Abstract
Aims
At the same location, variability among orchards may be high, which is reflected in fruit quality, and it may be associated mainly with growers’ practices. This study aimed to identify physicochemical variability within pears (Pyrus communis L. cv 'Rocha') from three orchards from the same location and under the same climate conditions and correlate it with mineral composition.
Methods
Fruits from three orchards harvested at the optimal harvest time were characterized during ripening under shelf-life conditions of 7 d at 20 ± 2 °C. The analyses performed included ethylene production, respiration rate, fruit quality (firmness, skin colour, soluble solids, and titratable acidity), macro and micronutrients, ethylene biosynthesis enzymes, esters, sugars, and organic acids. Principal component analysis was used to show the variability among fruits from the three orchards and to correlate the differences with the fruit mineral composition.
Results
Phosphorus (P) and potassium (K) were significantly correlated with esters and soluble solids content (r \(\sim\)0.4 for both minerals). Fruits with higher P and K concentrations were associated with a potential over-ripeness pattern considering their higher acetate production and lower sugar concentration. Esters, soluble sugars, and sorbitol were the dominant fruit quality factors responsible for the differences among the orchards.
Conclusion
With this study it is expected that a better understanding of the relationship between specific minerals and quality parameters can help growers manage their orchards more efficiently and achieve consumers’ quality demands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A good balance between high productivity, sustainable orchard practices, good fruit quality, and proper fruit storage is a challenge for growers when managing orchards and production. Growers constantly optimize their orchard management strategies to obtain high quality fruits that satisfy market goals and consumer needs and expectations. However, within the same cultivar, an orchard’s organoleptic variability, such as flavor, aroma, and texture, is high and often not controlled. This variability is mostly and significantly affected by two preharvest factors: agronomic grower practices and environmental conditions (Musacchi et al. 2021). Grower practices include soil management (irrigation and soil physicochemical properties), tree characteristics (rootstock and tree age), fertilization, fertigation, and foliar sprays. Environmental factors include temperature, wind, soil mineral quality and texture, soil pH, sunlight exposure, and soil water availability (Bramlage 1993; Link 2000). Like other crops, 'Rocha' pear productivity also depends on a combination of various factors like edaphoclimatic, pruning, among other cultural agronomic practices (Mendes 2017).
In the case of 'Rocha' pear, a native cultivar from Portugal with Protected Designation of Origin (PDO), there are several producer organizations from different municipalities in “West Region”, with Bombarral, Cadaval and Caldas da Rainha the most representative. Each grower organization is responsible for managing orchards to obtain pears according to market demand, mainly related to taste and firmness (Salta et al. 2010). However, even the same geographical region, there are dissimilarities among orchards. Since the variation in environmental conditions at the same location is not very high, it is not apparent whether differences among orchards are exclusively due to the grower’s practices, making orchard management difficult (Sansavini and Musacchi 2002). Growers’ practices variables significantly affected the unpredictability of orchards and fruit quality after harvest. Although this appears to be a complex of variables, Bramlage (1993) suggested that the mineral composition of fruit at harvest accounts for most fruit quality variability among orchards.
One of the primary effects on postharvest behavior and fruit quality is fruit mineral content, which depends, in part, on the orchard from which they are harvested (Sharples 1980; Viera and Winefield 2019). For example, storage-related disorders, particularly in pome fruits, have been correlated with plant nutrition and fruit mineral composition (Deuchande et al. 2017; Saquet et al. 2019; Xuan et al. 2005). Mineral nutrients are provided by the soil (Bonora et al. 2021), but they can also be supplied by fertigation and foliar applications (Mendes 2017). Fruit mineral characterization at harvest along with fruit physicochemical characterization can provide information about orchards and trees ecosystems, mainly how orchard factors interact with macro and micronutrients, and consequently explain some physicochemical and ripening differences in different orchards (Ali et al. 2014; Casero et al. 2004). The nutrients with the most notable effect on fruits are nitrogen (N), calcium (Ca), phosphorus (P), and potassium (K) (Marcelle 1995; Chiriboga et al. 2013; Janssens et al. 2015). For example, fruit acidity is positively influenced by K and P, and fruit firmness and flavor are positively correlated with Ca content (Bramlage 1993; Casero et al. 2004; Kalcsits 2016). In pome fruits, Ca and K are fundamental for quality and storage (Brunetto et al. 2015). According to Tagliavini et al. (2000) and Casero et al. (2004), fruit quality preservation during storage is favorably influenced by low N and high Ca levels. To the best of our knowledge, little information is available on the relationship between esters production, aroma and mineral composition.
In this research, data collected from the three orchards were subjected to PCA to understand which fruit physiological parameters differentiate orchards within the same geographical region and under similar climatic conditions. To this end, PCA was also used to understand whether there were interactions between physicochemical parameters/ripening and mineral composition at harvest.
