Abstract
Plants engage in many processes and relationships that appear to be wasteful of the high-energy compounds that they produce through carbon fixation and photosynthesis. For example, living trees keep leafless tree stumps alive (i.e. respiring) and support shaded understory trees by sharing carbohydrates through root grafts or mycorrhizal fungal networks. Plants exude a diverse array of organic compounds from their roots and leaves, which support abundant rhizosphere and phyllosphere microbiomes. Some plants release substantial amounts of sugar via extra-floral nectaries, which enrich throughfall and alter lichen communities beneath the canopy. Large amounts of photosynthetically fixed carbon are transferred to root associates such as mycorrhizal fungi and N-fixing micro-organisms. Plants also respire fixed C through an alternative pathway that does not generate ATP. Rates of each of these processes appear to be highest when plants are growing under mild-to-moderate deficiencies of nutrients or water. During this stage of deficiency, aboveground plant growth is curtailed more than photosynthesis, causing leaves to produce surplus carbohydrates. Each of the above phenomena provide a sink for these surplus carbohydrates, thereby preventing feedback inhibition of photosynthesis, and perpetuating the influx of C. Because these processes incur little cost to the source plant, they need not provide a benefit beyond the removal of surplus carbohydrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Under common environmental conditions such as deficiencies of water, nitrogen (N) or phosphorus (P), or low temperatures, aboveground plant growth is curtailed at an earlier stage of deficiency than is photosynthesis, and during this phase, leaves produce more photo-assimilates than they are able to use for primary metabolism (Prescott et al. 2020). This ‘surplus carbon’ phase occurs prior to the onset of severe or prolonged deficiencies which constrain photosynthesis (Körner 2013; Prescott et al. 2020, 2021). Nonstructural carbohydrates (soluble sugars and starch) also accumulate under high light intensity and elevated carbon dioxide concentrations, if growth is constrained by availability of water or nutrients (Sugiura et al. 2019; Prescott et al. 2020). Accumulation of carbohydrates is a key trigger for leaves to reduce photosynthetic C fixation rates (Drake et al. 1997; Ruiz-Vera et al. 2017), but removal of carbohydrates from sites of accumulation can forestall or postpone down-regulation of photosynthesis. This has been demonstrated by shading some of the leaves on a plant, which can prevent down-regulation of photosynthesis (Pieters et al. 2001) or may even increase photosynthetic rates in unshaded leaves (McCormick et al. 2006). Therefore, the presence of active sinks for surplus carbohydrates can cause plants to maintain rapid rates of photosynthesis when aboveground growth is restricted by resource supply. Several potential sinks for surplus carbohydrates exist (in addition to growth), including organs, physiological processes, and other organisms (either internal or external to the plant) that take up the surplus plant metabolites. In essence, any process that draws surplus carbohydrates away from sites of accumulation may serve as a sink under conditions of surplus C. Sinks for plant surplus carbohydrates may be behind several physiological and ecological phenomena that are difficult to explain, except through the lens of surplus C. Here, I discuss a few of these phenomena (alternative oxidase pathway, plant secondary metabolism, extra-floral nectaries, living stumps, and root exudates) with special emphasis on forest ecosystems and a few of the many scientific contributions of Hans Lambers to our understanding of plant physiological and ecological processes.
Alternative Oxidase Respiratory Pathway
Lambers (1980) investigated a non-phosphorylating respiratory pathway within plant mitochondria, which is not coupled to the production of ATP. Following analysis of experimental evidence, he concluded that the alternative pathway functions as an energy overflow to oxidize excess sugars (and NADH) that are not required for growth, maintenance, osmoregulation or storage. This ‘Alternative Oxidase Pathway’ (AOX) prevents the accumulation of reactive oxygen species in the mitochondrial electron transfer chain which would otherwise damage proteins, lipids and DNA (Lambers and Oliveira 2019). Alternative oxidase activity increases when carbon (NADH) supplies are plentiful and electron flow is restricted, which occurs under conditions such as high light intensity, prolonged water deficit, low temperature, low P supply or high CO2 concentration (Millenaar and Lambers 2003; Del-Saz et al. 2018; Selinski et al. 2018). These are also the conditions under which surplus fixed C is generated (Prescott et al. 2020), supporting the suggestion that under these environmental conditions, plants produce more carbohydrates through photosynthesis than they require for their primary metabolism at that time.
