Abstract
Purpose
Phosphorus (P) is a limiting nutrient in many managed forests. To further understand the risks and benefits of biochars as sustainable P source in forest management, an improved mechanistic understanding of its interactions in root systems is required.
Methods
A rhizobox experiment was conducted to observe root response of P. sylvestris (Scots pine) seedlings to different biochars in comparison to triple superphosphate (TSP) fertiliser as a P source. Three types of wood-derived biochar were compared: biochar from mixed softwood pellets (“Reference biochar”); from the vascular cambium zone of Picea sitchensis (“VCZ biochar”) and from mixed softwood pellets infused with TSP (“Processed biochar”). These biochars presented a range of available P from low to high. Seedling root development was spatially analysed using GIS software.
Results
The total length of P. sylvestris roots did not significantly differ between treatments. However, seedling roots showed strong preference for soil proximal to VCZ biochar and strong avoidance to TSP fertiliser. There was a milder avoidance effect for Processed biochar. Differences in root responses could be explained by available P: roots favored a moderate, sustained P source and avoided high available P sources. The avoidance effect can be attributed partially to lower soil pH around TSP fertiliser.
Conclusion
The extent concentration and duration of P availability affects the root response of P. sylverstris to P sources. Under P-deficient conditions, P. sylvestris root growth was markedly improved by introducing biochar with a certain P concentration, and VCZ biochar has potential as an effective source of P in forest establishment.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a key limiting nutrient in the growth and establishment of seedlings in forest ecosystems. The increasing atmospheric nitrogen (N) deposition and resulting N accumulation and saturation causes P to replace N as the limitation of tree growth (Attiwill and Adams 1993; Crowley et al. 2012). Under P-deficient conditions, a tight P ‘recycling system’ develops, closing the P cycling loop. This maximizes the use-efficiency of the P pool and sustains the P supply within mature forest systems (Lang et al. 2016). In a mycotrophic forest, a ‘recycling system’ depends on mycorrhizal fungi as well as root morphological alterations (Attiwill and Adams 1993; Lang et al. 2016). However, clear-fell silvicultural systems may disrupt the closed P cycling system, leading to net P loss from the ecosystem P pool by leaching and lateral transport, reducing the soil available P during replanting (Kaila et al. 2014; Nieminen 2003; Rodgers et al. 2010).
Soil P content and plants’ endogenous P status controls root morphology (Neumann et al. 2000), with enhanced root hair development and generate denser root clusters under deficient conditions (Lambers et al. 2006; Ma et al. 2001). Root clusters capture available P in soils by exudation of carboxylates through an anion channel which increases P availability (Lambers et al. 2013). In a mycotrophic forest, the P recycling system, mycorrhizal fungi as well as root morphological alterations play a role (Attiwill and Adams 1993; Lang et al. 2016). Mycorrhizal fungi in symbiosis with roots extend the P depletion zones and accelerate available P transformation through the excretion of organic acids (Cairney 2011) while accessing complex moieties in the soil organic matter (Cánovas 2019; Lindahl and Tunlid 2014).
Biochar is the manufactured product of thermochemical biomass conversion, mainly pyrolysis, and consists of various combination of carbon and mineral nutrients (Sohi et al. 2009). Biochar from woody sawmill residues contains a low concentration of P when compared to biochar produced from animal wastes and even arable crop residues (Hossain et al. 2020; Zhang et al. 2016). Relatively low concentrations of P could limit the agronomic value of wood-derived biochar, particularly when compared with mineral P fertilisers where concentrated P is rapidly disseminated in the soil. Biochar derived from ring debarking residues exhibits higher P concentration compared to other wood-derived biochars due to the relatively high nutrient concentration in the vicinity of the vascular cambium (Rathnayake et al. 2021). Generally, this particular feedstock may provide biochar with higher agronomic value than wood-derived biochars.
Leaving forest residues in-situ following clear-fell harvesting has been reported to mitigate P leaching at the same time as decreasing greenhouse gas emissions (Palviainen et al. 2004; Kaila et al. 2014). The application of wood-derived biochar following clear-fell harvesting has the advantage of stabilizing carbon as well as (Saarela et al. 2020) recycling P from harvested timber. Although there is volatilization of N during pyrolysis, the potential N gap is small and could be matched by N deposition (Crowley et al. 2012). The release mechanism of P from biochar is dependent on multiple factors (Qian et al. 2013; Uchimiya et al. 2010), affected by soil conditions as well as the self-promotion of root development (Abiven et al. 2015; Joseph et al. 2021; Lehmann et al. 2011; Xiang et al. 2017). Indeed, root promotion in pine (Pinus spp.) has been also observed for certain forest systems exposed to wildfire charcoal (Wardle et al. 1998).
Root interactions with the charosphere could be a key factor in plants accessing biochar-derived P (Chen et al. 2021). The porous structure of biochar derived from unprocessed woods makes it easier for roots and hyphae to penetrate and colonize biochar particles, increasing opportunity for nutrient transfer (Prendergast-Miller et al. 2014). The proliferation of mycorrhizal hyphae inside biochar through cracks and pores seems to stimulate root development as well as nutrient uptake (Ascough et al. 2010; Hammer et al. 2014; Jaafar et al. 2014; Solaiman et al. 2010).
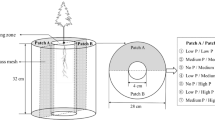
The present study investigates the potential of biochar as a sustainable fertiliser for seedling establishment in P-deficient forest ecosystems, focusing on the interactions between the charosphere and rhizosphere. Three types of biochar were assessed as P sources in the establishment of P. sylvestris seedlings alongside mineral P fertiliser. The response of P. sylvestris root systems to the presence of these biochars of increasing P concentration and availability was assessed for low-P conditions using rhizoboxes (Fig. 1) and a bespoke growing medium (Table 1). Root system responses to the biochars was compared to mineral P fertiliser and to no P addition at all. Our study tests the hypotheses that the acquisition of P affects the spatial distribution of new root growth in the early establishment phase of P. sylvestris seedlings.
