Abstract
Background and aims
The pasture legume Trifolium subterraneum ssp. yanninicum exhibits waterlogging tolerance. This study investigates diversity for waterlogging tolerance within ssp. yanninicum. We tested the hypotheses that (1) variation for waterlogging tolerance exists within ssp. yanninicum and (2) is related to phenotypic and growth trait differences, which (3) reflect eco-geographic variables at site of origin.
Methods
Twenty-eight diverse ssp. yanninicum ecotypes collected from the Mediterranean region and four cultivars were grown in a controlled environment glasshouse. Seedling traits were measured at 14 and 21 days after sowing. Waterlogged and free-draining (control) treatments were then imposed for 28 days. Relative distance and multivariate plasticity indices were calculated.
Results
Under waterlogging, shoot (87–108% of controls) and root (80–116% of controls) relative growth rates (RGRs) differed significantly among ssp. yanninicum. Waterlogging tolerance, as assessed by shoot RGR, had strong positive correlations with root RGR (r = 0.86; P < 0.001), petiole length (r = 0.59; P < 0.001) and leaf size (r = 0.55; P < 0.01) under waterlogging. The proportion of biomass as leaf increased under waterlogging, due to leaf size being maintained (mean 102% of controls), but petiole length decreased (mean 84% of controls). Petiole length was the most plastic trait. Seed size, seedling traits, maturity duration and eco-geographic variables at site of origin were not related to waterlogging tolerance.
Conclusions
Wide variation in waterlogging tolerance exists within ssp. yanninicum. Petiole length reduction, an easy-to-measure and non-destructive indicator, could be used as a preliminary selection tool when screening large numbers of ssp. yanninicum for waterlogging tolerance in a breeding program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil waterlogging, a widespread abiotic stress worldwide, can reduce crop production by 20–50% or more, depending on plant growth stage and duration and intensity of waterlogging (Bertholdsson 2013; Solaiman et al. 2007). Soil waterlogging occurs in the root-zone and is defined as the excess water saturation of soil pores affecting oxygen concentration, leading to hypoxia (low O2 concentration) and eventually to anoxia (absence of O2) (Armstrong 1980). Under waterlogging, the movement of gases, such as O2 and CO2, through water-filled soil pores and through roots into cells of plant organs dramatically decreases (gas diffusion is 10 000 times slower in water than in air) (Bailey-Serres and Voesenek 2008; Colmer 2003; Voesenek and Sasidharan 2013). Oxygen deficiency inhibits aerobic respiration, resulting in a shortage of carbohydrates and energy that leads to an ‘energy crisis’ and disrupts photosynthesis and the uptake of nutrients from soil (Colmer and Greenway 2011; Pang et al. 2004). In addition, waterlogging reduces the availability in soil of some essential nutrients (e.g. N), but other ions such as manganese (Mn2+), iron (Fe2+), sulphur (S2−) and carboxylic acids can accumulate to toxic levels due to microbial anaerobic metabolism (Ponnamperuma 1984). Thus, soil waterlogging adversely affects plant growth and function and can result in wilting, premature leaf senescence, and growth cessation, and premature death (Marschner 1995; Stoddard et al. 2006).
The most common approach to define waterlogging tolerance is to assess factors related to the maintenance of shoot growth and accumulated yield under waterlogging, relative to non-waterlogged conditions (Setter and Waters 2003). A given genotype copes with spatial and temporal environmental heterogeneity by producing a range of phenotypes (Grassein et al. 2010). This phenotypic variability is produced by two complementary mechanisms: genetic variability and phenotypic plasticity (Grassein et al. 2010). Genetic variation is modified during evolution by natural selection, with genotypes expressing the most favoured phenotypes being selected; this leads to development of diverse ecotypes in response to diverse environments (Erskine et al. 1989; Grassein et al. 2010; West-Eberhard 1989). Conversely, phenotypic plasticity is the ability of a genotype to be altered by environmental influences and is a more flexible and quicker response to changes in growing conditions (Grassein et al. 2010; Whitman et al. 2009). Hence, both genotypic variation and phenotypic plasticity in trait variation is important for a given genotype to deal with ambient conditions.
Mediterranean-climate zones are characterized by mild, wet winters and hot dry summers, with large intra- and inter-annual variability (Porqueddu et al. 2016). The five regions of the world with Mediterranean-type climates are the Mediterranean basin itself, southern Australia, the southern tip of South Africa, California and central Chile (Porqueddu et al. 2016). Plants in Mediterranean regions can experience ‘transient waterlogging’, which is waterlogging caused by excessive rainfall during the winter growing season, with the duration dependent on the amount of rain, evapotranspiration and soil structure (Malik et al. 2002). Although the upper soil levels dry out in subsequent spring and summer months, plants can suffer prolonged adverse effects from waterlogging after the water recedes (Colmer and Flowers 2008; Malik et al. 2002).
In southern Australia soil subject to transient waterlogging covers a large area of farmland with an estimated annual cost in terms of crop production lost of AU$90 million (Hamilton et al. 2000). While rainfall has decreased for this region over the past few decades and annual rainfall is projected to further decline, the intensity and frequency of heavy rainfall events are projected to increase with climate change, which is likely to increase the incidence of waterlogging (Chapman et al. 2012; Jarvis et al. 2010). Therefore, selection of crop and pasture plants with tolerance to waterlogging is paramount for maintaining productivity.
Waterlogging tolerance varies among genotypes of many crop and pasture legumes. A review of shoot growth in wheat (Triticum aestivum L.) during waterlogging found that tolerant varieties consistently showed about twice the shoot growth of intolerant varieties (Setter and Waters 2003). Rogers et al. (2011) examined diversity in waterlogging tolerance among 23 genotypes of messina (Melilotus siculus (Turra) Vitman ex B.D. Jacks.), an annual forage legume with high tolerance to waterlogging, and identified several genotypes with greater shoot growth in a deoxygenated, stagnant treatment than in an aerated control. Gibberd et al. (2001) explored waterlogging tolerance among 20 Trifolium species/subspecies and found nine genotypes had no reduction in shoot growth under waterlogging, and two genotypes had increased growth, compared to drained controls. Exploring waterlogging tolerance within species, therefore, could yield significant benefits for breeding programs by identifying genotypes with greater tolerance (Bailey-Serres and Voesenek 2008; Malik et al. 2015; Rogers et al. 2011).
Subterranean clover (Trifolium subterraneum L., commonly known as subclover), originates from the Mediterranean region and Western Europe (Nichols et al. 2013). It has been introduced to southern Australia, where it is the most widespread pasture legume, having been sown over 29 million ha (Nichols et al. 2013). Subclover forms the basis of many permanent or semi-permanent pastures in irrigated and high rainfall areas, while in low and medium rainfall areas it is commonly grown in rotation with cereal crops. It is highly valued in the livestock and grains industries as a source of high-quality forage and for its ability to fix atmospheric nitrogen (N) (Nichols et al. 2013). Subclover consists of three subspecies: ssp. subterraneum; ssp. brachycalycinum; and ssp. yanninicum (Katznelson and Morley 1965). Each subspecies is used in Australian agriculture and each has different soil preference. In particular, ssp. yanninicum exhibits a higher tolerance to waterlogging and is mostly grown in medium-high rainfall areas (450–1200 mm mean annual rainfall) with soils prone to waterlogging (Francis and Devitt 1969; Nichols et al. 2013; Reed et al. 1985). Evidence for the superior waterlogging tolerance of ssp. yanninicum comes from agronomic trials (Craig 1992; Peak and Morley 1973; Reed et al. 1985; Sandral et al. 2003), ecological surveys (Cocks 1994; Francis 1976; Katznelson and Morley 1965; Piano 1984) and glasshouse studies (Devitt and Francis 1972; Enkhbat et al. 2021b; Francis and Devitt 1969; Francis et al. 1974; Gibberd and Cocks 1997; Marshall and Millington 1967).