Secondary Plant Metabolites
Lambers (1993) explored the influence of plant ‘excess carbon’ in determining concentrations of secondary plant metabolites, particularly phenolic compounds, in leaves. He noted that there was already a wealth of evidence that under nutrient- or water-limiting conditions, plants accumulate non-structural carbohydrates and produce more secondary metabolites of a phenolic nature. Two metabolic mechanisms explain this accumulation of secondary metabolites: sucrose levels increasing beyond those needed for protein synthesis, and insufficient N to convert phenylalanine into protein, causing more of the phenylalanine to be diverted into pathways that generate phenolic compounds. Based on this ‘excess carbon’ hypothesis, Lambers (1993) predicted that elevated CO2 would only increase concentrations of secondary metabolites in plants if the higher CO2 concentration was not matched by increased uptake of nutrients or water. This prediction has been borne out in studies reporting negative correlations between levels of C-based secondary metabolites and N concentrations of plants exposed to high CO2 (Wu et al. 2011; Ibrahim and Jaafar 2012), and heightened effects of elevated CO2 on secondary metabolite levels in plants grown at low nutrient availability (Julkunen-Tiitto et al. 1993; Lavola and Julkunen-Tiitto 1994). The effect appears to be specific to a deficiency of N and limited to concentrations of phenylpropanoid-derived compounds (not hydrolyzable tannins or terpenoids) (Koricheva 1998). Along a 2-million-year soil-development chronosequence in south-western Australia, phenolics accumulated in plants growing on young soils in which N was limiting for primary productivity, but not on old soils, where P was limiting and silica accumulated (de Tombeur et al. 2021). Secondary metabolism also produces excess reducing equivalents, which can be oxidized through the AOX pathway (Sakano 2001). These findings support surplus fixed C as a causative link between nutrient deficiency, secondary metabolism and the AOX pathway.
Starch granules
Young western hemlock trees on northern Vancouver Island are deficient in N and P, with chlorotic foliage and slow growth (Prescott et al. 2013). Transmission electron microscopy of their needles revealed that a single large starch grain occupied 80% of the cross-sectional area of the chloroplasts (Fig. S1). The thylakoid membranes appeared to be mechanically distorted and the number of thylakoid membranes per granum stack was reduced relative to that in trees that had been fertilized with N and P (White 2001). Starch accumulates in chloroplasts of P-deficient plants and, like sucrose production in the cytosol, is attributed to the production of fixed C in excess of that required by the plant, i.e. when adequate sinks are not available (Hurewitz and Janes 1983; Fredeen et al. 1989). Accumulation of large, irregularly shaped starch grains that distorted grana stacks were also reported in response to elevated CO2 (Cave et al. 1981), further supporting a link to surplus fixed C. Starch is usually considered to function as an energy store for the plant, but C availability is unlikely to constrain production in nutrient-deficient trees. It is more likely that large accumulations of starch in leaves function as sinks where surplus carbohydrates can accumulate without osmotic consequences (Prescott et al. 2020). Cell-wall thickening as also been proposed as a mechanism by which plants consume surplus carbohydrates (Sugiura et al. 2020).
