Abstract
Aims
Fertilisating crops with zinc (Zn) is considered important to enhance agricultural productivity and combat human Zn deficiencies in sub-Saharan Africa. However, it is unclear on which soils Zn fertilisation can lead to higher yields and increased grain Zn concentrations. This study aimed to find soil properties that predict where soil Zn is limiting maize yields and grain Zn concentrations, and where these respond positively to Zn fertilisation.
Methods
Zinc omission trials were set up at multiple farm locations in Kenya (n = 5), Zambia (n = 4) and Zimbabwe (n = 10). Grain yields and tissue Zn concentrations were analysed from plots with a full fertiliser treatment as compared to plots where Zn was omitted.
Results
A positive maize yield response to soil Zn fertilisation was found at only two out of nineteen locations, despite soil Zn levels being below suggested critical concentrations at most locations. Soil properties nor plant concentrations were able to explain maize yield response to Zn fertilisation. However, positive responses in Zn uptake and grain Zn concentrations to Zn fertilisation were found at the majority of sites, especially in soils with low pH and organic carbon contents. Labile soil Zn measurements related more with Zn uptake (R2 = 0.35) and grain Zn concentrations (R2 = 0.26) than actual available Zn measurements.
Conclusions
We conclude that soil Zn fertilisation did not increase maize yields, but can increase maize grain Zn concentrations, especially in soils with low pH and organic carbon content. Predicting a yield response to Zn fertilisation based on soil properties remains a challenge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea Mays) is an important staple crop in sub-Saharan Africa (SSA). It provides a significant proportion of the human daily intake of calories and mineral nutrition (Goredema‐Matongera et al. 2021). The production of maize in SSA is dominated by smallholder farming, generally characterized by little use of inputs on soils with low fertility (Santpoort 2020; Ten Berge et al. 2019). As a result, maize yields are often limited by multiple nutrient deficiencies, which can be addressed by the use of mineral and organic fertilisers (Goredema‐Matongera et al. 2021; Ten Berge et al. 2019; Vanlauwe et al. 2015). It has been recognized decades ago that soils which have been cropped with little or no inputs for prolonged periods lack not only macronutrients but also micronutrients to sustain crop growth (Kang and Osiname 1985; Rodel and Hopley 1972). Nevertheless, soil fertility and crop nutrition research in SSA has mainly focused on macronutrients, i.e. nitrogen (N), phosphorus (P) and potassium (K) (Kihara et al. 2017; Stoorvogel et al. 1993; Vanlauwe et al. 2015). Research on micronutrient deficiencies in crops has received less frequent attention (Mutsaers et al. 2017).
With regard to maize, studies on yield response to micronutrient fertilisation in SSA have often been conducted for only limited sets of locations with either a positive or absent yield response (Abbas et al. 2007; Abunyewa and Mercer-Quarshie 2003; Botoman et al. 2022b; Chiezey 2014; Chilimba et al. 1999; Eteng et al. 2014; Njoroge et al. 2018; Osiname et al. 1973; Shehu et al. 2018; Yerokun and Chirwa 2014). Other studies have focused on the effect of micronutrient fertilisation on yields at the regional or global scale in order to understand where micronutrients may be yield-limiting. In 1990, Sillanpää (1990) published the results of 190 single-micronutrient omission field trials distributed over 15 countries. It was found that among all micronutrients, zinc (Zn) was most of the time yield-limiting, with a positive yield response to Zn fertilisation in 49% of all locations. More recently, maize nutrient omission trials including treatments with a mixture of secondary nutrients and micronutrients have been conducted across various countries in SSA. Kihara et al. (2017, 2016) concluded that application of secondary and micronutrients (calcium (Ca), magnesium (Mg), sulphur (S), boron (B) and zinc (Zn)) increased maize yields in several SSA countries by 0.8 Mg ha−1 on average, an increase of 25% compared to application of NPK alone. Similar results were found by Wortmann et al. (2019), who reported a mean increase in maize yields between 20 and 30% when S, Zn and B were fertilised. These studies suggest that secondary and micronutrient deficiencies limit maize yields across SSA. On the other hand, Rurinda et al. (2020) concluded that the overall maize yield response to secondary and micronutrients (S, Ca, Mg, Zn, and B) was small, i.e. between 0 and 0.3 Mg ha−1, across all studied sites in Nigeria, Tanzania and Ethiopia.
The aforementioned studies by Kihara et al. (2017) and Wortmann et al. (2019) suggest that deficiencies of secondary and micronutrients hamper maize yields across SSA. Since mixtures of secondary and micronutrients were used in these studies, it remains unclear which particular micronutrients are deficient at which locations. Furthermore, using mixtures of nutrients makes it challenging to identify soil properties that explain particular nutrient limitations for maize growth (Kihara et al. 2017). Such analyses are however indispensable for extending existing science-based fertiliser recommendation schemes that currently include only NPK, with secondary and micronutrients (Rurinda et al. 2020; Sattari et al. 2014).
Apart from enhancing yield quantity (Abbas et al. 2007; Abunyewa and Mercer-Quarshie 2003; Kihara et al. 2017; Manzeke et al. 2014; Njoroge et al. 2017; Sillanpää, 1990), Zn is also relevant for human health and insufficient intake can result in severe health issues. More than 17.3% of the global population are prone to insufficient Zn intake (Kiran et al. 2022) and 50% of all children in SSA are estimated to be at risk of Zn deficiency (Black et al. 2008). The risk of human Zn deficiency is considered high especially in Eastern and Southern African countries (Joy et al. 2014). Micronutrient deficiencies in humans are widespread in regions where crops are grown in soils with low micronutrient levels, as soil availability determines plant uptake and therefore micronutrient concentrations in the edible parts of plants (Cakmak 2004; Dimkpa and Bindraban 2016; Gashu et al. 2021; Manzeke et al. 2012). Berkhout et al. (2019) indeed found significant relations between soil concentrations of micronutrients such as Zn and Cu in SSA, and prevalence of child mortality, stunting, wasting and underweight, which are typical health problems associated with micronutrient deficiencies. However, current assessments of possible micronutrient deficiencies among humans are based on standard food composition tables and consequently do not take into account variability in soil properties and associated soil Zn availability, which can significantly affect grain Zn concentrations, and subsequent Zn intake by humans (Gashu et al. 2021; Manzeke et al. 2012). It has been shown that increasing soil Zn availability through fertilisation is a feasible strategy to increase grain Zn concentrations, and thereby reduce the risk for human Zn deficiency (Cakmak 2008; de Valença et al. 2017; Joy et al. 2015; Manzeke et al. 2012), also known as agronomic biofortification (Kiran et al. 2022). Next to soil Zn availability, genetic variation among cultivated maize varieties has great implications on Zn uptake from the soil, and the translocation of Zn to the edible parts (Brkic et al. 2004; Oikeh et al. 2007). Knowledge on the effect of soil properties and maize variety on total Zn uptake and associated grain Zn concentrations, and how these factors affect the effectiveness of agronomic biofortification, can enhance target-based intervention programs to combat human Zn deficiencies.
Soil Zn availability for plant uptake decreases with increasing pH, due to precipitation and increased adsorption to reactive surfaces such as soil organic matter and metal (hydr)oxides (Alloway 2009; Van Eynde et al. 2022). With increased amounts of soil organic matter, the availability of Zn may decrease due to increased adsorption (Van Eynde et al. 2022), or increase due to soil organic matter mineralization (Tella et al. 2016) or formation of soluble organic Zn complexes (Hernandez-Soriano et al. 2013). Different chemical extractions have been used to evaluate soil Zn availability for plant uptake, the associated yield response to Zn fertilisation (Chilimba et al. 1999; Duffner et al. 2013; Lindsay and Norvell 1978; Mertens and Smolders 2013) and Zn concentrations in the edible plant parts (Kihara et al. 2020; Manzeke et al. 2012). For example, a soil test with diethylenetriaminepentaacetic acid (DTPA) as chelating agent is widely used for near-neutral and calcareous soils (Lindsay and Norvell 1978), while others have used acidic soil extracts such as HCl or Mehlich-3 (M3) for more acidic soils (Alloway 2009; Mehlich 1984; Mertens and Smolders 2013). The DTPA and M3 soil extracts are currently most often used for Zn fertiliser recommendations and critical extractable soil Zn levels have been derived below which a positive maize yield response to Zn-fertilisation can be expected. Based on field and greenhouse experiments, these critical soil Zn levels range from 1 – 2.5 mg kg−1 Zn-M3 (Chilimba et al. 1999; Cuesta et al. 2021; Wendt 1995), or 0.5 – 1 mg kg−1 Zn-DTPA (Chilimba et al. 1999; Cuesta et al. 2021; Lindsay and Norvell 1978). Alternatively, weak salt extractions such as 0.01 M CaCl2 have been used for measuring soil available Zn (Houba et al. 2000), assuming that these extractions approximate more the directly available pool for plant uptake (Duffner et al. 2013; Menzies et al. 2007). Validation of soil extracts such as DTPA, M3 or 0.01 M CaCl2 as diagnostic criteria for Zn availability to field-grown maize, however, is limited.
Therefore, this study aims to test whether soil properties can be used to predict where Zn availability is limiting maize yields (quantity) and grain Zn concentrations (quality), and whether the application of Zn fertilisers increases yield quantity and/or quality. Using Zn fertiliser omission trials in several African countries, we aim to test the following hypothesis, namely that crop yield, Zn uptake and grain Zn, and their response to Zn fertilisation, can be predicted based on soil parameters that have been shown to predict Zn in the soil solution: pH, soil organic matter, the Zn quantity, and perhaps metal (hydr)oxides (Van Eynde et al. 2022). Findings from this study will help to understand under which circumstances Zn fertilisation can increase maize yields, as well as grain Zn concentrations in SSA.
Materials and methods
Field trials
Researcher-managed omission trials with maize were executed during one growing season at 19 locations in three countries: Kenya (5 locations with 5 replications), Zambia (4 locations with 4 replications) and Zimbabwe (10 locations with 6 replications). Based on soil maps (Fig. 1), soil Zn levels were expected to be generally low (i.e. below the potentially critical level of 2.5 mg kg−1 Zn-M3; Chilimba et al. 1999) at all locations.
Locations of the field trials in Kenya (A), Zambia (B) and Zimbabwe (C). The maps represent soil Zn concentrations in a Mehlich-3 (Zn-M3) extraction (Hengl et al. 2021)
The Zn fertiliser omission trials were executed as part of a larger experiment, in which zinc, copper and boron fertiliser omissions were studied. The plots with the different treatments were laid out as a randomized block design. In total, 101 blocks were analysed for the effect of micronutrient fertilization on maize. As the focus of this work is on Zn, only details of the relevant treatments are presented. These include a full treatment including all nutrients (hereafter denoted as “Full”) and a Zn omission treatment including all nutrients except Zn (hereafter denoted as “-Zn”).