The Australian Pastures Genebank (APG) contains the world’s largest subclover collection, with more than 9,000 subclover ecotypes collected from around 3,000 sites in its native habitat in the Mediterranean basin and surrounding areas (Smith et al. 2021). Of these, ssp. yanninicum is the rarest of the three subspecies, comprising only 2.2% of all subclover ecotypes; this reflects ssp. yanninicum being confined to latitudes 38.5–40.5 °N and hence having been collected in the wild from only: Greece, Italy, Spain, Turkey and Tunisia (Nichols et al. 2013). Its typical native habitat is coastal, or areas with mild winter temperatures, on soils prone to winter waterlogging where mean annual precipitation is > 450 mm (commonly > 750 mm) (Ghamkhar et al. 2015).
Nichols et al. (2013) reported the current cultivars of ssp. yanninicum were developed from a narrow genetic base. There is, therefore, a need to explore the diversity among ecotypes and to identify phenotypic traits for use in future breeding to improve productivity and resilience of ssp. yanninicum and broaden its genetic base. Enkhbat et al. (2021a) found large variation for 10 morphological traits, flowering time and leaf isoflavone content among 108 ecotypes of ssp. yanninicum. Furthermore, all traits (except stem diameter and leaf mark crescent size) were correlated with at least one of 22 eco-geographic variables from collection sites. However, neither ecotypic variation for waterlogging tolerance within ssp. yanninicum, nor its relationship with site of origin parameters, have been previously examined.
Genetic variation in subclover exists for highly heritable and readily measurable traits such as flowering time (maturity) and seed size which are considered crucial traits for adaptation of annual legumes. The optimum flowering time in subclover differs among environments to facilitate seed set and burr burial prior to onset of the dry summer, while maximising the period of vegetative growth (Piano 1984). Seeds tend to be smaller in hot and dry environments enabling them to be germinate successfully with less water at the start of the season and to be mature rapidly at the end of the season (Piano et al. 1996). The following studies in other species have demonstrated a relationship between these traits and waterlogging tolerance. Under field conditions in a Mediterranean-type climate, long-season wheat cultivars show a yield advantage over short-season cultivars (Gardner and Flood 1993; McDonald and Gardner 1987). Large-seeded pea and lentil genotypes demonstrated higher waterlogging tolerance than small-seeded genotypes, due to greater carbohydrate reserves and high early seedling vigour (Malik et al. 2015). Kabuli chickpea, with larger seed and more vigorous early growth than desi chickpea, is better adapted to transient waterlogging (Palta et al. 2010; Siddique et al. 2000). Sultana et al. (2013) also found large- and dark-seeded, long-season genotypes of pigeon pea (Cajanus cajan L.) had greater waterlogging tolerance at the seedling stage than light-coloured, small-seeded, short-season genotypes. The advantage of the long season genotype reflects capacity for early sowing and establishment enabling them to avoid waterlogging during the intolerant stages of germination and emergence, as well as their flowering time being late enough to avoid spring waterlogging damage (Gardner and Flood 1993; McDonald and Gardner 1987). Exploring relationships between waterlogging tolerance and readily measurable, highly heritable traits in subclover could provide a simple selection criterion for preliminary screening for waterlogging tolerance.
The survival of a single genotype when experiencing abiotic stress (e.g. waterlogging) is highly related of its ability to quickly express of alternative phenotypes, referred as ‘phenotypic plasticity’ (Pennacchi et al. 2021). Trifolium species can adjust their root phenotype to develop lateral roots emerging along and above the soil surface as a response to waterlogging (Enkhbat et al. 2021b; Gibberd and Cocks 1997; Rogers and West 1993). Production of large number of adventitious and young lateral roots enables enhanced porosity (a gas volume per unit of root volume) and improved transport of oxygen, and other gases, within plants (from aerial shoots to submerged roots) throughout newly formed aerenchyma (Armstrong 1980; Drew et al. 1979; Trought and Drew 1980). Aerenchyma, a plastic response to waterlogging, provides large interconnected gas channels improving internal aeration capacity (Colmer and Flowers 2008). But, measuring aerenchyma (i.e. root porosity) is unlikely to be suitable for large number of genotypes as it requires time and fast processing of fresh roots. Instead, the assessment of newly formed adventitious roots and lateral root biomass could be a simple and convenient approach.
Growth of leaves and petioles in subclover is sensitive to waterlogging and shows a highly plastic response (Enkhbat et al. 2021b). This is reflects the aerial part of plant, as the interface between the plant and above-ground physical environment, playing an important role in carbon and water metabolism under waterlogging (Colmer 2003; Pennacchi et al. 2021; Striker et al. 2005). Furthermore, species that often experience prolonged but shallow waterlogging events possess traits, such as hyponastic growth (towards a vertical position) and petiole elongation, that contributed to waterlogging tolerance (Herzog and Pedersen 2014). Striker (2012a) emphasized that hyponastic growth is a common response of species with high tolerance to transient waterlogging. Although subclover has a prostrate growth habit, hyponastic growth and inherently long petioles of ssp. yanninicum under waterlogging could be morphological features that confer specific adaptation to waterlogging (Enkhbat et al. 2021b; Francis et al. 1974). Hence, evaluating waterlogging tolerance among ssp. yanninicum by assessing phenotypic changes induced by the environment is crucial to identify genotypes with a higher tolerance to, and better performance under, waterlogged conditions.
The overall objective of this study was, therefore, to investigate diversity for waterlogging tolerance in ssp. yanninicum. We tested three main hypotheses: (i) variation for waterlogging tolerance exists within ssp. yanninicum; (ii) this variation is related to phenotypic and growth trait differences; and (iii) these trait differences are related to eco-geographic variables at site of origin.
Materials and methods
Plant materials
Seeds of 32 genotypes of ssp. yanninicum were obtained from the Australian Pastures Genebank (APG) in 2019 and grown at the University of Western Australia Shenton Park Field Station to obtain fresh seed. These comprised: (i) 28 ‘ecotypes’ (coded A-AB), originating from wild populations collected in their native Mediterranean basin habitat; and (ii) four cultivars: Yarloop, Larisa, Meteora and Trikkala (Table 1; Supplementary data Fig. S1). In this study, ‘genotype’ is a collective term for both ecotypes and cultivars. As T. subterraneum is a self-pollinated, and therefore true-breeding, species (Nichols et al. 2013), the plants of individual genotypes are identical. The subspecies identity of each genotype was confirmed on the basis of distinguishing morphological features of ssp. yanninicum described by Katznelson and Morley (1965) and Nichols et al. (2013). Further details of this process are provided in Enkhbat et al. (2021a).
The ecotypes were selected on the basis of diversity for (i) collection site ‘passport data’, which includes latitude, longitude, altitude and other collection site information; and (ii) flowering time, as determined by (Enkhbat et al. 2021a). Among these were four ecotypes (E, G, H and Q) with black-coloured seeds (as opposed to the usual amber/cream colour) described in Enkhbat et al. (2021a). Cultivars Larisa and Meteora are themselves ecotypes, originally collected from Greece as CPI 039313Y and CPI 039327YB, respectively (Nichols et al. 2013; Oram 1989) and have associated passport data. However, cv. Yarloop is a naturalized strain originally found growing in the Yarloop district of Western Australia (WA) (Nichols et al. 1996; 2013; Oram 1989) and cv. Trikkala is derived from cross breeding and so neither have passport data (Nichols et al. 2013; PBR 2021). Additionally, the waterlogging-susceptible cultivars Antas (ssp. brachycalycinum) and Seaton Park (ssp. subterraneum) (Enkhbat et al. 2021b) and the waterlogging-tolerant T. michelianum cv. Frontier (Nichols et al. 2007) were included as benchmarks for waterlogging susceptibility and tolerance, respectively. This gave a total of 35 genotypes (Table 1).
Climatic data
Climatic variables for collection sites of the ecotypes were extracted from the ‘WorldClim’ (Version 2) data base at 2.5 arc-minutes spatial resolution (Ghamkhar et al. 2015; Hijmans et al. 2005) on the basis of the passport data. This information was used to map a spatial model in the R software package, as described in Enkhbat et al. (2021b). Nineteen bioclimatic (BIOCLIM) variables (Supplementary data Table S1) were imported for all 30 sites. These are a standard set of eco-geographic variables that have been used in previous studies (Abdi et al. 2020; Enkhbat et al. 2021a; Ghamkhar et al. 2015). BIOCLIM variables for 29 ecotypes (including cv. Larisa and Meteora) were calculated (Table 2, Supplementary data Table S1). Ecotype E was the only ecotype collected from Tunisia (Supplementary data Fig. S1), a site with exceptionally high annual and winter precipitation (Table 1, Supplementary data Table S1), which greatly affected trait correlations with climatic variables. It was, therefore, excluded from these analyses.
