Abstract
Background and aims
Studies that consider the effects of screes plant communities on the soil properties are lacking and whether the plant cover could be used as an indicator of biotic and/or abiotic parameters of the scree colonization has never been assessed. Here we aimed to identify plant communities-soil relationships in the process of colonization of alpine limestone screes.
Methods
Plant communities and chemical-physical soil properties were studied on 50 plots from an area of the Italian Alps, and related to plant cover (colonization). Correlation and regression analysis were performed to determine the relationships and define the best trend models.
Results
At the varying of plant cover, plant communities and the soil properties changed greatly. Floristic analysis especially showed how the pioneer/early stages of colonization revealed a limited number of species (< 20) typical of unstable screes (Thlaspietea rotundifolii) while the late/final stage has a greater number of species (> 25), mainly of basophilic grasslands (Elyno-Seslerietea). Statistical analysis showed high correlation between plant cover and number of species, shear vane strength (τ), soil depth, pH, organic matter (OM), total nitrogen (TN), sand and medium gravel content. The number of species and the values of τ, soil depth, OM, TN and sand content increased over the colonization/succession, albeit with different trends.
Conclusion
This research allowed a better comprehension of the phenomenon of colonization of alpine limestone screes and showed how plant cover is a parameter useful to estimate some soil and vegetation features, therefore to facilitate the study/management of these environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scree slopes form an azonal habitat typical of mountain areas all around the world. Screes, also known as “talus”, especially in the United States of America (Luckman 2013) are “microcosms” dominated by physical weathering processes and gravitational processes carrying downslope rocks and debris (Pignatti and Pignatti 2014). These environments are inhabited by many living organisms including a wide range of plant species (Leuschner and Ellenberg 2017; Giupponi and Giorgi 2019).
Over the Alps, there are both siliceous screes (mostly in the inner-alpine areas), and calcareous-dolomitic screes (mostly in the pre-Alps, Dolomites and Carnic Alps) (Pfiffner 2014), everyone characterized by a specific flora. Among the open habitats, screes and rocks are the richest in endemic and rare plants. In the European Alps, the 35–40% of endemics of Europe were found (Nagy and Grabherr 2009). Some of them are species of community interest included in Annex II of the Habitat Directive (92/43/EEC) (Giupponi and Giorgi 2019), the most important European tool for the nature conservation.
The vegetation of the active and sliding screes (Thlaspietea rotundifolii phytosociological class) was differentiated into carbonate (Thlaspietalia rotundifolii) and silicate (Androsacetalia alpinae) scree communities, both characterized by many species (Biondi and Blasi 2015; Mucina et al. 2016; Leuschner and Ellenberg 2017). These species make up the communities of the pioneer/early stages of primary plant successions (Walker and Del Moral 2003) and they show many morpho-physiological adaptations to survive in extreme environments (Körner 2021). Some species of thereof plant communities colonizing the screes (mostly herbs and dwarf shrubs) are pioneer plants able to modify the chemical-physical characteristics of the substrata where they grow. They are known as “scree stabilizer” (Schröter 1926) because they trap the soil particles, especially the fine sediments, and as “scree accumulator” because they store organic matters and nutrients (Schröter 1926; Leuschner and Ellenberg 2017). The presence of these colonizing species causes a gradual but constant stabilization of the debris, which in turn favors the formation of a fertile fine earth stratus, process needed for increasing the plant cover and to reach the late/final stages of the plant succession. These natural processes, which can take place over hundreds of years, are the result of plant/plant community-soil interactions. Investigating all the natural mechanisms that regulate the bio-geosphere provides data, information and evidences useful to develop low-impact measures for soil stabilization and/or for ecological restoration (Walker and Del Moral 2008; Aronson and Alexander 2013; Giupponi and Leoni 2020a).
In the last decades, some “ecosystem engineer species”, intended as species that change their physical environment through their adapted traits and thereby create habitats for other species (Jones et al. 1994; Jones 2012) have been identified also for alpine screes/moraines. Dryas octopetala, an alpine dwarf shrub that grows on limestone debris/rocks, is a well-known engineer species that consolidate debris, and improve the soil fertility (Welker et al. 1997; Eichel et al. 2016; Körner 2021). Nevertheless, the study of a single “ecosystem engineer species” could be a reductive approach to assess the benefits on the screes environment, because the vegetation that colonizes the screes is generally constituted by a set of species (plant communities) interacting with each other and with the other components (biotic and abiotic) of the ecosystem.
The phytosociological and/or synecological approach has been widely used for the study of plant communities of screes, at least in Europe (Valachovič et al. 1997; Mucina et al. 2016; Leuschner and Ellenberg 2017; Nowak et al. 2021; Tomaselli et al. 2021; Trakić et al. 2021). Multidisciplinary studies considering the effects of screes plant communities on chemical-physical features of the soil (and conversely) are whereas limited, and even more limited are the ones considering slope stability linked to plant communities (Cislaghi et al. 2019; Greinwald et al. 2021). The stabilization of debris/rocks, together with the accumulation of fine earth, is essential to facilitate the expansion of plant colonization over the screes (Leuschner and Ellenberg 2017). This evidence, together with the further gradual increase of plant cover (and stratification), was clearly observed in similar habitats such as glacier forelands/moraines (Raffl et al. 2006; Eichel et al. 2013, 2016; Eichel 2019; Nagl and Erschbamer 2010; Greinwald et al. 2021). On contrary, no recent studies occur on the plant coverage of the different stages of colonization over the screes.
Nowadays, the plant cover is a variable easily measurable with modern remote sensing and/or digital image analysis techniques/tools (Patrignani and Ochsner 2015; Hu et al. 2018; Yu and Guo 2021), and could be used as an indicator of biotic and/or abiotic parameters of the screes that are more complicated to be measured (such as stability and soil fertility). This aspect should be investigated not only to facilitate the study of scree ecosystems but also to facilitate and improve the land monitoring and management of areas (especially remote areas) subject to imbalances due to climate and land use change such as the Alps (Cislaghi et al. 2019; Giupponi and Leoni 2020b; Körner 2021).
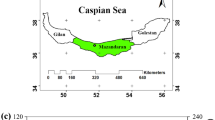
This research aims to analyze the plant communities that colonize the calcareous screes of a sub-alpine area of the Italian Alps (Mount Cavallo, Orobic Alps) and to evaluate the effects of vegetation colonization on the main chemical and physical characteristics of the soil (soil depth, shear vane strength, particle size distribution, pH, organic matter and nitrogen content). In particular, the main purpose is to assess if the plant cover is a reliable indicator for the biological and soil parameters of the screes communities. This goal could be obtained evaluating how the plant cover influences the floristic changes inside the plant communities that colonize the screes, and which correlations exist between the plant cover and the chemical-physical characteristics of the soil.