Maize varieties, planting densities, plot sizes, fertiliser application rates and number of replications, differed between countries based on the availability of resources and local practices. Details of each of these trials are specified below; rainfall data are presented in the supplementary information (Figure S1).
Kenya
Field trials in Kenya were set up in collaboration with the African Plant Nutrition Institute (APNI), Nairobi, Kenya. They were conducted in the long rainy season in 2018 (March — August) at five on-farm locations in Siaya county, Western Kenya (Fig. 1), in the humid cool tropics agroecological zone (Sebastian 2009). The soils of the field trials are classified as Haplic Acrisols (Hengl et al. 2017). During the ten preceding cropping seasons prior to this experiment, no inputs were applied besides chemical N, P and K fertilisers (Njoroge Kinyanjui 2019).
Short season maize variety DK8031 was used at all locations. Two seeds were planted per hole with a plant spacing of 25 cm × 75 cm. Two weeks after emergence the plants were thinned to one plant per planting station, resulting in a final plant density of 53,333 plants ha−1. Plot sizes were 4.5 m × 4.5 m, with five replicates per treatment at each location. Weeding and pest control were done when needed. The following fertiliser application rates (in kg ha−1) were used: 350 N, 180 P, 120 K, 59 Ca, 20 Mg, 31 S, 5 Zn, 5 copper (Cu) and 5 B. Nitrogen was applied as urea in three equal splits, with a basal application during planting, and two topdressings at stages V6 and V10 of plant growth. Phosphorus and Ca were applied as TSP, K as muriate of potash (KCl), Mg and S as MgSO4, Zn as ZnSO4, Cu as CuSO4 and B as Na2B4O7.5(H2O). The P and K fertilisers plus those supplying secondary and micronutrients were applied together in the planting hole during planting. At physiological maturity, a net plot of three rows of 3 m length (6.75 m2) was harvested from the inside of each plot, omitting border plants to avoid edge effects.
Zambia
Field trials in Zambia were set up in collaboration with agricultural students from the Foundations for Cross-cultural Education (FCE) training centre in Zambia. Micronutrient omission trials were conducted from November 2018 – April 2019 at the FCE training centre and at three on-farm locations in surrounding villages in the Masaiti district, Copperbelt, Central Zambia (Fig. 1). These locations are situated in the semiarid cool tropics agroecological zone (Sebastian 2009) and the soils were classified as Haplic Ferralsols (Hengl et al. 2017). At each location, legumes were cultivated in the preceding season. At the training centre, compost manure had been added annually to the field to conserve soil fertility. At the three on-farm locations, no organic or chemical inputs had recently been applied and crop residues had usually been burned in the fields. At each location, the open pollination maize variety Afric1 (Klein Karoo, South Africa) was used. Three seeds were planted per hole with a plant spacing of 60 cm × 75 cm. Two weeks after emergence the plants were thinned to two plants per planting station, resulting in a final plant density of 44,444 plants ha−1. Plot sizes were 4.5 m × 4.2 m. Weeding and pest control were done when needed. The following fertiliser application rates (kg ha−1) were used: 180 N, 35 P, 100 K, 26 Ca, 2.3 Mg, 5.6 S, 3 Zn and 3 B. Nitrogen was applied as urea, as one basal application with the other elements during planting, and two topdressings. Boron was applied as borax, mixed with the urea and applied only during the two topdressings at stages V6 and V10 of plant growth. Phosphorus and Ca were applied as TSP, K as muriate of potash (KCl), Mg as MgSO4 and Zn as ZnSO4. Copper was not applied given the high soil concentrations found during preliminary lab analysis. At physiological maturity, a net plot of 4 rows by 5 plants (9 m2) was harvested from the inside of each plot, omitting border plants to avoid edge effects.
Zimbabwe
Field trials in Zimbabwe were set up in collaboration with the Department of Plant Production Sciences of the University of Zimbabwe (UZ), Harare, Zimbabwe. Micronutrient omission trials were conducted from November 2019 – April 2020 at nine on-farm locations in several villages in the Goromonzi district as well as one location on UZ campus (Fig. 1). All locations are situated in the semiarid cool tropics (Sebastian 2009) and the soils were classified as Haplic Lixisols or Haplic Acrisols (Hengl et al. 2017). At each location, maize was cultivated in the preceding growing season. The selected locations are characterised by low NPK inputs, and at best received cattle manure once every 4 years at a dose of 2–4 Mg ha−1.
At each location, the hybrid maize variety SC637 (SeedCo) was used. Two seeds were planted per hole with a plant spacing of 25 cm × 90 cm. Two weeks after emergence the plants were thinned to one plant per planting station, resulting in a final plant density of 44,444 plants ha−1. Plot sizes were 5.4 m × 4 m. Weeding and pest control were done when needed. The following fertiliser application rates (kg ha−1) were used: 180 N, 80 P, 120 K, 61 Ca, 20 Mg, 26 S, 5 Zn, 5 Cu, 3 B and 0.3 Mn. Nitrogen was applied as ammonium nitrate, one basal application and two topdressings at 4 and 8 weeks after crop emergence. Phosphorus and Ca were applied as TSP, K as muriate of potash (KCl), Mg as MgSO4, Zn as ZnSO4, Cu as CuSO4, Mn as MnCl2 and B as Na2B4O7.5(H2O). All fertilisers were applied together in the planting hole during planting. At physiological maturity, a net plot of 3 rows by 2 m (5.4 m2) was harvested from the inside of each plot, omitting border plants to avoid edge effects.
Field data and sample collection
Field-dry stover and grain biomass were measured for each plot during harvest. A subsample was dried and the biomass measurements were converted to dry biomass. Throughout the manuscript, the grain yield data are reported using a standardized moisture content of 13%.
Composite topsoil samples (0–20 cm) from each block were collected during harvest. Soil samples were taken between the rows, as fertilisers were applied in the planting hole. From each individual plot, a total of 200 g of stover and grains were sampled from several plants. These samples were dried to determine dry matter content and shipped together with the soil samples to the soil chemical laboratory (CBLB, Wageningen, the Netherlands) for further analysis.
Plant analysis
Stover and grain samples were dried at 70 ºC until a constant dry weight was reached, and ground to < 1 mm size before analysis. Stover and grain samples were analysed for N, P, K, S, Mg, Ca, iron (Fe), Zn, B, Cu and manganese (Mn) concentrations. Nitrogen concentrations were measured after a 0.8 M H2SO4/Se/H2O2 digestion (Novozamsky et al. 1983) using a Segmented Flow Analyser. All other elements were extracted based on microwave digestion with concentrated HNO3 (Novozamsky et al. 1983) and measured using Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES, Thermo Scientific iCAP6500) or High Resolution Inductively Coupled Plasma—Mass Spectrometry (HR-ICP-MS, Element 2, Thermo Scientific), depending on their concentrations.
Soil analyses
Soil samples were air-dried and sieved over 2 mm prior to further analysis. Relevant soil properties were chosen based on previously obtained knowledge about the processes controlling soil Zn availability (Van Eynde et al. 2022): reactive surfaces for adsorption (i.e. soil organic matter, dissolved organic matter and micro-crystalline metal (hydr)oxide nanoparticles) and soil pH.
Total soil organic carbon (SOC) content was analysed using a wet oxidation method according to the Kurmies procedure and measured with a spectrophotometer (Walinga et al. 2008). An ammonium oxalate (AO) extraction with a solution-to-solid ratio of 20 L kg−1 and an equilibration time of 4 h (ISO 2012) was used to measure micro-crystalline Fe and Al (Fe-AO, Al-AO). The Fe and Al in the AO extractions were analysed using ICP-OES. Soil pH was measured with a glass electrode in a 0.01 M CaCl2 soil extract, with a solution-to-solid ratio of 10 L kg−1 and an equilibration time of 2 h (Houba et al. 2000). The dissolved total carbon and dissolved inorganic carbon concentrations were measured in the same CaCl2 extract after centrifugation and filtration with a 0.45 µm membrane filter, with a Segmented Flow Analyzer (SFA-TOC, San + + , Skalar) equipped with an IR detector that measures the amount of CO2(g) after an internal acidification and destruction step. The dissolved organic carbon (DOC) concentrations were calculated as the difference between total and inorganic carbon.
Soil Zn was measured in a 0.43 M HNO3 extraction, a Mehlich-3 (M3) extraction, a diethylenetriamine pentaacetate (DTPA) extract and a 0.01 M CaCl2 extraction. The first three tests are expected to approximate the Zn quantity or labile content, while the latter was considered to be an estimation of the intensity or the actual available concentration. The HNO3 soil extraction was done using a solution-to-solid ratio of 10 L kg−1 and an equilibration time of 4 h (ISO 2016). After centrifugation and filtration over a 0.45 µm membrane filter, Zn-HNO3 was measured in the supernatant with ICP-OES. The Zn-M3 was measured with ICP-OES in a centrifuged and filtered (0.45 µm) M3 extract. The M3 extract consisted of 0.1 M CH3COOH, 0.25 M NH4NO3, 0.015 M NH4F, 0.013 M HNO3 and 0.001 M EDTA. Samples were extracted for 5 min with a solution-to-solid ratio of 10 L kg−1 (Mehlich 1984). For the DTPA soil extraction, soils were extracted with a solution-to-solid ratio of 2 L kg−1 and an equilibration time of 2 h, using a solution consisting of 0.005 M DTPA, 0.1 M triethanolamine and 0.01 M CaCl2 that was buffered at a pH of 7.3 (Lindsay and Norvell 1978). The suspensions were centrifuged, filtered over a 0.45 µm membrane filter and analysed for Zn-DTPA using ICP-OES. In the same 0.01 M CaCl2 soil extraction as described before for DOC analysis, Zn-CaCl2 was measured in an acidified (0.14 M HNO3) subsample of the supernatant with HR-ICP-MS.
In addition, soil phosphorus was measured based on an extraction with sodium bicarbonate (Olsen et al. 1954), further denoted as P-Olsen.
Data analysis
The data analysis was done using the R software, version 4.0.2 (R Core Team and R Development Core Team, 2020). Results were visualized with the ggplot2 package (Wickham 2016).
