM) 0.33 (3 mM), 0.2 (5mM) and 0.1 (10mM) atom % 15N excess in the above nutrient solutions. Pots were fed 1 L of nutrient solution twice weekly until harvest.
Sampling
Plants were harvested on different dates due to different growth rates, with M. pruriens harvested 16 and 21, C. mucunoides 29 and 34 and P. phaseoloides 23 and 28 weeks after sowing. At harvest, plants were cut just above the sand surface and 7 stem pieces (12–15 cm long) excised and oven dried at 85oC for 48 h and weighed, as was the remainder of the shoot. Each pot was tipped over carefully to remove loose sand and ensure the root system plus nodules remained as intact as possible. Roots were washed clean using tap water and a 2-mm sieve used to capture the remaining roots and nodules from the wash bucket. Each intact root system was divided into 0–5 cm, and > 5 cm sections in relation to depth in the sand. Nodules were separated from the root and counted, before roots and nodules were oven-dried at 85oC for 48 h and weighed. All dried plant samples were ground in a ball mill.
Analyses of plant material
Dried samples of legume shoots, roots and nodules were analysed for total N and 15N using an automated combustion analyser linked to a mass spectrometer (Dawson and Brooks 2001). Ground stem samples were subject to solute extraction and the extracts stored in a freezer prior to analysis for ureide, amino and nitrate-N as described in Unkovich et al. (2008). Briefly, ureide-N was analysed using the Rimini-Schryver reaction (Young and Conway 1942), amino compounds were analysed using the ninhydrin method (Yemm and Cocking 1955) and nitrate was analysed using the salicylic acid method (Cataldo et al. 1975).
Calculations
The concentration of ureides in the stem solutes relative to the amino and nitrate N concentrations, which derive principally from soil nitrate uptake (Herridge and Peoples 2002a), can be expressed as relative ureide N (RUN). Given that there are four N atoms in the ureide molecule, the RUN (%) can then be represented as:
where a is the concentration of ureides; b nitrate-N and c amino-N (Herridge 1982).
The relationship between RUN (%) and the proportion of plant N derived from the atmosphere (pNdfa, %) has been shown to be close to unity for a range of legumes (Unkovich et al. 2008).
In the present study the pNdfa (%) was calculated using the 15N isotope dilution technique with the 15N analysis data and the following equation (Unkovich et al. 2008).
-
2.
Field survey of N2 fixation by legumes under oil palm
Site information
Field studies were carried out in thirteen plantations across three commercial oil palm estates (Kapiura, Mosa and Numundo) near Kimbe (5.5512o S, 150.1387o E), West New Britain Province, PNG. The climate in the region is tropical with rain every month and an average annual rainfall of 3600 mm. Oil palm ages in the 13 sampled plantations ranged from 2 to 25 years with each plantation approximately 27 ha. Fertiliser application in the year prior to sampling (2011) varied with age of palm and management approach (Table 1). Although a mix of legume cover species had generally been sown immediately after the palms were planted as per best practice recommendation (Fairhurst and Hardter 2003) there were only three plantations, two on the Kapiura estate and one on the Mosa estate, where more than one species (i.e. C. caeruleum with P. phaseoloides) contributed to the ground cover (Table 1), whereas at all other plantations only one species was present. (Table 1). The soils are volcanic ash in origin (Banabas et al. 2008). Sampled soils pHCaCl2 ranged from 4.3 to 5.6 (Table 1) and were sandy loam to clay texture in the top 30 cm.
Soil and plant sampling and analyses
Soil and plant sampling was conducted in January and February 2012. In Kapiura and Mosa estates, for each legume cover species present, ten replicate samples of above-ground biomass (green shoots and litter separately) within 1m2 quadrats were taken at each plantation. Quadrats were placed randomly in areas containing the specific legume cover. Stem segments (10 cm length) were also obtained from each quadrat. Sampling at the Numundo estate plantations was restricted to stem segments and assessment of legume ground cover. Shoot, stem and litter samples were oven dried (85oC), weighed, ground, subsampled and transported to Australia for analysis of total N and N solutes extracted from stem segments as described for the glasshouse study. Soil was sampled to a depth of 20 cm using an auger (15 cm diameter) in four of the quadrats at each plantation. The four samples were thoroughly mixed, subsampled (~ 500 g) and oven-dried at 60oC. Sub-samples of the dried soils (~ 100 g) were sent to Australia. To comply with Australian quarantine regulations all dried plant and soil samples were gamma irradiated at a dosage of 25kGray (Steritech Pty Ltd, Queensland), prior to release for analysis. Sub-samples of each soil were then extracted with 2 M KCl (40ml/10 g soil) for one hour and the extracts analysed on an Alpkem Flow Solution II Analyser for nitrate-N and ammonium-N using colorimetry (Rayment and Lyons 2011). Further sub-samples were extracted in 0.01 M CaCl2 (1:5) for one hour and after allowing sediment to settle for 30 min, pH in the supernatant was measured with a Thermo ORION 960 pH/conductivity electrode (Rayment and Lyons 2011).