Material and methods
Study area
Mount Cavallo is in Brembana Valley (Lombardy region, Northern Italy; latitude: 46°02′04.24″ N, longitude: 09°41′25.09″ E) within the Orobie Bergamasche Regional Park, that is one of the Italian sites richest in endemic plant species (Blasi et al. 2011; Martini et al. 2012; Giupponi and Giorgi 2017, 2019). Its geological substrate is limestone rocks of “Calcare di Esino” formation (Upper Anisian – Ladinian) that is almost pure limestone (Provincia di Bergamo 2002). The study area belongs to the group of massive calcareous and dolomitic substrates (pedogenetic value 2) (Comunità Montana Valle Brembana 2017). The group denotes a congenital permeability almost nil, and the acquired one is always attributable to fracturing or solution in the not frequent phenomena of karst and hypogeal circulation of water. The alterability is modest, and the major product of the alteration is clay. The stability is, in general, good and especially massive formations show excellent compactness characteristics (Comunità Montana Valle Brembana 2017).
On the North and East slopes of Monte Cavallo there are extensive calcareous screes colonized by vegetation at various degree (Fig. 1). On the loose screes, there are plant species of Thlaspion rotundifolii alliance (Thlaspietea rotundifolii class), which easily grow on the calcareous ones, in the supra- to cryorotemperate thermotypes of the Alps. On contrary, where the scree is more stable, there are mostly basophilic grasslands of Caricion austroalpinae and Caricion firmae (Elyno-Seslerietea class) (Sutter 1962; Giupponi and Giorgi 2017). The study area belongs to the South-Orobico Geobotanical District (Andreis 2002), characterized by a pre-Alpine sub-oceanic climate with an average annual precipitation higher than 1,500 mm. Considering the meteorological data collected by the climatic station of Foppolo (2005–2020), the average annual temperature of the study area is 6.7 °C and the average annual precipitation amounts to 1,776 mm. From December to March the average monthly temperature is below 0 °C and the soils are affected by cryoturbation phenomena.
According to the classification of the ecoregions of Italy, Mount Cavallo is part of the Central and Eastern Alps section of the Alpine Province where natural and semi-natural areas are dominant (77%) while agricultural areas are quite limited (16%) and artificial surface covers only 4% of the total area (Blasi et al. 2014). As for the vegetation dynamics, the study area belongs to the pre-Alpine Central and Eastern geosygmetum of Carex firma grasslands and Sesleria caerulea basophilic grasslands. These include the lithophile series of Potentillo-Telekietum speciosissimae and Thlaspion rotundifolii, the edaphic series of Tofieldio calyculatae-Caricetum firmae, and the climacical series of esalpic Sesleria caerulea grasslands (Caricion austroalpinae, Seslerio-Cytisetum emeriflori) and Rhodothamno-Rhododendretum hirsuti (Verde et al. 2010).
Sampling procedure
Field data and samples used for the vegetation and soil analysis were collected in 50 sites located along the North and East slope of Mount Cavallo, where there are loose and stabilized screes (Fig. 1) in the subalpine belt with an elevation range between 2,000 and 2,100 m a.s.l.. The sampling sites are situated at a slope range between 30° and 50° excluding the areas on the top and on the bottom of the screes where fine debris and large material accumulate. The sampling sites were selected according to a gradient of plant colonization (or plant cover) and for each one an area of 4 m2 (2 m × 2 m) was delimited and photographed from above using a digital camera (SONY DSC-W830) on a mobile pole/tripod. The photos were taken perpendicular to the ground and at a distance of 2 m from the ground. Then, the sampling procedure for each sampling site consisted in: (i) a vegetation analysis, including the evaluation of plant cover through an image analysis and a phytosociological relevés; (ii) a soil analysis, including the collection of five soil samples and five soil depth measurements, and 20 measures of undrained shear strength using the shear vane test (Fig. 1).
Vegetation data collection and analysis
Field data and samples used for the vegetation analysis were collected in 50 sites located along the North and East slope of Mount Cavallo, where there are loose and stabilized screes (Fig. 1) in the subalpine belt with an elevation range between 2,000 and 2,100 m a.s.l.. The sampling sites are situated at a slope range between 30° and 50° excluding the areas on the top and on the bottom of the screes where fine debris and large material accumulate. The sampling sites were selected according to a gradient of plant colonization (or plant cover) and for each one an area of 4 m2 (2 m × 2 m) was delimited and photographed from above using a digital camera (SONY DSC-W830) on a mobile pole/tripod. The photos were taken perpendicular to the ground and at a distance of 2 m from the ground. For each sampling site, the captured image was processed through an image analysis using Adobe Photoshop software to remove shadows, improve contrast between vegetation and rocks/bare soil and correct any image distortion due to the camera, and using the Easy Leaf Area software, an easy-to-use tool for rapid non-destructive measurement of canopy area, to estimate the percentage of plant cover (Easlon and Bloom 2014).
One phytosociological relevés was carried out on the same 4 m2 areas (Fig. 1) using the methods of Braun-Blanquet (1964). Species (tracheophytes) of the plant communities were identified using the dichotomous keys of Pignatti (2017), whereas their coverage was estimated using the conventional abundance/dominance scale of Braun-Blanquet (r, rare species in the relevé; + , coverage < 1%; 1, coverage 1–5%; 2, coverage > 5–25%; 3, coverage > 25–50%; 4, coverage > 50–75%; 5, coverage > 75 – 100%). The relevés were performed in July 2020 and the scientific names of the species are in accordance with Pignatti (2017). The data of the relevés were arranged in a matrix (relevés x species) where abundance/dominance indexes of the species were converted into percentage of coverage as proposed by Canullo et al. (2012) (r, 0.01%; + , 0.5%; 1, 3.0%; 2, 15.0%; 3, 37.5%; 4, 62.5%; 5, 87.5%). This transformation allowed to perform multivariate analysis. In fact, a hierarchical cluster analysis and detrended correspondence analysis (DCA) were performed to identify floristic-physiognomic similarities/differences among the relevés. Cluster analysis was performed using the Unweighted Pair Group Method with Arithmetic mean method (UPGMA) and the chord distance coefficient (Legendre and Gallagher 2001). The average number of species and the average plant cover were calculated for each type of vegetation (cluster) in order to describe the primary plant succession of the limestone screes of the study area. Cluster analysis and DCA were carried out using the “vegan” R package (Dixon 2003).