Extra-floral nectaries
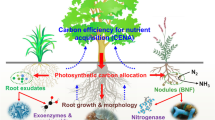
In sub-boreal spruce forests in British Columbia, foliose lichens with cyanobacterial bionts are disproportionately abundant and species-rich on conifer saplings beneath Populus trees (Campbell et al. 2010). The abundance of these lichens was linked to the fungal biont receiving sugars washed from poplar leaves (Campbell et al. 2013). These exogenous sugars allow the fungus to survive extended dry periods during which the cyanobacteria cannot produce sugars to support the symbiosis (Campbell et al. 2013). Populus species are among the 1–2 % of vascular plant species that produce extra-floral nectaries (EFN) at the base of their leaves (Weber and Keeler 2013). Extrafloral nectaries are plant glands that secrete sugar, water and amino-acids (Weber and Keeler 2013). High concentrations of saccharides have been measured in leaf wash and throughfall in Populus stands (Wildman and Parkinson 1981; Sanborn and Pawluk 1983). The sugars released from EFNs and removed via leaf wash may be surplus carbohydrates from leaves. Several lines of evidence support this interpretation: 1) leaching of carbohydrates from leaves is greatest during periods of high light intensity (Tukey et al. 1957; Bixenmann et al. 2010); 2) sugar is imported from older leaves into young leaves that generate EFNs (Radhika et al. 2008); and 3) rainfall washes the existing accumulation of sugar from the leaf surface and stimulates further release from EFNs (Trelease 1881, cited in Campbell et al. 2013). The finding that sugar release from EFN increases at the beginning of the rainy season (Calixto et al. 2021) could also result from sugar removal via rain stimulating its release from EFN. Phylloplane bacteria may also be an important sink for sugars and other metabolites released from leaves (Rodger and Blakeman 1984, Mercier and Lindow 2000) (Fig. 1).
Sinks for surplus plant carbon generated when growth is constrained by availability of N, P or water. In the leaves, surplus carbohydrates can be stored as starch granules in chloroplasts, transformed into secondary metabolites, respired via phosphorylating or alternative oxidase pathways (AOP), or exuded via extra-floral nectaries. On leaf surfaces, compounds can be removed via precipitation, assimilated by bacteria or consumed by insects. Surplus carbohydrates transported to roots can be stored, respired, exuded and assimilated by rhizosphere bacteria, or transferred to endobionts such as N2-fixing microorganisms or mycorrhizal fungi
Extra-floral nectaries are usually interpreted as adaptations for plant defense in that they entice invertebrates such as ants or wasps to act as “pugnacious bodyguards” (Bentley 1977) that protect the plants from herbivores (Koptur 1992). However, observations are not always consistent with this interpretation of the ‘purpose’ of EFNs. For example, in Populus tremuloides trees, concentrations of phenolic glycosides, which deter insect feeding, were about 30% greater on leaves bearing EFNs than on leaves without EFNs (Young et al. 2010). This observation required a rather convoluted explanation on the basis of plant defenses, but is not unexpected when viewed through a surplus C lens, as both sugar exudation and elevated concentrations of secondary metabolites are expected under plant growth-limiting conditions (Prescott et al. 2020). The finding that nectar production by EFNs is stimulated by phloem-sucking insects but not by mechanical damage by other insects (Escalante-Perez et al. 2012) also indicates strong sink control of nectar production in EFNs.
Belowground carbon flux
Up to half of the photosynthate produced by plants may be transported to belowground organs (Högberg and Högberg 2002; Pausch and Kuzyakov 2018), depending on environmental conditions. Much of it is used for root growth and maintenance or is stored, but 10-44% of photosynthetically fixed carbon is excreted by roots or transferred to mycorrhizal fungi (Bais et al. 2006; Pausch and Kuzyakov 2018). The C exuded from roots or from associated mycorrhizal fungi supports a large component of the soil biota, including micro-organisms and invertebrates (Pollierer et al. 2007; Drigo et al. 2008, Yarwood et al. 2009). Half or more of the soil activity in forests may be driven by photosynthate that is transported to mycorrhizal fungi and root-associated microbes within a few days of being fixed (Högberg et al. 2008). Why do trees export so much photosynthate to the belowground ecosystem?