Estimation of legume ground cover (%) and amount of fixed N in standing shoot biomass
Ground cover (%) for each legume species present in a plantation was assessed using a representative hexagonal ‘palm unit’ (Fig. 1) that accounted for the triangular layout of palms, a weeded circle, the cleared harvest path and the frond piles. A quadrat (1m2) was moved from place to place within the hexagonal area to assess the total area (m2) covered by the legume species. Cover was observed to grow across harvest paths and on top of frond piles but the weeded circle usually remained as bare soil. The amount of N fixed by the legumes in standing shoot biomass in the plantation was calculated as the product of estimated % legume cover, pNdfa (%) as determined from stem ureide analysis and application of the calibration equation generated in the glasshouse study, and measured N contents of the shoots. To calculate the total amount of above-ground fixed N (in standing biomass plus litter) a simple assumption was made that the pNdfa (%) measured for the shoots was also applicable to the N in the litter under those shoots.
Hexagonal ‘palm unit’ assessed for % legume species cover at each location. Palm spacing varied with planting density but at all locations was close to the standard triangular distance between adjacent palms of 9 × 9 × 9 m (Corley and Tinker 2008). The weeded circle diameter is 1 m radius at time of palm planting and is gradually expanded to 2 m radius by palm age 2 years
Legume nodulation assessment
Legume nodulation was visually assessed on randomly selected plants at all plantations on the Mosa and Kapiura estates using a scoring system based on number and position of nodules on the excavated root system, modified from Corbin et al. (1977). Categories were poor (score 0–2); fair (2–3), good (3–4) and excellent (5) nodulation, with the lowest considered indicative of little or no N2 fixation and the highest implying that N2 fixation potential is near optimal.
Statistical analysis of data
Analysis of variance was used to explore differences in species responses to NO3- supply in the glasshouse experiment. Linear regressions were established between RUN (%) and pNdfa (%) from the glasshouse experiment. Firstly a general linear homogeneity of slopes analysis was used to determine if there were differences between species in the slopes of the regressions (MANCOVA, P < 0.05). If there were no differences between species then a single linear regression was derived using the mean species data. All analyses were conducted with the Statistica™ software package (v13.5.0.17, TIBCO Software Inc., CA USA). For the field data, general relationships between variables were explored using visual lines of best fit (power curves) using Microsoft Excel. The field experiments were based on a need to gather quantitative data on N2 fixation and as such were descriptive rather than hypothesis driven.
Results
-
1.
Glasshouse experiment to calibrate the relative ureide N technique for cover legumes
Proportional dependence on nitrogen fixation (pNdfa, %) measured by 15N isotope dilution
Since the only source of N in the zero NO3− treatments was N2 fixation, these plants are assumed to have fixed 100% of their N. The NO3− supply regime was effective in providing a wide range of dependencies on N2 fixation (Fig. 2A). Across the two harvests pNdfa (%) decreased with increasing N supply, especially for C. mucunoides and P. phaseoloides (Fig. 2A) which appeared very similar in their response, with 5mM NO3− being sufficient to suppress N2 fixation by approximately 90%. In contrast, M. pruriens maintained a significantly (P < 0.01) higher dependence on N2 fixation compared with the other two species under all NO3− additions.
Nodule mass per plant (Fig. 2B) was much greater for M. pruriens than the other two species, and as for N2 fixation, was suppressed less than for the others under increasing NO3− supply. Nodule number (data not shown) also significantly decreased with increasing nitrate supply in P. phaseoloides, but not in the other two species.
Stem ureide N calibration
Stem RUN (%) indices were very similar in the two harvests (data not shown) and thus mean values across harvests within a species and NO3− supply are presented (Fig. 3). Mean RUN was the same (P < 0.01) for C. mucunoides, and P. phaseoloides at each rate of NO3− supply (Fig. 3A), except for the 1 mM treatment, in which C. mucunoides had a higher RUN (40%, P < 0.01) than the other two species. The RUN for M. pruriens for the zero and 1mM nitrate treatments was much lower than for the other species (Fig. 3A) and similar to values for the higher nitrate treatments, suggesting ureide production in this species is not necessarily related to nitrogen fixation.
Relative stem ureide N (RUN, %) for (C mucunoides, P. phaseoloides and M pruriens as a function of NO3- supplied in the glasshouse. Values are means across two harvests. Error bars are standard error of the mean for n = 8. A single (power) curve has been fitted across data for C mucunoides and P. phaseoloides
Plotting the 15 N-estimated Ndfa (%) against the measured RUN (%) for each NO3− concentration again showed the similarities between C mucunoides and P. phaseoloides (Fig. 4). Homogeneity of slopes analysis showed that the regressions for C mucunoides and P. phaseoloides were not significantly different from each other (P > 0.05 by analysis of variance), and thus they were combined to produce a single calibration curve relating Ndfa (%) to stem RUN (%) for use in the field study (viz. Ndfa (%) = 1.514 x RUN (%) + 4.9848). While the correlation between Ndfa and stem RUN for M. pruriens (data not shown) was no weaker than for the other two species (R2 = 0.8763), the slope was very much steeper (Ndfa (%) = 7.4857 x RUN (%) – 2.5601), meaning that an absolute change of 1% in stem RUN for M. pruriens resulted in a shift in Ndfa of 7.5% with the comparable shift for the other species of 1.5%. This further indicated that RUN is unlikely to be a useful indicator of pNdfa for M pruriens.
-
2.