Treatment effects
The effect of fertiliser treatment (i.e. Full and -Zn) on maize grain yields, Zn uptake and Zn grain concentrations was assessed with linear mixed effect models (LME) using the lme function from the nlme package (Pinheiro et al. 2013) with the REML method, and tested by analysis of variance (function Anova). Homogeneity of variances was tested with the Levene’s test, using the leveneTest function from the car package (Fox and Weisberg 2019). Normality of the residuals from the LMEs were checked with the Shapiro–Wilk test using the shapiro.test function from the stats package (R Core Team and R Development Core Team, 2020). This analysis was done for each location, taking all replications into account with treatment as fixed factor, and block as random factor (i.e. random = ~ 1|block). At country level, the differences between locations were also assessed using the same LME model but now with location as additional fixed factor.
The treatment effects on plot-level were also assessed by calculating the empirical cumulative distribution (ecdf function) of the response in yield, Zn uptake and Zn grain concentrations. To do so, the response ratio was calculated for each block as follows:
in which Y represents grain yield, Zn uptake or grain Zn concentrations in the full and the -Zn treatments.
Determination of yield-limiting nutrient
Zinc uptake depends on the soil Zn availability and on the availability of other nutrients. In situations where Zn is the most yield-limiting factor, Zn uptake by a crop equals the amount of Zn that a soil can supply during a growing season (Janssen et al. 1990) and good relations are expected between soil properties and Zn uptake.
Whether Zn is the most yield-limiting nutrient can be assessed based on the yield response to Zn fertilisation, or by the degree of Zn dilution in the maize crop (Janssen et al. 1990; Sattari et al. 2014; Witt et al. 1999). The latter refers to the internal efficiency (IE) of Zn in maize, which is the grain yield produced per amount of nutrient taken up in the above-ground plant biomass in kg dry weight kg−1 Zn (Witt et al. 1999). The IE ranges between a crop and nutrient specific physiological minimum and maximum. When the IE for Zn is close to its maximum, Zn is maximally diluted in the crop, and is most likely to be yield-limiting. The maximum and minimum IE can be derived from the relation between grain yield (kg ha−1) and nutrient uptake (kg nutrient ha−1), using data from large number of field trials (Witt et al. 1999). For the macronutrients N, P and K, these parameters have been derived for maize (Janssen et al. 1990; Sattari et al. 2014). In order to derive the physiological minimum and maximum Zn concentrations for maize, data from field trials in Nigeria (Rurinda et al. 2020) and Zimbabwe (Kurwakumire et al. 2015) were combined with data collected for this study. From these combined data, the upper and lower 2.5% of the datapoints were excluded and then the minimum Zn uptake (r) needed to produce any grain, and the maximum (d) and minimum (a) slopes or IE values were derived (Witt et al. 1999).
Using the IE parameters for N, P, K and Zn in combination with yield and nutrient uptake measurements, the relative dilution of each nutrient can be calculated as follows (Heinen 2020):
With Ui the uptake of nutrient i, Ui,D and Ui,A the uptake of nutrient i at maximum dilution and accumulation respectively, Y the actual yield, d the maximum IE and a the minimum IE and r the minimum nutrient uptake. The principle behind Eq. 2 is to estimate how the actual nutrient uptake differs from the uptake that belongs to the maximum physiological efficiency for the measured yield, relative to the maximum range in uptake. The nutrient for which the value obtained based on Eq. 2 is the smallest, is expected to be the most yield-limiting nutrient. Based on this analysis, a subset was created with only the -Zn plots for which Zn was found to be the most yield-limiting nutrient. This subset was subsequently used to derive soil–plant relations between yield, Zn uptake and grain Zn concentrations and soil properties.
Soil–plant relations
The relation between soil properties and grain yield, Zn uptake and grain Zn concentrations in the -Zn treatments was assessed using LME models based on maximization of the log-likelihood (method ML). This was also done for the relation between soil properties and the response in yield, Zn uptake and Zn grain concentrations. Soil properties were used as fixed effects and the effect of agroecological zone and maize variety were included as random effects, represented by a single variable namely country (i.e. random = ~ 1|Country). The soil properties that were included in the modelling were: pH, Zn-HNO3 Zn-DTPA, Zn-M3, Zn-CaCl2, the sum of Fe and Al in the AO extract and SOC. Since DOC data were not available for the soils in Zimbabwe, this soil property was not included in the analysis. The effect of potentially competing cations such as Cu-M3 and Ca-M3 on Zn uptake and Zn grain concentrations was also tested. The selection of variables was done based on the LME model with the lowest Akaike's Information Criterion (AIC) value (Webster and McBratney 1989) using the dredge function from the MuMIn package (Barton 2020). Normality of the residuals from the final LME model were checked with the Shapiro–Wilk test using the shapiro.test function from the stats package (R Core Team and R Development Core Team, 2020). The dependent and independent variables were log10 transformed when normality of the residuals was violated. Since the Zn measurements in the DTPA, M3 and HNO3 extracts were strongly correlated (see further in the results), the model selection analysis was done with each of these Zn pools separately as input in addition to the other soil properties (i.e. pH, SOC, Fe and Al, Zn-CaCl2), and final models were compared using the anova function. The final LME models were checked for multicollinearity between the independent variables, using the vif function from the car package in R (Fox and Weisberg 2019). The variance explained by the regression models was calculated using the r2 function from the performance package in R (Ludecke et al. 2021), which reports the variance of the fixed effects (R2fixed) and the variance explained by both the fixed and random effects (R2total). The relative contribution of different variables in the LME models to the total variation of the dependent variable, was tested using the r2beta function from the r2glmm package (Jaeger 2017) or the calc.relimp function from the relaimpo package (Gromping 2006) in case no contribution of country as random factor was found in the model.
Results
Soil properties
The soil properties are given in Table 1. The soils in this study are characterized by low SOC contents which do not exceed 20 g kg−1. In addition, the field trials covered a limited range in soil pH between 4 and 6.1. Within countries, the range in pH values was even more limited, with the Kenyan locations covering pH 4.4 – 5.4 and the Zambian locations covering pH 4.5 – 5.7. The pH values of the locations in Zimbabwe ranged between 4.0 and 6.1, covering the entire range in pH values reported in this study.
The soils in this study were characterized by low Zn levels (Table 1). The lowest soil Zn levels were found in Zambia and Zimbabwe, and the highest in Kenya. For the majority of soils, soil Zn levels were below the critical values reported in literature (Fig. 2) and in line with the soil maps (Fig. 1), pointing towards potential Zn deficiency for maize grown in these soils.
The relation between the yield response ratio, and Zn in a Mehlich-3 (A), DTPA (B), HNO3 (C) or 0.01 M CaCl2 (D) extract for all the different replicates for each site. Above the dotted horizontal lines, Zn fertilisation increased yields; below these, it decreased yields. The grey areas in Figures A and B show the range of critical values of Zn-M3 and Zn-DTPA below which it is expected that Zn fertilisation leads to an increase in maize yields (Chilimba et al. 1999; Cuesta et al. 2021; Lindsay and Norvell 1978; Wendt 1995)
Crop responses to Zn fertilisation
Yield
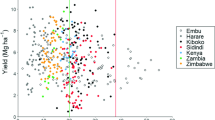
Maize yields ranged between 1.9 and 9.8 Mg ha−1, and were highest in Kenya, followed by Zimbabwe and Zambia (Fig. 3). Across countries, Zn fertilisation led to an average yield increase of 0.03 Mg ha−1 or 4% compared to the -Zn treatment, however, this effect was not significant (p = 0.97). It significantly increased maize yields at two out of 19 locations: one location in Zambia and one in Zimbabwe (Fig. 3). Zinc fertilisation however reduced maize yields at one location in Kenya and one in Zambia. Within each of the countries, significant differences in maize yields among locations were found (Fig. 3). The variation in maize yields was relatively large within a single location and treatment, ranging up to 2.7 Mg ha−1 in Kenya (Fig. 3).
Yields in the full (white) and the -Zn (grey) treatments at five locations in Kenya, four locations in Zambia and ten locations in Zimbabwe. The boxplots show the median (line), first and third quartiles (hinges), the minimum and maximum based on the interquartile range (whiskers) and the outliers (markers). Asterisks indicate a significant treatment effect. Locations within a country with the same letter do not differ significantly (p < 0.05; n = 5 for Kenya, n = 4 for Zambia, n = 6 for Zimbabwe)
The effect of Zn fertilisation was also assessed by calculating the yield response ratio for each block (Eq. 1). The cumulative distribution of this yield response ratio shows that yield responded positively to Zn fertilisation in 47 blocks (48%), while for 52 blocks (52%) a negative response was found (Fig. 4A). The majority of the blocks (61%) had a yield response ratio between 0.8 and 1.2, which may be considered as natural variation, considering the relatively large variation in yield within locations (Fig. 3). Similar results were obtained when looking at the response in total biomass production, i.e. stover and grains (Figure S3 and Figure S4).
The cumulative proportion of blocks in all three countries for the response ratio of yield (A), Zn uptake (B) and grain Zn concentration (C), calculated as the ratio of the full treatment over the -Zn treatment. A response ratio > 1 means a positive response of maize to Zn fertilisation. The horizontal lines and accompanying numbers in the figures show the cumulative proportion of all blocks with a response ratio of 0.8 (dashed), 1 (solid), and 1.2 (dashed), respectively
Zn uptake
Zinc fertilisation led to an average increase in Zn uptake of 177 g ha−1 or 175% compared to the -Zn treatment (p < 0.05). It significantly increased maize Zn uptake at ten out of the 19 locations: one location in Zambia and nine in Zimbabwe (Fig. 5). In Kenya, Zn uptake was rather constant among the five locations, and ranged between 200 and 300 g ha−1 (Fig. 5). In Zambia and Zimbabwe, a wider range in Zn uptake was found and significant differences among locations and treatments were found (Fig. 5). The effect of Zn fertilisation was also assessed by calculating the response ratio of Zn uptake for each block (Eq. 1). The cumulative distribution of this response ratio shows that a positive response in Zn uptake was found for 79% of all blocks (Fig. 4B).
Zn uptake in the full (white) and the -Zn (grey) treatments at five locations in Kenya, four locations in Zambia and ten locations in Zimbabwe. The boxplots show the median (line), first and third quartiles (hinges), the minimum and maximum based on the interquartile range (whiskers) and the outliers (markers). Asterisks indicate a significant treatment effect. Locations within a country with the same letter do not differ significantly (P < 0.05; n = 5 for Kenya, n = 4 for Zambia, n = 6 for Zimbabwe)
Grain Zn
Grain Zn concentrations ranged from 9 to 27 mg kg−1 across the three countries (Fig. 6). Similar to Zn uptake and stover Zn concentrations (Figure S5), grain Zn concentrations varied less in Kenya compared to Zambia and Zimbabwe.