The proportion of fixed C transported belowground is related to the relative availabilities of C versus growth-limiting resources (N, P or water). In forests with high nutrient availability, a greater proportion of the photosynthates produced annually is used for plant biomass production, compared with forests with low nutrient availability (58% vs 42% in a synthesis of 49 forests; Vicca et al. 2012). Increasing availability of C but not nutrients (via CO2 enrichment) often induces a C allocation shift towards belowground compartments (meta-analysis by Dieleman et al. 2010). In the sweetgum stand at the ORNL free-air CO2 enrichment (FACE) experiment, the only increases in NPP in elevated CO2 plots (after the first year) were in fine-root production (Iversen et al. 2008; Norby et al. 2021). They attributed this to progressive N limitation in the stand (Norby et al. 2010), which is consistent with the generation and belowground transport of surplus C under nutrient-deficient conditions. In a hardwood forest at the Swiss FACE experiment, elevated CO2 did not increase aboveground growth or respiration, but did increase C transport to below-ground sinks, as indicated by C transfer to ectomycorrhiza and neighbouring trees and by increased C export to soil (Klein et al. 2016a). In the loblolly pine stand at the Duke free-air CO2 enrichment (FACE) experiment, elevated CO2 conditions led to increases in total belowground C flux, root production, biomass and respiration, exudation and fungal allocation, microbial biomass, heterotrophic respiration and soil CO2 efflux (Drake et al. 2011). Increasing soil N availability through N fertilization reduced the belowground C flux in both ambient and elevated CO2 plots (Drake et al. 2011).
In boreal pine forests, aboveground productivity is strongly limited by N and about 50% of tree photosynthate is transferred belowground and respired from the soil (Högberg and Högberg 2002). Nitrogen additions to a boreal pine forest reduced the flux of tree photosynthate to roots and soil biota, including ectomycorrhizal (ECM) fungi, by as much as 60% (Högberg et al. 2010). Belowground C flux returned to pre-fertilization levels after N additions ceased, coincident with increased abundance of mycorrhizal fungi (Högberg et al. 2011). Belowground C flux (as a proportion of gross primary productivity, GPP) also increases under conditions of high light (Smith and Reynolds 2015), elevated CO2 (Jackson et al. 2009; Drake et al. 2011) or reduced availability of P (Keith et al. 1997) or water (Stape et al. 2008; Preece and Peñuelas 2016; Hasibeder et al. 2015; Ledo et al. 2018). These observations are consistent with the amount of plant C transported belowground being strongly influenced by the amount of surplus fixed C in plant leaves. Indeed, the rapid (1-day) link between GPP and respiration from roots and mycorrhizal fungi in forests prompted Heinemeyer et al. (2007) to propose that the mycorrhizal CO2 flux component represents an overflow ‘CO2 tap’ through which surplus plant carbon can be returned directly to the atmosphere.
A strong seasonal pattern of photosynthate fluxes in boreal pine forests was also noted by Högberg et al. (2010). Belowground flux of photosynthate in August was 500% greater than that in June. They attributed this to developing leaves being a sink for fixed C early in the growing season (Horwath et al. 1994; Kagawa et al. 2006). Once leaves had fully expanded and no longer served as a sink, the fixed C was translocated belowground, much of it to mycorrhizal fungi. Sporocarp production of ECM fungi was totally dependent on recent photosynthate in the late season. This late-summer flux of surplus photosynthate may underly the autumn peak in sporocarp production in boreal forests. Mycorrhizal fungi may therefore function as an alternate sink for surplus carbohydrates once leaf expansion is complete.
Living stumps and carbon sharing among living trees
Leafless tree stumps, which are nevertheless ‘alive’ in the sense that they respire, have been observed in many forests. These stumps have a living root system, which is connected to that of other trees through root grafts and/or mycorrhizal fungal hyphae. These connections give them access to carbohydrates from the root systems of living trees, which sustains the remaining tissues of the leafless tree (Bader and Leuzinger 2019). Evolutionary rationales for the living plants investing carbohydrates in non-photosynthesizing neighbors are challenging, but it has been suggested that the stumps provide an extended root system for mechanical stability and uptake of water and nutrients (Bormann 1966, Keeley 1988, but see Loehle and Jones 1990). Alternatively, living stumps may result from surplus carbohydrates from living trees traveling to them through the phloem of connected roots, driven by the difference in hydrostatic pressure provided by phloem unloading and respiration in the surviving tissues of the stump tree. Sharing of surplus carbon does not entail a cost to the source tree, so this explanation does not require there to be a benefit to the living trees of maintaining a non-living neighbor.