Field Survey of legume cover species in oil palm plantations
Legume cover shoot and litter dry matter and N content
Shoot dry matter for total legume cover sampled in each plantation ranged from 144 g/m2 to 792 g/m2 (mean 347 g/m2) across all plantations (Fig. 5A). Shoot biomass was greater in plantations with young oil palm, especially where cover was a mixture of two species (P phaseoloides and C caeruleum), and less in plantations with older (7–25 year) palms where cover was mainly C. caeruleum. As a result, there was a strong correlation (R2 = 0.67) between legume cover shoot dry matter and age of palm across plantations (Fig. 5A), with shoot biomass decreasing rapidly in the first 5–10 years, after which it remained at around 150 g/m2. Litter collected directly under the legume shoots averaged 762 g/m2 in plantations 6 years old or less and 139 g/m2 for those with palms 7–25 years old. The litter mass was almost always greater than the standing shoot biomass, regardless of palm age or legume species present, and was also strongly correlated with oil palm plantation age (Fig. 5B).
Correlations between age of oil palm plantation and A shoot or B litter dry matter (DM), and C shoot or D litter N content for total legume cover sampled from different aged palm plantations in West New Britain Province, PNG. Power curves have been fitted between age of oil palm and total legume cover DM or N per plantation
Shoot N concentration for legumes ranged from 2.2% (C. caeruleum, Kapiura 25 year old plantation) to 2.9% (M. pruriens, Mosa 6 year old). There were no significant differences (P = 0.44) between shoot N concentrations of C. caeruleum and P. phaseoloides, however species differences remain unclear because P. phaseoloides was only found in young palm plantations and there was a strong negative correlation (Adj R2 = 0.64, P < 0.01) between the age of the stand and the shoot N concentration. The litter N concentrations for individual species (1.6 to 3.2%) were marginally lower than those of the associated legume shoots, and more markedly so in older plantations. Legume shoot N content was 3.6 (C. caeruleum, Kapiura, 25 year old) to 18.6 (P. phaseoloides, Mosa 2 year old) g/m2 and legume litter N content was 2.0 (C. caeruleum, Kapiura, 25 year old) to 21.8 (M pruriens, Mosa 6 year old) g/m2. There were good correlations between legume shoot N content and palm age (Fig. 5C, R2 = 0.71) and litter N content and palm age (Fig. 5D, R2 = 0.65), as reported for dry matter.
Stem RUN
C. caeruleum generally had higher stem RUN than P. phaseoloides, ranging from 17 to 43% (average 28%), while the range for P. phaseoloides was 13 to 38% (average 18%). Furthermore, in the glasshouse study RUN in C. mucunoides obtained at zero N and 10mM N were very similar to those reported for fully nodulated (80% RUN) and fully nitrate-dependent (10% RUN) C. caeruleum (Gault and Peoples 1993), suggesting the two species of that genera are very similar in ureide activity. This gave confidence for applying the calibration derived for RUN in C. mucunoides to the C. caeruleum cover present in the field. RUN for the single M. pruriens sampled in the field survey was 28%, exceeding the maximum (12%) recorded for that species in the glasshouse experiment.
Legume proportional dependence on N2 fixation (pNdfa, %) for cover species
Mean pNdfa was significantly higher (P < 0.05) for C. caeruleum (49%) than for P. phaseoloides (32%), although for both species a wide range of values were observed (Table 2). There was no correlation between plantation age and pNdfa for P. phaseoloides or C. caeruleum (Fig. 6A) although P. phaseoloides, which was only present in younger plantations, generally had low pNdfa (14–27%) and consistently appeared to be deriving a larger proportion of its N from soil (72–82%) than C. caeruleum (25–72%, Table 2).
Soil extractable N
Concentrations of nitrate-N in the soil extracts ranged from 2 to 25 mg NO3−-N/kg. The highest NO3−-N occurred in soil from under P. phaseoloides in a 3 year old plantation on the Kapiura estate and the lowest NO3−-N in soil under C. caeruleum from a 25 year old plantation on the same estate. There was a strong negative relationship (R2 = 0.55) between soil nitrate concentration and pNdfa (%) of individual cover legumes (Fig. 6B), with a marked decline in dependence on N2 fixation as nitrate increased above 5 mg NO3−-N/kg.
There were exceptionally high concentrations of ammonium-N (95–292 mg NH4+-N/kg) in all the soil extracts and a poor correlation between pNdfa (%) and ammonium-N concentration in soil (R2 = 0.02). It was suspected that ammonium concentrations in the soils may have been influenced by the irradiation treatment of the samples on import to Australia.
Nodulation score
A high nodulation score (4 or 5) indicating an excellent potential for N2 fixation was recorded for both P. phaseoloides and C. caeruleum in plantations that were 5 years old or less (Table 2). A nodulation score of 4 was also recorded for C. caeruleum in a 13 year old Mosa plantation, whereas in the majority of plantations with older palms the nodulation scores for all three legumes were 2–3 indicating fair nodulation but reduced N2 fixation potential. A nodule score of 1 was assigned to C. caeruleum in the 18 year old Kapiura plantation suggesting little or no N2 fixation activity by this cover under mature trees. However, there were no obvious correlations between nodule score and either soil nitrate-N or pNdfa (%) of individual legume species (Table 2).
