Abstract
Purpose
The symbiosis between plants and arbuscular mycorrhizal (AM) fungi can increase soil carbon storage by contributing to glomalin-related soil protein (GRSP) and increasing plant biomass. However, increased plant available soil phosphorus (P) can disrupt the AM fungal symbiosis, which could reduce the potential for carbon storage in systems experiencing high nutrient inputs. Using two complementary approaches, we examined whether GRSP production and the ability of AM fungi to affect plant growth varied in soils sampled along a nutrient gradient.
Methods
First, GRSP and soil nutrients were sampled in a field survey of 10 grasslands to determine whether GRSP content was negatively correlated with soil P. Second, a common garden experiment evaluated GRSP production and the biomass response of Trifolium pratense to soil biota sampled from each of the 10 field sites. To evaluate the influence of AM fungi, two fractions of soil biota were used in the experiment: a whole soil inoculum than contained AM fungi along with other soil microbes and a microbial wash inoculum in which AM fungi had been removed by filtration, but which still contained other microbes.
Results
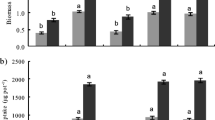
We found that GRSP content differed between field sites, but was not correlated with soil P. In the common garden experiment, GRSP production did not vary among sources of soil biota nor was it correlated with soil P from field sites for either the whole soil or microbial wash treatments. GRSP production was also higher in the microbial wash treatment, which lacked AM fungi, compared to the whole soil inoculum treatment. In addition, plant biomass was significantly higher in the whole soil vs. microbial wash treatments, and plant biomass was highest when inoculated with soil biota from sites with the highest P content.
Conclusions
The results of this study suggest that elevated soil P does not necessarily negatively impact the ability of soil microbes to produce GRSP nor does it have a negative impact on the ability of AM fungi to stimulate plant growth. Our findings are also consistent with recent work suggesting that AM fungi may not necessarily contribute to GRSP production.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available in the supplemental material.
References
Agnihotri R, Bharti A, Ramesha A, Prakash A, Sharma MP (2021) Glomalin related protein and C16:1ω5 PLFA associated with AM fungi as potential signatures for assessing the soil C sequestration under contrasting soil management practices. Eur J Soil Biol 103:103286
Ames RN, Mihara KL, Bethlenfalvay GJ (1987) The establishment of microorganisms in vesicular-arbuscular mycorrhizal and control treatments. Biol Fertil Soils 3:217–223
Bedini S, Avio L, Argese E, Giovannetti M (2007) Effects of long-term land use on arbuscular mycorrhizal fungi and glomalin-related soil protein. Agric Ecosyst Environ 120:462–466
Berend K, Haynes K, MacKenzie CM (2019) Common garden experiments as a dynamic tool for ecological studies of alpine plants and communities in northeastern North America. Rhodora 121:174–212
Bever JD (1994) Feedback between plants and their soil communities in an old field community. Ecol 75:1965–1977
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Burrows RL (2014) Glomalin production and infectivity of arbuscular-mycorrhizal fungi in response to grassland plant diversity. Am J Plant Sci 5:103–111
Camenzind T, Hempel S, Homeier J, Horn S, Velescu A, Wilcke W, Rillig MC (2014) Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob Chang Biol 20:3646–3659
Cissé G, Essi M, Kedi B, Mollier A, Staunton S (2021) Contrasting effects of long term phosphorus fertilization on glomalin-related soil protein. Eur J Soil Biol 107:103363
Dai J, Hu J, Lin X, Yang A, Wang R, Zhang J, Wong MH (2013) Arbuscular mycorrhizal fungal diversity, external mycelium length, and glomalin-related soil protein content in response to long-term fertilizer management. J Soils Sediments 13:1–11
Detheridge A, Brand G, Fychan R, Grotty FV, Sanderson R, Griffith GW, Marley CL (2016) The legacy effect of cover crops on soil fungal populations in a cereal rotation. Agri Ecosyst Environ 228:49–61