Transfer of carbohydrates among living trees through root grafts (Fraser et al. 2006) or mycorrhizal fungi (Simard et al. 1997; Klein et al., 2016b) has received considerable attention. Carbon fluxes have been traced from source trees growing in full light to sink trees growing in low light conditions (Fraser et al. 2006; Teste et al. 2009). The adaptive ‘purpose’ of the transfers through common mycorrhizal networks has been explained through kin selection, as neighboring trees have a high probability of being related (Gorzelak et al. 2015). Alternatively, these fluxes may represent the movement of plant surplus carbohydrates along pressure gradients through roots and fungal hyphae driven by phloem loading in source trees and phloem unloading in sink trees. Sharing surplus carbohydrates with sink trees is of little cost to the source tree (Corrêa et al. 2012), and may actually benefit the source tree by providing a sink for surplus photo-assimilates.
Carboxylate exudation
Roots of plants growing under conditions of low P availability often exude more carboxylates, especially organic anions such as citrate and malate (Lambers et al. 2011, 2013). In the rhizosphere, carboxylates compete with inorganic and organic P for binding sites which increases the availability of P for plant uptake (Lambers et al. 2011). Release of carboxylates is therefore widely considered to be part of a P-acquisition strategy that allows plants to survive in low-P environments (Lambers et al. 2006, 2011). However, several observations are inconsistent with this interpretation, such as increased carboxylate exudation at low N availability (Zhu et al. 2016) and inconsistent relationships between rates of carboxylate release and both P uptake and plant growth (Huang et al. 2017; He et al. 2021; Wang and Lambers 2020). Carboxylate exudation by roots of alfalfa (Medicago sativa) growing in an alkaline soil low in both N and P was studied by He et al. (2020, 2021). Root exudation of carboxylates (particularly tartrate) decreased with increasing P availability but also increased exponentially with increasing shoot N concentration. The closer association of root carboxylate release with N than with P concentration prompted He et al. (2021) to suggest that N addition resulted in increased production of photosynthates, which could not be used for primary metabolism and growth due to the lack of P, and so were discharged as carboxylates.
Metabolite profiling of root exudates (as well as of shoots and roots) of P-deficient and P-sufficient plants also supports the hypothesis that root exudates can be a means of removing surplus metabolites. Relative to P-sufficient soybean plants, root exudates of P-deficient plants had higher concentrations of TCA cycle intermediates and amino acids, and lower concentrations of phosphate esters (Tawaraya et al. 2014). Shoot and root extracts of P-deficient plants also had low levels of P-containing metabolites such as adenosine 5’-monophosphate and glycerol 3-phosphate and elevated concentrations of adenine, cytosine and adenosine, reflecting inhibition of nucleotide synthesis induced by P starvation. Remobilization of P from phosphate esters is common in P-deficient plants (Tawaraya et al. 2014). The higher concentrations of TCA cycle intermediates such as organic acids in root exudates from P-deficient plants may be a consequence of their accumulation in root cells as surplus metabolites. Plants exposed to very low P supply have very low concentrations of Pi and ADP which restricts the cytochrome pathway and causes TCA-cycle intermediates such as organic acids – especially citrate – to accumulate (Selinski et al. 2018). Increased carbon supply for organic acid synthesis in the TCA cycle in P-deficient roots has been demonstrated through transcriptome (Wasaki et al. 2003; Li et al. 2010) and proteome (Fukuda et al. 2007) analysis; this would lead to higher concentrations of organic acids in P-deficient roots (Tawaraya et al. 2014). Therefore, a primary function of organic acid exudation may be the disposal of surplus metabolites. Particular morphological and physiological traits such as releasing carboxylates in exudative bursts from specialized structures such as cluster roots or dauciform roots are more likely adaptations for P acquisition (Lambers et al. 2006).