Plantation legume ground cover (%) and total standing biomass (DM), N and fixed N (kg/ha)
The proportion of ground covered by legumes in different plantations was highly variable and ranged from 0.6% in the oldest plantation sampled (C. caeruleum, Kapiura 25 year old) to 44% (P. phaseoloides, Mosa 2 year old). There were insufficient samples in the survey (Table 2) to be able to ascertain if there were trends in species presence with age of oil palm plantation, particularly as M. pruriens was only encountered in one plantation. However, it was evident from the restricted dataset (Table 2) that greater legume ground cover was recorded in plantations that were 5 years old or less, and that only C. caeruleum was present under the older plantations. Generally the percentage legume ground cover decreased as oil palm age increased (R2 = 0.67).
Assuming legume ground cover (%) across the entire plantation was similar to that in the sampled regions then the total biomass of above-ground legume cover (standing shoot plus litter) could be 1.2–5.6 Mg DM/ha containing 30–135 kg N/ha in plantations five years old or less (Table 2). This includes three plantations where mixtures of cover species occurred. Whereas in plantations older than five years standing shoot plus litter biomass (16–670 kg DM/ha) and N (< 1-21 kg N/ha) would be markedly lower, corresponding with observations of less cover and lower shoot/litter N concentrations in these plantations.
The amount of fixed N in shoots plus litter ranged from 2 to 34 kg N/ha (Table 2) but was not correlated with plantation age. Nevertheless, amounts of fixed N in total legume above-ground biomass (sum of standing shoot plus litter of all species present) were generally higher in the younger plantations where dry matter was greater due to more legume cover, and in some cases two cover species present (Table 2). Fixed N in total legume above-ground biomass was partitioned between standing shoots and litter in a ratio of 1:1 or 1:>1 in plantations less than 6 years old and in older plantations (with one exception) at a ratio of 1:1 or 1:<1. The proportion of N in total legume above-ground biomass derived from N2 fixation was greater than 30% for 6 of 9 plantations across two estates (Table 2), partly due to relatively high pNdfa for C. caeruleum (Table 2).
Discussion
A calibrated ureide technique was used, for the first time globally as far as we are aware, to estimate proportional dependence on N2 fixation (pNdfa) by cover legumes in oil palm plantations. Stem RUN for P. phaseoloides and C. caeruleum in a field survey in PNG were within the range (11–76%) reported for these species from a Malaysian rubber plantation study (Norhayati et al. 1988). Dependence on N2 fixation by the legume covers in this study ranged from 18% (P. phaseoloides) to 75% (C. caeruleum) and was highly variable between and within species, although overall C. caeruleum exhibited higher pNdfa than the other species. The pNdfa obtained for P. phaseoloides at 4 sites in this field survey in PNG were generally also lower than reported for this species in other tropical field studies using 15 N-based methods (Cadisch et al. 1989; Sanginga et al. 1996a, b; Giller 2001b; Fosu 2003; Sanginga 2003). The glasshouse study provided a robust calibration of the ureide technique (using 15N isotope dilution) for C. mucunoides and P. phaseoloides, and individual calibrations for C. mucunoides and P. phaseoloides were similar to those of pigeonpea (Peoples et al. 1989) and common bean (Unkovich et al. 2008). The limited range of ureide production in the glasshouse study for M. pruriens was similar to that observed for the tree legume species Gliricidia sepium which is considered a non-ureide exporter with respect to nitrogen fixation (Herridge et al. 1996). Indeed, it is possible that the colorimetric assay (Young and Conway 1942) used for analysing ureide in stem extracts of M pruriens in this present study could have given false positive results due to interference by non-ureide compounds, as has been shown for xylem sap extracts of Sesbania grandiflora (Herridge et al. 1996). The potential occurrence of this could be determined in future by checking the absorbance spectra of the final coloured products of the assay (Peoples et al. 1991). Such false data would render the apparent strong linear relationship between pNdfa and RUN for M pruriens invalid. Furthermore, the higher RUN value obtained for the single sample of M. pruriens in the field may have been related to time of sampling since significant diurnal fluctuation in ureide production has been reported, even for legumes where ureide production is not linked to pNdfa (Herridge et al. 1996). Overall we conclude that the RUN technique may not be suitable for estimating pNdfa in M pruriens Nevertheless, the ureide calibrations for the other two species from this study do provide an avenue to more widely use this reliable and fairly simple technique in future N2 fixation studies of legume covers in oil palm plantations or other agroecosystems.
It was apparent from the field survey that pNdfa for legume covers did not show any relationship with age of oil palm plantation, and hence age of these perennial legume covers. However, as one might expect given evidence in the literature (cited in the introduction) and data from the glasshouse study derived using 15N isotope dilution, pNdfa was negatively correlated with NO3− concentration in the soil under the cover legumes. Across all species and ages of plantation pNdfa was significantly lower where soil NO3− -N concentrations were above 5 mg/kg. Data from the glasshouse study also indicates that N2 fixation in C. mucunoides and P. phaseoloides was very sensitive to low concentrations of available NO3−. M pruriens on the other hand appears less sensitive and may continue to fix N2 under conditions of higher available N in the root zone. Thus, where mineral N in the soil is high, C. mucunoides and P. phaseoloides are likely to be more dependent on uptake of NO3− from soil than M. pruriens and this may decrease the potential for loss of N by leaching or through denitrification under these two species. Studies of P. phaseoloides as a cover crop with fruit trees in an Amazonian rubber plantation, using 15N-labelled fertiliser, similarly concluded that it was a good scavenger of applied N (Lehmann et al. 2000) and reduced N leaching losses compared to under the trees (Lehmann et al. 1999).