Material and methods
Biochar production and properties
The three biochars applied in this study were “Reference biochar” (low in P), “VCZ biochar” (derived from vascular cambium, naturally higher in P) and “Processed biochar” (high in P, artificially enhanced P content). All three biochars used were derived from woody biomass feedstock and produced by the UK Biochar Research Centre (UKBRC; University of Edinburgh, Edinburgh, UK). The equipment used was the Stage III system, a horizontal continuous feed pyrolysis kiln, manufactured by Ansac Pvt Ltd (Bunbury, Australia). The feedstock was pyrolyzed at a nominal highest heating temperature (HTT) of 550 ± 5 °C with a heating rate of 78 °C min−1. Mean residence time in the kiln was 12 min, with 3.9 min at HTT. Details of the Stage III pyrolysis system can be found in Mašek et al. (2018).
The feedstock for VCZ biochar was the product of ring debarking of Picea sitchensis (Sitka spruce) (Rathnayake et al. 2021). This is a sawmill process that removes the outer 5 mm of timber logs, yielding 40% wood and 60% bark by volume, incorporating the vascular cambium. The VCZ feedstock from this study was obtained from a sawmill in south-east Scotland (BSW, Petersmuir, Scotland).
Reference biochar was prepared using standard biochar SWP550, a material widely adopted by the research community and readily available from UKBRC (Mašek et al. 2018). The SWP550 biochar was produced from mixed softwood pellets (Puffin Wood Fuels, Insch, Scotland) using the Stage III pyrolysis system previously mentioned. To provide comparison with Processed biochar that was soaked in P solution and dried afterwards, the SWP550 biochar was soaked in deionized water for 72 h and oven dried at 50 °C.
To create Processed biochar, the SWP550 biochar was infused with dissolved P solution with a concentration of 13.2 mol P l−1 prepared from commercial TSP fertiliser (46% phosphate, Progreen Ltd, Bourne, England). The infusion was undertaken (1:2 w/v) over a period of 72 h to ensure even distribution through the biochar particles. Processed biochar was oven dried at 50 °C.
The composition and properties of the biochars and TSP fertiliser are shown alongside those of the growing medium used in the rhizoboxes in Table 1.
Rhizobox experiment design
Rhizoboxes were made of 0.6 cm thickness acrylic (sides and back) and polycarbonate (front) sheets. The effective size was 40 cm height × 30 cm width × 0.6 cm deep (Fig. 1). The separation was customized for one-year-old P. sylvestris seedlings, considering the diameter of the root collar and the expected length and density of their root systems. The rhizobox comprised of five parts in total including the sides and base, which had machined grooves along joins to provide additional strength and prevent potential slippage. The transparent front sheet was detachable and designed for observation and photographic imaging. Black opaque sheets were used for the other sides and were secure along the exterior joins using polyurethane grab adhesive (Gorilla Glue, Sharonville, Ohio). Rhizoboxes were set for 24 h to ensure that the skeleton of each rhizobox was stable. All rhizoboxes were rinsed, alcohol-sterilized and dried before use.
Bespoke growing medium was prepared and sterilized (at 85 °C) 2 weeks prior to the set-up. It comprised 60% peat and 40% washed quartz sand by volume with no nutrients added, to ensure that key nutrients were initially at a deficient level (Table 1). These conditions represent the nutrient regime of poor forest soil (Pyatt et al. 2001) and typical P-poor plantation forests in the UK (Lang et al. 2017). The growing medium was passed through a 2-mm sieve prior to filling the prepared rhizoboxes. If the measured pH (McLean 1983) was outside the range 4.5 ± 0.5 it was adjusted using 0.01 mol l−1 NaOH solution and 0.01 mol l−1 H2SO4 sprayed on the surface of growing medium and mixed afterwards. Approximately 720 g of growing medium was added into each rhizobox. The variation of added growing medium was no more than 10% to maintain a bulk density of 1.0 ± 0.10 kg m−3 to provide a comparable growing environment at the outset.
The 30 cm × 40 cm frontal area of the rhizoboxes was divided into twelve unit-sectors with a size of 10 cm width × 10 cm height which were marked onto the front sheet to create a visual grid. After the growing medium was added, the four treatment materials with complete structures were applied at differential mass dose based on the recommended dose of P fertilisation for conifer seedlings in P deficient soils (Taylor 1991). These amounts were 45 g rock phosphate/m2, adjusted for targeted application and growing medium volume, to maintain equivalent TSP:growing medium ratio: 0.96 g TSP fertiliser, 4.56 g Processed biochar, 3.60 g Reference biochar and 3.60 g VCZ biochar. The difference in mass dose of Processed biochar compared to other biochar was due to the mass of infused TSP within the material: 3.60 g Reference biochar + 0.96 g TSP fertiliser. The treatments were introduced centrally in the lower left sector without any mesh exclusion. No movement of treatment areas occurred during the experiment. The diameter of the treatment area (TA) for TSP fertiliser, Processed biochar, Reference biochar and VCZ biochar was 2 cm, 4 cm, 5.5 cm and 7.8 cm, respectively and implemented using prepared paper templates. The original growing medium was removed from the TA before the treatment materials were added. Treatment materials were mixed with growing medium (1:1) prior to application in order to prevent airspaces between treatment particles and simulate forest fertilisation conditions. Each treatment was arranged with five replicates. A blank control (“Blank”) comprising growing medium was included, resulting in 25 rhizoboxes in total. The growing medium and treatment materials in the rhizoboxes were fully watered (until point of saturation) using distilled water and allowed to equilibrate for 48 h before planting the seedlings.