Materials and methods
Plant material
'Rocha' pears (Pyrus communis L.) were harvested on August 2020 from three commercial orchards (O1 (orchard 1), O2 (orchard 2), and O3 (orchard 3)) located in the Portuguese “West Region” of Cadaval (N 39° 25’; W 8° 54’; 120 m) within a radius of 10 km from each other. The three orchards followed the same cultivation procedure as reported in the PDO protocol as described bellow. The region has a moderately rainy climate with a moderate annual average temperature between 15 and 16 °C. The orchards were installed on sandy-loam soils and chosen among those that were well managed. 'Rocha' pear rootstocks were the same in the three orchards and soil fertigation was tailored to a standard soil composition of: 100 kg/ha N, 50 kg/ha P, 200 kg/ha K, 100 kg/ha Ca and 50 kg/ha Mg. O1 and O2 are in flat areas and are around 20 years old, O3 is the oldest one with around 35 years and is in half hill-flat area. In O1 and O2 plants are distributed 1,5 m apart in the row and in orchard 3 they are placed 2 m apart. Distance between rows is 4 m, in all orchards. No cultivation is done between rows. To standardize the fruit maturity stage, pears from the three orchards were harvested at the optimal harvest period (firmness between 54 and 64 N, soluble solids content (SSC) between 11 and 13%, titratable acidity (TA) between 2 and 3 g L− 1 malic acid and starch index between 5 and 7) according to the European regulation PDO-PT-0160 for PDO “Pera Rocha do Oeste” (National Association of “Rocha” pear Grower, 2003). After harvest, the fruit was stored at 4 °C, for no more than 4 days (d), until further analysis. Fruit was removed from storage and transferred to shelf-life conditions during 7 d at 20 ± 2 °C. Ten fruits per time-point and per orchard were analysed across 0 and 7 d of shelf-life.
Quality parameters
Firmness, skin colour, SSC, and TA were measured to infer about fruit quality. Firmness (expressed as Newtons (N) was determined by penetration text on a texturometer (T.A. XT plus Texture Analyser, Stable Micro Systems, Cardiff, UK) fitted with an 8 mm diameter probe. On two opposite equatorial sides of each fruit, firmness was measured in ten fruits per time-point and orchard. The skin colour of pears was measured on opposite sides of the widest part of pears using a CR-400 colourimeter (Konica Minolta, Osaka, Japan) with the D65 illuminant and the CIE (Commission Internationale de l’Eclairage) parameters (L*, a*, b*). Results were obtained as hue angle (h °= arctan(b*/a*), a coordinate that monitor colour changes from green hue of unripe fruit to yellow hue of ripe fruit (McGuire 1992).
SSC was evaluated by digital refractometry (PR1ATAGO CoLTD (Japan)) in juice from three fruits (three replicates of three fruits each). TA was measured by titration with 0.1 M NaOH until pH 8.1 after homogenization of 10 g of pear with 90 mL of distilled water (blend of three fruits per replicate and three replicates per time-point and per orchard). Results are expressed as g of malic acid equivalents per kg of pear on a fresh weight basis (g kg − 1).
Ethylene and respiration rate measurements
Ethylene and respiration rates were measured according to Saquet and Almeida (2017), with some modifications. At each time-point, ethylene (µg kg-1 h-1) was measured in three replicates of two fruits each. Fruits were closed inside 1.5 L glass jars at 23 °C for 2 h. After that, a headspace of 1 mL was removed with an air-tight syringe via a rubber septum and injected into a Varian CP-3380 gas chromatograph (Walnut Creek, CA, USA) fitted with a capillary column TG bond alumina (Na2SO4) 50 m length and 0.53 mm i.d. (Thermo Fisher Scientific Inc., Marietta, USA). Hydrogen was used as carrier gas at a flow rate of 15 mL min-1. The temperature of the injector was set at 160 °C, a flame ionization detector (FID) at 180 °C, and the following oven temperature program: hold time of 1 min at 40 °C, followed by 20 °C min-1 to reach 100 °C, and a holding time of 2 min at 100 °C.
Respiration rate (mg CO2 kg-1 h-1) was assessed in the same fruit samples used for ethylene, with an infrared sensor (Dansensor CheckMate 3, METEK, USA) in a close circulation circuit.
Macro and micronutrients analysis
Samples of pear pulp tissue (three replicates of three fruits each) from the three orchards were dried in an oven at 70°C until dryness and ground into powder. For total N determination, samples were subjected to dry combustion using a ThermoQuest NA 1500 elemental analyzer (Carlo Erba, Milano, Italy). A calibration curve with atropine was performed, and results were expressed as % of the sample weight. For mineral analysis (Na, Mg, K, Ca, Mn, Fe, Cu, Zn and P), ground pulp samples, 300 mg, were digested by a microwave digestor system (Anton Paar MULTIWAVE-ECO) in Teflon tubes filled with 10 mL of 65% HNO3 by applying a one-step temperature ramp (the temperature increased to 180°C in 20 min and maintained for 10 min.). After 20 min of cooling time, the mineralized samples were transferred to polypropylene test tubes. The final extraction volume was registered. Then samples were properly diluted (1:40) in MILLI-Q water. The concentration of minerals was measured by ICP-MS (BRUKER Aurora-M90 ICP-MS). To check the nebulization performance, an aliquot of a 2 mg L-1 of an internal standard solution (72Ge, 89Y, 159Tb) was added to samples and calibration curve to give a final concentration of 20 µg L-1. Potential polyatomic analysis interferences were removed using CRI (Collision-Reaction-Interface) with a H2 flow of 80 mL min-1 flow through the skimmer cone. Results were presented as µg per g of pear on a dry weight basis (µg g-1).
Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) and ACC oxidase activity (ACO)
Fruit flesh tissue (three replicates of three fruits each) was frozen in liquid nitrogen at each time-point and stored at − 80°C until further measurements. 1-aminocyclopropane-1-carboxylic acid (ACC) was extracted following Bulens et al. (2011) protocol with some alterations. Frozen tissue, 6 g, was homogenized with 8 mL of 80% ethanol, gently shaken for 30 min in melted ice and centrifuged for 10 min at 12000 g and 4°C. The extract was then mixed with 10 mmol L-1 of HgCl2 and NaOCl (5% v/v)-NaOH (6 mol L-1) (2:1) in a vial with an air-tight septum containing cap and incubated in melted ice for 4 min. 1 mL headspace gas sample was extracted from the vial and injected into a GC fitted with an FID detector. ACC concentration (nmol ACC kg-1 of fresh weight) was determined indirectly by ethylene production.
ACC oxidase activity (ACO) was measured by homogenizing 5 g of frozen flesh tissue with 10 mL of a buffer containing 400 mmol L-1 at pH 7.2, 10% glycerol, 30 mmol L-1 ascorbic acid sodium salt and 2% PVPP. The homogenate was gently shaken for 10 min in melted ice flowed by centrifugation for 30 min at 28000 g and 4°C. The supernatant was stored at – 80°C until analysis. The mixture was incubated for 60 min at 30°C, after which 1 mL of headspace gas sample was injected into a gas chromatograph. Enzyme activity was determined according to Bulens et al. (2011). ACO activity was calculated by ethylene production (nmol C2H4 per kg-1 L-1 on a fresh weight basis).
Esters determination by SPME-GC-MS
Extraction and concentration of volatile compounds
Extraction and concentration of the volatile compounds were performed by HS-SPME technique using an SPME fibre with a 50/30 µm thickness of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS; Supelco Co., Bellefonte, PA, USA). Fibres were pre-activated following manufacturer’s instruction. Esters’s concentration was determined in three replicates of one fruit each at each time-point and per orchard. Pear was randomly sealed in a 1.5 L air-tight glass jar fitted with a rubber septum in the lid. Jars were closed and kept overnight at room temperature to reach equilibrium. Previously, 20 µL of 500 mg L− 1 3-octanol was added as internal standard. After stability, jars were immersed in a water bath (25.0 ± 0.1 °C), and the DVB/CAR/PDMS SPME fibre was inserted in the headspace through the septum for 1 h. During this time, the volatiles are absorbed on SPME fibre coating. After the extraction/concentration step, SPME fibre was manually introduced into the port at 220 °C for 5 min for analytes desorption in a splitless mode.
GC-MS conditions
Volatiles were identified and measured using a Varian 240-IT-MS mass selective detector coupled with a Varian 450-GC gas chromatograph (Walnut Creek, CA, USA) equipped with a 50 m x 0.32 mm x 0.25 μm BP-21 column. Helium at a flow of 1.0 mL min− 1 was used as carrier gas. The oven temperature program: 40 °C for 1 min, increased at 2 °C min− 1 to 220 °C, 220 °C for 30 min. Mass spectra were recorded at 70 eV in electron impact (EI) ionization mode. The ion source temperature was 210 °C, and the transfer line temperature was 160 °C. Mass spectra was scanned in the m/z range of 33–350. Tentative identification of the esters compounds was made with the data system library (NIST 98). Quantification was done by the internal standard method, where the concentration of each volatile was normalized to that of 3-octanol. The results were presented in nmol of ester per kg of pear on a fresh weight basis (nmol kg− 1).
Extraction and quantification of malic acid and sugars
Free sugars and malic acid composition were obtained by extracting three replicates of three fruits, following the methodology described by Lindo-García et al. (2019) and Giné-Bordonaba et al. (2017). First, 2 mL of 62.5% (v/v) aqueous methanol solvent was added to 300 mg freeze-dried pulp tissue and heated in a thermostatic bath to 55 °C for 15 min, mixing the solution every 5 min to prevent layering. Samples were centrifuged at 20,000 g for 7 min at 20 °C, and the supernatant was recovered, followed by solvent evaporation using a speed vacuum and subsequent dissolution in water. The HPLC analysis was performed using a Beckman Coulter System Gold HPLC (Knauer, Berlin, Germany) coupled to RI and UV detector. The malic acid quantification was obtained using an Aminex 37-H column (Bio-Rad, Berkeley, USA) at 40 °C and 5 mM H2SO4 as mobile phase (flow rate: 0.6 mL min− 1). The free sugar profile was determined using an Aminex HPX-87P column (Bio-rad, Berkeley, USA) at 85 °C and ultra-pure water as mobile phase at a 0.6 mL min− 1. The quantification of malic acid and free sugars was achieved using standard calibration curves (0.1–40 g L− 1). The results are represented as grams of the sugar or organic acid per kg of pear on a fresh weight basis (g kg− 1).