A decrease in nodule mass for all three legume cover species in response to increasing NO3−-N supplied was observed in the glasshouse study. This is consistent with many observations that legume nodulation, as well as legume-rhizobium symbiotic function, is inhibited by NO3− (Murray et al. 2016). The results for C. mucunoides contrasted with the observation of Camargos and Sodek (2010) that nodule mass and N2 fixation in this species were unaffected by NO3−-N, although that was after only a 5 day exposure to NO3−, and the same study did report a reduction in nodule number for C. mucunoides after 35 days exposure. A simple scoring method to assess nodulation of cover legumes in the field survey indicated poor nodulation for legume cover plants in a few of the older plantations, but generally fair to excellent nodulation across most plantations, irrespective of age and soil nitrate concentration. This was not necessarily expected given the highly acidic nature of the soils (Table 1), and that negative effects of low pH in the root zone on both growth of the legume and activity of rhizobia are well documented (Hungria and Vargas 2000). It is possible that inoculation practices using appropriately adapted rhizobium strains may have been implemented, but it is more likely that the indigenous rhizobia are effective at nodulation in these soils as has been observed for C. mucunoides in low pH soils of the Cerrado in Brazil (Ferreira et al. 2016). Nodule scoring is a useful rapid assessment of the potential N2 fixing ability of a legume but it is subjective and does not provide any indication of whether nodules are active or moribund. Perhaps this explains why there was no relationship between Ndfa (%) of legume species and nodule score for this field survey. Indeed, it can be concluded from the results of the glasshouse study that nodule mass is probably a better indicator than nodule score or nodule number in relation to pNdfa (%) and the response of legume N2 fixation to mineral N.
The measures of ammonium in these soils were extremely high and are considered to be an artefact from the mandatory gamma irradiation rather than representative of field conditions. While irradiation is thought to have little effect on NO3−concentrations in soils (McNamara et al. 2003), an increase in ammonium might result from amino N released from soil organisms which are all killed during irradiation, with no subsequent conversion to nitrate due to the absence of any active nitrifying bacteria (Lensi et al. 1991). A substantial increase (5 fold) in ammonium concentration in soils has been reported over a 55 day period following similar irradiation (Lensi et al. 1991), particularly where soils were moist. Although the soils for this study had been oven dried at 60oC in PNG it is possible that the samples retained some moisture during that process, or under the humid atmospheric conditions prevalent in PNG the stored samples re-absorbed moisture prior to transport and subsequent treatment in Australia. Delayed release of the imported samples from the Australia irradiation facility meant it was 3 months post-irradiation before the soils were analysed. The effects of ammonium on BNF are not as well defined as those for nitrate (Murray et al. 2016) and may merit consideration in future studies, although clearly valid measures need to be obtained using non-irradiated soils.
The standing shoot biomass (443–792 g/m2) obtained for legume cover from the field survey for the four plantations 5 years or less in age is similar to that recorded for 3–4 months growth of the same legume cover species in other tropical field environments (Broughton 1977; Sylvester-Bradley 1984; Zaharah et al. 1986; Sanginga et al. 1996a, b; Tian et al. 1999; Giller 2001a; Fosu 2003; Sanginga 2003; Franke et al. 2008; Samedani et al. 2015). Therefore, it could be considered reasonable to assume that amounts of fixed N in standing shoot biomass for each of these four plantations represents 4 months production, and could thus be multiplied by a factor of 3 to obtain annual N2 fixation estimates of 48–102 kg N /ha/year for legume cover in young PNG plantations. These quantities of fixed N in standing shoot biomass are less than the 150–200 kg N /ha/year reported for the same legume covers under oil palm and rubber plantations in Malaysia (Broughton 1977; Agamuthu and Broughton 1985; Zaharah et al. 1986), but are similar to that for C. mucunoides and M. pruriens cover crops in Ghana (Fosu 2003). Values reported in the literature appear to be from studies with close to 100% legume cover compared with the lower cover (17–44%) recorded in the present field survey in PNG. A reduced proportion of legume groundcover in plantations is not only due to shading as palm canopy closes, but also the result of other management factors, including poor cover crop establishment, slashing of cover crop vegetation to create harvest paths and removal of plants in the weeded circle. In the PNG survey total standing above-ground biomass (DM) and N content of cover legumes, and by default calculated amounts of N fixed, decreased in plantations more than five years old because legume ground cover markedly decreased in the older plantations. C caeruleum was the sole species surviving in plantations > 5 years old, perhaps indicating a greater tolerance to shade and other management influences.