Driver JD, Holben WE, Rillig MC (2005) Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol Biochem 37:101–106
Egerton-Warburton LM, Allen EB (2000) Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10:484–496
Fornara DA, Tilman D (2012) Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 93:2030–2036
Franklin JB, Hockey K, Maherali H (2020) Population-level variation in host plant response to multiple microbial mutualists. Am J Bot 107:1389–1400
Gillespie AW, Farrell RE, Walley FL, Ross AR, Leinweber P, Eckhardt KU, Regier TZ, Blyth RI (2011) Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials. Soil Biol Biochem 43:766–777
Gougoulias C, Clark JM, Shaw LJ (2014) The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2371
Grman E (2012) Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93:711–718
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Havlin JL, Kissel DE, Maddux LD, Claassen MM, Long JH (1990) Crop rotation and tillage effects on soil organic carbon and nitrogen. Soil Sci Soc of Am J 54:448–452
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GW (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Hoeksema JD, Bever JD, Chakraborty S, Chaudhary VB, Gardes M, Gehring CA, Hart MM, Housworth EA, Kaonongbua W, Klironomos JN, Lajeunesse MJ, Meadow J, Milligan BG, Piculell BJ, Pringle A, Rúa MA, Umbanhowar J, Viechtbauer W, Wang Y et al (2018) Evolutionary history of plant hosts and fungal symbionts predicts the strength of mycorrhizal mutualism. Commun Biol 1:116
Holátko J, Brtnickký M, Kučerík J, Kotianová M, Elbl J, Kintl A, Kynický J, Benada O, Datta R, Jansa J (2021) Glomalin – truths, myths, and the future of this elusive soil glycoprotein. Soil Biol Biochem 153:108116
Hurisso TT, Moebius-Clune DJ, Culman SW, Moebius-Clune BN, Thies JE, van Es HM (2018) Soil protein as a rapid soil health indicator of potentially available organic nitrogen. Agri Enviro Letters 3:180006
Jansa J, Treseder KK (2017) Introduction: mycorrhizas and the carbon cycle. In: Johnson NC, Gehring C, Jansa J (eds) Mycorrhizal mediation of soil: fertility, structure and carbon storage. Elsevier, Cambridge, pp 343–355
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism parasitism continuum. New Phytol 135:575–585
Keymer A, Pimprikar P, Wewer V, Huber C, Brands M, Bucerius SL, Delaux PM, Klingl V, von Roepenack-Lahaye E, Wang TL, Eisenreich W (2017) Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 6:1–33
Koide RT, Li M (1989) Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytol 111:35–44
Konvalinková T, Püschel D, Řezáčová V, Gryndlerová H, Jansa J (2017) Carbon flow from plant to arbuscular mycorrhizal fungi reduced under phosphorus fertilization. Plant Soil 419:219–333
Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, Mellado-Vázquez PG, Malik AA, Roy J, Scheu S, Steinbeiss S (2015) Plant diversity increases soil microbial activity and soil carbon storage. Nature Comm 6:6707
Liu Y, Johnson NC, Mao L, Shi G, Jiang S, Ma X, Du G, An L, Feng H (2015) Phylogenetic structure of arbuscular mycorrhizal community shifts in response to increasing soil fertility. Soil Biol Biochem 89:196–205
Lovelock CE, Wright SF, Clark DA, Ruess RW (2004) Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J Ecol 92:278–287
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Maherali H, Oberle B, Stevens PF, Cornwell WK, McGlinn DJ (2016) Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am Nat 188:E113–E125
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Moragues-Saitua L, Merino-Martín L, Stokes A, Staunton S (2019) Towards meaningful quantification of glomalin-related soil protein (GRSP) taking account of interference in the Coomassie blue (Bradford) assay. Eur J Soil Sci 70:727–735
Olsson PA, Rahm J, Aliasgharzad N (2010) Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol Ecol 72:123–131
Paré MC, Chagnon P, Plourde J, Legendre-Guillemin V (2019) Apatite stimulates the deposition of glomalin-related soil protein in a lowbush blueberry commercial field. Agricul 9:52
Primieri S, Magnoli SM, Koffel T, Stürmer SL, Bever JD (2022) Perennial, but not annual legumes synergistically benefit from infection with arbuscular mycorrhizal fungi and rhizobia: a meta-analysis. New Phytol 233:505–514