Statistical analysis
A principal component analysis (PCA) using SPSS 24 for Windows® (SPSS Inc., Chicago,USA) was carried out to evaluate the parameters more related to orchards differentiation and their association to mineral composition. The Varimax method was used to produce orthogonal transformations to the reduced factors to identify the high and low correlations among variables. Problematic variables with an anti-image correlation value below 0.5 were identified and removed based on Kaiser and Rice’s (1974) criteria (data not shown). Loadings represent the importance of the variable in explaining the data variability in the respective component. Therefore, variables with a loading threshold of 0.7 (absolute value) were dominant in the respective component (Nunnally 1978). The scores represent the distribution of the data in the rotated system composed by the principal components (Nunnally 1978).
Data were analysed using analysis of variance (one-way ANOVA) to assess the statistical significance of differences among orchards in each study. Significant differences were determined by calculating Tukey’s post-test significant difference at p < 0.05. Additionally, correlations between minerals and physicochemical parameters were assessed using the Pearson correlation coefficient using as significance level, p = 0.05.
Results
A fundamental decision to decrease fruit orchards’ variability is harvest time. According to Vanoli and Buccheri (2012), both early and late harvest can have a negative impact on fruit quality. In this way, to exclude harvest maturity influence from orchards variability evaluation, pears from the three orchards were harvested at the optimal harvest time according to the specifications of PDO 'Rocha' pear (National Association of “Rocha” pear Grower, 2003).
To exclude the influence of shelf-life on the distribution of the variables and samples within the PCA, we analysed time 0 and 7 d of shelf-life separately. Figure 1 shows the PCA at 0 d which explains 78.8% of the total variation in the data with two principal components (PC1 = 43.3%; PC2 = 35.5%). Figure 2 shows the PCA at 7 d, with the two components explaining 60.7% of the variance observed (PC1 = 41.1%; PC2 = 19.6%).
In both figures (Figs. 1 and 2), a clear differentiation among the three orchards was observed, particularly pears from O3 which revealed a clear separation, specially, along PC1 from the other two orchards. It is evident from both PCA’s, that physicochemical parameters, such as ester emission, SSC, sucrose, malic acid, and the minerals P, Cu, K, and Ca, mainly distinguish O3 from O1 and O2. About O1 and O2 could not be distinguished in PC1, but along PC2, they were separated mainly due to respiration rate, sorbitol, fructose, glucose, ethylene, and the minerals Na, Fe, and Zn.
To validate the differentiation among the orchards, each parameter was statistically analysed. As shown in Figs. 3, 4, and 5, pears from O3 clearly revealed, across ripening, higher ester production, lower soluble solids content, lower sugars (particularly sucrose), and lower acidity concentration, which were in general significantly different from O1 and O2 across ripening. O2 had the highest soluble solids content and acidity. The malic acid concentration was significantly different among three orchards. Moreover, O2 and O3 showed higher sorbitol concentrations.
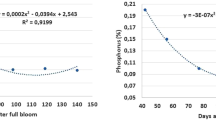
In Table 1 it is represented the mineral concentration of the three orchards at harvest. The O3 revealed a significant higher concentration of P, K, and Cu minerals. Additionally, O2 had significant lower concentration of Ca and Fe. Manganese (Mn) and N concentration did not also differ in the three orchards (Table 1). Higher esters production is related to higher mineral concentration in P and K (Table 1), which was confirmed by the significant correlation (r \(\sim\) 0.4, for each mineral). Besides, SSC was also correlated with P, Ca, and Fe ( -0.4 >r< -0.5, for each mineral) and malic acid was significantly correlated to P and Ca (r = -0.470, for P and Ca).
Additionally, Ca was significantly negatively correlated with the SSC, malic acid, and fructose (r= -0.509; r= -0.467; and r = -0.398, respectively). Zn did not correlate with any of the parameters analysed in this study though in both PCA’s it appears associated with respiration rate (Figs. 1 and 2). In relation to Fe, it was negatively correlated with SSC (r = -0.531).
Regarding ethylene production (Fig. 6), O2 had the lowest ethylene production and at the same time the lowest respiration rate, while O1 has the higher respiration rate (Fig. 6) after 7 days at 20 °C. Also, no statistical differences were found for ACC and ACO concentration among the three orchards (Fig. 6).
Although firmness and colour represent two important quality attributes (Calouro et al. 2008), no relevant changes among orchards were detected (data not shown).
Discussion
High esters, low sugar content, and low organic acids content are often concomitant with the senescence of fruits and, thus, low quality (Zlatić et al. 2016). Esters are responsible for the fruity aroma of pear and reflect their ripening stage (Plotto et al. 1999). According to Zlatić et al. (2016) and Hendges et al. (2018), elevated levels of ethyl are correlated with alcoholic fermentation and over-ripeness. Alcoholic fermentation is associated with the fermentation of sugars, resulting in their consumption and ethanol accumulation (Flikweert et al. 1999). Ethanol is then used in the biosynthesis of ethyl esters (Lara et al. 2003), to promote off-flavor and senescence. Additionally, fermentation is a biological process strongly influenced by the concentration of minerals, particularly Mg, P, K, Fe, and Mn ions (Comelli et al. 2016; Kounbesiou et al. 2010). Additionally, over-ripeness is complemented by the degradation of organic acids, as these metabolites play an essential role in energy generation and are necessary to respond to nutritional deficiency and metal ion stress (Chen et al. 2013). Considering that O3 demonstrated a higher production of esters (Fig. 3), lower TA and malic acid (Fig. 4), and lower sugar content (Fig. 5), which may help in predicting the fastest ripening and quality loss compared to other orchards.