It has been pointed out that the few reported amounts of N fixed by cover legumes in oil palm plantations do not include N in litter (Giller and Fairhurst 2003). Data from the field survey in PNG demonstrate that in plantations less than six years old there can be as much or more DM and N in the litter as there is in the corresponding standing shoot biomass of cover legumes. Indeed, assuming the same pNdfa for litter as shoots, the estimated amount of fixed N in legume litter for young plantations in this study either equalled (5.1-7 kg N/ha, Kapiura estate plantations) or was greater than that in shoots (15.5–19.3 kg N/ha, Mosa estate plantations). Accounting for this fixed N in litter would substantially increase annual estimates of BNF by cover legumes. Furthermore, this litter represents a significant pool of potentially mineralisable N in the warm moist climate of PNG, and is likely to be a factor in the observed accumulation of soil NO3− in the field survey that was related to reduction of pNdfa. Fixed N in legume litter sampled under older plantations in the survey was substantially lower at only 0.1–3.4 kg N/ha, often due to poor groundcover. It should be remembered that these amounts of N are for litter sampled at a single point in time and not annual values which are likely to be greater. Nitrogen transfer to litter from living biomass of cover legumes in plantations (oil palm and rubber) varies with age of cover and has been estimated at 120–160 kg N/ha/year for the first 3 years to less than 40 kg N /ha/year over years 4–7 (Agamuthu and Broughton 1985; Pushparajah and Chew 1998). However, annual litter accumulation and turnover cannot be quantified from the limited measures in this study and clearly requires further research to improve estimates of BNF inputs by cover legumes. Furthermore, a commonality between this study and all others that quantify BNF by tropical perennial cover legumes is that N accumulated below-ground in roots has not been measured. Studies with annual crop legumes have indicated that on average 30% of total plant N may be located below-ground (Herridge et al. 2008), and where legumes are dependent on BNF this represents input of fixed N to the system that needs to be included when considering the effect of cover legumes on the N cycle of oil palm plantations.
Conclusions
This study used a calibrated ureide technique for the first time in oil palm plantations to estimate pNdfa by cover legumes. Proportional dependence on N2 fixation by legume cover in oil palm plantations in PNG varied widely and was not related to age of the plantation but was strongly negatively correlated with increasing soil nitrate. Total amount of N derived from N2 fixation in above-ground biomass of legume cover was positively correlated with legume dry matter production which was greatest under young plantations. Legume cover declined with age, due to shading and other management influences, and C caeruleum was the only cover species present in plantations that were seven years or older. Further research is recommended, using the calibrated RUN technique to obtain annual estimates of BNF inputs over those obtained simply from standing shoot biomass by quantifying total legume biomass production over time, including litter and root accumulation. With this knowledge, more informed decisions can be made regarding the effective management of N inputs from fertilisers and legumes, to better synchronise fluxes of N in the system, and move towards more sustainable oil palm cultivation.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Agamuthu P, Broughton WJ (1985) Nutrient cycling within the developing oil palm-legume ecosystem. Agric Ecosyst Environ 13:111–123
Allen M, Bourke RM, McGregor A (2009) Cash income from agriculture in ‘Food and Agriculture in Papua New Guinea.‘ (Bourke RM, Harwood T eds .) pp. 283–424. The Australian Natoinal University Canberra
Banabas M, Turner MA, Scotter DR, Nelson PN (2008) Losses of nitrogen fertiliser under oil palm in Papua New Guinea: 1. Water balance, and nitrogen in soil solution and runoff. Aust J Soil Res 46:332–339
Broughton WJ (1977) Effect of various covers on soil fertility under Hevea brasiliensis muell. Arg. And on growth of the tree. Agro-Ecosyst 3:147–170
Byerlee D, Falcon WP, Naylor RL (2016) The tropical oil crop revolution: food, feed, fuel, and forests. Oxford University Press Available at https://doi.org/10.1093/acprof:oso/9780190222987.001.0001
Cadisch G, Sylvester-Bradley R, Nösberger J (1989) 15 N-Based estimation of nitrogen fixation by eight tropical forage-legumes at two levels of P:K supply. Field Crops Res 22:181–194
Camargos L, Sodek L (2010) Nodule growth and nitrogen fixation of Calopogonium mucunoides L. show low sensitivity to nitrate. Symbiosis 51:167–174
Cataldo D, Maroon M, Schrader L, Youngs V (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Chiu SB (2004) Mucuna bracteata - dry matter conversion and decay rate of litter. Planter 80:461–464
Choo YM, Muhamad H, Hashim Z, Subramaniam V, Puah CW, Tan Y (2011) Determination of GHG contributions by subsystems in the oil palm supply chain using the LCA approach. Int J Life Cycle Assess 16:669–681