Plant growth
The experiment was conducted in a naturally-lit glasshouse set at 20/15 \(^\circ\)C day/night at The University of Western Australia (31\(^\circ\)98’S, 115\(^\circ\)50’E) from June to August 2020. Plants were grown in sterilised, free-draining plastic pots (2.8 L) containing 3.2 kg of steam-pasteurised soil which had been dried at 40 \(^\circ\)C. Weedstop® matting (Gale Pacific Limited) was placed in the bottom of each pot to prevent soil loss. The soil was a 1:1 mix of washed river sand and loam with a pH of 6.2 in 0.01 M CaCl2 (Soil pH measurement in CaCl2 is usually preferred over H2O, as it is less affected by soil electrolyte concentration and provides a more consistent measurement (Minasny et al. 2011)). The water content (w w− 1) at field capacity (FC) was 15.5%. Soil analyses were performed by CSBP Laboratories, Bibra Lake, Western Australia (Supplementary data Table S2). Two days prior to transplanting seeds, pots were watered to 60% of FC and all essential nutrients were applied (mg kg− 1 soil− 1): P 20.5; K 88.7; S 34.2; Ca 41.0; Cl 72.5; Mg 3.95; Mn 3.26; Zn 2.05; Cu 0.51; B 0.12; Co 0.11 and Mo 0.08, according to Enkhbat et al. (2021b). These nutrients were reapplied in the same amounts at 14 days after sowing (DAS).
Seeds were germinated as described by Enkhbat et al. (2021b). Prior to sowing, seeds were scarified with a Kimseed Venables Seed Scarifier (Kimberley Seeds, Western Australia). Seeds were imbibed in Petri dishes containing double layers of moistened Whatman No 1 filter paper for 36–39 h in a darkened temperature-controlled room at 15 °C (referred here as the day of sowing). Six seeds with newly-emerged radicles were transplanted into each pot (radicle placed downwards) at 10 mm depth and covered with soil. All pots were watered to 80% FC with 40 mL of Rhizobium leguminosarum bv. trifolii strain WSM1325 (Group C) inoculum (mixture of 2.8 g of rhizobia in 1 L of water). Plants were grown for 21 days under free-draining conditions and were re-watered to 80% FC every 2–3 days. Pots were re-arranged weekly to minimise positional effects. Plants were randomly thinned to three per pot at 14 DAS with the removed plants used to measure seedling phenotypic traits.
Experimental design and treatments
The experiment had a factorial randomised block design with two factors (treatment and genotype), comprising two treatments and 35 genotypes in four replications. Each pot was an experimental unit. The two treatments consisted of: (i) a free-draining (control) treatment, which was watered to 80% of FC for duration of the experiment; and (ii) a waterlogged treatment which commenced after 21 DAS and was maintained for 28 days. To impose the waterlogged treatment, pots were placed inside the same sized pots, sealed by plastic bags, and the water level was maintained 10 mm above the soil surface by daily watering.
Harvests
Two harvests were undertaken: an initial harvest at 21 DAS and a final harvest at 49 DAS. For each harvest, soil was gently washed with flowing water from the roots of all three plants in the pot onto a sieve (3 mm mesh). At both harvests, plants were separated into shoots and roots and oven-dried at 60 °C for four days and weighed. Shoot and root relative growth rates (RGR) were calculated using the standard equation of Hunt (1982):
where: DW1 = dry weight (g) at initial harvest (t1), DW2 = dry weight (g) at final harvest (t2); and t2-t1 = number of days between harvests (28 days).
Measurements
Seed measurements
Mean seed weight
Four replicates of 50 random seeds per genotype were counted using a Condator seed counter (Preuffer, Germany) and weighed using a balance to calculate mean seed weight.
Measurements at 14 DAS
Hypocotyl length and cotyledon size/weight
Hypocotyl length and the size and weight of an individual cotyledon of each genotype were measured in 12 seedlings of each genotype (three seedlings from each pot). Intact seedlings were gently pulled from the soil. Hypocotyl length was measured by ruler from the junction of the cotyledons until the joint with the radicle (Supplementary data Fig. S2). Cotyledon size was estimated by comparison with the photographic plates and conversion formulae in Williams et al. (1964). Cotyledon weight was determined by cutting cotyledons at the junction with the shoot and oven-drying at 60 °C for three days before weighing.
Measurements at 21 DAS (Initial harvest)
Leaf size and petiole length
Leaf size (three leaflets combined) and petiole length of the first trifoliate leaf of each plant were measured for each ssp. yanninicum genotype. Leaf size was estimated by comparison with the photographic leaf size standards for subclover of Williams et al. (1964) and using their conversation formulae. Petiole length of the same leaves was measured by ruler.
Measurements at 49 DAS (Final harvest)
Leaf size and petiole length
Leaf size and petiole length of the fourth trifoliate leaf of each plant were measured using the same methods as the initial harvest for all subclover genotypes. Because T. michelianum has a different leaf shape to subclover, leaf size of cv. Frontier was measured using a portable leaf area meter (LI 3000; LI-COR Biosciences, Lincoln, USA).
Chlorophyll concentration
Relative changes in chlorophyll concentration were measured by a Soil Plant Analysis Development (SPAD) chlorophyll meter (Konica Minolta SPAD-502 Plus, Osaka, Japan), which produces relative SPAD meter values that are proportional to the amount of chlorophyll present in leaf (Ling et al. 2010). SPAD values of the youngest fully-opened leaf of all plants were determined at 44 DAS. Specific leaf weight (SLW) for 16 randomly selected genotypes was estimated, according to Nichols et al. (2009), to determine consistency of leaf thickness (a factor affecting correlation strength between SPAD value and chlorophyll concentration) among genotypes under control and waterlogged treatments.
Surface root proliferation
Relative differences in the amount of surface roots growing above the soil surface under waterlogged conditions were assessed for each pot using a 1–10 visual rating scale, where 1 was the lowest and 10 was the most vigorous growth.
Flowering time
Days to first flowering
Data for days to first flowering (DFF), measured as the number of days from sowing to the appearance of the first open flower, for the 32 ssp. yanninicum genotypes are from Enkhbat et al. (2021a) recorded at Shenton Park, Western Australia (31°57’S, 115°5’E) from a sowing date of 21 May 2019.
Phenotypic plasticity
Phenotypic plasticity was evaluated for traits measured at 49 DAS by a quantitative estimation of the phenotypic change induced by the environment, using (i) relative distance plasticity index (RDPI) and (ii) the multivariate plasticity index (MVPi).
The relative distance plasticity index (RDPI)
The relative distance plasticity index (RDPI) provides unbiased estimation of phenotypic variation and strong statistical power to test plasticity (Valladares et al. 2006). RDPI was used to quantify and compare levels of phenotypic plasticity for individual traits, based on phenotypic distances among all pairs of replicates of each genotype grown in control and waterlogged treatments. RDPI ranges from 0 (no plasticity) to 1 (maximal plasticity) and was calculated according to Valladares et al. (2006):
where Xc and Xwl are the phenotypic values for a particular trait of a single genotype in the control and waterlogged treatments, respectively, and n is the total number of distances.
Multivariate plasticity index (MVPi)
Multivariate plasticity index (MVPi) is a multivariate-based plasticity index, used to assess general plasticity; genotypes with higher MVPi values have higher phenotypic plasticity (Pennacchi et al. 2021). The MVPi of each genotype was calculated as the average of total observed Euclidian distances (ED) for all treatment combinations (total of 16 treatment combinations: 2 treatments each with 4 replicates) where:
and n and m are the number of replicates for each combination of treatments.