As stated before, the PCA results indicated the connection between ester production and P and K, which was confirmed by the significant correlation between these two parameters. Besides, the negative correlations of SSC and malic acid with P, Ca, and Fe were also demonstrated by Marcelle (1995) in ‘Jonagold’ apples and strengthen the importance of these minerals in fruit quality. P and K are two of the most critical minerals in pears (Casero et al. 2004), because an imbalance of these minerals is generally associated with fruit quality problems. P is involved in several energy metabolic processes responsible for the biosynthesis of several key cellular metabolites that store cellular energy, such as ATP and ADP, which are associated with the appearance of physiological problems and senescence (Mendes 2017; Veltman et al. 2003). It is essential for enzymatic activation, such as carbohydrate catabolism (Walker 2004). It has been shown that a high K concentration in proportion to the Ca concentration increases the susceptibility of fruit to pathogens and senescence (Bramlage 1993; Casero et al. 2004). In fact, fruits from O3 had a K concentration 20 times higher than the Ca concentration.
Thus, the higher concentrations of P, K, and Ca in O3 compared to O1 and O2 may indicate faster senescence and fruit quality loss of O3 associated with the fastest alcoholic fermentation, promoting a higher decrease in sugars and organic acids, a higher increase in ester production since harvest, and persistence throughout ripening.
Cu is a co-factor involved in several redox processes as an activator of several enzymes responsible for metabolic changes during ripening (Broadley et al. 2012). Cu is an important component of ethylene receptors, and plays an important role in ethylene sensitivity at a cellular level (Binder et al. 2010; Hoppen et al. 2019). Thus, the higher Cu concentration in O3 may be associated with higher fruit ethylene sensitivity and faster senescence. It is well established that Ca is involved in retarding ripening and senescence, by decreasing the ACC content, respiration, and ethylene production (Kalcsits 2016; Marcelle 1995). However, this is not clear in this study because O1 and O3 have the highest Ca content but not the lowest ethylene production (Fig. 6), and O1 has a higher respiration rate (Fig. 6).
Furthermore, one of the many roles of Fe is as an intermediate in the formation of reactive oxygen species, causing oxidative damage, and is also involved in alcoholic fermentation. Thus, combining the observation that O3 was the second orchard with the highest Fe concentration with other observations, this orchard may be associated with faster quality deterioration and senescence (Broadley et al. 2012).
Since the three orchards had the same standard soil composition, the combination of this evidence may suggest a different nutrient uptake by fruits from O3, mainly caused by higher tree age. Although all the orchards are in full production, O3 has ten more years than the other two orchards. O3 age can be one of the reasons for the lower quality of fruits. According to other studies, older trees do not show the same nutrient uptake as younger trees (Khalid et al. 2012;Webster 2002). This deficiency influences flowering and fruit quality (Tahir et al. 2007). In addition, the impact of different grower practices, such as irrigation frequency and water availability, can affect the results observed. According to the PDO protocol of 'Rocha' pear, fertigation should be applied to all orchards during fruit development until two weeks before harvest. However, other irrigation periods depend on the water availability. Nevertheless, the postharvest secondary metabolism of pears from O3 seems to have been affected, and consequently, the quality of the pears, mainly in terms of higher acetates release and lower sugars content and acidity. These results suggest that K and P are essential components of fruit, but any excess should be prevented because it negatively affects the postharvest quality of pear fruit.
Fe is an intermediate mineral in several oxidation processes (Broadley et al. 2012), and since it was negatively correlated with SSC, the lower Fe concentration in O2 (Table 1) may be associated with better fruit quality. The higher sugar content and acidity (Fig. 4), and the lower ethylene production and respiration rate observed (Fig. 6) in fruits belonging to O2 may strengthen this association. According to literature, a high K/Ca ratio is associated with good eating quality (Marcelle 1995). In fact, O2 showed the highest K/Ca ratio, which is in agreement with the significant higher acidity and soluble sugars observed (Figs. 4 and 5). However, as previously stated, a high K/Ca ratio is also associated with poor storage quality, mainly the incidence of physiological disorders. Indeed, in a study by Saquet et al. (2019), the ratio value calculated in this study is within the range of disordered 'Rocha' pear after 22 weeks of storage. Sorbitol is a strong chelator of Cu, and the increased levels of sorbitol in O2 and O3 may limit the role of Cu in several redox-systems, such as respiration and protection against oxidative stress (Fields et al. 1989). In this way, O2 and O3´s higher sorbitol levels may be associated with the observed lower respiration (Fig. 6).
In fact, as the orchards belong to the same geographical region and are installed in soil with similar characteristics, there are some external factors, such as light exposure and field inclination, that can influence the behaviour of the orchards and consequently the quality of the fruits. In a study performed on peaches, it was shown that light influenced flavour and fruit quality at harvest within fruits of the same orchard (Génard and Bruchou 1992). Despite being in the same geographical region and under the same climate, the ability of light to penetrate tree canopies can be different and is influenced by pruning, tree spacing, and row orientation canopy architecture, which producers should also be aware of (Bonora et al. 2021) because it can influence fruit maturity and mineral uptake (Musacchi et al. 2021).