One-year-old container grown P. sylvestris seedlings were obtained from Forest Research, Northern Research Station, Roslin, Scotland. P. sylvestris was selected as it is the most tolerant and suitable conifer species in sandy P-deficient soils in the UK and has a high commercial profit and ecological benefits (Savill 2019). These seedlings had been grown from seed and all seedlings were in a dormant state. The seedlings had been grown under non-deficient nutrient conditions and all displayed root systems 20–30 cm in length. Seedlings were carefully removed from their containers, growing medium manually removed, and roots carefully rinsed to avoid transfer of nutrients. The seedlings were planted into the rhizoboxes in the central position, gently separating entangled roots. To diminish the possibility of ‘transplant shock’, the roots were not sterilized or trimmed. To ensure roots could consistently detect the added P sources, one root in each rhizobox was positioned 2 cm adjacent to the edge of the TA, oriented towards the TA center. Each rhizobox was watered weekly by removing the front sheet and evenly spraying the surface medium with distilled water using a mist sprayer. The watering regime was flexible to ensure that the growing medium was moist but with no excess water or vertical water movement or pooling, preventing lateral P movement from TAs.
The front (colorless) polycarbonate sheets were clipped to the treated, planted rhizoboxes. Polystyrene sheets cut to the same size and shape were used to exclude light and maintain consistent internal conditions. They were also used to loosely cover the surface of the growing medium to reduce soil surface light penetration and temperature gradients, while ensuring respiration was not impeded. The temperature and relative humidity in the glasshouse were between 17.0–27.2 °C and 36.5–100.0%, respectively during the 10 weeks experimental period, detailed data can be found in Supplementary Information 2. The experiment duration was determined by the time required for full colonisation of the rhizoboxes by the seedling roots.
The experiment was arranged using a randomized block design, with one replicate of each treatment randomly placed in each block. The rhizoboxes in each block were stacked at an angle of 60°, slightly staggering the boxes (vertically and horizontally) to ensure the above ground parts of the seedlings did not interfere (Fig. 1).
Sampling and chemical analysis
At the conclusion to the experiment (after 10 weeks), the root system was separated from the seedling at the first root branch and was gently rinsed, dried in an oven at 90 °C and weighed on an electronic balance. New leader needles were picked individually from the top and oven dried at 90 °C before further total P analysis. The needles were digested with concentrated H2SO4 and 30% H2O2 and analysed by autoanalyser (Bran Luebbe AA3, Seal Analytical, Norderstedt, Germany).
Each treatment material was analysed before use. The same analysis was conducted on the growing medium at the outset and on TA material at the beginning and end of the growing period (Table 1). For the initial analysis of TA, material was pooled across replicates. After 10 weeks the TA was sampled, together with TA3 (the zone at the edge of the TA extending outwards into the growing medium for 3 cm, Fig. S3). The growing medium were sampled separately from each replicate rhizobox, using prepared paper templates to ensure correct positioning. The material sampled within each TA was then thoroughly mixed to reduce intra-sample variation and transferred to a refrigerator below 4 °C. Part of each fresh sample was used for NH4+-N, NO3—N and available P analysis. The rest of each samples was subsequently oven dried at 90 °C and milled in a ball grinder before further analysis for Fe, Al and Ca.
Chemical analysis of the growing medium and TA was undertaken to elucidate potential factors influencing root-biochar interactions. For the pooled pre-planting samples, the extraction or digestion procedure was replicated 3 times (as technical replicates). The final samples (after 10 weeks) were only extracted or digested once, i.e. without technical replicates. Extraction for NH4+-N and NO3−-N was undertaken using 1:10 w/v 1 M KCl and analysed by autoanalyser (as above). Available P was analysed through water extraction (Prendergast-Miller et al. 2014) and subsequent analysis of the extracts by autoanalyser (as above). Fe, Al and Ca was assessed using a modified dry-ash method for biochar. Samples were heated in a muffle furnace (500 °C, 8 h) and placed into a steam bath upon cooling. Concentrated nitric acid (HNO3) was added and evaporated to dryness, followed by 1:4 HNO3 and H2O2 which was evaporated to dryness. All residues were transferred through Whatman Grade 4 filter paper, using HNO3 and deionized water, into a volumetric flask. The elemental composition of the filtrate was measured by inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo-iCAP 6300, Thermo Electron, Waltham, USA). The pH of the growing medium was assessed for TA3 after the 10-week experiment period using a soil:water ratio of 1:2 (McLean 1983).
Morphological monitoring and analysis of root growth
The root system in each rhizobox was imaged photographically once each week (7 days interval). A lightbox was constructed from PVC sheet enveloped with thick photographic curtain. A port was created at the top of the box to provide access for a camera lens. The box was illuminated internally with LED symmetrical lighting strips orientated in four evenly distributed parallel lines across the top of the box, while thick black felt was used to line the base and sides to eliminate the ingress of natural light. The rhizoboxes were imaged in turn, with a fixed camera position in the light sealed port and with a precisely determined position for the rhizobox that ensured consistency in image positions. The rhizoboxes were imaged weekly using a DSLR camera at a native resolution of 7360 × 4912 pixels.
A method to spatially track and analyse root growth was developed using ArcGIS Pro 2.6 software (ESRI, Redlands, USA). The first step was to map each image to a referenced 30 cm × 40 cm physical area (the effective rhizobox area). This provided the coordination required for ArcGIS Pro 2.6 to define the absolute position feature layer which was created to manually track the growth of individual roots, assisted by the association between root color and increasing root age. A line feature layer along with corresponding point feature layer was created afterwards. The “Summarize Within” tool in ArcGIS was used to calculate the length and density of new roots. Kernel density is widely used in visualizing spatial data (Kalinic and Krisp 2018). Since root development in the interior surface of rhizoboxes could be regarded as a two-dimensional space, Kernel Density analysis was assessed in ArcGIS to analyse the cluster of root data (Kalinic and Krisp 2018; Okabe et al. 2009). Both the cell size and search radius used were default values calculated by the tool. The Gi* Hot Spot Analysis was conducted along with Kernel Density using similar procedure (Kalinic and Krisp 2018) (Fig. S2), visualising statistical information. New roots developed on the interior surface of the rhizoboxes owing to geotropism and the availability of water and air. Only new roots growing on the surface of front sheet were assessed and counted.