Furthermore, Pearson's phi coefficient (Φ) was used to identify the species significantly associated with the different type of vegetation (diagnostic species), equalizing the size of relevés groups, according to procedure proposed by Chytrý et al. (2002) and Tichý and Chytrý (2006). The coefficient Φ was calculated as follows:
where N is the number of relevés in the data set, Np is the number of the relevés in a target group of relevés (equalizing the size of relevés groups), n is the number of occurrences of the species in the data set and np is the number of occurrences of the species in the target group of relevés. This coefficient can assume values from –1 to 1: higher values indicate that species occurrences are concentrated in the target relevés group, whereas lower values indicate that they are under-represented in the target relevés group.
Phytosociological analysis of vegetation was performed using the hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities of the European vegetation (Mucina et al. 2016). Therefore, the phytosociological classes of the diagnostic species for each plant community were reported to determine the ecological features of the vegetation. Only the classes of the Alpine vegetation were considered. The names of the syntaxa are in accordance with Mucina et al. (2016). The identification of the diagnostic species was conducted using the “indicspecies” R package (De Cáceres 2013).
Soil samples collection and analysis
Soil samples were collected and the topsoil depth was measured for each sampling area and shear vane tests for each sampling site were performed as shown in Fig. 1.
Five holes were dug to measure the topsoil depth (the upper horizon where most of roots and organic matter are localized) and collect topsoil sample (~ 500 g). In cases where there was no topsoil (scree slightly colonized by vegetation and without organic horizon), samples of debris were collected at a depth of 0–5 cm. Then, the five soil samples were brought to the laboratory, air-dried and uniformly mixed to obtain a unique soil sample representative of each sampling site.
The particle size distribution was determined by sieving the coarse fractions (skeleton) (particle size > 2 mm) using sieve shaker (Octagon 2000) and by sedimentation of fine earth (particle size < 2 mm) with the Esenwein levigators for determining proportions of sand and mud (silt and clay). All the air- dried samples were preliminarily treated with a freshly prepared dilute solution (3%) of hydrogen peroxide in order to remove organic matter (Papuga et al. 2022). The percentage of the following five particle size classes was calculated for each soil sample: coarse gravel (> 20 mm), medium gravel (5–20 mm), fine gravel (2–5 mm), sand (0.05–2 mm) and silt–clay (< 0.05 mm). On the same samples, a series of chemical analysis was performed. A digital pH meter (Eutech pH 510) was used to measure the pH of the soil samples with distilled water (soil: water = 1: 2.5). The total organic carbon was determined according to the Walkley and Black (1934) method and organic matter percentage was calculated by multiplying the concentration of total organic carbon by the coefficient of Van Bemmelen (1.724). The total nitrogen content of each soil sample was determined according to the Kjeldahl method (Bremner 1960) using Kjeldhal apparatus (Buchi KC530). Chemical-physical analyses of the soil were performed in triplicate.
Soil shear vane strength is a soil parameter that indicates the internal resistance per unit area that a soil mass provides to contrast failure mechanisms along any plane (Sharifounnasab and Ullrich 1985; Morris and Williams 1993). This geotechnical parameter reflects how much shear resistance the rooted-soil matrix provides (higher than soil or roots separately) (Simon and Collison 2002). Shear vane strength was measured in situ using a field vane shear test (S057, Matest S.r.l.), a T-handle cylindrical body ending with a four-bladed vane with a height (h) of 0.40 m and a diameter (d) of 0.20 m. The test consists in placing it in the soil and rotating it from the surface to determine a torsional force T (in N m) by the vane. Thus, T is related to shear vane strength (τ in Pa) as follows:
In each sampling sites, 20 vane shear tests were conducted (Fig. 1). The rod with vanes mounted to it was placed at a depth of 10–15 cm after saturating the soil with water (in order to standardize the soil moisture content), then the average value of all measures was calculated.
Statistical correlation and regression analysis
Vegetation and soil analysis provided a wide dataset containing observations of vegetation traits (i.e., plant community, plant cover, and number of species), and physical–chemical measurements (i.e., shear vane strength, soil depth, distribution of particle size classes, pH, organic matter, and total nitrogen). Once identified the plant communities through the phytosociological relevés and the clustering analysis, one-way ANOVA test was conducted on the dataset in order to determine significant differences (p < 0.05) among different plant communities. Before performing ANOVA test, the assumptions of normality of residuals and homogeneity of variances were verified using the Shapiro–Wilk test and Levene’s test, respectively. When significant differences were found among plant communities, the post hoc Tukey test was carried out. Then, Pearson's correlation coefficient (r) that measures the linear correlation between two variable was applied to detect statistical relationships between the plant cover and the other observations of the dataset. When r assumed an absolute value higher than 0.55, a regression analysis was performed to define the model that can best interpret the relationship. Regression analysis was carried out using “basicTrendline” R package (Mei et al. 2018). The most commonly used models were: (i) linear: y = ax + b; (ii) 2nd order polynomial: y = ax2 + bx + c;(iii) logarithmic: y = a ln(x) + b; and (iv) exponential: y = a ebx + c (where x is the independent variable, i.e. the plant cover, y is the dependent variable, i.e. the number of species and the physical–chemical soil measurements). The fitting performance for each model was evaluated by the F-test, the coefficient of determination (R2) and Akaike Information Criterion (AIC) (Akaike 1974). The lowest value of AIC identifies the best performance fitting. The best model defines the change of biodiversity and soil properties during the colonization of the Alpine limestone screes. Statistical analyzes were performed using R software (R Development Core Team 2021).
Results
Vegetation traits
The plant cover values obtained by the image analysis were distributed in a fairly homogeneous way within a range from 0.53% to 96.6% (Fig. 2) and 105 species were identified (Table S1). The minimum number of species was 7, whereas the maximum one was 35. Most of them are commonly widespread over the Alps, except for some rare endemic species such as Linaria tonzigii, Moehringia concarenae and Allium insubricum. The cluster analysis produced a dendrogram (Fig. 3) that assembled the phytosociological relevés into 5 groups (clusters) (a1, a2, b1, b2 and b3) corresponding to 5 different vegetation types (plant communities).
Dendrogram of phytosociological relevés: the number in black indicate the relevés code while the code of the main clusters is in red. The range of plant cover was highlighted (with different shades of green as reported in Fig. 2) for each relevés
Table 1 shows the diagnostic species of each plant community. Most diagnostic species of plant community a1 and a2 are of Thlaspietea rotundifolii phytosociological class, whereas the other plant communities (b1, b2 and b3) are characterized by species of other class, mainly Elyno-Seslerietea, Carici rupestris-Kobresietea bellardii and Rhododendro hirsuti-Ericetea carneae.