Grain Zn concentrations in the full (white) and the -Zn (grey) treatments at five locations in Kenya, four locations in Zambia and ten locations in Zimbabwe. The boxplots show the median (line), first and third quartiles (hinges), the minimum and maximum based on the interquartile range (whiskers) and the outliers (markers). Asterisks indicate a significant treatment effect. Locations within a country with the same letter do not differ significantly (P < 0.05; n = 5 for Kenya, n = 4 for Zambia, n = 6 for Zimbabwe)
Across countries, Zn fertilisation led to an average increase in grain Zn concentrations of 2.4 mg kg−1 or 20% compared to the -Zn treatment, which had a mean Zn concentration of 14.8 mg kg−1 (p < 0.05). It significantly (p < 0.05) increased maize grain Zn concentrations at nine out of the 19 locations: one in Zambia and eight in Zimbabwe (Fig. 6). Except for one location in Zimbabwe, these were the same locations at which Zn fertilisation increased Zn uptake (Fig. 5). The effect of Zn fertilisation was also assessed by calculating the response ratio of grain Zn concentration for each block (Eq. 1). The cumulative distribution of this response ratio shows that a positive response in grain Zn concentrations was found for 77% of all blocks (Fig. 4C). For the 23% of bocks with a negative response, the ratio varied between 0.8 and 1, which can be considered natural variation.
Determination of yield limiting nutrient
Before studying the soil–plant relations in the next section, we analysed in which plots Zn was the most yield-limiting nutrient since good relations between soil properties and Zn uptake are expected especially in these situations. Based on literature data and results of this study, the maximum and minimum IE of Zn for maize were 71 and 8 kg grain g−1 Zn (Figure S4 and Table S1). Using these parameters, as well as those for N, P and K that have been previously derived (Janssen et al. 1990), the most yield-limiting nutrient was determined (Eq. 2; Fig. 7A). Including both the full and -Zn treatment, Zn appeared to be the most yield-limiting nutrient in 85 blocks (42%), followed by P (67 blocks or 33%), N (33 blocks or 16%) and K (13 blocks or 6%). Of the 101 plots that did not receive any Zn fertiliser, Zn was the most yield-limiting nutrient in 61 blocks (60%) (Fig. 7A). These plots were mainly located in Zimbabwe (38) followed by Kenya (13) and Zambia (10). For the other -Zn plots, a macronutrient was found to be more yield-limiting than Zn, despite the applied NPK fertilisers.
A Grain yield as dependent on Zn uptake. The lines represent the maximum dilution and accumulation of Zn in the maize crop. The colours refer to the most yield-liming nutrient in the full and -Zn treatment. B The yield response ratio in relation to stover Zn concentrations for the 101 plots that did not receive Zn fertilisation. Above the dotted horizontal lines, Zn fertilisation increased yields; below these lines, it decreased yields
Of the 47 blocks with a positive yield response to Zn fertilisation, Zn was identified as the most yield-limiting nutrient in 34 blocks. In the other 27 blocks for which Zn was identified as the most yield-limiting nutrient, a negative yield response was observed. So the degree of Zn dilution in the maize crop was not consistently associated with a positive yield response to Zn fertilisation.
The maximum dilution of Zn in maize was found to be large, as shown by the steep line in Fig. 7A. With a relatively low Zn uptake, yields up to 8 Mg ha−1 were found (Fig. 7A). Low Zn concentrations in maize were found, i.e. below 10 mg kg−1 (Fig. 7B), without finding a clear yield response to Zn fertilisation (Fig. 7B).
Soil properties predicting crop performance and response to Zn fertilisation
For the analyses below, two different subsets are used: the -Zn plots for which Zn was defined as the most yield-limiting nutrient based on the analysis in the previous section (n = 61) and all the -Zn plots (n = 101).
Yield
The best model predicting grain yields for the 61 -Zn plots in which Zn was found to be the most growth- limiting nutrient, included solely pH as fixed variable. Grain yields increased with pH, but the model explained limited variation, as illustrated by low R2 of 0.06 and this model was not significantly different from the model with only the intercept (p = 0.06).
The locations at which Zn fertilisation decreased or increased grain yields could not be separated from the other locations based on soil properties and nutrient concentrations in the maize crop: There was no relation between maize yield response to Zn fertilisation and soil Zn test concentrations in the M3, DTPA, HNO3 or CaCl2 soil extracts (Fig. 2). In the critical range of Zn-M3 and Zn-DTPA concentrations, an equal number of plots showed a positive or a negative response to Zn fertilisation (Fig. 2).
Linear mixed effects modelling was also used to assess the soil properties explaining variation in maize yield response to Zn fertilisation at the block level. Based on model selection with pH, SOC, FeAl-AO, and Zn pools as input parameters, a model with these soil properties was not significantly different from a model with only an intercept (p = 0.06). The residuals of this model were significantly correlated with the grain yield in the -Zn treatments (Pearson correlation coefficient of -0.47, p < 0.05) The plots with low yields in the -Zn treatments, showed a higher response to Zn fertilisation (Fig. 8).
Yields obtained in the full treatment in relation to the yields in the -Zn treatment. The solid black line represents the 1:1 line, the grey line shows the local polynomial regression line using the loess method in R (Vanlauwe et al. 2016)
Zn uptake
The best model predicting Zn uptake in the 61 -Zn plots in which Zn was found to be the most growth- limiting nutrient, included solely Zn-HNO3 as fixed variable. Zinc uptake increased with increased soil Zn-HNO3 concentrations (Fig. 9 and Table 2). None of the other soil parameters explained any additional variation. The Zn-HNO3 explained 35% of the variation in Zn uptake (Table 2). Model residuals were normally distributed (p = 0.9). The effect of country, representing agroecological zone and/or variety, did not explain any variation in Zn uptake, as illustrated by the identical total and fixed R2 (Table 2). The inclusion of possibly competitive nutrients, such as Cu, K or Ca, did not have a significant negative effect on Zn uptake. When the model was applied to all 101 -Zn plots, model coefficients for the intercept and the slope for Zn-HNO3 were similar to those of the model based on the subset of plots for which Zn was found to be the most yield-limiting nutrient (Fig. 9). The relation between Zn uptake and soil Zn-M3 or Zn-DTPA was also significant, in contrast to Zn-CaCl2 (Fig. 9). Based on AIC criteria, the model with Zn-HNO3 as input variable explained more variation than Zn-M3 or Zn-DTPA.
The relation between Zn uptake and Zn measured in the Mehlich-3 (A), DTPA (B), HNO3 (C) and CaCl2 (D) extraction for the plots without Zn fertilisation. The circles show the plots for which Zn was identified as the most yield-limiting nutrient while the triangles show the plots for which N, P or K was identified as most yield-limiting nutrient. The dotted line shows the regression line based on the linear mixed effects model with country as random factor and the respective soil Zn measurement as fixed variable (n = 61), the R2fixed represents the explained variation of the regression model attributed to the soil Zn measurement
With LME models, we assessed the relation between Zn uptake response ratio and soil properties, using country as random variable. The analysis was done for all 101 -Zn plots. The final model explained the Zn uptake response ratio based on pH and SOC (Fig. 10), with pH having a larger contribution in explaining the variation. Model residuals were normally distributed (p = 0.23). The response in Zn uptake to Zn fertilisation was largest in soils with low pH and SOC contents. Country as a random factor contributed to the model, illustrated by a higher total than fixed R2 value (Table 2). With similar pH and SOC levels, the response in Zn uptake to Zn fertiliser is the highest in Zimbabwe, followed by Kenya and Zambia (Fig. 10). This means that underlying variables such as maize variety and/or agro-ecological zone have an effect on the Zn uptake response to Zn fertilisation. Using only the 61 plots for which Zn was identified as the most yield-limiting nutrient instead of all 101 plots, gave a model with similar coefficients for pH and SOC.
The relation between the log10 of the uptake response ratio as explained by Eq. 1, and the pH (x-axis) and soil organic carbon (SOC) content for the locations in Kenya, Zambia and Zimbabwe. A log10 (uptake response ratio) > 0 (dotted line) means a positive response in Zn uptake by maize when Zn fertiliser is applied
Grain Zn
Variation in grain Zn concentrations for the -Zn plots in which Zn was the most yield-limiting nutrient (Sect. 3.2) was best explained by Zn-HNO3, with a positive coefficient (Table 2). Including country as a random variable, the model explained 56% of the variation in grain Zn concentrations. Model residuals were normally distributed (p = 0.5). A model with Zn-M3 or Zn-DTPA instead of Zn-HNO3 as independent variable performed similarly, albeit with a higher AIC value (difference of ~ 2–3). Grain Zn concentrations increased with Zn-HNO3 (Fig. 11). In contrast to what was found for Zn uptake, including country as random variable increased the explained variation in grain Zn concentrations (Table 2 and Fig. 11), indicating that agro-ecological zone, maize variety, or country-dependent management factors affected within-plant Zn allocation to the grain. For a similar soil Zn-HNO3, highest grain Zn concentrations were found in Kenya, followed by Zambia and Zimbabwe. When using all -Zn plots to calibrate the model for grain Zn concentrations, similar coefficients for the intercept and slope were found.
The relation between grain Zn concentrations and Zn measured in the HNO3 extraction. The circles show the plots for which Zn was identified as the most yield-limiting nutrient while the triangles show the plots for which N, P or K was identified as most yield-limiting nutrient. The black solid line shows the linear mixed effects model with parameters for all three countries while the dashed lines show the different intercept of the model that includes country as random factor
When relating the grain Zn concentration response to fertilisation to soil properties (n = 101), a similar model was found as for the response in Zn uptake, namely a negative contribution of SOC and soil pH to the response in grain Zn concentrations (Table 2). Model residuals were normally distributed (p = 0.4). There was a contribution of country as random variable, with the highest increase in Zn grain concentrations for Zimbabwe, followed by Kenya and Zambia, i.e., the same order as found for Zn uptake.
Discussion
Zn fertilisation does not result in higher maize yields
Field trials on 19 locations in three different countries showed that Zn fertilisation led to significant increases in maize yields at only two locations. These two locations did not differ from the other locations in terms of soil properties, nutrient uptake or tissue nutrient concentrations. A positive yield response above 20% to Zn fertilisation was only observed for a minority of all replicates.