Statistical analyses
Data were analysed using R software (version 3.6.3) and graphed as mean \(\pm\)standard error (\(\pm\)SE) using SigmaPlot 14 (Systat Software, Inc.). Genotypes were ordered in the graphs based on their tolerance, defined as maintenance of shoot RGR relative to controls, and this order was used for consistency in all other graphs. Among genotypes of ssp. yanninicum, data were analysed by two-way ANOVA, with genotype and treatment as main factors. The assumptions of equal group variance and the normality of residual distribution were verified by Bartlett’s and Shapiro-Wilk tests, respectively. A robust ANOVA test with the robust covariance matrix was conducted when heteroskedasticty was a problem. Dependent variables were log-transformed when errors were not normally distributed. As the experiment was a factorial design in a randomised block with four replicates, a two-factor model with a nested structure (randomised genotypes and replicates) for errors, was used by the ‘lme’ function in the ‘nlme’ package in R. Least significant difference (LSD) Fisher’s protected tests were used to test treatment effects for each genotype and to compare the means of phenotypic plasticity index values with the package ‘agricolae’.
One-way ANOVA was used to compare genotypic variation of ssp. yanninicum mean seed weight, days to first flowering and seedling traits measured at 14 and 21 DAS. It was also used to assess genotypic differences for surface root proliferation under waterlogging and for RDPI and MVPi plasticity indices, in addition to trait differences between amber and black-seeded ssp. yanninicum genotypes. The assumption of constant variance of one-way ANOVA were satisfied, but non-parametric tests (e.g. Kruskal-Wallis) were used when data were not normally distributed. Broad-sense heritability (H2), the proportion of variation in a given trait attributable to genotypic variation, was estimated according to Falconer (1989) for mean seed weight, DFF and seedling traits measured at 14 and 21 DAS across the ssp. yanninicum genotypes.
Multivariate analysis was conducted using hierarchical clustering analysis on principal components (HCPC) to perform clustering, and used complementarities between clustering and principal component analysis (PCA), to describe genotype similarities and features of the data set (Husson et al. 2010; Lê et al. 2008). To classify genotypes of ssp. yanninicum, a hierarchical clustering and partitioning on the first two principal components was first performed using the HCPC function of the ‘FactoMiner’ package in R on the outputs ‘res.pca’ of the PCA function. The number of clusters was chosen at the suggested level of tree cut based on the gain in inertia. Second, a description of each cluster group was achieved by the ‘desc.var’ object in the HCPC function which provided ‘v-test’ values indicating the average of a variable in the cluster (mean in category) as lower or greater than the average of the variable for the whole data set (overall mean). All variables in the data set measured at 14 and 21 DAS (traits under free-draining) and 49 DAS (traits under waterlogging) were used to describe clustered groups. A variable correlation plot of the first two principal components (PC1 and PC2) was produced to visualize the interrelationships of the variables and to sort them by importance for waterlogging tolerance. Finally, a PCA biplot was produced to visualize the interrelationship of eco-geographic variables of collection sites (passport data and BIOCLIM) in clustered groups.
Pearson correlation coefficients and their levels of significance were calculated between all traits measured at 14 and 21 DAS and traits under waterlogged conditions at 49 DAS among ssp. yanninicum genotypes. They were also calculated between plant traits and eco-geographic (passport data and BIOCLIM) variables of collection sites.
Results
Variation for seed and seedling traits measured at 14 and 21 DAS
Broad variation with highly significant genotype differences was observed for mean seed weight, DFF and all seedling traits measured at 14 and 21 DAS (0.01 < P < 0.001) (Table 3; Supplementary data Table S3). Mean seed weight was highly heritable (H2 = 94%) and ranged from 8.6 to 13.6 mg. All seedling traits measured at 14 and 21 DAS also showed high heritability H2 ≥ 47%, except root DW at 21 DAS. At 14 DAS, cotyledon size ranged from 0.5 to 0.6 cm2, cotyledon DW from 1.7 to 2.8 mg and hypocotyl length from 9.1 to 13.3 mm, while at 21 DAS leaf size ranged from 2.5 to 3.6 cm2, petiole length from 3.6 to 5.7 cm, shoot DW from 74.9 to 111.2 mg and root DW from 37.6 to 52.2 mg (Table 3).
Variation between genotypes with amber and black-coloured seeds
The only significant differences between amber and black-seeded genotypes were for leaf size and petiole length at 21 DAS (both P < 0.05) and surface root proliferation at 49 DAS (P < 0.01) under waterlogging (Supplementary data Table S4). Surface root proliferation in black-seeded genotypes was significantly higher than amber-seeded genotypes, largely attributable to Ecotypes G and H (Supplementary data Table S4).
Variation for traits measured at 49 DAS (after 28 days of treatment)
Shoot and root RGR
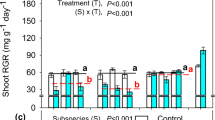
There was a significant treatment × genotype interaction for both shoot and root RGRs (both P < 0.05) for 32 ssp. yanninicum genotypes (Fig. 1). Shoot RGRs under waterlogging ranged from 87 to 108% of controls, while root RGRs ranged from 80 to 116% of controls (Supplementary data Table S5).
Relative growth rates (RGR) of (a) shoots and (b) roots for 32 ssp. yanninicum genotypes, consisting of 4 cultivars (hatched) and 28 ecotypes, along with ssp. subterraneum cv. Seaton Park, ssp. brachycalycinum cv. Antas and T. michelianum cv. Frontier after 28 days of treatment (mean \(\pm\)SE n = 4). Treatments were imposed at 21 days after sowing: free-draining (control) and waterlogged (WL, water level kept 10 mm above the soil surface). Two-way ANOVA results are given in each panel. The single cultivars of ssp. subterraneum, ssp. brachycalycinum and T. michelianium are not included in the statistical analyses. The significant differences (P < 0.05; Fisher LSD test) between control and WL treatments of each genotype are shown: *P < 0.05; **P < 0.01; ***P < 0.001 (black indicates the control treatment was higher and red indicates that the WL treatment was higher). Genotypes are ordered from negative to positive impact of WL on shoot relative growth rate
Waterlogging reduced RGR compared to controls for four for shoot RGR and 13 genotypes for root RGR (Fig. 1; Supplementary data Table S5). Several genotypes showed higher RGRs of either shoots and roots under waterlogging, but only Ecotype D showed significantly higher RGR of both shoots (P < 0.05) and roots (P < 0.01) among all other genotypes. In contrast, both shoot and root RGRs of ssp. subterraneum cv. Seaton Park (82% and 73% of controls, respectively) and brachycalycinum cv. Antas ssp. (87% and 85%, respectively) were severely decreased by waterlogging compared to their respective controls (Fig. 1; Supplementary data Table S5). For T. michelianum cv. Frontier, both shoot and root RGRs were unaffected by waterlogging (99% and 97% of controls, respectively).
Shoot and root DW
There was a significant treatment × genotype interaction for both shoot (P < 0.05; Fig. 2a) and root (P < 0.001, Fig. 2b) DWs among the ssp. yanninicum genotypes. Marked variation was observed under waterlogging for both shoot (66–120% of controls) and root (58–137%) DWs (Supplementary data Table S5).
Dry weights (DW) of (a) shoots and (b) roots for 32 ssp. yanninicum genotypes, consisting of 4 cultivars (hatched) and 28 ecotypes, along with ssp. subterraneum cv. Seaton Park, ssp. brachycalycinum cv. Antas and T. michelianum cv. Frontier after 28 days of treatment (mean \(\pm\)SE n = 4). Treatments were imposed at 21 days after sowing: free-draining (control) and waterlogged (WL, water level kept 10 mm above the soil surface). Two-way ANOVA results are given in each panel. The single cultivars of ssp. subterraneum, ssp. brachycalycinum, and T. michelianium are not included in the statistical analyses. The significant differences (P < 0.05; Fisher LSD test) between control and WL treatments of each genotype are shown: *P < 0.05; **P < 0.01; ***P < 0.001 (black indicates the control treatment was higher and red indicates that the WL treatment was higher). Genotypes are ordered from negative to positive impact of WL on shoot relative growth rate (see Fig. 1)
Waterlogging significantly reduced shoot DWs in only four genotypes compared to controls, but reduced root DWs in 13 genotypes. Ecotype D again showed superior tolerance to waterlogging with both shoot (P < 0.05) and root (P < 0.01) DWs significantly higher under waterlogging (115% and 133% of controls, respectively) (Fig. 2). In contrast, both shoot and root DWs of ssp. subterraneum cv. Seaton Park (58% and 49% of controls, respectively) and ssp. brachycalycinum cv. Antas (64% and 68%) were severely decreased by waterlogging. For T. michelianum cv. Frontier, both shoot and root DWs were unaffected by waterlogging (92% and 87% of controls, respectively).