Once classified the sampling areas, a trend of vegetation traits in function of the plant communities was evident. Indeed, plant cover and number of species significantly increase from a1 to b3 (Fig. 4). In particular, a1 is the plant community with lowest plant cover (7.35% ± 5.97) and lowest species richness (13.00 ± 3.56), whereas b3 is the plant community where the vegetation cover and the number of species are the highest (75.53% ± 21.31 and 27.67 ± 5.02, respectively). Such trend is statistically confirmed by the one-way ANOVA (Table S3) and describes the progression in screes colonization by the vegetation and, at the same time, an evolution of the plant community of the screes. Furthermore, the biplot of DCA (Fig. 5) showed a clear delimitation of plant communities in terms of botanic features. Figure 5 shows how plant cover is one of the main variables that differentiates plant communities. Plant cover decreased along the first axis (DCA1), explaining most of the total variance in the dataset (39.09%). Moreover, the species of the pioneer (a1) and early stages (a2) were mostly herbaceous species of unstable screes where movement of debris (abiotic disturbance) are frequent. The plants of the intermediate stage of the succession (b1 and b2) were mainly species of basophilic alpine grasslands (such as Carex firma, Festuca pumila, Sesleria caerulea and Juncus monanthos) with some dwarf shrubs (as Dryas octopetala and Arctostaphylos alpinus). Instead, various shrubs (as Rhododendron hirsutum, Salix glabra and Erica carnea), plants of basophilic alpine grasslands and herbaceous species that require soils rich in nutrients (as Trollius europaeus and Bistorta vivipara), characterized the vegetation of the late/final stage (b3) (Fig. 5 and Table S1). In addition, Fig. 5 allows to define the ranges of plant cover within which the plant communities / stages of succession are placed: pioneer stage (a1), plant cover < 20%; early stage (a2), 10–50%; intermediate stages (b1 and b2), 30–70%; late/final stage (b3), 60–100%. The average number of species increased along the succession: there were about 13 species in the initial stage while there were about 27 in the final stage (Fig. 4).
Vegetation traits in function of the five plant communities detected by the cluster analysis: plant cover on the upper panel, whereas number of species on the lower panel. Different letters above the error bars indicate significant differences (p < 0.05) among plant communities obtained by the one-way ANOVA and the Tukey post-hoc test
DCA biplot of phytosociological relevés overlapped with plant cover contour lines (green lines with percentage of plant cover). In the DCA biplot the species are indicated with crosses and some of them were highlighted: Achillea_c, Achillea clavennae; Adenostyles_a, Adenostyles alpina; Arctostaphylos_a, Arctostaphylos alpinus; Asplenium_v, Asplenium viride; Biscutella_l, Biscutella laevigata; Carex_f, Carex firma; Cystopteris_a, Cystopteris alpina; Doronicum_g, Doronicum grandiflorum; Festuca_p, Festuca pumila; Gypsophyla_r, Gypsophyla repens; Horminum_p, Horminum pyrenaicum; Hornungia_a, Hornungia alpina; Juncus_m, Juncus monanthos; Larix_d, Larix decidua; Linaria_t, Linaria tonzigii; Primula_g, Primula glaucescens; Pulsatilla_a, Pulsatilla alpina subsp. austroalpina; Rhododendron_h, Rhododendron hirsutum; Salix_g, Salix glabra; Salix_r, Salix retusa; Saxifraga_a, Saxifraga aizoides; Saxifraga_h, Saxifraga hostii subsp. rhaetica; Sesleria_c, Sesleria caerulea; Valeriana_s, Valeriana saxatilis; Veronica_a, Veronica aphylla
Physical–chemical soil properties
The physical analysis of the soils (Table S2) showed that soil samples are composed by at least 50% of skeleton (particles > 2 mm), except for 3 samples. In all samples, the medium gravel represented the largest portion; however, the particle size distribution differed in function of plant community. This evidence is confirmed by the one-way ANOVA (Fig. 6 and Table 2), despite the particle size distribution of soils into the 5 plant communities significantly differed only for the content of medium gravel and sand. However, aggregating the gravel percentages and calculating the skeleton of the soil samples, this last variable showed a statistical difference among the plant communities. In fact, the percentage of skeleton decreased from the early (a1 and a2) to the final stages (b3) of succession (while the fine earth increased). The sand content of the soils of the early stages was lower than that of the intermediate and final stages, whereas the percentage of gravel was higher in the soils of plant communities a1 and a2 than in those of communities b1, b2 and b3 although the results of the Tukey post-hoc test did not show a significant difference between a1 and b3 (Fig. 6).
The soils of the 5 plant communities also significantly differed in the other physical–chemical properties investigated: shear vane strength, soil depth, organic matter, total nitrogen content, and pH (Table 2). The values of shear vane strength and soil depth increased gradually from a1 (shear vane strength: 20.24 ± 6.13 kPa; depth: 0.59 ± 0.79 cm) to b3 (shear vane strength: 61.53 ± 17.38 kPa; depth: 12.35 ± 5.45 cm) as shown in Fig. 7. Contrary, pH values decreased from a1 to b3 (Fig. 8). The values of organic matter and total nitrogen had the same trend and differed significantly between the communities of the pioneer/early stages, a1 in particular (organic matter: 0.97 ± 0.93%; total nitrogen: 0.15 ± 0.10 g kg−1), and b3 (organic matter: 7.75 ± 5.92%; total nitrogen: 1.47 ± 1.19 g kg−1).
Vegetation traits and physical–chemical soil properties vs. plant cover
Pearson correlation analysis provided the linear relationships between plant cover and the other measurements. The results showed that all the vegetation traits and the physical–chemical soil properties have a medium–high correlation (|r|> 0.55), except for coarse gravel (r = 0.30), fine gravel (r = -0.20), and silt and clay (r = -0.03). In particular, a high correlation (r > 0.80) was found with shear vane strength, soil depth and number of species. Most of correlations were positive, whereas plant cover vs. pH, medium gravel and skeleton were negative.
Pearson correlation analysis underlined how plant cover increasing (from a1 to b3) runs together with increasing of shear vane strength, soil depth, organic matter, total nitrogen and sand content, whereas pH, medium gravel, and skeleton percentage decrease (Fig. 9). Furthermore, the trend analysis showed that the trend of the shear vane strength the soil depth, and the number of species are the most robust with R2 > 0.70 (Table S4). The relationship between shear vane strength and plant cover was expressed as a linear regression, whereas the other ones as an exponential regression. Then, sand percentage, organic matter and total nitrogen showed a positive linear relationship with the plant cover, moderately robust (R2 > 0.35). Then, percentage of medium gravel and pH have a negative exponential trend with plant cover increasing (R2 > 0.37).