The belowground ecosystem
The abundant microorganisms in the plant rhizosphere provide a large and dynamic sink for surplus plant metabolites. The low-molecular-weight organic compounds exuded from roots are rapidly taken up and transformed by rhizosphere bacteria (Treonis et al. 2004; Ostle et al. 2003; Kaštovská and Santruckova 2007), which are grazed by rhizosphere protists (Gao et al. 2019; Ceja-Navarro et al. 2021) and further transformed through the soil food web (Pollierer et al. 2007). As a result, exudates in the soil solution have turnover times in the order of minutes or hours (Nguyen, 2009; Butler et al. 2004). This maintains a steep concentration gradient immediately outside root tips, which promotes exudation (Canarini et al. 2019) and generates a strong sink for plant C. Root microbionts can also stimulate photosynthesis, as evident in the faster photosynthetic rates of plants inoculated with rhizobia and/or mycorrhizal fungi (Wright et al. 1998; Kaschuk et al. 2009) and the decline in photosynthesis rates following removal of arbuscular mycorrhizal fungi (Gavito et al. 2019). These observations are consistent with root microbionts serving as active carbohydrate sinks, which prevents the accumulation of surplus carbohydrates and attendant suppression of photosynthesis in source leaves.
Conclusions
The physiological and ecological phenomena discussed in this paper are challenging to explain if fixed C is invariably viewed as a scarce resource that plants must use efficiently. However, under conditions such as mild-to-moderate deficiencies of N, P or water that reduce leaf growth more so than photosynthesis, plants tend to produce more fixed C than they can use at that time. Accumulation of surplus fixed C in the absence of the growth sink can generate photo-oxidative damage and induce end-product inhibition of photosynthesis, but these outcomes are prevented by the activities of multiple alternative sinks for fixed C such as the alternative oxidase pathway, starch synthesis, secondary metabolites, extra-floral nectaries, root exudation and mycorrhizal fungi. Activities of these sinks increase under the same conditions that result in generation of surplus C (mild-to-moderate deficiencies of N, P or water and high light and CO2). Under these conditions, C ‘allocated’ to these sinks is not really an ‘investment’ as it does not entail a cost to the plant. Instead, these phenomena can be viewed as downstream consequences of C flow from sites of accumulation to sites of removal.
References
Bader MK, Leuzinger S (2019) Hydraulic coupling of a leafless auri tree remnant to conspecific hosts. iScience 19:255
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. Annu Rev Ecol Syst 8:407–427
Bixenmann RJ, Coley PD, Kursar TA (2010) Is extrafloral nectar production induced by herbivores or ants in a tropical facultative ant–plant mutualism? Oecologia 165:417–425
Bormann FH (1966) The structure, function, and ecological significance of root grafts in Pinus strobus L. Ecol Monogr 36:1–26
Butler JL, Bottomley PJ, Griffith SM, Myrold DD (2004) Distribution and turnover of recently fixed photosynthate in ryegrass rhizospheres. Soil Biol Bioch 36:371–382
Calixto ES, Novaes LR, Santos DFB, Lange D, Moreira X, Del-Claro K, Züst T (2021) Climate seasonality drives ant–plant–herbivore interactions via plant phenology in an extrafloral nectary-bearing plant community. J Ecol 109:639–651
Campbell J, Bengtson P, Fredeen AL, Coxson DS, Prescott CE (2013) Does exogenous carbon extend the realized niche of canopy lichens? Evidence from sub-boreal forests in British Columbia. Ecology 94:1186–1195
Campbell J, Bradfield GE, Prescott CE, Fredeen AL (2010) The influence of overstorey Populus on epiphytic lichens in subboreal spruce forests of British Columbia. Can J For Res 40:143–154