Weekly images were analysed as separate segments for the same rhizobox image. Overlaying the images in this way allowed an integrated root growth feature layer to be created and summarized for each rhizobox. This provided the calculated length and density of cumulative root growth. The analysis also allowed length, density and growth rate to be visualised and analysed. Root growth was separately assessed for the whole rhizobox, the TA and TA3.
Statistical analysis
All data were tested for normality (K-S test) and homogeneity of variance (Levene test, p = 0.05). One-way analysis of variance (ANOVA) was conducted using Python 3.8 (Scotts Valley, CA) and with p = 0.05 as the threshold for statistical significance to compare the root density, root density difference, available P, and total Al, Fe and Ca between treatments. Post-hoc pairwise comparison was conducted using Tukey tests. Considering the F-test results and the distribution of residuals, a square root transformation-linear model was applied to root density data to distribute slight skew and reduce heteroscedasticity of the residuals (Osborne 2002) (Fig. 2). Both the simple linear model and linear model after square root transformation (Osborne 2002) were developed using Python 3.8 (Scotts Valley, CA).
Square root transformation-linear model fitting root results for different treatments over 10 weeks. Root density in the whole rhizobox for (a) Blank; (b) TSP; (c) Processed; (d) Reference and (e) VCZ treatments. Root density of TA3 in (f) Blank; (g) TSP; (h) Processed; (i) Reference and (j) VCZ treatments. Detailed results see Table S3, n = 4 in Blank and n = 5 in other treatment. Plots with no trend line indicate non-significance according to the linear regression model
Results
Root morphological responses to different treatment materials
After 10 weeks, there was no statistically significant effect of treatment material on total growth of new roots or total root biomass (Table S2). One seedling in the Blank treatment did not survive transplanting and this replicate was excluded from further analyses. All roots within 2 cm of TSP TA exhibited complete death of root tissues. Similar root mortality was found in one rhizobox where the treatment patch contained Processed biochar.
Linear regression revealed similar growth trends of roots throughout the rhizobox between the Blank, Reference and VCZ biochar treatments after 10 weeks (Fig. S1). Root growth in Blank treatment was much higher than Processed biochar and TSP treatment. In TA3, root growth was significantly higher for rhizoboxes with TAs containing Reference biochar or VCZ biochar. Detailed fitting data was listed in Table S3. Total root growth between treatments was quite similar at the beginning (< 4 weeks). The slope between treatments was fairly consistent and no significant difference could be observed in the short term, which was in accordance with One-way ANOVA results (Table S1). The limitations of available nutrients and rhizobox space in the rhizoboxes preclude prediction in the long term. In TA3 (Fig. 2f–j), the root growth for Reference biochar and VCZ biochar treatments was consistent with the growth trend in the whole rhizoboxes and much greater than that in Processed biochar and TSP fertiliser treatments. Root growth for Processed biochar and TSP fertiliser was at a consistent, extremely low level. The higher slope k of linear fitting indicates that the root growth for TA3 in Reference biochar (0.075) and VCZ biochar (0.077) treatments was greater than other treatments (0.036 in Blank treatment, 0.026 in Processed biochar treatment) prior to Week 8.
According to model fitting results (Fig. 2) as well as Kernel Density analysis results (Fig. 3), P. sylvestris seedling roots have a distinct growth preference for VCZ biochar TA. The mean root density in the 10th week in the VCZ TA and TA3 was 4.2 mm cm−2 and 4.1 mm cm−2 greater than the Blank treatment, respectively. The TA in TSP treatment showed a clear limiting effect on seedling root density, with a complete absence of root growth in 4 replicates. A similar but milder limiting effect on root development was observed in Processed biochar treatment. P. sylvestris roots show undifferentiated growth in the Blank treatment. It was notable that the area of densest root growth for VCZ biochar was located at the boundary of the TAs and that root density was significantly higher than the corresponding areas in the Blank treatment.
By comparing root density in TA3 with the mirrored area on the opposite side of the rhizobox, a quantitative left-to-right comparison can be made throughout the experimental period for each treatment based on weekly images (Fig. 4). During the first 7 weeks, there was no significant preference for roots associated with any TAs, although avoidance of TSP was visually apparent. From week 8, the left-to-right comparison became significant for TSP fertiliser and VCZ biochar treatments. Subsequently, from week 8, the trends began to clearly diverge (Fig. 4). In VCZ biochar and Reference biochar treatments, root growth became concentrated within the TA indicating the emergence of seedling root development preferences. Conversely, an avoidance emerged in Processed biochar and TSP fertiliser treatments. In the Blank treatment, a weak imbalance emerges during the initial growth (week 0–7), but convergence on a left-to-right equilibrium indicates that the contribution of new roots in the Blank treatment gradually becomes uniform and undifferentiated (Fig. 4). Overall, the differences demonstrate that there was a strong preference for P. sylvestris roots to forage around VCZ biochar and a milder corresponding preference for Reference biochar. There was a strong avoidance for TSP fertiliser and a milder avoidance for Processed biochar. Around Processed biochar, a high density of new roots was concentrated on the outer edge of the TA (Fig. 3).
Cumulative weekly root density difference between TA3 and corresponding areas on the opposite side of the rhizobox for 10 weeks. A positive number means that the density in TA3 was greater, while a negative number means density in TA3 was lower. A positive number indicates greater new root growth in TA3, a negative number indicates greater new growth in the opposite side. Data are mean ± SE, n = 5 except for Blank where n = 4
Roots response to different pH
The growing medium was alkalised by VCZ biochar and Reference biochar, and acidified by Processed biochar and TSP fertiliser (Table 1). After 10 weeks, pH in areas within 3 cm of the TA stabilized between 5.2 to 6.2, with pH 5.7–6.2 (approx.) in TA3 of the Blank, VCZ biochar and Reference biochar treatments, and pH 5.2–5.7 in TA3 of Processed biochar and TSP fertiliser treatments. The difference between the Blank treatment and both Processed biochar and TSP fertiliser was significant (Table S5), even in areas at a greater distance (4.5 cm) from TA3 (Fig. S3).