Leaf size and petiole length
For leaf size, there was a significant treatment × genotype interaction (P < 0.001; Fig. 3a) among genotypes of ssp. yanninicum. Under waterlogging, leaf size ranged from 76 to 130% of controls (Supplementary data Table S5). Waterlogging significantly reduced leaf size in only one genotype (Ecotype E), compared to its control (P < 0.01) (Fig. 3a; Supplementary data Table S5). In contrast, five ecotypes had significantly larger leaves than their respective controls. Leaf sizes for both ssp. subterraneum cv. Seaton Park (49% of control) and brachycalycinum cv. Antas (67% of control) were severely reduced by waterlogging, whereas leaf size in T. michelianum cv. Frontier (94% of control) was unchanged by the waterlogged treatment.
(a) Leaf size and (b) petiole length for 32 ssp. yanninicum genotypes, consisting of 4 cultivars (hatched) and 28 ecotypes, along with ssp. subterraneum cv. Seaton Park, ssp. brachycalycinum cv. Antas and T. michelianum cv. Frontier after 28 days of treatment (mean \(\pm\)SE n = 4). Treatments were imposed at 21 days after sowing: free-draining (control) and waterlogged (WL, water level kept 10 mm above the soil surface). Two-way ANOVA results are given in each panel. The single cultivars of ssp. subterraneum, ssp. brachycalycinum, and T. michelianium are not included in the statistical analyses. The significant differences (P < 0.05; Fisher LSD test) between control and WL treatments of each genotype are shown: *P < 0.05; **P < 0.01; *P < 0.001 (black indicates the control treatment was higher and red indicates that the WL treatment was higher). Genotypes are ordered from negative to positive impact of WL on shoot relative growth rate (see Fig. 1). Note the y-axis scale change between the panels
For petiole length, there was a significant treatment × genotype interaction (P < 0.01; Fig. 3b) among ssp. yanninicum genotypes. Petiole length ranged from 63 to 105% of controls (Supplementary data Table S5). Waterlogging reduced petiole length in 13 out of 32 genotypes compared to controls including cultivars Meteora, Yarloop and Trikkala. Petiole length in the remaining 19 ssp. yanninicum genotypes was unaffected by waterlogging and did not differ from their controls. Petiole lengths for ssp. subterraneum cv. Seaton Park (58%), ssp. brachycalycinum cv. Antas (77%) and T. michelianum cv. Frontier (77%) were significantly reduced compared to their respective controls. Furthermore, a visual observation indicated a hyponastic growth response of all ssp. yanninicum under waterlogged conditions (Fig. 4), but this effect was not quantified.
Surface root proliferation
There was a significant genotype difference (P < 0.001) for surface root density under waterlogging among genotypes of ssp. yanninicum (Fig. 5; Supplementary data Table S5). Surface roots were observed in all genotypes, irrespective of their waterlogging tolerance. The highest surface root proliferation scores occurred in two of the black-seeded ecotypes: H (score 10.0) and G (score 9.4). The proliferation scores of surface roots of the other genotypes ranged from 1 to 7.
Surface root proliferation after 28 days of waterlogging for 32 ssp. yanninicum genotypes, consisting of 4 cultivars (hatched) and 28 ecotypes, along with ssp. subterraneum cv. Seaton Park, ssp. brachycalycinum cv. Antas and T. michelianum cv. Frontier grown under waterlogging (WL, mean \(\pm\)SE n = 4). The WL treatment was imposed at 21 days after sowing (water level kept 10 mm above the soil surface). Surface roots were scored on a 1–10 visual rating scale with 1 the lowest and 10 the most vigorous growth. The one-way ANOVA result is given in the panel. Genotypes are ordered from negative to positive impact of WL on shoot relative growth rate (see Fig. 1)
Chlorophyll concentration
There was a significant treatment × genotype interaction (P < 0.001; Fig. 6) for SPAD values among genotypes of ssp. yanninicum. SPAD values under waterlogging were reduced significantly (0.01 < P < 0.001) for all genotypes compared to their controls (Supplementary data Table S5). SLW in 16 genotypes (except Ecotype K) remained constant between control and waterlogged treatments (Supplementary data Table S6).
SPAD values for 32 ssp. yanninicum genotypes, consisting of 4 cultivars (hatched) and 28 ecotypes, along with ssp. subterraneum cv. Seaton Park, ssp. brachycalycinum cv. Antas and T. michelianum cv. Frontier after 28 days of treatment (mean \(\pm\)SE n = 4). Treatments were imposed at 21 days after sowing: free-draining (control) and waterlogged (WL, water level kept 10 mm above the soil surface. Two-way ANOVA results are given in the panel. The single cultivars of ssp. subterraneum, ssp. brachycalycinum, and T. michelianium are not included in the statistical analyses. Genotypes are ordered from negative to positive impact of WL on shoot relative growth rate (see Fig. 1)
Multivariate analysis: hierarchical clustering on principal components (HCPC) and interrelationships of traits
The output of the HCPC function produced by the two first principal components (PC) suggested partitioning of the ssp. yanninicum genotypes into three cluster groups: Group I (cv. Larisa, cv. Trikkala and Ecotypes A, D, O, P, S and AB), Group II (cv. Meteora and Ecotypes B, C, F, H, I, J, K, L, T, U, V, W, X, Y, Z and AA) and Group III (cv. Yarloop and Ecotypes E, G, M, N, Q and R) (Fig. 7). This clustering explains 58% of the total variance in the dataset. A dendrogram of hierarchical clustering (showing the optimal level of division suggested by the HCPC function in a solid black line), contribution of variables to PC1 and PC2 and scree plot for the dimensionality of the data are shown in Supplementary data Fig. S4.
Descriptions of each cluster group with characterized variables (the output of the v-test) are given in Table 4. Genotypes in Group I had the highest waterlogging tolerance, with above average means for both shoot and root RGRs and DWs (Fig. 7; Table 4). Notably, Ecotype AB in Group I is distinctive from the other genotypes in Group I. Group II is characterized by below average means for seed weight and seedling traits measured at 14 and 21 DAS, but had average means for waterlogging traits (49 DAS). Group III is characterized by higher than average mean seed weights and seedling traits (14 and 21 DAS), but lower than average means for waterlogging traits (49 DAS).
Among all measured traits, shoot and root RGRs had the strongest positive correlation (r = 0.86; P < 0.001) (Fig. 8; Supplementary data Table S7). Under waterlogging, petiole length had strong positive correlations to both shoot (r = 0.59; P < 0.001) and root (r = 0.57; P < 0.001) RGRs, while leaf size had a high positive correlation with shoot RGR (r = 0.55; P < 0.01). Surprisingly, neither SPAD value nor surface root proliferation were significantly correlated with shoot and root RGRs under waterlogging.