Discussion
As shown by the results, as plant cover increases (progression of screes colonization) vegetation traits and chemical-physical properties of the soil change as resumed in Fig. 10. Floristic analysis showed how the pioneer/early stages, usually having a low plant cover (< 50%), display also a limited number of species (< 20) typical of unstable screes (Thlaspietea rotundifolii). Some of these species, in the case analysed, are steno-endemic of Alps (as Linaria tonzigii, Moehringia concarenae and Allium insubricum) and their presence in the study area is ascribable to the fact that part of the Southern Alps, as the study area, are hotspots of endemic species for historic and evolutionary circumstances (Pawlowski 1970; Ozenda 1995; Andreis et al. 2017).
Species of pioneer/early stages lessen with the increasing of plant cover, representing the intermediate and late/final stages of colonization. Intermediate and late/final stages are in fact characterised by the presence of species of other environments/phytosociological classes, among which basophils grasslands of the supratemperate thermotype and cryorotemperate thermotype (Elyno-Seslerietea) (Biondi and Blasi 2015; Mucina et al. 2016). The grasslands of Elyno-Seslerietea represent the “current potential vegetation” (Biondi 2011) of the study area (Verde et al. 2010). The colonization of the mobile screes by pioneer species able to modify their environment (“reaction”) and favour the incoming of other species better fitting the modified environment (“facilitation”), so replacing the pioneer species, is a concept at the base of most accredited succession models as Clements (1928) “facilitation-based succession model” and Connell and Slatyer (1977) “facilitation, tolerance, inhibition’-model”. In our case study, it was possible to see how the early/pioneer stages can modify their growth substrate making it more stable and progressively deeper and richer in organic substance and nitrogen. In this way, the substrate becomes more suitable especially for species of basophiles grasslands and shrubs of Rhododendro hirsuti-Ericetea carneae class. These changes, together with an increasing of the number of species of the communities (species richness), were observed also in other succession models in similar environments as for example the glacier forelands/moraines (Caccianiga et al. 2006; Eichel et al. 2013, 2016; Eichel 2019; Greinwald et al. 2021).
Eichel (2019) observed a gradual increase of species in the plant communities of pioneer/early stages in the biotic successional trends for primary successions in glacier forelands, until the achievement of a certain stability (or even a slight decrease) in the intermediate/late stages. In our study case as well, we could observe this trend in the species richness (Fig. 9a). Community a1 has 13.00 ± 3.56 species in 4 m2 while community b3 has 27.67 ± 5.02 and from model in Fig. 9a is possible to see how in the early stages of a succession the number of species increases faster than in intermediate and final stages. This trend can be justified through Matthews (1992) “geoecological succession model” that combines physical (abiotic) and biological (biotic) processes and describes their consequence in the successional sequence. In this model, at the start of the plant succession abiotic factors are more important than biotic factors and biotic processes control succession later (Matthews 1992; Raab et al. 2012). In screes, principal abiotic factors affecting pioneer/early stages of successions are debris movements and the lack of a soil layer able to provide water and nutrients. On that note, only species able to survive in this kind of environment (those of Thlaspietea rotundifolii) can grow without developing, at least at the early stages, competition, provided that disturbance (debris flow) is not too intense, preventing plants from taking root. In this phase, the number of species can grow fast precisely because there is space to colonize without competition, and because Alpine species colonising screes are not few (Leuschner and Ellenberg 2017) and their seeds have high germinability (Giupponi and Leoni 2020a). With the evolvement of the colonization (in the intermediate and final stages), the importance of abiotic factors decreases, and the effect of biotic factors (as the species competition) increases, becoming the most important in the succession development and the most responsible of the stabilising (or even slight decrease) of the species richness of the community (Matthews 1992; Raab et al. 2012; Eichel 2019). Thus, from pioneer stages to the final ones of primary successions also the functional strategy of the various species diversifies in accordance with the model of Grime (2001). Following this model, when disturbance intensity decreases and resource availability increases, the disturbance-adapted (ruderal) strategist is succeeded by the competitor. Resource availability and disturbance intensity decreases thereafter, and the stress-tolerator dominates. The validity of this model was confirmed by studies conducted by Caccianiga et al. (2006) in peri-glacial areas and should be tested also for screes colonization.
In addition to changes in the plant communities, this research showed how at the increasing of plant cover (colonization) also some chemical-physical features of the soil change, as the shear vane strength, the soil depth, the availability of organic matter and nitrogen, pH and percentage of medium gravel and sand (Fig. 10). A high correlation (r > 0.80) between plant cover and shear vane strength and soil depth was found, even if with different trend models (Fig. 9b and c). From this study we can affirm that shear vane strength increases linearly with plant cover and that, while soil substrate of pioneer stages has values of 20.24 ± 6.13 kPa, the one of late/final stages has values three times higher (61.53 ± 17.38 kPa). These values are in accordance with the ones of Liu et al. (2014) collected in natural restoration areas on cut-slope (soil shear strength: ~ 20 kPa; plant cover: 1.3%) and in grass and shrub areas on fill-slope (soil shear strength: ~ 60 kPa; plant cover: 92%), while they are somewhat lower than the ones found by Cislaghi et al. (2019) in Alpine pastures. The increase in the soils shear strength during colonization is justified by the reinforcement/stabilizing role of roots (Vergani et al. 2012, 2016; Chiaradia et al. 2016; Cislaghi et al. 2017; Bordoni et al. 2020). In early stages, when few plants are present, the role of roots is marginal while in the last stages of colonization the action of roots becomes more marked, since the density of roots, one of the factors determining the stability of debris in alpine environments (Greinwald et al. 2021), increases. Vegetation, then, also in calcareous subalpine screes colonization, has a greater slope stabilising effect the higher is the grade of plant colonization (plant cover). Although, there is not much information about the morphological/architectural, mechanical or phytochemical features of many herbaceous and/or dwarf species of Alps (Kutschera et al. 1997; Giupponi et al. 2017, 2019; Cislaghi et al. 2019).