Roots response to different available P
The available P in TA ranged from extremely low in the Blank treatment to extremely high in TSP fertiliser treatments. TSP fertiliser has extremely high available P (and low pH).
Regardless of source, the P available in TAs declined drastically after 10 weeks (Fig. 5a). Proportional available P decline was not significantly different between TSP fertiliser, Processed biochar and Reference biochar treatments, while the difference between the Blank treatment and other treatments was significant. The VCZ biochar treatment showed the lowest proportional decline (92.3%) in available P over the duration of the experiment (apart from the Blank treatment, where the decrease was 44%). The decrease in available P for VCZ biochar is significantly lower than in TSP fertiliser treatments (97.9%). The mean available P decline in Reference biochar and Processed biochar treatments was 95.6% and 96.1%, respectively.
After 10 weeks experimental period, (a) Available P reduction in TA, p < 0.05. Data are mean ± SE, n = 3 (technical replicates) at the beginning, n = 5 after 10 weeks (except for Blank where n = 4); (b-d) Ca, Fe and Al concentration in TA. Data are mean ± SE, n = 3 (technical replicates) at the beginning (except for Blank where n = 5)
There was significantly higher Ca, Fe and Al in TAs of TSP treatment (Fig. 5b). The Fe and Al concentration in the TA of VCZ biochar treatment was lower than that in TSP fertiliser treatment, but similar to Reference biochar treatment. Processed biochar treatment showed a significantly higher Al concentration in the TA than that of the Blank treatment.
Total P concentration in new growth leader needles was significantly higher for TSP fertiliser treatments than the Blank and VCZ biochar treatments, but did not differ between the Blank and VCZ biochar or Reference biochar treatments (Table S6).
Discussion
P. sylvestris seedlings show altered root development strategies in response to distinct P sources. The morphological root response in the acquisition of P from different sources determines the spatial distribution of new root growth in the early establishment phase. There are several potential mechanisms to account for these differences, which either enhance or limit root development.
Morphological and spatial root responses to sources of phosphorus
The length of roots is known to be affected by many factors, including soil nutrient content (López-Bucio et al. 2003), pH (Hinsinger et al. 2003; Vanguelova et al 2007) and soil physical structure (Dexter 2004). Some effects of P-deficient conditions are the stimulation of primary root growth and the exploration of a larger soil volume (Ma et al. 2001). Roots under these conditions may also exude carboxylates to facilitate the mobilization of P (Lambers et al. 2013). Calvaruso et al. (2013) showed that P. sylvestris Scots pine can accelerate apatite dissolution through organic acid. In the present study, the roots of P. sylvestris showed clear preference for VCZ biochar compared to the Blank treatment, and a milder preference for Reference biochar (Fig. 3). These preferences indicate high delivery of P present in the biochar. Deliverable P could be expressed as the available P in soil which plants can actually access, which is limited by root health and root accessibility to available P-rich zones. Mineral nutrients associated with the interior and exterior surfaces of biochar may be mobilized by exposure to soil solution, providing a supply of assimilable P in otherwise deficient soils (Joseph et al. 2021). Phosphorus in biochar originates from the feedstock biomass and in general, displays low concentrations. The vascular cambium has a higher concentration (Risopatron et al. 2010), extending to the zones used to create the VCZ biochar from Picea sitchensis (Sitka spruce) biomass.
Root aggregation occurs around the boundary of the VCZ biochar TA (Fig. 3e), suggesting that VCZ biochar could provide more available P to seedlings compared with Reference biochar and growing medium without additional P source. VCZ biochar concentrated root development in TA and limited foraging in areas that are low in P. Antithetical to VCZ biochar, Reference biochar provided low available P, resulting in a sparser but wider root system in order to explore a large volume of growing medium to satisfy P demand (Fig. 3d).
In addition to the negative effect of low pH on root density and biomass of P. sylvestris seedlings (Vanguelova et al. 2007), alteration of soil pH can result in phosphorus fixation in growing medium, where Al, Fe and Ca competitively bind to free phosphate compounds, decreasing the P that is available in soils (Schlesinger 2005). The P pool in the Blank treatment was probably at equilibrium with respect to pH. The TSP fertiliser lowered soil pH and contributed to accelerating decline of available P after application as the soluble phosphate may be sorbed by Al, Fe and Ca within comparatively a short period of time (Penn and Camberato 2019) (Fig. 5a). Although acidic conditions have a negative long-term impact on P acquisition provision in a forest system, extremely high available P can directly inhibit root growth and potentially cause root mortality (Ma et al. 2001). While TSP fertilisers guarantee P supply for P. sylvestris, the concentration of P can still inhibit wider foraging, leading to root structures that are potentially detrimental to mature stands. This may adversely impact the deliverability of P to the seedling and limit the potential uptake of P from bulk soil once the additional P was sorbed, due to poorly developed root systems. The actual effect of TSP fertiliser on root development and foraging ability is related to the application regime and rates which should be carefully considered when applied to natural stands as indirect topical application of a 1:1 ratio of TSP to growing medium has caused root mortality and root forage inhibition (Fig. 2g).
Application of biochars showed variable impacts on the pH of the external growing medium, conversely, the external pH will have also affected the availability of the P present in different amounts in different biochars. These factors interact to determine the P that is available inside and around biochar TA. High levels of Ca could limit the release of P from the high-pH interior of VCZ and Reference biochars (Buss et al. 2018). The proportional decrease in available P from VCZ biochar treatments was, however, significantly smaller than for TSP fertiliser (Fig. 5a). The pH conditions at the interface of alkaline biochar and acidic growing medium could be critical, providing for the mobilization of P bonded with Ca but without Fe phosphate that form below pH ~ 6 (Penn and Camberato 2019). It is likely that the availability of P was affected indirectly by pH, altering the labile P pool in the growing medium and the total P released from the TA. Comparison of root biomass in the TA of contrasting pH revealed no significant difference, suggesting that direct effects of pH were not a major factor.