The lack of yield responses to fertilisation was not expected, given that the majority of these locations had soil Zn levels below critical values reported in literature. Critical soil Zn levels, below which a positive maize yield response to Zn fertilisation is expected, have been derived previously from field and greenhouse experiments (Chilimba et al. 1999; Cuesta et al. 2021; Lindsay and Norvell 1978; Wendt 1995). Both positive and negative yield responses were found for soils that had soil Zn concentrations below these critical levels (Fig. 2). In addition, no extraction method was capable of predicting the yield response to Zn fertilisation (Fig. 2). This result points towards the challenges associated with the use of soil extractions as diagnostic criteria for nutrient deficiencies and corresponding fertiliser recommendations (Schut and Giller 2020).
The relative dilution of Zn in maize was also found to be a poor indicator of yield response to Zn fertilisation. For the majority of the -Zn plots, Zn was found to be highly diluted in the maize crop, approaching its maximal IE indicating Zn may have been yield-limiting. This was however not the case, since no positive response to Zn fertilisation was observed. One explanation may be that other factors and/or nutrients may be still more growth-limiting than Zn, despite low soil Zn levels and despite the applied fertilisation with a range of macro-and micronutrients. Another explanation can be that this IE approach may not be suitable for Zn. Generally, it has been shown that Zn deficiency in maize rapidly decreases with increasing Zn availability, after which the yields remain constant with increasing Zn supply (Singh and Banerjee 1987). This may suggest that a critical threshold may exist, above which Zn uptake does not determine maize grain yields but that Zn uptake is merely driven by grain (and stover) yields, despite strong Zn dilution in the crop. Our results have shown that Zn can indeed be highly diluted in the crop, resulting in tissue Zn concentrations below 10 mg kg−1 which is lower than previously reported critical tissue concentrations (Reuter and Robinson 1997; Singh and Banerjee 1987). It must be noted that these critical tissue concentrations have often been derived based on measurements of plant parts during the growing season and not after harvest. Tissue concentrations measured in this study were, however, found to be a poor indicator of a positive yield response to Zn fertilisation (Fig. 7).
The most important diagnostic criteria of Zn deficiency for maize growth that can be derived from this study, is the yield in the control treatment that received optimal fertilisation with macro-and micronutrients except Zn. This effect of yield in the control treatment on the yield response has been previously observed when soil fertility treatments were tested in the field (Ichami et al. 2019; Vanlauwe et al. 2016). Generally, the plots with yields below ~ 6 Mg ha−1, had the highest probability for a positive yield response to Zn fertilisation (Fig. 8). Kihara et al. (2017) reported a similar trend for the results from field trials in various SSA countries, where the yield response to secondary and micronutrients decreased with increasing maize yields in the plots that received only NPK fertilisers. Combining literature data from field trials in SSA countries (Kihara et al. 2017, 2016; Rurinda et al. 2020) shows that the yield level below which there is a high probability that maize shows a positive response to secondary and micronutrients also points at a diagnostic threshold value of around 6 Mg ha−1 (Figure S5), similar to what has been found in this study (Fig. 8). In addition, previous studies with Zn omission trials in SSA that reported a positive yield response of maize to Zn fertilisation, are also characterized by relatively low yields compared to the yields in this study (i.e. below 6 Mg ha−1) in the plots receiving no Zn fertiliser (Abbas et al. 2007; Eteng et al. 2014; Manzeke et al. 2014; Nziguheba et al. 2009).
The application of NPK fertilisers was given to all treatments in this study and the quantities, ranging between 180–350 kg N ha−1, 35–180 kg P ha−1 and 100–120 kg K ha−1, were relatively high in comparison with (for example) Kihara et al. (2016). These high NPK doses, in combination with a range of secondary and other micronutrients, were applied in order to assure that other nutrients than Zn were not yield-limiting and that possible Zn deficiencies would become visible. However, these high quantities of NPK fertilisers could also have masked the incidence of micronutrient deficiencies, such as Zn. For example, the work of Manzeke et al. (2014) in Zimbabwe showed that maize yields responded less to Zn fertilisation when larger quantities of N and P were applied. The application of relatively high amounts of macronutrient and secondary fertilisers in our study may have led to healthier crops, whose root systems were able to explore a larger soil volume. This may have led to sufficient Zn uptake even when Zn was not fertilised and despite low soil Zn availability as previously suggested by Pasley et al. (2019) based on field trials in Kenya and Zimbabwe. Others have also demonstrated the significant response in root traits and associated increased Zn uptake, due to macronutrient fertilisation (Ma et al. 2014), but the underlying processes are still unclear.
Since the experiments were only run for one growing season, we cannot draw any conclusions on the residual effects of the applied fertiliser Zn. However, since most studies show that the recovery of soil Zn fertiliser decreases in time (Boawn 1974), we do not expect an effect of yield due to residual Zn fertiliser during subsequent growing seasons.
It has been stated that the supply of secondary and micronutrients is vital for enhancing agricultural productivity in SSA (Kihara et al. 2017; Wortmann et al. 2019). However, these conclusions are based on an average positive maize yield response to secondary and micronutrient fertilisation. One may argue whether it is justified to use an average yield response as a basis for such recommendations (Vanlauwe et al. 2016), given the large variation in yield responses found in these studies (Figure S6) as well as for the 19 locations in this study and the relatively low chance of a positive yield response to Zn fertilisation (Fig. 4). In addition, caution should be made when such averages are used to calculate the economic return of micronutrient fertiliser application (Kihara et al. 2020), given the high probability for absent or even negative yield responses as illustrated by our results (Fig. 4).
Soil Zn availability
Soil Zn availability was measured using four different extractions, namely DTPA, HNO3, M3 and CaCl2. The first three extraction methods are considered to approximate the available Quantity (Q), which represents the directly available Zn in the soil solution as well as the Zn adsorbed to the soil solid particles, which can become available throughout a growing season (Groenenberg et al. 2017; Lindsay and Norvell 1978; Mehlich 1984; Robson 1993). The CaCl2 solution is more related to the Intensity (I) or the Zn in the solution phase, which represents the Zn directly available for plant uptake (Houba et al. 2000). The Zn concentrations measured in DPTA, HNO3 and M3 soil extracts were strongly correlated (Figure S2). The Zn measured in CaCl2 can be derived from the Zn measured in HNO3 and soil pH (Van Eynde et al. 2022).
The results from our study have shown that Q-tests, and more specifically the HNO3 extraction, performs best in quantifying the soil available Zn for the unfertilised plots, based on the significant relation with Zn uptake (Fig. 9). In terms of practical applications, this is a promising result, since soil Zn data available for SSA mostly comprises Q-tests (Hengl et al. 2021) rather than I-tests (Keskinen et al. 2019).
In literature, contrasting results have been found on whether I-or Q-tests are the best approximation of the soil available Zn content. Based on a review, Kim et al. (2015) recommended the use of I-tests to quantify bioavailability of relatively mobile metals such as Zn in contaminated soils, in line with other studies (Impellitteri et al. 2003; Nolan et al. 2005). For low Zn soils, both Q-tests (Tian et al. 2008) and I-tests (Duffner et al. 2013; Menzies et al. 2007) have been found to be related to plant Zn concentrations and uptake. Due to the relatively low pH and low SOC content of the soils in this study, the adsorption affinity for Zn in the solid phase is relatively low as illustrated by the fact that a large proportion of the Zn measured in the HNO3 is also extracted by the CaCl2 solution (Table 1). This low adsorption affinity for Zn also explains the significant response in Zn uptake and grain Zn concentrations since fertilised Zn is readily available as it stays in solution (see next section). In terms of Zn adsorption affinity, the soils from this study differ from the typical calcareous soils that are often associated with Zn deficiency, as investigated for example by Duffner et al. (20142013). Their soils are characterized by a pH above 6, and by higher Zn-HNO3 concentrations (up until 318 µmol kg−1) and lower Zn-CaCl2 concentrations (mostly below 1 µmol kg−1) than found for the soils from this study (Table 1). In soils with high Zn adsorption affinity, Duffner et al. (2013) found that Zn-CaCl2, in combination with pH-CaCl2, related better with Zn shoot concentrations in wheat than Zn-DTPA. We hypothesize that the relatively low adsorption affinity for Zn in the soils in this study explains why Q-tests relate better with Zn uptake than I-tests, similar to what has been found and discussed previously for phosphorus by Nawara et al. (2017). The low adsorption affinity may imply that not the concentration in the soil solution (~ Zn-CaCl2) is limiting Zn uptake by maize, but the buffering capacity of the soil to provide Zn to maize during the whole growing season, as reflected by Zn-HNO3 (or DTPA or M3).
Soil Zn-HNO3 explained only 35% of the variation in the total Zn uptake for the plots receiving no Zn fertiliser (Fig. 9). The relatively low explanatory power for Zn uptake may be attributed to the fact that Zn was in many cases not the most yield-limiting factor, as illustrated by the absence in yield response to Zn fertilisation and the IE analysis. In addition, soil Zn concentrations from samples taken at lower depth could have given more information about Zn availability, in line with the previously suggested hypothesis about the maize root system. No clear effect of country or maize variety was found on Zn uptake. However, differences in Zn uptake among maize varieties have been reported earlier (Bender et al. 2013).
Response in Zn uptake
Our data show that the Zn uptake response to fertilisation decreases with pH and SOC content. Previous studies have shown the importance of pH and SOC for the solid-solution partitioning of Zn in similar soils from SSA countries, with SOC being the most important adsorption surface and a strong increase in Zn adsorption with increasing pH (Groenenberg et al. 2017; Van Eynde et al. 2022). Our results indicate that the highest increase in Zn uptake can be expected in soils with a low adsorption capacity (i.e. where fertiliser-Zn remains mainly in solution). However, for the response in Zn uptake, a contribution of country as a random factor was found, with the highest response in Zimbabwe (Fig. 10 and Table 2). There may be several explanations for this observation, as country represents differences in both maize variety as well as in agro-ecological zone. At low Zn availability (i.e. in the unfertilised plots), the three different varieties may explore the same volume of soil for Zn, and effectively translocate this Zn supply from the root to the shoots. In a situation of excess Zn (i.e. in the fertilised plots), varieties can differ in the reduction of active Zn transport from roots to shoots, and may be less effective in coping with relatively higher Zn tissue concentrations (White and Broadley 2011). Next to variety, the agro-ecological zone may play a role in the Zn uptake response ratio. Not only soil properties, but weather events associated with the different agroecological zones may also affect the fertiliser use efficiency. Analysis of the rainfall data (Figure S1) shows that the cumulative rainfall surplus is the highest in Kenya (348 mm), and the lowest in Zimbabwe (144 mm). The latter may result in a higher nutrient use efficiency of fertiliser Zn due to reduced leaching in these soils with relatively low Zn adsorption capacity, explaining the higher Zn uptake response ratio in Zimbabwe.