However, in the present study, regarding the information obtained from the soil and climate, which were not different among the studied orchard, it is believed that the orchards variability may be mostly related to the tree age differences and to different growers’ practices. The time of application of nutrients and their accumulation in different tree parts can also influence fruit quality, since nutrients can be transported only in subsequent years. Thus, plants respond to both direct and residual fertilization (Zia et al. 2006).
Conclusion
In the current work, general relationships among mineral nutrition and fruit quality were demonstrated with particular emphasis on 'Rocha' pear fruits.
Extreme variability in terms of quality and ripening differences among pears from different orchards in the same geographical region. Different ripening characteristics among pears from the three orchards were related to mineral composition and potentially to slower or faster senescence and quality loss. The impact of minerals on harvest persists throughout ripening, affecting fruit quality.
Because orchards were very similar in terms of soil properties and environmental conditions, agronomic management, including tree age, may explain most of the ripening differences observed among pears. Using PCA, our work highlighted the correlation between fruit quality, senescence, and mineral composition. In general, the factors that contributed the most to the variation among orchards were ester production, sugar concentration, and acidity. These, in turn, were correlated with K and P, which may be used to predict fruit quality and senescence mainly in terms of aroma and sugars concentration.
In our opinion, this study provides helpful information to discriminate fruit quality variations that can be found in orchards from the same location. It was evident that fruit quality was related to mineral nutrition, especially by connecting esters production with mineral concentration. To the best of our knowledge, there is no information regarding the association between aroma and minerals. Thus, it may be useful for fruit growers to understand these associations to contribute for a greater agronomic management and standardization of fruit quality.
Further sensorial analysis will be conducted to link the high production of acetates and lower sugar concentrations with off flavours production and lower quality perceived by consumers.
References
Ali A, Khattak M, Shah S, Shah M, Zaheer S, Bibi S (2014) Comparative effect of foliar application on micronutrients content in peach leaf and fruit. Int J farming allied sci 3:382–388
Binder BM, Rodríguez FI, Bleecker AB (2010) The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. J Biol Chem 285(48):37263–37270. https://doi.org/10.1074/jbc.M110.170027
Bonora A, Muzzi E, Franceschini C, Boini A, Bortolotti G, Bresilla K, …, Grappadelli LC (2021) Preharvest factors affecting quality on “Abate Fetel” pears: study of superficial scald with multivariate statistical approach. J Food Qual 2021:1–11. https://doi.org/10.1155/2021/9921834
Bramlage WJ (1993) Interactions of orchard factors and mineral nutrition on quality of pome fruit. Acta Hortic 326(326):15–28. https://doi.org/10.17660/ActaHortic.1993.326.1
Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F (2012) Function of nutrients. In: Marschner’s Mineral Nutrition of Higher Plants. Elsevier, Amsterdam, p 191–248. https://doi.org/10.1016/B978-0-12-384905-2.00007-8
Brunetto G, Bastos De Melo GW, Toselli M, Quartieri M, Tagliavini M (2015) The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev Bras Frutic 37(4):1089–1104. https://doi.org/10.1590/0100-2945-103/15
Bulens I, Van de Poel B, Hertog ML, De Proft MP, Geeraerd AH, Nicolaï BM (2011) Protocol: an updated integrated methodology for analysis of metabolites and enzyme activities of ethylene biosynthesis. Plant Methods 7(1):17. https://doi.org/10.1186/1746-4811-7-17
Calouro F, Jordão P, Duarte L (2008) Characterization of the mineral composition of pears of the Portuguese cultivar ‘Rocha.’ Acta Hortic 800:587–590. https://doi.org/10.17660/ActaHortic.2008.800.78
Casero T, Benavides A, Puy J, Recasens I (2004) Relationships between leaf and fruit nutrients and fruit quality attributes in golden smoothee apples using multivariate regression techniques. J Plant Nutr 27(2):313–324. https://doi.org/10.1081/PLN-120027656
Chen M, Xie X, Lin Q, Chen J, Grierson D, Yin X, …, Chen K (2013) Differential expression of Organic Acid degradation-related genes during Fruit Development of Navel oranges (Citrus sinensis) in two habitats. Plant Mol Biol Rep 31(5):1131–1140. https://doi.org/10.1007/s11105-013-0583-2
Chiriboga M-A, Schotsmans WC, Larrigaudière C, Dupille E, Recasens I (2013) Responsiveness of ‘Conference’ pears to 1-methylcyclopropene: the role of harvest date, orchard location and year. J Sci Food Agric 93(3):619–625. https://doi.org/10.1002/jsfa.5853