In addition to the chemical mobilization of P that occurs along a pH gradient, physical changes could be relevant. VCZ biochar is friable, has a low bulk density, high porosity and a large internal surface area (Rathnayake et al. 2021). This supports relatively easy access by roots and ectomycorrhizal fungi that facilitate P acquisition. The folding, compression and heating that occurs in the pelleting of biomass for Reference biochar and Processed biochar creates structures that are less conducive to such interactions. Pelletisation of biomass destroys the cellular structure of wood, decreasing the porosity and surface area of potential biochar, while conversely increasing its potential bulk density (Stelte et al. 2012). Surface colonization by ectomycorrhizal fungi was visually apparent on VCZ and Reference biochar particles (Fig. S4). These fungi could provide a mycotrophic alternative for the access and acquisition of P by P. sylvestris (Joseph et al. 2021). On the other hand, the potential for these microorganisms to assist in the uptake of P within the timeframe of the rhizobox study is uncertain.
Analysis for metals revealed concentrations of Cd in TSP fertiliser (Table 1) that could potentially limit the root growth and levels of Cd in plant tissue that present toxicity issues (Yazici et al. 2021). Although direct toxic effects on P. sylvestris root systems and above-ground depends on the free Cd2+ concentration in plant tissues, which was not analysed, the Cd concentration measured in TSP fertiliser was approximately an order of magnitude higher than the 5 mg kg−1 threshold proposed by Ismael et al. (2019) and set for fertiliser products in the Netherlands (7 mg kg−1) (Crommentuijn et al. 2000), although within the threshold of 60 mg kg−1 set by the EU (Council regulation 2019). Since most of the P added in Processed biochar originated from TSP, it was not surprising that Cd concentrations in Processed biochar also exceeded some fertilizer thresholds.
Potential of biochar in phosphorus management in temperate forest systems
The available P in bulk soil is critical to seedling establishment success in the first stage of forest regeneration (Ceccon et al. 2003) and is often the limiting factor during seedling establishment in sandy upland planting sites in the UK. P. sylvestris is widely planted in semi-natural and commercial forests (Savill 2019) as a tolerant species that can survive and grow in nutrient poor sandy soils where other species would be unsuitable. Seedling root development is important as it influences the root architecture of the mature tree, with high lateral root growth of improving tree stability and mature growth. Wind damage is the primary factor in determining the terminal height of trees in Scotland and the primary cause of tree damage in the UK. Targeted application of fertiliser P can lead to under-developed or asymmetric root architecture in mature trees, affecting crop stability (Krišāns et al. 2020) and deliverability of P. The stimulation of root development from biochar amendment could potentially improve the structure of roots in mature stands, although further research is required. Improved crop stability may be important to the future resilience of forests in the face of climate change (Alongi 2008).
Biochar has been recognized as an option for improved management of agricultural systems, including the UK (Dobbie et al. 2011). The specific use of biochar in silviculture has been less widely considered. The results of the present study indicate that biochar can be selected to efficiently deliver small doses of P to tree seedlings, manipulating pH in the charosphere and potentially stimulating symbioses with fungi, VCZ biochar has similar available P and porous structure to charcoal collected in coniferous forests after wildfires (Gundale and DeLuca 2007; Santín et al. 2017) and Wardle et al. (1998) which have demonstrated positive effects on germination and growth of trees in pine forest. Pinus spp. are generally fire-adapted and other studies have shown a significant improvement of plant growth after applying charcoal derived from wildfires (DeLuca et al. 2006; Gundale and DeLuca 2007).
Co-products of timber processing such as the cambial zone of P. sitchensis contain nutrient P that could be usefully and effectively cycled back into forestry at nutrient limited establishment sites. Supplying wood-derived P to P-deficient forest using biochar would expand local P pools and assist in closure of the P cycle, contributing to the sustainability of forestry operations (Palviainen et al. 2004; Kaila et al. 2014).
Conclusion
Growth of roots in Pinus sylvestris (Scots pine) seedlings showed strong preference for growing medium proximal to biochar made from the vascular cambial zone (VCZ) of Picea stichensis (Sitka spruce) and strong avoidance to TSP fertiliser. The preference and avoidance effects for biochar from softwood pellets with and without additional infused phosphorus were milder. These different preferences reflected the concentration and duration of P available from the materials, which was probably a function of pH at the soil interface, led to suppression of root growth and decrease the availability and deliverability of P. VCZ biochar has good potential as a source of P in establishment of forests on P-deficient soils.
Data availability
The dataset generated during this study are available from the corresponding author on reasonable request.