Considering the depth of the top-soil, our research showed how in the early colonization stages top soil is almost inexistent, while it reaches a depth of 12 cm in the late/final stage (Fig. 7). The build-up of a more or less deep soil can be ascribed similarly to the vegetation activity during time and it is strictly linked as well to the stability of the mountain side (Fig. 9b). Roots/stems of some plants colonising screes and stabilising the substrate, are able to favour the accumulation in the soil of minerals particles. The deposit of organic matter and nitrogen derived from the plant materials decomposition (and/or from nitrogen fixation operated by symbiotic and free bacteria) (Pignatti and Pignatti 2014; Körner 2021; Eichel 2019) joins this particulate material. An example is Dryas octopetala, an “ecosystem engineer species” (Eichel 2019) found in many relevés realized especially in plant community b2, of which it results a diagnostic species (Table 1). Among the various species constituting the communities colonising screes there are probably other “ecosystem engineer species” able to stabilise the soil and improve its fertility. These kinds of species should be identified with auto-ecological ad hoc studies as done by Hao et al. (2022). Anyway, independently from the presence of single “ecosystem engineer species”, during the colonization of calcareous screes “ecosystem engineer communities”, able to stabilise and increase the soil fertility (depth and nitrogen and matter content), follow one another.
The decrease of pH and the increase of fine earth (meaning the soil particles of sand granulometric class) observed with the evolution of colonization, are as well features depending directly or indirectly on vegetation development. The increase in the organic matter (mainly imputable to the decomposition of plant material) would justify the decrease in pH due to the dissociation of soluble organic acids released into the soil (Ritchie and Dolling 1985). pH difference resulted significant between the early and intermediate/late stages of the plant communities (Fig. 8) and intermediate/final stages soil resulted mainly neutral-alkaline or slightly acid. This is justified by the presence also in the final stages of a moderate percentage (~ 60%) of calcareous gravel (Fig. 6) that prevent partially the increasing of acidity. In late/final stages of succession a significant decrease in the medium gravel percentage and a considerable increase in the sand (fine earth then) was observed (Fig. 6). This is probably due by both biotic agents (as vegetation) and abiotic agents causing the disaggregation of debris and gravel in particles with a smaller diameter (sand). This process should be investigated with further studies to understand the respective roles in the process.
Conclusion
This research allowed a better comprehension of the phenomenon of colonization of calcareous alpine screes by vegetation. Further, it showed how plant cover is a useful parameter to infer some soil features as shear vane strength, depth, sand and medium gravel content, availability of organic matter and total nitrogen as well as some vegetation features as the number of species and ecology of the plants of the alpine screes communities. This is an important result in the framework of environmental and applied botany because it provides researchers with an easy method to evaluate/monitor the features of alpine limestone screes communities and, more importantly, to obtain information on the soil properties, often difficult to measure in the field. Models combining plant cover, community features and soil characteristics found in this study could find application in the field of nature conservation (for example monitoring the increase/decrease of the habitat of endangered/rare screes species) or in the monitoring of environmental hazards (as landslides, debris flow etc.) and/or ecological restoration or slope stabilization works through low impact techniques, current topics in the management of mountain territories in the Alps and all around the world.
Data availability
The raw data supporting the conclusions of this manuscript will be made available by the corresponding author, without undue reservation, to any qualified researcher.
Code availability
Not applicable.
References
Akaike H (1974) A new look at the statistical model identification. IEE Trans Automat Contr 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Andreis C (2002). I distretti geobotanici. In: Del Favero R (ed) I tipi forestali della Lombardia. Cierre, Verona, pp 36–40
Andreis C, Armiraglio S, Caccianiga M, Cerabolini B, Ravazzi C (2017) Alpi Orobie e Prealpi Carbonatiche: un hot spot di endemicità. In: Blasi C, Biondi E (eds) La flora in Italia. Sapienza Università Editrice, Roma, pp 100–103
Aronson J, Alexander S (2013) Ecosystem restoration is now a global priority: time to roll up our sleeves. Restor Ecol 21:293–296. https://doi.org/10.1111/rec.12011
Biondi E (2011) Phytosociology today: methodological and conceptual evolution. Plant Biosyst 145(suppl 1):19–29. https://doi.org/10.1080/11263504.2011.602748
Biondi E, Blasi C (2015) Prodromo Della Vegetazione d’Italia. Available online. https://www.prodromo-vegetazione-italia.org. Accessed 05 February 2022
Blasi C, Capotorti G, Copiz R, Guida D, Mollo B, Smiraglia D, Zavattero L (2014) Classification and mapping of the ecoregions of Italy. Plant Biosyst 148:1255–1345. https://doi.org/10.1080/11263504.2014.985756
Blasi C, Marignani M, Copiz R, Fipaldini M, Bonacquisti S, Del Vico E, Rosati L, Zavattero L (2011) Important Plant Areas in Italy: From data to mapping. Biol Conserv 144:220–226. https://doi.org/10.1016/j.biocon.2010.08.019
Bordoni M, Cislaghi A, Vercesi A et al (2020) Effects of plant roots on soil shear strength and shallow landslide proneness in an area of northern Italian Apennines. Bull Eng Geol Environ 79:3361–3381. https://doi.org/10.1007/s10064-020-01783-1
Braun-Blanquet J (1964) Pflanzensoziologie, 3rd edn. Springer, Wien
Bremner JM (1960) Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci 55:11–33. https://doi.org/10.1017/S0021859600021572
Caccianiga M, Luzzaro A, Pierce S et al (2006) The functional basis of a primary succession resolved by CSR classification. Oikos 112:10–20. https://doi.org/10.1111/j.0030-1299.2006.14107.x