Comelli RN, Seluy LG, Isla MA (2016) Optimization of a low-cost defined medium for alcoholic fermentation – a case study for potential application in bioethanol production from industrial wastewaters. New Biotechnol 33(1):107–115. https://doi.org/10.1016/j.nbt.2015.09.001
Deuchande T, Carvalho SMP, Larrigaudière C, Vasconcelos MW (2017) Mineral concentrations at harvest as novel markers to predict internal browning disorders in ‘Rocha’ pear during storage under high CO2. Sci Hortic 220:102–106. https://doi.org/10.1016/j.scienta.2017.03.037
Fields M, Lewis CG, Beal T (1989) Accumulation of sorbitol in copper deficiency: dependency on gender and type of dietary carbohydrate. Metabolism 38(4):371–375. https://doi.org/10.1016/0026-0495(89)90127-3
Flikweert MT, Kuyper M, van Maris AJA, Kötter P, van Dijken JP, Pronk JT (1999) Steady-state and transient-state analysis of growth and metabolite production in a Saccharomyces cerevisiae strain with reduced pyruvate-decarboxylase activity. Biotechnol Bioeng 66(1):42–50. https://doi.org/10.1002/(SICI)1097-0290(1999)66:1<42::AID-BIT4>3.0.CO;2-L
Génard M, Bruchou C (1992) Multivariate analysis of within-tree factors accounting for the variation of peach fruit quality. Sci Hortic 52(1–2):37–51. https://doi.org/10.1016/0304-4238(92)90006-X
Giné-Bordonaba J, Echeverria G, Ubach D, Aguiló-Aguayo I, López ML, Larrigaudière C (2017) Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol Biochem 111:216–225. https://doi.org/10.1016/j.plaphy.2016.12.002
Hendges MV, Neuwald DA, Steffens CA, Vidrih R, Zlatić E, do Amarante CVT (2018) 1-MCP and storage conditions on the ripening and production of aromatic compounds in conference and Alexander Lucas pears harvested at different maturity stages. Postharvest Biol Technol 146(February):18–25. https://doi.org/10.1016/j.postharvbio.2018.08.006
Hoppen C, Müller L, Hänsch S, Uzun B, Milić D, Meyer AJ, Groth G, Hoppen C, Müller L, Hänsch S, Uzun B, Milić D, Meyer AJ, Weidtkamp-Peters S, Groth G (2019) Soluble and membrane-bound protein carrier mediate direct copper transport to the ethylene receptor family. Sci Rep 9(1):10715. https://doi.org/10.1038/s41598-019-47185-6
Janssens P, Odeurs W, Elsen A, Verjans W, Deckers T, Bylemans D, Vandendriessche H (2015) Relations between taste quality of “Conference” pear and mineral contents in fruit, leaf and soil. Acta Hortic 1094(1094):333–340. https://doi.org/10.17660/ActaHortic.2015.1094.42
Kaiser HF, Rice J (1974) Little Jiffy, Mark Iv. Educ Psychol Meas 34(1):111–117. https://doi.org/10.1177/001316447403400115
Kalcsits LA (2016) Non-destructive measurement of calcium and potassium in apple and pear using handheld X-ray fluorescence. Front Plant Sci 7(APR2016):1–8. https://doi.org/10.3389/fpls.2016.00442
Khalid S, Malik AU, Saleem BA, Khan AS, Khalid MS, Amin M (2012) Tree age and canopy position affect rind quality, fruit quality and rind nutrient content of ‘Kinnow’ mandarin (Citrus nobilis Lour×Citrus deliciosa Tenora). Sci Hortic 135:137–144. https://doi.org/10.1016/j.scienta.2011.12.010
Kounbesiou M, Savadogo A, Barro N, Thonart P, Sabadenedy A (2010) Effect of minerals salts in fermentation process using mango residues as carbon source for bioethanol production. Asian J Ind Eng 3(1):29–38. https://doi.org/10.3923/ajie.2011.29.38
Lara I, Miró RM, Fuentes T, Sayez G, Graell J, López ML (2003) Biosynthesis of volatile aroma compounds in pear fruit stored under long-term controlled-atmosphere conditions. Postharvest Biol Technol 29(1):29–39. https://doi.org/10.1016/S0925-5214(02)00230-2
Lindo-García V, Larrigaudière C, Echeverría G, Murayama H, Soria Y, Giné-Bordonaba J (2019) New insights on the ripening pattern of ‘Blanquilla’ pears: a comparison between on- and off-tree ripened fruit. Postharvest Biol Technol 150(October):112–121. https://doi.org/10.1016/j.postharvbio.2018.12.013
Link H (2000) Significance of flower and fruit thinning on fruit quality. Plant Growth Regul 31(1–2):17–26. https://doi.org/10.1023/a:1006334110068
Marcelle R (1995) Mineral nutrition and fruit quality. Acta Hortic 383:219–226. https://doi.org/10.17660/ActaHortic.1995.383.22
McGuire RG (1992) Reporting of objective color measurements. HortScience 27(12):1254–1255. https://doi.org/10.21273/HORTSCI.27.12.1254
Mendes RDB (2017) Pools de nutrientes em pomares e sua relação com a incidência de acastanhamentos internos em pera “Rocha” Engenharia Agronómica-Especialidade em Hortofrutícultura e Viticultura. Universidade de Lisboa. Retrieved from https://www.repository.utl.pt/bitstream/10400.5/14841/1/Mendes,R.D.2017_VersãoDefinitiva_F.pdf. Accessed Dec 2022
Musacchi S, Iglesias I, Neri D (2021) Training systems and sustainable orchard management for european pear (Pyrus communis L.) in the Mediterranean area: a review. Agronomy 11(9):1765. https://doi.org/10.3390/agronomy11091765