A variable correlation plot of the first two principal components (PC1 and PC2) for 32 ssp. yanninicum genotypes, consisting of 4 cultivars and 28 ecotypes. The length of eigenvectors in the plot indicates the relative importance of each variable to PC1 and PC2. Variables: 1, shoot relative growth rate (RGR, 49 days after sowing, DAS); 2, root RGR (49 DAS); 3, shoot dry weight (DW, 49 DAS); 4, root DW (49 DAS); 5, leaf size (49 DAS); 6, petiole length (49 DAS); 7, chlorophyll content (49 DAS); 8, surface root proliferation (49 DAS); 9, days to first flowering; 10, mean seed weight; 11, cotyledon size (14 DAS); 12, cotyledon DW (14 DAS), 13, hypocotyl length (14 DAS); 14, leaf size (21 DAS); 15, petiole length (21 DAS); 16, shoot DW (21 DAS); and 17, root DW (21 DAS)
None of the seedling traits measured at 14 and 21 DAS contributed to waterlogging tolerance, that is, they did not show a significant positive correlation with any of the waterlogging traits measured at 49 DAS (Fig. 8; Supplementary data Table S7). On the contrary, seedling traits had negative correlations with waterlogging traits. Thus, genotypes of ssp. yanninicum with larger seeds (and hence, bigger cotyledons) and vigorous seedlings under free-draining conditions with larger leaves and longer petioles at 21 DAS showed a low RGR under waterlogging (Fig. 8; Supplementary data Table S7). Furthermore, DFF, SPAD value and surface root proliferation had low contributions to the first two PCs (Fig. 8). Therefore, DFF, all seedling traits measured at 14 and 21 DAS, SPAD value and surface root proliferation at 49 DAS were excluded from analyses of phenotypic plasticity to assess waterlogging tolerance among ssp. yanninicum genotypes. Furthermore, shoot and root DWs were also excluded, as the calculation of both shoot and root RGRs were based on dry weights. Hence, traits used for phenotypic plasticity analyses were shoot and root RGRs at 49 DAS, leaf size and petiole length at 49 DAS.
Phenotypic plasticity in response to waterlogging
The relative distance plasticity index (RDPI)
Shoot RGR, leaf size and petiole length, but not root RGR, expressed significant differences (0.01 < P < 0.001) in RDPI among 32 ssp. yanninicum genotypes (Fig. 9). The highest level of plasticity was observed for petiole length, followed by leaf size.
Relative distance plasticity index (RDPI) at 49 days after sowing (DAS) for 32 ssp. yanninicum genotypes, consisting of 4 cultivars and 28 ecotypes. Treatments were imposed at 21 DAS: free-draining (control) and waterlogged (WL, water level kept 10 mm above the soil surface). Boxplots show a visual representation of the maximum value (upper whisker), minimum value (lower whisker), interquartile range (lower and upper quartile) and the median (horizontal line) of the dataset. Significant differences (P < 0.05) among genotypes are shown: *P < 0.05; **P < 0.01; ***P < 0.001
Among all genotypes, the RDPI values in Group III (in particular Ecotypes E, R and N) and Ecotypes AB and S in Group I, consistently showed high plasticity for shoot and root RGRs, leaf size and petiole length (Supplementary data Table S8). Ecotype AB was more plastic than the other genotypes in Group I, especially for shoot RGR, leaf size and petiole length (Supplementary data Table S8).
Multivariate plasticity index
MVPi values calculated for the combination of shoot and root RGRs, leaf size and petiole length measured at 49 DAS varied significantly among ssp. yanninicum genotypes (Fig. 10 and Supplementary data Table S8). Genotypes in Group III (except Ecotype M) tended to have high MVPi values (higher phenotypic plasticity), while genotypes in Group I (except Ecotypes AB and S) and Group II (except Ecotypes AA and U) tended to have with low MVPI values. Ecotypes in Group III (except Ecotype M) and Ecotype AB in Group I showed similarly large plasticity, but the direction of response to waterlogging was opposite (positive for Group I and negative for Group III) (Table 4).
The multivariate plasticity index (MVPi) calculated for the combination of shoot relative growth rate (RGR), root RGR, leaf size and petiole length at 49 days after sowing (DAS) for 32 ssp. yanninicum genotypes, consisting of 4 cultivars and 28 ecotypes (mean ± SE, n = 4). Treatments were imposed at 21 DAS: free-draining (control) and waterlogged (WL, water level kept 10 mm above the soil surface). Cluster groups are indicated by colour: Group I (red), Group II (green) and Group III (blue). Different letters indicate significant differences among genotypes (P < 0.05; Fisher LSD test) and result of one-way ANOVA is given in panel. Genotypes are ordered from negative to positive impact of WL on shoot relative growth rate (see Fig. 1)
Relationships of variation for waterlogging tolerance with eco-geographic variables among ssp. yanninicum ecotypes
The relationships between ecotypes and 22 eco-geographic variables are shown in Fig. 11. The first two PCs in the biplot explain 65% of the total variance in the dataset. There was no distinct partitioning for cluster Groups I, II and III in terms of eco-geographic variables at the site of origin (Fig. 11). Neither shoot nor root RGRs correlated with any eco-geographic variables (Fig. 11; Supplementary data Table S9 and Fig S5), except weak positive correlations of shoot RGR with temperature variables: BIO5 (r = 0.42; P < 0.05), BIO9 (r = 0.43; P < 0.05) and BIO10 (r = 0.44; P < 0.05). These were associated with only a small number of genotypes in Group I (including AB, D and A) with high tolerance to waterlogging (Fig. 11).
PCA biplot of eco-geographic variables and for 29 ssp. yanninicum genotypes, consisting of two cultivars (Trikkala and Yarloop excluded) and 27 ecotypes (Ecotype E excluded). Cluster groups are indicated by colour: group I (red), group II (green) and group III (blue). The eigenvectors (length indicates relative importance) are: 1–19, BIO1-BIO19 (See Table 2 for a code definitions of BOCLIM variables); 20, latitude; 21, longitude; 22, altitude
Discussion
High variation for waterlogging tolerance, as assessed by the maintenance of shoot RGR relative to free-draining conditions, was evident among ssp. yanninicum genotypes, supporting the first hypothesis of this study. It is noteworthy that the highest tolerance to waterlogging was observed among wild ecotypes rather than commercialized cultivars, with several ecotypes displaying similar or greater growth under waterlogging than free-draining conditions. Ecotypes D (CPI 039315 YC) and AB (LO0751) showed a superior waterlogging tolerance among genotypes. The high tolerance in Ecotype AB is attributable to its large plastic response, demonstrating its enhanced fitness to waterlogged conditions. Overall, many genotypes of ssp. yanninicum tolerated transient waterlogging that had relatively stable growth with low MVPi values, a general plasticity, showing little distance between waterlogged and free-draining conditions, particularly genotypes in cluster Groups I and II. In contrast, high MVPi values for genotypes in cluster Group III demonstrated that these genotypes were vulnerable to waterlogging display large phenotypic changes in response to waterlogging stress. The study identified genetic resources with a range of ecotypes that could be used as parents in plant breeding programs to improve tolerance to waterlogging in subclover.
Relationships of variation for waterlogging tolerance with phenotypic traits
The second hypothesis, that the variation in waterlogging tolerance is related to phenotypic and growth trait differences, was supported. High genetic variation existed among ssp. yanninicum genotypes in seed and seedling traits measured at 14 and 21 DAS as well as maturity duration. However, these highly heritable, readily measureable traits (e.g. seed size, seed colour, seedling traits and maturity duration) did not contribute to waterlogging tolerance. Palta et al. (2010) also found that vigorous early growth of seedlings is unrelated to tolerance to waterlogging in chickpea. This may reflect genotypes with lower initial shoot biomass needing to improve their growth rates above the soil surface in order to respond to soil waterlogging. An increased proportion of aerial organs enables higher oxygen uptake and greater exchange of gases under waterlogging (Colmer 2003; Striker et al. 2005; Striker and Colmer 2017). It also could be that a genotype with high initial shoot biomass saves reserves (energy and carbohydrates) for later use, since resources already accumulated in biomass (aerial organs) might be enough to respond waterlogging stress. Striker (2012b) highlighted that plant growth responses under waterlogging do not fully define tolerance to the stress. Little or absence of growth under waterlogging can save reserves and deliver benefits for rapid growth following alleviation of waterlogging stress (Setter and Waters 2003; Striker 2012b). Further research is, therefore, required to investigate performance of ssp. yanninicum after alleviation of waterlogging stress comparing their growth under waterlogging to evaluate tolerance to waterlogging of ssp. yanninicum.
The variation in waterlogging tolerance among ssp. yanninicum was expressed by the capacity for phenotypic plasticity in traits at 49 DAS (after 28 days of treatment) when plants were grown under waterlogging. Waterlogging tolerance (e.g. shoot RGR) and traits that contributed to waterlogging tolerance or explained sensitivity to waterlogging are now discussed in more detail.