Canullo R, Allegrini MC, Campetella G (2012) Reference field manual for vegetation surveys on the CONECOFOR LII network, Italy (National Programme of Forest Ecosystems Control—UNECE, ICP Forests). Braun-Blanquetia 48:5–65
Chiaradia EA, Vergani C, Bischetti GB (2016) Evaluation of the effects of three European forest types on slope stability by field and probabilistic analyses and their implications for forest management. Forest Ecol Manag 370:114–129. https://doi.org/10.1016/j.foreco.2016.03.050
Chytrý M, Tichý L, Holt J, Botta-Dukát Z (2002) Determination of diagnostic species with statistical fidelity measures. J Veg Sci 13:79–90. https://doi.org/10.1111/j.1654-1103.2002.tb02025.x
Cislaghi A, Bordoni M, Meisina C, Bischetti GB (2017) Soil reinforcement provided by the root system of grapevines: quantification and spatial variability. Ecol Eng 109:169–185. https://doi.org/10.1016/j.ecoleng.2017.04.034
Cislaghi A, Giupponi L, Tamburini A, Giorgi A, Bischetti GB (2019) The effects of mountain grazing abandonment on plant community, forage value and soil properties: observations and field measurements in an alpine area. Catena 181:104086. https://doi.org/10.1016/j.catena.2019.104086
Clements FE (1928) Plant succession and indicators: a definitive edition of plant succession and plant indicators. The Wilson Company, New York
Comunità Montana Valle Brembana (2017) Piano d’Indirizzo Forestale Alta Valle Brembana, relazione di accompagnamento. Comunità Montana Valle Brembana, Piazza Brembana
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
De Cáceres M (2013) How to use indicspecies package (ver. 1.7.1). Centre Tecnologic Forestal de Catalunya, Solsona
Dixon P (2003) Vegan, a package of R functions for community ecology. J Veg Sci 14:927–930. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x
Easlon HM, Bloom AJ (2014) Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl Plant Sci 9:1400033. https://doi.org/10.3732/apps.1400033
Eichel J (2019) Vegetation Succession and Biogeomorphic Interactions in Glacier Forelands. In: Heckmann T, Morche D (eds) Geomorphology of Proglacial Systems, Geography of the Physical Environment. pp 327–349. https://doi.org/10.1007/978-3-319-94184-4_19
Eichel J, Corenblit D, Dikau R (2016) Conditions for feedbacks between geomorphic and vegetation dynamics on lateral moraine slopes: a biogeomorphic feedback window. Earth Surf Process Landf 41:406–419. https://doi.org/10.1002/esp.3859
Eichel J, Krautblatter M, Schmidtlein S, Dikau R (2013) Biogeomorphic interactions in the Turtmann glacier forefield, Switzerland. Geomorphology 201:98–110. https://doi.org/10.1016/j.geomorph.2013.06.012
Giupponi L, Borgonovo G, Giorgi A, Bischetti GB (2019) How to renew soil bioengineering for slope stabilization: some proposals. Landsc Ecol Eng 15:37–50. https://doi.org/10.1007/s11355-018-0359-9
Giupponi L, Giorgi A (2017) Mount Cavallo Botanical Path: A proposal for the valorization of an area of the Orobie Bergamasche Regional Park (Southern Alps). J Prot Mt Areas Res Manag 9:5–15. https://doi.org/10.1553/eco.mont-9-2s5
Giupponi L, Giorgi A (2019) A contribution to the knowledge of Linaria tonzigii Lona, a steno-endemic species of the Orobie Bergamasche Regional Park (Italian Alps). J Prot Mt Areas Res Manag 11:16–24. https://doi.org/10.1553/eco.mont-11-1s16
Giupponi L, Leoni V (2020a) Alpine pioneer plants in soil bioengineering for slope stabilization and restoration: results of a preliminary analysis of seed germination and future perspectives. Sustainability 12:7190. https://doi.org/10.3390/su12177190
Giupponi L, Leoni V (2020b) VegeT: An easy tool to classify and facilitate the management of seminatural grasslands and dynamically connected vegetation of the Alps. Land 9:473. https://doi.org/10.3390/land9120473
Giupponi L, Pentimalli D, Manzo A, Panseri S, Giorgi A (2017) Effectiveness of fine root fingerprinting as a tool to identify plants of the Alps: results of preliminary study. Plant Biosyst 152:464–473. https://doi.org/10.1080/11263504.2017.1306003
Greinwald K, Gebauer T, Treuter L et al (2021) Root density drives aggregate stability of soils of different moraine ages in the Swiss Alps. Plant Soil 468:439–457. https://doi.org/10.1007/s11104-021-05111-8
Grime JP (2001) Plant strategies, vegetation processes and ecosystem properties. Wiley & Sons, Chichester
Hao G, Liu X, Zhang Q et al (2022) Optimum Selection of Soil-Reinforced Herbaceous Plants Considering Plant Growth and Distribution Characteristics. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00768-1
Hu JB, Dai M, Peng ST (2018) An automated (novel) algorithm for estimating green vegetation cover fraction from digital image: UIP-MGMEP. Environ Monit Assess 190:1–15. https://doi.org/10.1007/s10661-018-7075-7
Jones CG (2012) Ecosystem engineers and geomorphological signatures in landscapes. Geomorphology 157–158:75–87. https://doi.org/10.1016/j.geomorph.2011.04.039
Jones CG, Lawton JH, Shachack M (1994) Organisms as ecosystem engineers. Oikos 69:373–386 https://doi.org/10.2307/3545850
Körner C (2021) Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems, 3rd ed. Springer Nature, Cham
Kutschera L, Lichtenegger E, Sobotik M, Haas D (1997) Wurzeln. Bewurzelung von Pflanzen in der verschiedenen Lebensräumen. 5 Band der Wurzelatlas-Reihe. Herausgegeben vom OÖ Landesmuseum, Linz
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. https://doi.org/10.1007/s004420100716
Leuschner C, Ellenberg H (2017) Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats. Springer Nature, Cham
Liu Y-J, Wang T-W, Cai C-F, Li Z-X, Cheng D-B (2014) Effects of vegetation on runoff generation, sediment yield and soil shear strength on road-side slopes under a simulation rainfall test in the Three Gorges Reservoir Area, China. Sci Total Environ 485–486:93–102. https://doi.org/10.1016/j.scitotenv.2014.03.053
Luckman BH (2013) Talus Slopes. In: Elias SA (ed) The Encyclopedia of Quaternary Science. Elsevier, Amsterdam, pp 566–573 https://doi.org/10.1016/B978-0-444-53643-3.00090-X
Martini F, Bona E, Federici G, Fenaroli F, Perico G, et al. (2012) Flora vascolare della Lombardia centro-orientale. Lint, Trieste
Matthews JA (1992) The ecology of recently-deglaciated terrain: a geoecological approach to glacier forelands. Cambridge University Press, Cambridge
Mei W, Yu G, Lai J, Rao Q, Umezawa Y (2018) BasicTrendline: add trendline and confidence interval of basic regression models to plot. R Package Version 2:3