National Association of “Rocha” pear grower (2003) Caderno de especificações Pera Rocha do Oeste. Retrieved on November 2022 from https://ec.europa.eu/info/food-farming-fisheries/food-safety-and-quality/certification/quality-labels/geographical-indications-register/details/EUGI00000013606
Nunnally J (1978) Psychometric theory, 2nd edn. McGraw-Hill, New York
Plotto A, McDaniel MR, Mattheis JP (1999) Characterization of `Gala’ apple aroma and flavor: differences between controlled atmosphere and air storage. J Am Soc Hortic Sci 124(4):416–423. https://doi.org/10.21273/JASHS.124.4.416
Salta J, Martins A, Santos RG, Neng NR, Nogueira JMF, Justino J, Rauter AP (2010) Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars – a comparative study. J Funct Foods 2(2):153–157. https://doi.org/10.1016/j.jff.2010.02.002
Sansavini S, Musacchi S (2002) European pear orchard design and HDP managment: a review. Acta Hortic 596:589–601. https://doi.org/10.17660/ActaHortic.2002.596.103
Saquet A, Almeida D (2017) Internal disorders of ‘Rocha’ pear affected by oxygen partial pressure and inhibition of ethylene action. Postharvest Biol Technol 128:54–62. https://doi.org/10.1016/j.postharvbio.2017.02.005
Saquet AA, Streif J, Almeida DPF (2019) Mineral composition and distribution within ‘Rocha’ pear in relation to internal storage disorders. Postharvest Biol Technol 158(August):111002. https://doi.org/10.1016/j.postharvbio.2019.111002
Sharples RO (1980) The influence of orchard nutrition on the storage quality of apples and pears grown in the United Kingdom. Acta Hortic 92(92):17–28. https://doi.org/10.17660/ActaHortic.1980.92.3
Tagliavini M, Zavalloni C, Rombolà AD, Quartieri M, Malaguti D, Mazzanti F, …, Marangoni B (2000) Mineral nutrient partitioning to fruits of deciduous trees. Acta Hortic (512):131–140. https://doi.org/10.17660/ActaHortic.2000.512.13
Tahir II, Johansson E, Olsson ME (2007) Improvement of quality and storability of apple cv. Aroma by adjustment of some pre-harvest conditions. Sci Hortic 112(2):164–171. https://doi.org/10.1016/j.scienta.2006.12.018
Vanoli M, Buccheri M (2012) Overview of the methods for assessing harvest maturity. Stewart Postharvest Rev 8(1):1–11. https://doi.org/10.2212/spr.2012.1.4
Veltman R, Lenthéric I, Van der Plas LH, Peppelenbos H (2003) Internal browning in pear fruit (Pyrus communis L. cv Conference) may be a result of a limited availability of energy and antioxidants. Postharvest Biol Technol 28(2):295–302. https://doi.org/10.1016/S0925-5214(02)00198-9
Viera W, Winefield C (2019) Genetic parameters for fruit mineral content in an interspecific pear (Pyrus spp.) population. New Z J Crop Hortic Sci 47(2):125–141. https://doi.org/10.1080/01140671.2019.1596958
Walker GM (2004) Metals in yeast fermentation processes. 197–229. https://doi.org/10.1016/S0065-2164(04)54008-X
Webster AD (2002) Factors influencing the flowering, fruit set and fruitgrowth of european pears. Acta Hortic (596):699–709. https://doi.org/10.17660/ActaHortic.2002.596.121
Xuan H, Streif A, Römheld V, Bangerth F (2005) Application of boron with calcium affects respiration and ATP/ADP ratio in ‘Conference’ pears during controlled atmosphere storage. J Hortic Sci Biotechnol 80(5):633–637. https://doi.org/10.1080/14620316.2005.11511990
Zia MH, Ahmad R, Khaliq I, Ahmad A, Irshad M (2006) Micronutrients status and management in orchards soils: applied aspects. Soil and Environ 25(1):6–16
Zlatić E, Zadnik V, Fellman J, Demšar L, Hribar J, Čejić Ž, Vidrih R (2016) Comparative analysis of aroma compounds in ‘Bartlett’ pear in relation to harvest date, storage conditions, and shelf-life. Postharvest Biol Technol 117:71–80. https://doi.org/10.1016/j.postharvbio.2016.02.004
Acknowledgements
Cindy Dias: Conceptualization, Methodology, Data analysis; Writing- original draft; Writing - review & editing, Investigation; Tânia Ribeiro: Data analysis; review; Ana Cristina Rodrigues: Validation; António Ferrante: Supervision, Methodology, Validation; Marta W Vasconcelos: Supervision, Methodology, Validation; Manuela Pintado: Conceptualization, Supervision, Methodology, and Validation.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was co-supported by the European Fund for the Regional Development (FEDER), through the Internationalization and Competitiveness Operational Program (POCI), within the project: RE-EAT ROCHA PEAR (POCI-01-0247-FEDER-040016). We would also like to thank the scientific collaboration under the FCT project UIDB/50016/2020 and FCT individual Ph.D. grant (SFRH/BD/143560/2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Ciro A. Rosolem.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dias, C., Ribeiro, T., Rodrigues, A.C. et al. Relationship between minerals and physicochemical parameters with fruit quality in ‘Rocha’ pear orchards. Plant Soil 496, 243–255 (2024). https://doi.org/10.1007/s11104-023-06137-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06137-w