Grain Zn concentrations
Soil Zn availability (Zn-HNO3) only explained 20% of the variation in grain Zn concentrations in the -Zn plots, compared to 35% of the variation in Zn uptake. In contrast to Zn uptake, maize variety and/or agro-ecological zone, represented by the random country variable, significantly contributed to the model. Although the effect of variety and agro-ecological zone cannot be separated, both effects are feasible. Strong variation in maize grain Zn concentrations, ranging from 4 – 96 mg kg−1, have been found among genotypes (Prasanna et al. 2020). In addition, environmental factors can also affect grain Zn concentrations. For example, it has been shown that the maximum grain Zn concentration can be increased by increasing N availability (Manzeke et al. 2020).
Gashu et al. (2021) collected around 2000 maize samples in Malawi and Ethiopia and found that grain Zn concentrations increased with increasing soil pH and SOC content. With regard to SOC, our results are in agreement with those from Gashu et al. (2021). In our study, Zn-HNO3 was the most important variable explaining grain Zn concentrations and Zn-HNO3 was strongly correlated with SOC (Figure S2). However, the relationships between grain Zn and soil properties found by Gashu and other authors (Bevis and Hestrin 2021; Gashu et al. 2021) were not always clear and straightforward, as opposite trends were found. Based on our findings in this study, we question the general feasibility of using soil properties as proxy for grain Zn concentrations and the associated likelihood of human Zn deficiencies, since soil properties only explained 26% of the variation in grain Zn concentrations. Giller and Zingore (2021) posed the same question after reading the study by Gashu et al. (2021), particularly with regard to challenges associated with estimating soil bioavailable Zn and the effect of management practices that may weaken the relation between soil and grain Zn concentrations. Our analyses raise additional challenges with regard to this question. First of all, soil–plant relations in terms of Zn uptake may only be significant when Zn is the most yield-limiting nutrient (Janssen et al. 1990). Secondly, we have shown that there is a strong effect of variety and/or agro-ecological zone on grain Zn concentrations. Thirdly, tissue concentrations such as grain Zn, may also depend on the relative dilution of Zn in the maize, and thus on the availability of other nutrients that affect biomass production. These factors may all lead to a weak relationship between soil properties and grain Zn concentrations, thus challenging the assignment of areas with high risk of Zn deficiency in humans due to low grain Zn concentrations based only on soil properties (Botoman et al. 2022a).
Agronomic biofortification
Goredema‐Matongera et al. (2021) argued that the application of soil Zn fertilisers may benefit the crop by increasing its yield, but without increasing grain Zn concentrations, because of the low soil Zn content for most countries in SSA. Based on our results, we argue that the opposite is true, and that the application of Zn fertilisers may be beneficial for grain Zn content while it does not increase yields despite low soil Zn levels. Our results have demonstrated that the application of 5 kg ha−1 Zn fertiliser can lead to an average increase of 20% in grain Zn concentrations. This finding is in line with a review of Joy et al. (2015), who found an average increase in maize grain Zn concentration of 28% with fertilisation of ~ 16 kg ha−1 Zn. In general, this increase in grain Zn concentrations of maize is lower than what has been found for other cereal crops (Cakmak and Kutman 2018). However, despite Zn fertilisation, grain Zn concentrations were still below the target level of 38 mg kg−1 of the HarvestPlus program to combat human Zn deficiencies(Bouis and Welch 2010). We have shown that soil properties affect the effectiveness of agronomic biofortification through soil fertilisation. The increase in Zn grain concentrations by fertilisation was the largest for soils with low pH and SOC content, similarly as found for Zn uptake. Next to soil properties that are related to the adsorption of fertiliser-Zn, country as random variable was also found to affect agronomic biofortification, with the largest increase in grain Zn concentrations found in Zimbabwe (Table 2). Similarly as discussed for uptake, this result can be explained by variety and/or agro-ecological zone effects. Finally, the effectiveness of agronomic biofortification may also depend on the availability of other nutrients, such as nitrogen (Manzeke et al. 2020; Pasley et al. 2019) and phosphorus (Amanullah et al. 2020). The results of this study provide important information for future assessment of environmental influences on agronomic biofortification with new high Zn varieties.
Conclusions
Zinc fertilisation did not lead to higher yields in 17 out of 19 sites in sub-Saharan Africa. It requires further research to find out why an increase in Zn uptake does not generally lead to higher yields, even when Zn is strongly diluted in the maize crop. Conclusions with regard to micronutrient fertilisation should not be based on average yield responses, given the large variability that was observed in this study and previous work.
The application of Zn fertilisers can be a feasible strategy to combat human Zn deficiencies in communities that are heavily reliant on maize as a staple crop since we found that Zn fertilisation improved Zn uptake and grain Zn concentrations. However, grain Zn concentrations were still below target values, pointing towards the use of more efficient fertiliser strategies such as foliar application when improvement of the nutritional quality is the main objective.
Existing critical levels based on commonly used soil tests, such as DTPA and Mehlich-3, failed to predict Zn deficiencies and a positive yield response of maize to Zn fertilisation. Other soil extractions such as a 0.43 M HNO3 or 0.01 M CaCl2 were also not able to predict maize yield responses to Zn fertilisation.
Soil tests could reasonably predict Zn uptake, albeit that only 40% of the variation was explained. Soil tests that measured the Zn quantity performed better in predicting Zn uptake than soils tests that measure the Zn intensity. We explained this observation by the relatively low adsorption affinity for Zn of the soils used in this study. The response in both grain Zn concentrations and Zn uptake to Zn fertilisation was explained by the soil properties associated with the Zn adsorption affinity of these soils, namely soil organic carbon and pH. Grain Zn concentrations were found to be less related to soil properties than aboveground Zn uptake, with only 20% explained by the soil Zn levels estimated by a 0.43 M HNO3 extraction. An effect of variety and/or agroecological zone was found to contribute to the variation in grain Zn levels, but not Zn uptake. Our results show that the identification of areas in which crop and human Zn deficiencies may be problematic, based on soil properties, remains challenging.
Data availability
Data available on demand.
References
Abbas AE, Hamad ME, Babiker HM, Nour AE (2007) Effects of added phosphorus and zinc on yield and its components of corn. Gezira J Agric Sci 5(2)
Abunyewa A, Mercer-Quarshie H (2003) Response of Maize to Magnesium and Zinc Application in the Semi Arid Zone of West Africa. Asian J Plant Sci 3:1–5. https://doi.org/10.3923/AJPS.2004.1.5
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548. https://doi.org/10.1007/S10653-009-9255-4
Alwahibi MS, Elshikh MS, Alkahtani J, Muhammad A, Khalid S, Ahmad M, Khan N, Ullah S, Ali I (2020) Phosphorus and zinc fertilization improve zinc biofortification in grains and straw of coarse vs. fine rice genotypes. Agron 10:1155. https://doi.org/10.3390/AGRONOMY10081155
Barton K (2020) MuMIn: Multi-model inference. R Packag Version 1(43):17
Bender RR, Haegele JW, Ruffo ML, Below FE (2013) Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron J 105:161–170. https://doi.org/10.2134/AGRONJ2012.0352
Berkhout ED, Malan M, Kram T (2019) Better soils for healthier lives? An econometric assessment of the link between soil nutrients and malnutrition in Sub-Saharan Africa. PLoS One 14:e0210642. https://doi.org/10.1371/journal.pone.0210642
Bevis LE, Hestrin R (2021) Widespread heterogeneity in staple crop mineral concentration in Uganda partially driven by soil characteristics. Environ Geochem Health 43:1867–1889. https://doi.org/10.1007/S10653-020-00698-W/TABLES/19
Black R, Allen L, Bhutta Z, Caulfield L (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371:243–260
Boawn LC (1974) Residual Availability of Fertilizer Zinc. Soil Sci Soc Am J 38:800–803. https://doi.org/10.2136/SSSAJ1974.03615995003800050031X
Botoman L, Chagumaira C, Mossa AW, Amede T, Ander EL, Bailey EH, Chimungu JG, Gameda S, Gashu D, Haefele SM, Joy EJM, Kumssa DB, Ligowe IS, McGrath SP, Milne AE, Munthali M, Towett E, Walsh MG, Wilson L, Young SD, Broadley MR, Lark RM, Nalivata PC (2022) Soil and landscape factors influence geospatial variation in maize grain zinc concentration in Malawi. Sci Rep 121(12):1–13. https://doi.org/10.1038/s41598-022-12014-w
Botoman L, Chimungu JG, Bailey EH, Munthali MW, Ander EL, Mossa A, Young SD, Broadley MR, Lark RM, Nalivata PC (2022b) Agronomic biofortification increases grain zinc concentration of maize grown under contrasting soil types in Malawi. Plant Direct 6:e458. https://doi.org/10.1002/PLD3.458
Bouis HE, Welch RM (2010) Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci 50:S20–S32. https://doi.org/10.2135/cropsci2009.09.0531
Brkic I, Simic D, Zdunic Z, Jambrovic A, Ledencan T, Kovacevic V, Kadar I (2004) Genotypic variability of micronutrient element concentrations in maize kernels. Cereal Res Commun 32:107–112