Morris PH, Williams DJ (1993) A new model of vane shear strength testing in soils. Géotechnique 43:489–500. https://doi.org/10.1680/geot.1993.43.3.489
Mucina L, Bültmann H, Dierßen K et al (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci 19(Suppl. 1):3–264. https://doi.org/10.1111/avsc.12257
Nagl F, Erschbamer B (2010) Pflanzliche Sukzessionen im Gletschervorfeld. Vegetation und Besiedlungsstrategien. In: Erschbamer B, Koch EM (eds) Glaziale und periglaziale Lebensräume im Raum Obergurgl. Innsbruck University Press, Innsbruck, pp 121–142
Nagy L, Grabherr G (2009) The biology of alpine habitats. Oxford University Press, New York
Nowak A, Świerszcz S, Nowak S et al (2021) Vegetation Diversity of Screes and Taluses of The Pamir and South-Western Tian Shan in Middle Asia. Folia Geobot 56:43–67. https://doi.org/10.1007/s12224-021-09392-w
Ozenda P (1995) L’endémisme au niveau de l’ensemble du Système alpin. Acta Bot Gallica 142:753–762. https://doi.org/10.1080/12538078.1995.10515302
Papuga K, Kaszubkiewicz J, Kawałko D, Kreimeyer M (2022) Effect of organic matter removal by hydrogen peroxide on the determination of soil particle size distribution using the dynamometer method. Agriculture 12:226. https://doi.org/10.3390/agriculture12020226
Patrignani A, Ochsner TE (2015) Canopeo: A Powerful New Tool for Measuring Fractional Green Canopy Cover. Agron J 107:2312–2320. https://doi.org/10.2134/agronj15.0150
Pawlowski B (1970) Remarques sur l’endémisme dans la flore des Alpes et des Carpates. Vegetatio 21:181–243. https://doi.org/10.1007/BF02269663
Pfiffner OA (2014) Geology of the Alps. Wiley & Sons, Chichester
Pignatti E, Pignatti S (2014). Plant life of the Dolomites. Vegetation Structure and Ecology. Springer Nature, Heidelberg/Berlin
Pignatti S (2017) Flora d’Italia. Edagricole, Bologna
Provincia di Bergamo (2002) Carta geologica della Provincia di Bergamo 1:50.000 Grafica Monti s.n.c., Bergamo
R Development Core Team (2021) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/. Accessed 17 Jan 2021
Raab T, Krümmelbein J, Schneider A et al (2012) Initial Eecosystem processes as key factors of landscape development - a review. Phys Geogr 33:305–343. https://doi.org/10.2747/0272-3646.33.4.305
Raffl C, Mallaun M, Mayer R, Erschbamer B (2006) Vegetation succession pattern and diversity changes in a glacier valley, Central Alps, Austria. Arct Antarct Alp Res 38:421–428. https://doi.org/10.1657/1523-0430(2006)38%5b421:VSPADC%5d2.0.CO;2
Ritchie GSP, Dolling PJ (1985) The role of organic matter in soil acidification. Aust J Soil Res 23:569–576. https://doi.org/10.1071/SR9850569
Schröter C (1926) Das Pflanzenleben der Alpen. Verlag von Albert Raustein, Zürich
Sharifounnasab M, Ullrich CR (1985) Rate of shear effects on vane shear strength. J Geotech Eng 111:135–139. https://doi.org/10.1061/(ASCE)0733-9410(1985)111:1(135)
Simon A, Collison AJC (2002) Quantifying the mechanical and hydrologic effects of riparian vegetation on streambank stability. Earth Surf Process Landf 27:527–546. https://doi.org/10.1002/esp.325
Sutter R (1962) Das Caricion austroalpinae ein neuer insubrisch-sudalpiner Seslerietalia-verband. Mitt Ostalp Din Pflanzensoz Arbeitsgem 2:18–22
Tichý L, Chytrý M (2006) Statistical determination of diagnostic species for site groups of unequal size. J Veg Sci 17:809–818. https://doi.org/10.1111/j.1654-1103.2006.tb02504.x
Tomaselli M, Carbognani M, Foggi B, Adorni M, Petraglia A, Forte TGW, Segadelli S, Rossi G, Gennai M (2021) Scree vegetation in the northern Apennines (N-Italy). Phytocoenologia 51:39–94. https://doi.org/10.1127/phyto/2021/0391
Trakić S, Bakić V, Đug S (2021) Vegetation of alpine screes on Bjelašnica Mt. - syntaxonomy and ecology. Ecol Montenegrina 42:62–84 https://doi.org/10.37828/em.2021.42.3
Valachovič M, Dierssen K, Dimopoulos P et al (1997) The vegetation on screes - A synopsis of higher syntaxa in Europe. Folia Geobot 32:173–192. https://doi.org/10.1007/BF02803739
Verde S, Assini S, Andreis C (2010). Le serie di vegetazione della regione Lombardia. In: Blasi C (ed) La vegetazione d’Italia. Palombi and Partner S.r.l., Roma, pp 181–203
Vergani C, Chiaradia EA, Bischetti GB (2012) Variability in the tensile resistance of roots in Alpine forest tree species. Ecol Eng 46:43–56. https://doi.org/10.1016/j.ecoleng.2012.04.036
Vergani C, Schwarz M, Soldati M et al (2016) Root reinforcement dynamics in subalpine spruce forests following timber harvest: a case study in Canton Schwyz, Switzerland. Catena 143:275–288. https://doi.org/10.1016/j.catena.2016.03.038
Walker LR, Del Moral R (2003) Primary Succession and Ecosystem Rehabilitation. Cambridge University Press, Cambridge
Walker LR, Del Moral R (2008) Lessons from primary succession for restoration of severely damaged habitats. Appl Veg Sci 12:56–67. https://doi.org/10.1111/j.1654-109X.2009.01002.x
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Welker JM, Molau U, Parsons AN et al (1997) Responses of Dryas octopetala to ITEX environmental manipulations: a synthesis with circumpolar comparisons. Glob Change Biol 3:61–73. https://doi.org/10.1111/j.1365-2486,1997.gcb143.x
Yu X, Guo X (2021) Extracting Fractional Vegetation Cover from Digital Photographs: A Comparison of In Situ, SamplePoint, and Image Classification Methods. Sensors 21. https://doi.org/10.3390/s21217310
Acknowledgements
We would like to thank Alessia Rodari and Luigi Abram for the help provided in analyzing soil samples.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This research was supported by the “Montagne: Living Labs di innovazione per la transizione ecologica e digitale” and “Agritech National Research Center” projects.
Author information
Authors and Affiliations
Contributions
LG, and AC conceived and designed the study and interpreted the results. LG performed floristic analyses. LG, DP, VL and MZ performed chemical-physical analysis of soil. LG and AC carried out statistical analysis. LG, AC and VL wrote the manuscript. LG is the project administrator. All authors have read and agreed to this version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Hans Lambers.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giupponi, L., Leoni, V., Pedrali, D. et al. Plant cover is related to vegetation and soil features in limestone screes colonization: A case study in the Italian Alps. Plant Soil 483, 495–513 (2023). https://doi.org/10.1007/s11104-022-05760-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05760-3