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363
Rillig MC, Maestre FT, Lamit LJ (2003a) Microsite differences in fungal hyphal length, glomalin, and soil aggregate stability in semiarid Mediterranean steppes. Soil Biol Biochem 35:1257–1260
Rillig MC, Ramsey PW, Morris S, Paul EA (2003b) Glomalin, an arbuscular-mycorrhizal fungi soil protein, responds to land-use change. Plant Soil 253:293–299
Ryan MH, van Herwaarden AF, Angus JF, Kirkegaard JA (2005) Reduced growth of autumn-sown wheat in a low-P soil is associated with high colonisation by arbuscular mycorrhizal fungi. Plant Soil 270:275–286
Sainz MJ, Arines J (1988) Effect of indigenous and introduced vesicular-arbuscular mycorrhizal fungi on growth and phosphorus uptake of Trifolium pratense and on inorganic phosphorus fractions in a cambisol. Biol Fertil Soils 6:55–60
Sherrard ME, Maherali H (2012) Local adaptation across a fertility gradient is influenced by soil biota in the invasive grass, Bromus inermis. Evol Ecol 26:529–544
Smith SE, Read D (2008) Mycorrhizal Symbiosis. Academic Press, New York
Stanescu S, Maherali H (2017) Arbuscular mycorrhizal fungi alter the competitive hierarchy among old-field plant species. Oecologia 183:479–491
Staunton S, Saby NPA, Arrouays A, Quiquampoix H (2020) Can soil properties and land use explain glomalin-related soil protein accumulation? A nationwide survey in France. Catena 193:104620
Steinberg PD, Rillig MC (2003) Differential decomposition of arbuscular mycorrhizal fungal hyphae and glomalin. Soil Biol Biochem 35:191–194
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK (2013) The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 371:1–13
Treseder KK, Allen MF (2000) Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition. New Phytol 147:189–200
Treseder KK, Turner KM (2007) Glomalin in ecosystems. Soil Sci Soc Am J 71:1257–1266
Treseder KK, Turner KM, Mack MC (2007) Mycorrhizal responses to nitrogen fertilization in boreal ecosystems: potential consequences for soil carbon storage. Glob Change Biol 13:78–88
Ven A, Verbruggen E, Verlinden MS, Olsson PA, Wallander H, Vicca S (2019) Mesh bags underestimated arbuscular mycorrhizal abundance but captured fertilization effects in a mesocosm experiment. Plant Soil 446:563–575
Vierheilig H, Coughlan AP, Wyss URS, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Wang W, Zhong Z, Wang Q, Wang H, Fu Y, He X (2017) Glomalin contributed more to carbon, nutrients in deeper soils, and differently associated with climates and soil properties in vertical profiles. Sci Reports 7:1–13
Weber SE, Diez JM, Andrews LV, Goulden ML, Aronson EL, Allen MF (2019) Responses of arbuscular mycorrhizal fungi to multiple coinciding global change drivers. Fungal Ecol 40:62–71
Wright SF, Upadhyaya A (1996) Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci 161:575–586
Xu M, Li X, Cai X, Li X, Christie P, Zhang J (2017) Land use alters arbuscular mycorrhizal fungal communities and their potential role in carbon sequestration on the Tibetan plateau. Sci Reports 7:1–11
Acknowledgements
The authors thank K. MacColl and K. Hicks for assistance with soil collection in the field, and M. Mucci and L. Illman for assistance with maintaining plants in the greenhouse. We also thank K.E. Dunfield and C.M. Caruso for comments on the manuscript.
Funding
This work was funded by a Discovery grant (NSERC RGPIN-2018-04620) and a Discovery Accelerator Supplement grant (NSERC RGPAS-2018-522480) from the Natural Sciences and Engineering Research Council of Canada to HM.
Author information
Authors and Affiliations
Contributions
SML and HM were responsible for conceptualization, methodology, data curation, manuscript preparation, reviewing, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Stavros D. Veresoglou.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Law, S.M., Maherali, H. Variation in glomalin-related soil protein and plant growth response to arbuscular mycorrhizal fungi along a nutrient gradient in temperate grasslands. Plant Soil 487, 623–637 (2023). https://doi.org/10.1007/s11104-023-05958-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05958-z