Comte I, Colin F, Whalen JK, Grünberger O, Caliman J-P (2012) Chapter three - agricultural practices in oil palm plantations and their impact on hydrological changes, nutrient fluxes and water quality in Indonesia: A Review. In ‘Advances in Agronomy.‘ (Donald LS ed. ) Vol. 116 pp. 71–124. Academic Press
Corbin E, Brockwell J, Gault R (1977) Nodulation studies on chickpea (Cicer arietinum). Aust J Exp Agric 17:126–134
Corley RHV (2009) How much palm oil do we need? Environ Sci Policy 12:134–139
Corley RHV, Tinker PB (2008) The Oil Palm, Fourth Edition. Wiley-Blackwell, Chichester UK
Corley RHV, Tinker PBH (2016) Chap. 12 Mineral Nutrition of Oil Palms. The Oil Palm. John Wiley and Sons Ltd, Chichester UK), pp 329–398
Cramb R, Curry GN (2012) Oil palm and rural livelihoods in the Asia–Pacific region: an overview. Asia Pac Viewp 53:223–239
Dawson T, Brooks PD (2001) Fundamentals of stable isotope chemistry and measurement In ‘Application of stable isotope techniques to study biological processes and functioning of ecosystems.‘ (Unkovich MJ, Pate JS, McNeill A, Gibbs DJ eds.) pp. 1–18. Kluwer Academic: Dordrecht
Fairhurst T, Hardter R (eds) (2003) Oil palm: management for large and sustainable yields. IPNI and IPI, Singapore
Ferreira TC, Aguilar JV, Souza LA, Justino GC, Aguiar LF, Camargos LS (2016) pH effects on nodulation and biological nitrogen fixation in Calopogonium mucunoides. Brazilian J Bot 39:1015–1020
Fosu M (2003) Nitrogen accumulation and release by Sunn Hemp, Calopo, Mucuna and devil bean in semi-arid Ghana. Agric Food Sci J Ghana 2:141–153
Franke A, Laberge G, Oyewole BD, Schulz S (2008) A comparison between legume technologies and fallow, and their effects on maize and soil traits, in two distinct environments of the west african savannah. Nutr Cycl Agrosyst 82:117–135
Galloway JN, Leach AM, Bleeker A, Erisman JW (2013) A chronology of human understanding of the nitrogen cycle. Philosophical Trans Royal Soc B: Biol Sci 368:20130120
Gault R, Peoples MB (1993) Development of on-farm methods for measuring N2 fixation. Proceedings of XVII International Grassland Congress. New Zealand and Australia, pp 1577–1579
Giller KE (2001a) Legumes as green manures and cover crops. In: Giller KE (ed) Nitrogen fixation in tropical cropping systems. CAB International Publishing, pp 168–186
Giller KE (2001b) Nitrogen fixation in tropical cropping systems. CABI Publishing, London
Giller KE, Fairhurst T (2003) Legume cover plants. In: Fairhurst T, Hardter R (eds) Oil palm: management for large and sustainable yields. Potash & Phosphate Institute (PPI)/Potash, Singapore, p. 384
Giller KE, Wilson KJ (1991) Nitrogen fixation in tropical cropping systems. C.A.B International, London
Herridge DF (1982) Relative abundance of ureides and nitrate in plant tissues of soybean as a quantitative assay of nitrogen fixation. Plant Physiol 70:1–6
Herridge DF, Palmer B, Nurhayati DP, Peoples MB (1996) Evaluation of the xylem ureide method for measuring N2 fixation in six tree legume species. Soil Biol Biochem 28:281–289
Herridge DF, Peoples MB (2002a) Calibrating the xylem-solute method for nitrogen fixation measurement of ureide-producing legumes: cowpea, mungbean, and black gram. Commun Soil Sci Plant Anal 33:425–437
Herridge DF, Peoples MB (2002b) Timing of xylem sampling for ureide analysis of nitrogen fixation. Plant Soil 238:57–67
Herridge DF, Peoples MB, Boddey R (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Research 65:151–164
Lehmann J, Pereira da Silva J, Schroth G, Gebauer G, Da Silva LF (2000) Nitrogen use in mixed tree crop plantations with a legume cover crop. Plant Soil 225:63–72
Lehmann J, Da Silva J, Trujillo L, Uguen K (1999) Legume cover crops and nutrient cycling in tropical fruit tree production. Proceedings of 2nd ISHS Conference on Fruit Production in the Tropics and Subtropics. Bonn-Rottgen, Germany, pp 65–72
Lensi R, Lescure C, Steinberg C, Savoie J-M, Faurie G (1991) Dynamics of residual enzyme activities, denitrification potential, and physico-chemical properties in a γ-sterilized soil. Soil Biology and Biochemistry 23:367–373
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Applied Soil Ecology 24:117–132
Mendham DS, Kumaraswamy S, Balasundaran M, Sankaran KV, Corbeels M, Grove TS, O’Connell AM, Rance SJ (2004) Legume cover cropping effects on early growth and soil nitrogen supply in eucalypt plantations in south-western India. Biol Fertil Soils 39:375–382
Murray JD, Liu C-W, Chen Y, Miller AJ (2016) Nitrogen sensing in legumes. J Exp Bot 68:1919–1926
Norhayati M, Noor S, Chong K, Faizah A, Herridge DF, Peoples MB, Bergersen F (1988) Adaptation of methods for evaluating N2 fixation in food legumes and legume cover crops. Plant Soil 108:143–150
Pardon L, Bessou C, Nelson PN, Dubos B, Ollivier J, Marichal R, Caliman J-P, Gabrielle B (2016) Key unknowns in nitrogen budget for oil palm plantations. Rev Agron Sustainable Dev 36:20
Pardon L, Huth NI, Nelson PN, Banabas M, Gabrielle B, Bessou C (2017) Yield and nitrogen losses in oil palm plantations: main drivers and management trade-offs determined using simulation. Field Crops Research 210:20–32
Peoples MB, Atkins CA, Pate JS, Chong K, Faizah AW, Suratmini P, Nurhayati DP, Bagnall DJ, Bergersen FJ (1991) Re-evaluation of the role of ureides in the xylem transport of nitrogen in Arachis species. Physiol Plant 83:560–567