References
Abiven S, Hund A, Martinsen V, Cornelissen G (2015) Biochar amendment increases maize root surface areas and branching: a shovelomics study in Zambia. Plant Soil 395:45–55. https://doi.org/10.1007/s11104-015-2533-2
Alongi DM (2008) Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76:1–13. https://doi.org/10.1016/j.ecss.2007.08.024
Ascough PL, Sturrock CJ, Bird MI (2010) Investigation of growth responses in saprophytic fungi to charred biomass. Isotopes Environ Health Stud 46:64–77. https://doi.org/10.1080/10256010903388436
Attiwill PM, Adams MA (1993) Nutrient cycling in forests. New Phytol 124:561–582. https://doi.org/10.1111/j.1469-8137.1993.tb03847.x
Buss W, Assavavittayanon K, Shepherd JG, Heal KV, Sohi S (2018) Biochar phosphorus release is limited by high ph and excess calcium. J Environ Qual 47:1298–1303. https://doi.org/10.2134/jeq2018.05.0181
Cairney JW (2011) Ectomycorrhizal fungi: the symbiotic route to the root for phosphorus in forest soils. Plant Soil 344:51–71. https://doi.org/10.1007/s11104-011-0731-0
Calvaruso C, Turpault MP, Frey-Klett P, Uroz S, Pierret MC, Tosheva Z, Kies A (2013) Increase of apatite dissolution rate by Scots pine roots associated or not with Burkholderia glathei PML1 (12) Rp in open-system flow microcosms. Geochim Cosmochim Acta 106:287–306. https://doi.org/10.1016/j.gca.2012.12.014
Cánovas FM (2019) Molecular Physiology and Biotechnology of Trees. Academic Press, San Diego
Ceccon E, Huante P, Campo J (2003) Effects of nitrogen and phosphorus fertilization on the survival and recruitment of seedlings of dominant tree species in two abandoned tropical dry forests in Yucatán, Mexico. For Ecol Manag 182:387–402. https://doi.org/10.1016/S0378-1127(03)00085-9
Chen X, Lewis S, Heal KV, Lin Q, Sohi SP (2021) Biochar engineering and ageing influence the spatiotemporal dynamics of soil pH in the charosphere. Geoderma 386:114919. https://doi.org/10.1016/j.geoderma.2020.114919
Council regulation (2019) Council regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products. Official Journal L170. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009&from=EN. Accessed 21 Sept 2022
Crommentuijn T, Sijm D, de Bruijn J, van den Hoop M, van Leeuwen K, van de Plassche E (2000) Maximum permissible and negligible concentrations for metals and metalloids in the Netherlands, taking into account background concentrations. J Environ Manage 60:121–143. https://doi.org/10.1006/jema.2000.0354
Crowley KF, McNeil BE, Lovett GM et al (2012) Do nutrient limitation patterns shift from nitrogen toward phosphorus with increasing nitrogen deposition across the northeastern United States? Ecosystems 15:940–957. https://doi.org/10.1007/s10021-012-9550-2
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453. https://doi.org/10.2136/sssaj2005.0096
Dexter AR (2004) Soil physical quality: Part I. Theory, effects of soil texture, density, and organic matter, and effects on root growth. Geoderma 120:201–214. https://doi.org/10.1016/j.geoderma.2003.09.004
Dobbie KE, Bruneau PMC, Towers W (2011) The State of Scotland's Soil. Natural Scotland. http://www.sepa.org.uk/land/land_publications.aspx. Accessed 21 Sept 2022
Gundale MJ, DeLuca TH (2007) Charcoal effects on soil solution chemistry and growth of Koeleria macrantha in the ponderosa pine/Douglas-fir ecosystem. Biol Fert Soils 43:303–311. https://doi.org/10.1007/s00374-006-0106-5
Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SL, Rillig MC (2014) A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol Biochem 77:252–260. https://doi.org/10.1016/j.soilbio.2014.06.012
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59. https://doi.org/10.1023/A:1022371130939
Hossain MZ, Bahar MM, Sarkar B et al (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2:279–420. https://doi.org/10.1007/s42773-020-00065-z
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2019) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11:255–277. https://doi.org/10.1039/c8mt00247a
Jaafar NM, Clode PL, Abbott LK (2014) Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J Integr Agr 13:483–490. https://doi.org/10.1016/S2095-3119(13)60703-0
Joseph S, Cowie AL, Van Zwieten L et al (2021) How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 13:1731–1764. https://doi.org/10.1111/gcbb.12885
Kaila A, Sarkkola S, Laurén A et al (2014) Phosphorus export from drained Scots pine mires after clear-felling and bioenergy harvesting. Forest Ecol Manag 325:99–107. https://doi.org/10.1016/j.foreco.2014.03.025
Kalinic M, Krisp JM (2018) Kernel density estimation (KDE) vs. hot-spot analysis–detecting criminal hot spots in the City of San Francisco. The 21st Conference on Geo-Information Science
Krišāns O, Samariks V, Donis J, Jansons A (2020) Structural Root-plate characteristics of wind-thrown Norway spruce in hemiboreal forests of Latvia. Forests 11:1143. https://doi.org/10.3390/f11111143
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Lambers H, Clements JC, Nelson MN (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100:263–288. https://doi.org/10.3732/ajb.1200474
Lang F, Bauhus J, Frossard E et al (2016) Phosphorus in forest ecosystems: new insights from an ecosystem nutrition perspective. J Plant Nutr Soil Sci 179:129–135. https://doi.org/10.1002/jpln.201500541
Lang F, Krüger J, Amelung W, Willbold S, Frossard E, Bünemann EK et al (2017) Soil phosphorus supply controls P nutrition strategies of beech forest ecosystems in Central Europe. Biogeochemistry 136:5–29. https://doi.org/10.1007/s10533-017-0375-0
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – A review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Lindahl DB, Tunlid A (2014) Ectomycorrhizal fungi – potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447. https://doi.org/10.1111/nph.13201
López-Bucio J, Cruz-Ramırez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287. https://doi.org/10.1016/S1369-5266(03)00035-9
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 24:459–467. https://doi.org/10.1046/j.1365-3040.2001.00695.x
Mašek O, Buss W, Roy-Poirier A et al (2018) Consistency of biochar properties over time and production scales: A characterisation of standard materials. J Anal Appl Pyrol 132:200–210. https://doi.org/10.1016/j.jaap.2018.02.020