Shoot and root growth
Root growth was more affected by waterlogging than shoot growth in ssp. yanninicum. This supports the assertions of Colmer and Voesenek (2009), Herzog et al. (2016), Nakai et al. (2009) and Striker (2012a) that soil waterlogging directly effects root system growth and function. A high positive correlation between root and shoot growth rate under waterlogging in the present study demonstrated the primary role of root systems to waterlogging tolerance (Armstrong et al. 1983). All genotypes developed surface roots in response to waterlogging, as also found by Francis and Devitt (1969) and Enkhbat et al. (2021b). Enkhbat et al. (2021b) demonstrated that under waterlogging subclover forms lateral roots positioned near the better-aerated soil surface with improved porosity. Root proliferation near to soil surface demonstrates that roots are highly plastic and this plasticity strength enables ssp. yanninicum to cope with waterlogging by enhancing genotype fitness.
Leaf size and petiole length
Leaf growth among genotypes of ssp. yanninicum was little affected by waterlogging, while petiole length was often reduced. Francis and Devitt (1969) found no reduction in leaf size among 25 genotypes of ssp. yanninicum under waterlogging. The relative stability of leaf growth might be mediated through substantial plasticity of physiological characters (Schlichting 1986) as demonstrated in this study by a significant reduction in SPAD value, with characteristic yellowing of the leaves and reduction of SPAD values. Observed high positive correlations between both leaf and petiole growth with shoot growth under waterlogging could be explained as a snorkelling effect (part of the shoot protruding into the air), by enhancing internal aeration whereby the air-exposed tissues conduct oxygen from the atmosphere to oxygen-deprived below-ground tissues (Herzog and Pedersen 2014). Reduction of petiole length under waterlogging is a relatively simple, inexpensive and non-destructive measurement, can be used as an indicator of waterlogging stress in ssp. yanninicum and can be used as preliminary selection tool to screen large number of genotypes for waterlogging tolerance in plant breeding programs.
Relationships of variation for waterlogging tolerance with eco-geographic variables
Variation in waterlogging tolerance of ssp. yanninicum showed no relationship with any of the eco-geographic variables. Thus, the third hypothesis was not supported. This result could be attributed to the restricted distribution of ssp. yanninicum as its natural habitat is reported to be low altitude (below 1400 m a.s.l) coastal areas (often flat meadows) with high mean annual rainfall (> 450 mm) and mild winter temperatures (Francis 1976; Ghamkhar et al. 2015). The distribution of ssp. yanninicum in lower profiles of the landscape, which tend to collect more water, could be a reason for ssp. yanninicum having inherent features conferring adaptation to waterlogging compared to ssp. brachycalycinum and ssp. subterraneum, which are found in elevations up to 2190 and 2940 m a.s.l, respectively (Ghamkhar et al. 2015).
Plant breeding implications
This study has identified promising material for plant breeders as new parents for crossing to enhance waterlogging tolerance and increase the genetic base in this globally important annual legume. However, as results could differ in field environments (Villemereuil et al. 2015), field studies under waterlogged conditions are required to validate our results. Our approach could be strengthened with focus on other key responses to waterlogging such as shoot physiological responses, and changes in root anatomical and morphological responses. Finally, exploring post-waterlogging stress among genotypes of ssp. yanninicum is crucial to identify genotypes with better performance after waterlogging subsides.
References
Abdi AI, Nichols PGH, Kaur P, Wintle BJ, Erskine W (2020) Morphological diversity within a core collection of subterranean clover (Trifolium subterraneum L.): lessons in pasture adaptation from the wild. PLoS ONE 15:e0223699–e0223699
Armstrong W (1980) Aeration in higher plants. In: Woolhouse HW (ed) Advances in botanical research, vol 7. Elsevier Science & Technology, Amsterdam, pp 225–332
Armstrong W, Healy MT, Lythe S (1983) Oxygen diffusion in pea. II. Oxygen concentrations in the primary pea root apex as affected by growth, the production of laterals and radial oxygen loss. New Phytol 94:549–559
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Bertholdsson N (2013) Screening for barley waterlogging tolerance in nordic barley cultivars (Hordeum vulgare L.) using chlorophyll fluorescence on hydroponically-grown plants. Agron (Basel) 3:376–390
Chapman SC, Chakraborty SM, Dreccer MF, Howden SM (2012) Plant adaptation to climate change-opportunities and priorities in breeding. Crop Pasture Sci 63:251–268
Cocks P (1994) Colonization of a South Australian grassland by invading Mediterranean annual and perennial pasture species. Aust J Agric Res 45:1063–1076
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Flowers TJ (2008) Flooding tolerance in halophytes. New Phytol 179:964–974
Colmer TD, Greenway H (2011) Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J Exp Bot 62:39–57
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Craig A (1992) Pasture production of two cultivars of Trifolium subterraneum at Kybybolite, South Australia. Aust J Exp Agric 32:611–617
Devitt A, Francis C (1972) The effect of waterlogging on the mineral nutrient content of “Trifolium subterraneum”. Aust J Exp Agric 12:614
Drew MC, Jackson MB, Giffard S (1979) Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147:83–88
Enkhbat G, Nichols PGH, Foster KJ, Ryan MH, Inukai Y, Erskine W (2021a) Diversity for morphological traits, flowering time and leaf isoflavone content among ecotypes of Trifolium subterraneum L. ssp. yanninicum and their relationships with site of origin. Crop Pasture Sci 72:1022–1033
Enkhbat G et al (2021b) Large variation in waterlogging tolerance and recovery among the three subspecies of Trifolium subterranean L. is related to root and shoot responses. Plant Soil 464:467–487
Erskine W, Adham Y, Holly L (1989) Geographic distribution of variation in quantitative traits in a world lentil collection. Euphytica 43:97–103
Falconer DS (1989) Introduction to quantitative genetics, 3rd edn. Longman, Scintific & Technical Burnt Mill, Harlow
Francis C, Devitt A (1969) The effect of waterlogging on the growth and isoflavone concentration of Trifolium subterraneum L. Aust J Agric Res 20:819–825
Francis CM (1976) Observations on the ecology of subterranean clover in Greece and Crete: a report based on a seed collection tour 1976. Western Australian Dept. of Agriculture, Perth
Francis CM, Devitt AC, Steele P (1974) Influence of flooding on the alcohol dehydrogenase activity of roots of Trifolium subterraneum L. Funct Plant Biol 1:9–13
Gardner WK, Flood RG (1993) Less waterlogging damage with long season wheats. Cereal Res Commun 21:337–343
Ghamkhar K, Nichols PGH, Erskine W, Snowball R, Murillo M, Appels R, Ryan MH (2015) Hotspots and gaps in the world collection of subterranean clover (Trifolium subterraneum L.). J Agric Sci 153:1069–1083
Gibberd MR, Cocks PS (1997) Effect of waterlogging and soil pH on the micro-distribution of naturalised annual legumes. Aust J Agric Res 48:223–230
Gibberd MR, Gray JD, Cocks PS, Colmer TD (2001) Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation and ‘aerotropic rooting’. Ann Bot 88:579–589
Grassein F, Till-Bottraud I, Lavorel S (2010) Plant resource-use strategies: the importance of phenotypic plasticity in response to a productivity gradient for two subalpine species. Ann Bot 106:637–645
Hamilton G, Bakker D, Houlebrook D, Spann C (2000) Raised beds prevent waterlogging and increase productivity. Aust J Soil Res 43:575–585
Herzog M, Pedersen O (2014) Partial versus complete submergence: snorkelling aids root aeration in Rumex palustris but not in R. acetosa. Plant. Cell Environ 37:2381–2390
Herzog M, Striker GG, Colmer TD, Pedersen O (2016) Mechanisms of waterlogging tolerance in wheat -a review of root and shoot physiology. Plant Cell Environ 39:1068–1086
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hunt R (1982) Plant growth curves: the functional approach to plant growth analysis. Edward Arnold, London