Cakmak I (2008) Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 302:1–17. https://doi.org/10.1007/S11104-007-9466-3/FIGURES/9
Cakmak I (2004) Identification and correction of widespread zinc deficiency in Turkey: a success story. International Fertiliser Society, p 28
Cakmak I, Kutman UB (2018) Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci 69:172–180
Chiezey UF (2014) Field Performance of Quatity Protein Maize With Zinc and Magnesium Fertilizers in the Sub-Humid Savanna of Nigeria. J Agric Sci 6:84. https://doi.org/10.5539/JAS.V6N3P84
Chilimba ADC, Mughogho SK, Wendt J (1999) Mehlich 3 or Modified Olsen for soil testing in Malawi. Commun Soil Sci Plant Anal 30:1231–1250. https://doi.org/10.1080/00103629909370280
Cuesta NM, Wyngaard N, SaínzRozas H, ReussiCalvo N, Carciochi W, Eyherabide M, Colazo JC, Barraco M, Guertal EA, Barbieri P (2021) Determining Mehlich-3 and DTPA extractable soil zinc optimum economic threshold for maize. Soil Use Manag 37:736–748. https://doi.org/10.1111/sum.12657
de Valença AWW, Bake A, Brouwer IDD, Giller KEE (2017) Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob Food Sec 12:8–14. https://doi.org/10.1016/j.gfs.2016.12.001
Dimkpa CO, Bindraban PS (2016) Fortification of micronutrients for efficient agronomic production: a review. Agron Sustain Dev 36:7. https://doi.org/10.1007/s13593-015-0346-6
Duffner A, Hoffland E, Weng L, van der Zee SEATM (2013) Predicting zinc bioavailability to wheat improves by integrating pH dependent nonlinear root surface adsorption. Plant Soil 373:919–930. https://doi.org/10.1007/s11104-013-1845-3
Duffner A, Weng L, Hoffland E, van der Zee SEATM (2014) Multi-surface modeling to predict free zinc ion concentrations in low-zinc soils. Environ Sci Technol 48:5700–5708. https://doi.org/10.1021/es500257e
Eteng EU, Asawalam DO, Ano AO (2014) Effect of Cu and Zn on Maize (<i>Zea mays</i> L.) Yield and Nutrient Uptake in Coastal Plain Sand Derived Soils of Southeastern Nigeria. Open J Soil Sci 04:235–245. https://doi.org/10.4236/OJSS.2014.47026
Fox J, Weisberg S (2019) An R companion to Applied Regression, Third, Edit. Sage, Thousand Oaks CA
Gashu D, Nalivata PC, Amede T, Ander EL, Bailey EH, Botoman L, Chagumaira C, Gameda S, Haefele SM, Hailu K, Joy EJMM, Kalimbira AA, Kumssa DB, Lark RM, Ligowe IS, McGrath SP, Milne AE, Mossa AW, Munthali M, Towett EK, Walsh MG, Wilson L, Young SD, Broadley MR (2021) The nutritional quality of cereals varies geospatially in Ethiopia and Malawi. Nature 594:71–76. https://doi.org/10.1038/s41586-021-03559-3
Giller KE, Zingore S (2021) Mapping micronutrients in grain and soil unearths hidden hunger in Africa. Nat 594:31–32. https://doi.org/10.1038/D41586-021-01268-5
Goredema-Matongera N, Ndhlela T, Magorokosho C, Kamutando CN, van Biljon A, Labuschagne M (2021) Multinutrient Biofortification of Maize (Zea mays L) in Africa: Current Status. Oppor Limitations Nut 13:1039. https://doi.org/10.3390/NU13031039
Groenenberg JE, Römkens PFAM, Zomeren AV, Rodrigues SM, Comans RNJ (2017) Evaluation of the single dilute (0.43 M) nitric acid extraction to determine geochemically reactive elements in soil. Environ Sci Technol 51:2246–2253. https://doi.org/10.1021/acs.est.6b05151
Gromping U (2006) Relative Importance for Linear Regression in R: The package relaimpo. J Stat Softw 17:1–27
Hengl T, Mendes de Jesus J, Heuvelink GBM, Ruiperez Gonzalez M, Kilibarda M, Blagotić A, Shangguan W, Wright MN, Geng X, Bauer-Marschallinger B, Guevara MA, Vargas R, MacMillan RA, Batjes NH, Leenaars JGB, Ribeiro E, Wheeler I, Mantel S, Kempen B (2017) SoilGrids250m: Global gridded soil information based on machine learning. PLoS One 12:e0169748. https://doi.org/10.1371/journal.pone.0169748
Hengl T, Miller MAE, Križan J, Shepherd KD, Sila A, Kilibarda M, Antonijeví O, Glušica L, Glušica G, Dobermann A, Haefele SM, Mcgrath SP, Acquah GE, Collinson J, Parente L, Sheykhmousa M, Saito K, Johnson J-M, Chamberlin J, Silatsa FBT, Yemefack M, Macmillan RA, Wheeler I, Crouch J (2021) African Soil Properties and Nutrients Mapped at 30-m Spatial Resolution using Two-scale Ensemble Machine Learning. Sci Rep 11:6130
Hernandez-Soriano MC, Peña A, Mingorance MD (2013) Soluble metal pool as affected by soil addition with organic inputs. Environ Toxicol Chem 32:1027–1032. https://doi.org/10.1002/ETC.2159
Houba VJG, Temminghoff EJM, Gaikhorst GA, van Vark W (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal 31:1299–1396
Ichami SM, Shepherd KD, Sila AM, Stoorvogel JJ, Hoffland E (2019) Fertilizer response and nitrogen use efficiency in African smallholder maize farms. Nutr Cycl Agroecosystems 113:1–19
Impellitteri CA, Saxe JK, Cochran M, Janssen GMCM, Allen HE (2003) Predicting the bioavailability of copper and zinc in soils: modeling the partitioning of potentially bioavailable copper and zinc from soil solid to soil solution. Environ Toxicol Chem 22:1380–1386
ISO (2016) Soil quality 17586:2016 - Extraction of trace elements using dilute nitric acid
ISO (2012) ISO 12782–3:2012 Soil quality - Parameters for geochemical modelling of leaching and speciation of constituents in soils and materials - Part 3: Extraction of aluminium oxides and hydroxides with ammonium oxalate/oxalic acid
Jaeger B (2017) r2glmm: Computes R Squared for Mixed (Multilevel) Models. R package version 0.1.2. [WWW Document]. URL https://cran.r-project.org/package=r2glmm
Janssen BH, Guiking FCT, van der Eijk D, Smaling EMA, Wolf J, van Reuler H (1990) A system for quantative-evaluation of the fertility of tropical soils (QUEFTS). Geoderma 46:299–318. https://doi.org/10.1016/0016-7061(90)90021-Z
Joy EJM, Ander EL, Young SD, Black CR, Watts MJ, Chilimba ADC, Chilima B, Siyame EWP, Kalimbira AA, Hurst R, Fairweather-Tait SJ, Stein AJ, Gibson RS, White PJ, Broadley MR (2014) Dietary mineral supplies in Africa. Physiol Plant 151:208–229. https://doi.org/10.1111/PPL.12144
Joy EJM, Stein AJ, Young SD, Ander EL, Watts MJ, Broadley MR (2015) Zinc-enriched fertilisers as a potential public health intervention in Africa. Plant Soil 389:1–24. https://doi.org/10.1007/s11104-015-2430-8
Heinen M (2020) Wageningen Environmental Research
Kang BT, Osiname OA (1985) Micronutrient problems in tropical Africa. Fertil Res 7:131–150
Keskinen R, Nyambura M, Heikkinen J, Sila A, Eurola M, Towett E, Shepherd K, Esala M (2019) Readily available concentrations of selected micronutrients and harmful metals in soils of Sub-Saharan Africa. Geoderma 347:203–209. https://doi.org/10.1016/J.GEODERMA.2019.04.014
Kihara J, Bolo P, Kinyua M, Rurinda J, Piikki K (2020) Micronutrient deficiencies in African soils and the human nutritional nexus: opportunities with staple crops. Environ Geochem Health 42:3015–3033. https://doi.org/10.1007/s10653-019-00499-w
Kihara J, Nziguheba G, Zingore S, Coulibaly A, Esilaba A, Kabambe V, Njoroge S, Palm C, Huising J (2016) Understanding variability in crop response to fertilizer and amendments in sub-Saharan Africa. Agric Ecosyst Environ 229:1–12. https://doi.org/10.1016/j.agee.2016.05.012
Kihara J, Sileshi GW, Nziguheba G, Kinyua M, Zingore S, Sommer R (2017) Application of secondary nutrients and micronutrients increases crop yields in sub-Saharan Africa. Agron Sustain Dev 37:25. https://doi.org/10.1007/s13593-017-0431-0
Kim R-Y, Yoon J-K, Kim T-S, Yang JE, Owens G, Kim K-R (2015) Bioavailability of heavy metals in soils: definitions and practical implementation—a critical review. Environ Geochem Health 37:1041–1061. https://doi.org/10.1007/s10653-015-9695-y
Kiran A, Wakeel A, Mahmood K, Mubaraka R, Haefele SM (2022) Biofortification of Staple Crops to Alleviate Human Malnutrition: Contributions and Potential in Developing Countries. Agron 12:452. https://doi.org/10.3390/AGRONOMY12020452
Kurwakumire N, Chikowo R, Zingore S, Mtambanengwe F, Mapfumo P, Snapp S, Johnston A (2015) Nutrient management strategies on heterogeneously fertile granitic-derived soils in subhumid Zimbabwe. Agron J 107:1068–1076. https://doi.org/10.2134/agronj14.0466
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Ludecke D, Ben-Sachar MS, Patil I, Waggoner P, Makowski D (2021) performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J Open Source Softw 6:3139. https://doi.org/10.21105/joss.03139
Ma Q, Wang X, Li H, Li, H, Cheng L, Zhang F, Rengel Z, Shen J (2014) Localized application of NH4+-N plus P enhances zinc and iron accumulation in maize via modifying root traits and rhizosphere processes. F Crop Res 164:107–116. https://doi.org/10.1016/J.FCR.2014.05.017
Manzeke GM, Mapfumo P, Mtambanengwe F, Chikowo R, Tendayi T, Cakmak I (2012) Soil fertility management effects on maize productivity and grain zinc content in smallholder farming systems of Zimbabwe. Plant Soil 361:57–69. https://doi.org/10.1007/s11104-012-1332-2
Manzeke GM, Mtambanengwe F, Nezomba H, Mapfumo P (2014) Zinc fertilization influence on maize productivity and grain nutritional quality under integrated soil fertility management in Zimbabwe. F Crop Res 166:128–136. https://doi.org/10.1016/j.fcr.2014.05.019
Manzeke MG, Mtambanengwe F, Watts MJ, Broadley MR, R Murray L, Mapfumo P (2020) Nitrogen effect on zinc biofortification of maize and cowpea in Zimbabwean smallholder farms. Agron J 112:2256–2274. https://doi.org/10.1002/AGJ2.20175
Mehlich A (1984) Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Menzies NW, Donn MJ, Kopittke PM (2007) Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ Pollut 145:121–130. https://doi.org/10.1016/J.ENVPOL.2006.03.021
Mertens J, Smolders E, (2013) Zinc. Zinc. In: Alloway B (eds) Heavy Metals in Soils. Environmental Pollution, vol 22. Springer, Dordrecht, pp 465–493. https://doi.org/10.1007/978-94-007-4470-7_17
Mutsaers H, Coyne D, Hauser S, Huising J, Kamara A, Nziguheba G, Pypers P, Taulya G, van Asten P, Vanlauwe B (2017) Soil and soil fertility management research in sub-Saharan Africa: Fifty years of shifting visions and chequered achievements. Routledge, p 376. https://doi.org/10.4324/9781315518855