McLean EO (1983) Soil pH and lime requirement. In: Page AL (ed) Methods of soil analysis: Part 2 Chemical and microbiological properties, 2nd edn. American Society of Agronomy, Soil Science Society of America, Madison, pp 199–224
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919. https://doi.org/10.1006/anbo.2000.1135
Nieminen M (2003) Effects of clear-cutting and site preparation on water quality from a drained Scots pine mire in southern Finland. Boreal Environ Res 8:53–59
Okabe A, Satoh T, Sugihara K (2009) A kernel density estimation method for networks, its computational method and a GIS-based tool. Int J Geogr Inf Sci 23:7–32. https://doi.org/10.1080/13658810802475491
Osborne J (2002) Notes on the use of data transformations. Prac Assess Res Evaluation 8:6. https://doi.org/10.7275/4vng-5608
Palviainen M, Finér L, Kurka AM, Mannerkoski H, Piirainen S, Starr M (2004) Decomposition and nutrient release from logging residues after clear-cutting of mixed boreal forest. Plant Soil 263:53–67. https://doi.org/10.1023/B:PLSO.0000047718.34805.fb
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120. https://doi.org/10.3390/agriculture9060120
Prendergast-Miller MT, Duvall M, Sohi SP (2014) Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur J Soil Sci 65:173–185. https://doi.org/10.1111/ejss.12079
Pyatt G, Ray D, Fletcher J (2001) An Ecological Site Classification for Forestry in Great Britain. Bulletin 124. Forestry Commission, Edinburgh
Qian T, Zhang X, Hu J, Jiang H (2013) Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 93:2069–2075. https://doi.org/10.1016/j.chemosphere.2013.07.041
Rathnayake D, Creber H, Van Poucke R, Sohi SP, Meers E, Mašek O, Ronsse F (2021) Biochar from sawmill residues: characterization and evaluation for its potential use in the horticultural growing media. Biochar 3:201–212. https://doi.org/10.1007/s42773-021-00092-4
Risopatron JPM, Sun Y, Jones BJ (2010) The vascular cambium: molecular control of cellular structure. Protoplasma 247:145–161. https://doi.org/10.1007/s00709-010-0211-z
Rodgers M, O’Connor M, Healy MG et al (2010) Phosphorus release from forest harvesting on an upland blanket peat catchment. For Ecol Manag 260:2241–2248. https://doi.org/10.1016/j.foreco.2010.09.037
Saarela T, Lafdani EK, Laurén A, Pumpanen J, Palviainen M (2020) Biochar as adsorbent in purification of clear-cut forest runoff water: Adsorption rate and adsorption capacity. Biochar 2:227–237. https://doi.org/10.1007/s42773-020-00049-z
Santín C, Doerr SH, Merino A et al (2017) Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci Rep 7:11233. https://doi.org/10.1038/s41598-017-10455-2
Savill PS (2019) The silviculture of trees used in British forestry, 3rd edn. CABI, Boston
Schlesinger WH (2005) Biogeochemistry. Elsevier, Amsterdam
Sohi S, Lopez-Capel E, Krull E, Bol R (2009) Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci Rep 5:17–31
Solaiman ZM, Blackwell P, Abbott LK, Storer P (2010) Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Soil Res 48:546–554
Stelte W, Sanadi AR, Lei S, Holm JK, Ahrenfeldt J, Henriksen UB (2012) Recent developments in biomass pelletization-A review. BioResources 7:4451–4490
Taylor CMA (1991) Forest Fertilisation in Britain. Forestry Commission, London
Uchimiya M, Lima IM, Klasson KT, Wartelle LH (2010) Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere 80:935–940. https://doi.org/10.1016/j.chemosphere.2010.05.020
Vanguelova EI, Nortcliff S, Moffat AJ, Kennedy F (2007) Short-term effects of manipulated increase in acid deposition on soil, soil solution chemistry and fine roots in Scots pine (Pinus sylvestris) stand on a podzol. Plant Soil 294:41–54. https://doi.org/10.1007/s11104-007-9225-5
Wardle DA, Zackrisson O, Nilsson MC (1998) The charcoal effect in Boreal forests: mechanisms and ecological consequences. Oecologia 115:419–426. https://doi.org/10.1007/s004420050536
Xiang Y, Deng Q, Duan H, Guo Y (2017) Effects of biochar application on root traits: a meta-analysis. GCB Bioenergy 9:1563–1572. https://doi.org/10.1111/gcbb.12449
Yazici MA, Asif M, Tutus Y, Ortas I, Ozturk L, Lambers H, Cakmak I (2021) Reduced root mycorrhizal colonization as affected by phosphorus fertilization is responsible for high cadmium accumulation in wheat. Plant Soil 468:19–35. https://doi.org/10.1007/s11104-021-05041-5
Zhang H, Chen C, Gray EM, Boyd SE, Yang H, Zhang D (2016) Roles of biochar in improving phosphorus availability in soils: a phosphate adsorbent and a source of available phosphorus. Geoderma 276:1–6. https://doi.org/10.1016/j.geoderma.2016.04.020
Acknowledgements
We would like to thank Dr. Thijs de Boer from IBED, University of Amsterdam for their suggestions on data analysis. John Morman in the School of GeoSciences, University of Edinburgh, assisted in chemical analysis, Sophie Haupt, Billy Adams and Pat Watson in the School of Biological Sciences, University of Edinburgh, provided support during the rhizobox experiment. Dr. Ondřej Mašek, University of Edinburgh, produced the biochars. We are grateful to three anonymous reviewers for improvements arising from their earlier helpful comments.
Funding
This study was supported by IBED, University of Amsterdam and UKBRC, School of GeoSciences, the University of Edinburgh. Additional funding was provided by NERC E3 DTP.
Author information
Authors and Affiliations
Contributions
All authors conttributed to the study conception, espically Hamish Creber. The biochars, seedlings and growing medium were prepared by Hamish Creber and Kaiyu Lei. The rhizoboxes were designed and assembled by Hamish Creber and Kaiyu Lei. Specimen collection and analysis was performed by Kaiyu Lei and Hamish Creber. Kaiyu Lei collected and analysed the data and worte the first draft of the manuscript. All authors commented on previous verisons and did some modifications. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
We declare there is no competing financial interest.
Additional information
Responsible Editor: Tim S. George.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lei, K., Creber, H., Bol, R. et al. Preferences of Pinus sylvestris seedling roots for different phosphorus sources under phosphorus-deficient conditions. Plant Soil 482, 229–244 (2023). https://doi.org/10.1007/s11104-022-05682-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05682-0