Peoples MB, Hebb DM, Gibson AH, Herridge DF (1989) Development of the xylem ureide assay for the measurement of nitrogen fixation by pigeonpea (Cajanus cajan (L.) Millsp). J Exp Bot 40:535–542
Peoples MB, Unkovich MJ, Herridge DF (2009) Measuring symbiotic nitrogen fixation by legumes. In: Emerich DW, Krishnan HB (eds) Nitrogen fixation in crop production. American Society of Agronomy: Monograph, United States of America, pp 125–170
Philip A, Abraham J (2009) Litter chemistry and decomposition in rubber plantations. Nat Rubber Res 22:10–16
Pushparajah E, Chew PS (1998) Integrated nutrient management for sustaining high yield of plantation tree crops in tropical Asia. CD ROM Proceedings of 16th World Congress of Soil Science 20-26th August 1998 Montpellier, France
Rayment GE, Lyons DJ (2011) Soil chemical methods Australasia. Melbourne, (CSIRO
Samedani B, Juraimi A, Rafii M, Sheikh Awadz S, Anwar M, Anuar A (2015) Effect of cover crops on weed suppression in oil palm plantation. Int J Agric Biol 17:251–260
Sanginga N (2003) Role of biological nitrogen fixation in legume based cropping systems; a case study of West Africa farming systems. Plant Soil 252:25–39
Sanginga N, Ibewiro B, Houngnandan P, Vanlauwe B, Okogun JA, Akobundu IO, Versteeg M (1996a) Evaluation of symbiotic properties and nitrogen contribution of mucuna to maize grown in the derived savanna of West Africa. Plant Soil 179:119–129
Sanginga N, Okogun A, Akobundu IO, Carsky RJ, Tian G, Wirkom LE (1996b) Nodulation and estimation of symbiotic nitrogen fixation by herbaceous and shrub legumes in Guinea savanna in Nigeria. Biol Fertil Soils 23:441–448
Sayer J, Ghazoul J, Nelson P, Boedhihartono AK (2012) Oil palm expansion transforms tropical landscapes and livelihoods. Glob Food Sec 1:114–119
Streeter JG, Wong PP (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. CRC Crit Rev Plant Sci 7:1–23
Sylvester-Bradley R (1984) Rhizobium inoculation trials designed to support a tropical forage legume selection programme. Plant Soil 82:377–386
Teoh CH (2010) Key sustainability issues in the palm oil sector. World Bank Group, Washington
Tian G, Kolawole GO, Salako FK, Kang BT (1999) An improved cover crop-fallow system for sustainable management of low activity clay soils of the tropics. Soil Sci 164:671–682
Turner P, Gillbanks R (2003) Oil palm management and cultivation. The Incorporated Society of Planters, Kuala Lumpur
Unkovich MJ, Herridge DF, Peoples MB, Cadisch G, Boddey B, Giller KE, Alves BJR, Chalk P (2008) Measuring plant-associated nitrogen fixation in agricultural systems. Australian Centre for International Agricultural Research (ACIAR) Monograph No 136, p 258
Vesterager JM, Osterby S, Jensen ES, Schjoerring JK (1995) Symbiotic N-2-fixation by the cover crop Pueraria phaseoloides as influenced by litter mineralization. Plant Soil 177:1–10
Wilson KJ, Peoples MB, Jefferson RA (1995) New techniques for studying competition by Rhizobia and for assessing nitrogen fixation in the field. Plant Soil 174:241–253
Yemm EW, Cocking EC (1955) The determination of amino-acids with ninhydrin. Analyst 80:209–214
Young EG, Conway CF (1942) On the estimation of allantoin by the Rimini-Schryver reaction. J Biol Chem 142:839–853
Zaharah AR, Sharifuddin HAH, Razley MN, Mohd Saidi AK (1986) Measurement of nitrogen fixed by Pueraria phaseoloides by N-15 dilution technique. Pertanika 9:45–49
Acknowledgements
Staff and students at The University of Adelaide, South Australia and the Oil Palm Research Association in Papua New Guinea (PNG) who generously assisted with sampling and other support. Mark Peoples and David Herridge for advice. The Biogeochemistry Centre at The University of Western Australia for isotope analyses. The Australian Centre for International Agricultural Research provided a John Allwright Fellowship for Rachel Pipai and technical support in PNG via Project SMCN-2009-013 ‘Sustainable management of soil and water resources for oil palm production systems in PNG’.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions The Australian Centre for International Agricultural Research funded a John Allwright Fellowship for Rachel Pipai and technical support in PNG via Project SMCN-2009-013 ‘Sustainable management of soil and water resources for oil palm production systems in PNG’. Research infrastructure support was provided by The University of Adelaide and the Oil Palm Research Association in PNG.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Set up of experiments, data collection and analysis were performed by Rachel Pipai with supervision and input by Murray Unkovich and Ann McNeill. The first draft of the manuscript was written by Ann McNeill and all authors commented on the initial and subsequent versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Euan K. James.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pipai, R., McNeill, A., Unkovich, M. et al. Biological nitrogen fixation by legume cover plants in oil palm plantations: calibration of the ureide technique and effects of plantation age and soil nitrate. Plant Soil 491, 665–680 (2023). https://doi.org/10.1007/s11104-023-06147-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06147-8