Nawara S, Van Dael T, Merckx R, Amery F, Elsen A, Odeurs W, Vandendriessche H, Mcgrath S, Roisin C, Jouany C, Pellerin S, Denoroy P, Eichler-Löbermann B, Börjesson G, Goos P, Akkermans W, Smolders E (2017) A comparison of soil tests for available phosphorus in long-term field experiments in Europe. Eur J Soil Sci 68:873–885. https://doi.org/10.1111/ejss.12486
Njoroge Kinyanjui S (2019) Feed the crop, not the soil! Explaining variability in maize yield responses to nutrient applications in smallholder farms of western Kenya. PhD thesis. Wageningen University. https://doi.org/10.18174/503185
Njoroge R, Otinga AN, Okalebo JR, Pepela M, Merckx R (2018) Maize (Zea mays L.) Response to secondary and micronutrients for profitable N, P and K fertilizer use in poorly responsive soils. Agronomy 8:49. https://doi.org/10.3390/agronomy8040049
Njoroge R, Otinga AN, Okalebo JR, Pepela M, Merckx R (2017) Occurrence of poorly responsive soils in western Kenya and associated nutrient imbalances in maize (Zea mays L.). F Crop Res 210:162–174. https://doi.org/10.1016/j.fcr.2017.05.015
Nolan AL, Zhang H, McLaughlin MJ (2005) Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J Environ Qual 34:496–507
Novozamsky I, Houba VJG, van Eck R, van Vark W (1983) A novel digestion technique for multi-element plant analysis. Commun Soil Sci Plant Anal 14:239–249
Nziguheba G, Tossah BK, Diels J, Franke AC, Aihou K, Iwuafor ENO, Nwoke C, Merckx R (2009) Assessment of nutrient deficiencies in maize in nutrient omission trials and long-term field experiments in the West African Savanna. Plant Soil 314:143–157. https://doi.org/10.1007/s11104-008-9714-1
Oikeh SO, Menkir A, Maziya-Dixon B, Welch R, Glahn RP (2007) Genotypic differences in concentration and bioavailability of kernel-iron in tropical maize varieties grown under field conditions. J Plant Nutr 26:2307–2319. https://doi.org/10.1081/PLN-120024283
Olsen SR, Cole CV, Watanabe FS (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Dep Agric Circ 939:19
Osiname OA, Kang BT, Schulte EE, Corey RB (1973) Zinc response of maize (Zea mays L.) grown on sandy inceptisols in Western Nigeria. Agron J 65:875–877. https://doi.org/10.2134/AGRONJ1973.00021962006500060008X
Pasley HR, Cairns JE, Camberato JJ, Vyn TJ (2019) Nitrogen fertilizer rate increases plant uptake and soil availability of essential nutrients in continuous maize production in Kenya and Zimbabwe. Nutr Cycl Agroecosystems 115:373–389. https://doi.org/10.1007/S10705-019-10016-1
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–108
Prasanna BM, Palacios-Rojas N, Hossain F, Muthusamy V, Menkir A, Dhliwayo T, Ndhlela T, San Vicente F, Nair SK, Vivek BS, Zhang X, Olsen M, Fan X (2020) Molecular Breeding for Nutritionally Enriched Maize: Status and Prospects. Front Genet 10:1392. https://doi.org/10.3389/FGENE.2019.01392/BIBTEX
R Core Team, R Development Core Team (2020) A language and environment for statistical computing
Reuter D, Robinson J (1997) Plant analysis: an interpretation manual. CSIRO Publishing, Clayton
Robson AD (1993) Zinc in soils and plants. In: Proceedings of the International Symposium on ‘Zinc in Soils and Plants’ held at The University of Western Australia, 27–28 September, 1993. Springer Science + Business Media, pp 208
Rodel MGW, Hopley JDH (1972) Investigations into systems of farming suitable for tribal trust land. Rhod Agr J 70, no 1.
Rurinda J, Zingore S, Jibrin JM, Balemi T, Masuki K, Andersson JA, Pampolino MF, Mohammed I, Mutegi J, Kamara AY, Vanlauwe B, Craufurd PQ (2020) Science-based decision support for formulating crop fertilizer recommendations in sub-Saharan Africa. Agric Syst 180:102790. https://doi.org/10.1016/j.agsy.2020.102790
Santpoort R (2020) The drivers of maize area expansion in sub-saharan Africa. How policies to boost maize production overlook the interests of smallholder farmers. Land 9:68. https://doi.org/10.3390/LAND9030068
Sattari SZ, van Ittersum MK, Bouwman AF, Smit AL, Janssen BH (2014) Crop yield response to soil fertility and N, P, K inputs in different environments: Testing and improving the QUEFTS model. F Crop Res 157:35–46. https://doi.org/10.1016/j.fcr.2013.12.005
Schut AGT, Giller KE (2020) Soil-based, field-specific fertilizer recommendations are a pipe-dream. Geoderma 380:114680. https://doi.org/10.1016/J.GEODERMA.2020.114680
Sebastian K (2009) Agro-ecological zones of Africa | IFPRI : International Food Policy Research Institute [WWW Document]. Geospatial data . URL http://hdl.handle.net/1902.1/22616 (accessed 1.6.22)
Shehu BM, Merckx R, Jibrin JM, Kamara AY, Rurinda J (2018) Quantifying Variability in Maize Yield Response to Nutrient Applications in the Northern Nigerian Savanna. Agron 8:18. https://doi.org/10.3390/AGRONOMY8020018
Sillanpää M (1990) Micronutrient assessment at the country level: an international study. FAO Soil Bull 63:208
Singh K, Banerjee NK (1987) Critical levels of zinc in corn (Zea mays L.) At different stages of growth. India J Plant Physiol XXX:7–12
Stoorvogel JJ, Smaling EMA, Janssen BH (1993) Calculating soil nutrient balances in Africa at different scales - I Supra-national scale. Fertil Res 35:227–235. https://doi.org/10.1007/BF00750641
Tella M, Bravin MN, Thuriès L, Cazevieille P, Chevassus-Rosset C, Collin B, Chaurand P, Legros S, Doelsch E (2016) Increased zinc and copper availability in organic waste amended soil potentially involving distinct release mechanisms. Environ Pollut 212:299–306. https://doi.org/10.1016/J.ENVPOL.2016.01.077
Ten Berge HFM, Hijbeek R, Van Loon MP, Rurinda J, Tesfaye K, Zingore S, Craufurd P, Van Heerwaarden J, Brentrup F, Schröder JJ, Boogaard HL, De Groot HLE, Van Ittersum MK (2019). Maize Crop Nutrient Input Requirements for Food Security in Sub-Saharan Africa. https://doi.org/10.1016/j.gfs.2019.02.001
Tian Y, Wang X, Luo J, Yu H, Zhang H (2008) Evaluation of holistic approaches to predicting the concentrations of metals in field-cultivated rice. Environ Sci Technol 42:7649–7654. https://doi.org/10.1021/ES7027789/SUPPL_FILE/ES7027789_SI_001.PDF
Van Eynde E, Groenenberg JE, Hoffland E, Comans RNJ (2022) Solid-solution partitioning of micronutrients Zn, Cu and B in tropical soils: Mechanistic and empirical models. Geoderma 414:115773. https://doi.org/10.1016/J.GEODERMA.2022.115773
Vanlauwe B, Coe R, Giller KE (2016) Beyond averages: new approaches to understand heterogeneity and risk of technology success or failure in smallholder farming. Exp Agric 55:84–106. https://doi.org/10.1017/S0014479716000193
Vanlauwe B, Descheemaeker K, Giller KE, Huising J, Merckx R, Nziguheba G, Wendt J, Zingore S (2015) Integrated soil fertility management in sub-Saharan Africa: Unravelling local adaptation. Soil 1:491–508. https://doi.org/10.5194/soil-1-491-2015
Walinga I, Novozamsky I, Houba VJG, Kithome M, van der Lee JJ (2008) Spectrophotometric determination of organic carbon in soil. Commun Soil Sci Plant Anal 23:1935–1944. https://doi.org/10.1080/00103629209368715
Webster R, McBratney AB (1989) On the Akaike Information Criterion for choosing models for variograms of soil properties. J Soil Sci 40:493–496. https://doi.org/10.1111/j.1365-2389.1989.tb01291.x
Wendt JW (1995) Evaluation of the Mehlich 3 soil extractant for upland Malawi soils. Commun Soil Sci Plant Anal 26:687–702. https://doi.org/10.1080/00103629509369328
White PJ, Broadley MR (2011) Physiological limits to zinc biofortification of edible crops. Front Plant Sci 2:80. https://doi.org/10.3389/FPLS.2011.00080/BIBTEX
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Witt C, Dobermann A, Abdulrachman S, Gines HC, Guanghuo W, Nagarajan R, Satawatananont S, Thuc Son T, Sy Tan P, Van Tiem L, Simbahan GC, Olk DC (1999) Internal nutrient efficiencies of irrigated lowland rice in tropical and subtropical Asia. F Crop Res 63:113–138. https://doi.org/10.1016/S0378-4290(99)00031-3
Wortmann CS, Kaizzi KC, Maman N, Cyamweshi A, Dicko M, Garba M, Milner M, Senkoro C, Tarfa B, Tettah F, Kibunja C, Munthali M, Nalivata P, Nkonde D, Nabahungu L, Ouattara K, Serme I (2019) Diagnosis of crop secondary and micro-nutrient deficiencies in sub-Saharan Africa. Nutr Cycl Agroecosystems 113:127–140. https://doi.org/10.1007/s10705-018-09968-7
Yerokun OA, Chirwa M (2014) African Journal of Agricultural Research Soil and Foliar Application of Zinc to Maize and Wheat Grown on a Zambian Alfisol 9:963–970. https://doi.org/10.5897/AJAR2013.7623
Acknowledgements
This work was supported by NWO (grant number 14688, ‘‘Micronutrients for better yields”). We are grateful to the Foundation for Cross-cultural education (FCE) for facilitating the field trials in Zambia, and to Simon and Eline Futerman for setting up and managing them. We thank the staff of the CBLB laboratory and of Unifarm (Wageningen, the Netherlands) for helping with the analyses of the soil and plant samples.
Funding
This work was supported by NWO (grant number 14688, ‘‘Micronutrients for better yields”).
Author information
Authors and Affiliations
Contributions
RNJC and EH designed the research idea; MB, SN and RC organized and conducted the field research; MB with inputs from RNJC, EH and EVE designed and organized the chemical analysis; EVE with inputs from MB analysed the data; EVE drafted the paper. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or non-financial competing interests.
Additional information
Responsible Editor: Hans Lambers.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Eynde, E., Breure, M.S., Chikowo, R. et al. Soil zinc fertilisation does not increase maize yields in 17 out of 19 sites in Sub-Saharan Africa but improves nutritional maize quality in most sites. Plant Soil 490, 67–91 (2023). https://doi.org/10.1007/s11104-023-06050-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06050-2