Husson F, Josse J, Pages J (2010) Principal component methods-hierarchical clustering-partitional clustering: why would we need to choose for visualizing data. Technical Report 17
Jarvis A, Ramirez J, Anderson B, Leibing C, Aggarwal P (2010) Scenarios of climate change within the context of agriculture. In: Reynolds MP (ed) Climate change and crop production. CABI, Wallingford
Katznelson J, Morley FHW (1965) A taxonomic revison of sect. Calycomorphum of the genus Trifolium. I. The geocarpic species. Isr J Bot 14:112–134
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Ling Q, Huang W, Jarvis P (2010) Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynthesis Res 107:209–214
Malik AI, Ailewe TI, Erskine W (2015) Tolerance of three grain legume species to transient waterlogging. AoB Plants 7:plv040
Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M (2002) Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol 153:225–236
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Marshall T, Millington A (1967) Flooding tolerance of some Western Australian pasture legumes. Aust J Exp Agric 7:367–371
McDonald GK, Gardner WK (1987) Effect of waterlogging on the grain yield response of wheat to sowing date in south-western Victoria. Aust J Exp Agric 27:661–670
Minasny B, McBratney AB, Brough DM, Jacquier D (2011) Models relating soil pH measurements in water and calcium chloride that incorporate electrolyte concentration. Eur J Soil Sci 62:728–732
Nakai A, Yurugi Y, Kisanuki H (2009) Growth responses of Salix gracilistyla cuttings to a range of substrate moisture and oxygen availability. Ecol Res 24:1057–1065
Nichols PG, Cocks PS, Francis CM (2009) Evolution over 16 years in a bulk-hybrid population of subterranean clover (Trifolium subterraneum L.) at two contrasting sites in south-western Australia. Euphytica 169:31–48
Nichols PGH, Collins WJ, Barbetti MJ (1996) Registered cultivars of subterranean clover -their characteristics, origin and identification. Chief executive officer, Western Australia
Nichols PGH, Foster KJ, Piano E, Pecetti L, Kaur P, Ghamkhar K, Collins WJ (2013) Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop Pasture Sci 64:312–346
Nichols PGH et al (2007) New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution. Field Crops Res 104:10–23
Oram RN (1989) Register of Australian herbage plant cultivars. 3rd ed. / compiled by R.N. Oram edn. Australian Herbage Plant Registration Authority, Division of Plant Industry, CSIRO, Melbourne
Palta JA, Ganjeali A, Turner NC, Siddique KHM (2010) Effects of transient subsurface waterlogging on root growth, plant biomass and yield of chickpea. Agric Water Manage 97:1469–1476
Pang J, Zhou M, Mendham N, Shaballa S (2004) Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust J Agric Res 55:895–895
PBR (2021) Plant breeders rights database. Commonwealth Government of Australia. https://www.ipaustralia.gov.au/plant-breeders-rights. Accessed 12 Jan 2021
Peak JW, Morley FHW (1973) Comparison of introduced lines of Trifolium subterraneum subsp. yanninicum with cultivars of T. subterraneum. 3 Irrigated and dryland sward trials at Canberra. Australian Capital Territory vol Field station record, vol 12. Division of Plant Industry, CSIRO, Melbourne
Pennacchi JP, Lira JMS, Rodrigues M, Garcia FHS, Mendoncą AMdC, Barbosa JPRAD (2021) A systemic approach to the quantification of the phenotypic plasticity of plant physiological traits: The multivariate plasticity index. J Exp Bot 72:1864–1878
Piano E (1984) Preliminary observations on the structure and variability of Sardinian populations of subterranean clover. Genet Agr 38:75–90
Piano E, Pecetti L, Carroni AM (1996) Climatic adaptation in subterranean clover populations. Euphytica 92:39–44
Ponnamperuma FN (1984) Effects of flooding on soils. In: Kozlowski TT (ed) Flooding and plant growth. Academic, San Diego, pp 9–45
Porqueddu C et al (2016) Grasslands in ‘Old World’ and ‘New World’ Mediterranean-climate zones: past trends, current status and future research priorities. Grass Forage Sci 71:1–35
Reed K, Schroder P, Eales J, McDonald R, Chin J (1985) Comparative productivity of Trifolium subterraneum and T. yanninicum in south-western Victoria. Aust J Exp Agric 25:351–361
Rogers ME et al (2011) Salinity and waterlogging tolerance amongst accessions of messina (Melilotus siculus). Crop Pasture Sci 62:225–235
Rogers ME, West DW (1993) The effects of rootzone salinity and hypoxia on shoot and root growth in Trifolium species. Ann Bot 72:503–509
Sandral GA, Peoples MB, Wilson BCD, Taylor JN, Rodham CA (2003) Growth, seed set and nitrogen fixation of 28 annual legume species on 3 Vertosol soils in southern New South Wales. Anim Prod Sci 43:1101–1115
Schlichting CD (1986) The evolution of phenotypic plasticity in plants. Annu Rev Ecol Syst 17:667–693
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Siddique KHM, Brinsmead RB, Knight R, Knights EJ, Paull JG, Rose IA (2000) Adaptation of chickpea (Cicer arietinum L.) and faba bean (Vicia faba L.) to Australia. In: Knight R (ed) Linking Research and Marketing Opportunities for Pulses in the 21st Century: Proceedings of the Third International Food Legumes Research Conference. Springer, Dordrecht, pp 289–303
Smith RW, Harris CA, Cox K, McClements D, Clark SG, Hossain Z, Humphries AW (2021) A history of Australian pasture genetic resource collections. Crop Pasture Sci 72:591–612
Solaiman Z, Colmer TD, Loss SP, Thomson BD, Siddique KHM (2007) Growth responses of cool-season grain legumes to transient waterlogging. Aust J Agric Res 58:406–412
Stoddard FL, Balko C, Erskine W, Khan HR, Link W, Sarker A (2006) Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 147:167–186
Striker G (2012a) Flooding stress on plants: anatomical, morphological and physiological responses. In: Mworia JK (ed) Botany. IntechOpen
Striker G, Insausti P, Grimoldi A, Ploschuk E, Vasellati V (2005) Physiological and anatomical basis of differential tolerance to soil flooding of Lotus corniculatus L. and Lotus glaber Mill. Plant Soil 276:301–311
Striker GG (2012b) Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants. Ecol Res 27:983–987
Striker GG, Colmer TD (2017) Flooding tolerance of forage legumes. J Exp Bot 68:1851–1872
Sultana R et al (2013) Waterlogging tolerance in pigeonpea (Cajanus cajan (L.) Millsp.): genotypic variability and identification of tolerant genotypes. J Agric Sci 151:659–671
Trought MCT, Drew MC (1980) The development of waterlogging damage in young wheat plants in anaerobic solution cultures. J Exp Bot 31:1573–1585
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
Villemereuil PD, Gaggiotti OE, Mouterde M, Till-Bottraud I (2015) Common garden experiments in the genomic era: new perspectives and opportunities. Heredity 116:249
Voesenek LACJ, Sasidharan R (2013) Ethylene- and oxygen signalling-drive plant survival during flooding. Plant Biol 15:426–435
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst 20:249–278
Whitman DW, Agrawal AA, Ananthakrishnan T (2009) What is phenotypic plasticity and why is it important. Phenotypic plasticity of insects: mechanisms and consequences. Science Publishers, Enfield
Williams RF, Evans LT, Ludwig LJ (1964) Estimation of leaf area for clover and lucerne. Aust J Agric Res 15:231–233
Acknowledgements
This research was conducted as part of the Annual Legume Breeding Australia (ALBA) Joint Venture between UWA and PGG Wrightson Seeds. The project is also under the International Collaborative Program in Agricultural Sciences between Nagoya University and UWA. G.E. is supported by an Australian Government Research Training Program (RTP) scholarship and a Science Industry PhD Fellowship from the Government of Western Australia. We gratefully acknowledge Robert Creasy, João Paulo Pennacchi, Bill Piasini, Joanne Wisdom, Evonne Walker, Elise Robinson and Robert Jeffery for technical advice and help. We also thank three anonymous referees, whose comments have improved this paper.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Gustavo Gabriel Striker.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 4.03 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enkhbat, G., Ryan, M.H., Nichols, P.G.H. et al. Petiole length reduction is an indicator of waterlogging stress for Trifolium subterraneum ssp. yanninicum. Plant Soil 475, 645–667 (2022). https://doi.org/10.1007/s11104-022-05404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05404-6