Canarini A, Kaiser C, Merchant A, Richter A, Wanek W (2019) Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:157
Cave G, Tolley LC, Strain BR (1981) Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneum leaves. Physiol Plantarum 51:171–174
Ceja-Navarro JA, Wang Y, Ning D, Arellano A, Ramanculova L, Yuan MM, Byer A, Craven KD, Saha MC, Brodie EL, Pett-Ridge J, Firestone MK (2021) Protist diversity and community complexity in the rhizosphere of switchgrass are dynamic as plants develop. Microbiome 9:96–18
Corrêa A, Gurevitch J, Martins-Loução MA, Cruz C (2012) C allocation to the fungus is not a cost to the plant in ectomycorrhizae. Oikos 121:449–463. https://doi.org/10.1111/j.1600-0706.2011.19406.x
de Tombeur F, Laliberté E, Lambers H, Faucon M, Zemunik G, Turner BL, Cornelis J, Mahy G, van der Putten W (2021) A shift from phenol to silica-based leaf defences during long-term soil and ecosystem development. Ecol Lett 24:984–995
Del-Saz NF, Ribas-Carbo M, McDonald AE, Lambers H, Fernie AR, Florez-Sarasa I (2018) An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci 23:206–219
Dieleman WIJ, Luyssaert S, Rey A, De Angelis P, Barton CVM, Broadmeadow MSJ, Broadmeadow SB, Chigwerewe KS, Crookshanks M, Dufrêne E, Jarvis PG, Kasurinen A, Kellomäki S, Le Dantec V, Liberloo M, Marek M, Medlyn B, Pokorný R, Scarascia-Mugnozza G et al (2010) Soil [N] modulates soil C cycling in CO2-fumigated tree stands: a meta-analysis. Plant, Cell Environ 33:2001–2011
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48:609–639
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, DeLucia EH, Finzi AC (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357
Drigo B, Kowalchuk GA, van Veen JA (2008) Climate change goes underground: effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biol Fert Soils 44:667–679
Escalante-Perez M, Jaborsky M, Lautner S, Fromm J, Müller T, Dittrich M, Kunert M, Boland W, Hedrich R, Ache P (2012) Poplar extrafloral nectaries: two types, two strategies of indirect defenses against herbivores. Plant Physiol 159:1176–1191
Fraser EC, Lieffers VJ, Landhäusser SM (2006) Carbohydrate transfer through root grafts to support shaded trees. Tree Physiol 26:1019–1023
Fredeen AL, Madhusudana Rao I, Terry N (1989) Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol 89:225–230
Fukuda T, Saito A, Wasaki J, Shinano T, Osaki M (2007) Metabolic alterations proposed by proteome in rice roots grown under low P and high Al concentration under low pH. Plant Science 172:1157–1165
Gao Z, Karlsson I, Geisen S, Kowalchuk G, Jousset A (2019) Protists: puppet masters of the rhizosphere microbiome. Trends Plant Sci 24:165–176
Gavito ME, Jakobsen I, Mikkelsen TN, Mora F (2019) Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol 223:896–907
Gorzelak MA, Asay AK, Pickles BJ, Simard SW (2015) Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 7:plv050
Hasibeder R, Fuchslueger L, Richter A, Bahn M (2015) Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol 205:1117–1127
He H, Zhang Z, Peng Q, Chang C, Su R, Cheng X, Li Y, Pang J, Du S, Lambers H (2021) Increasing nitrogen supply to phosphorus-deficient Medicago sativa decreases shoot growth and enhances root exudation of tartrate to discharge surplus carbon dependent on nitrogen form. Plant Soil 469:193–211
He H, Wu M, Guo L, Fan C, Zhang Z, Su R, Peng Q, Pang J, Lambers H (2020) Release of tartrate as a major carboxylate by alfalfa (Medicago sativa L.) under phosphorus deficiency and the effect of soil nitrogen supply. Plant Soil 449:169–178
Heinemeyer A, Hartley IP, Evans SP, Carreira de la Fuente JA, Ineson P (2007) Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Change Biol 13:1786–1797
Högberg MN, Briones MJI, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, Näsholm T, Högberg P (2010) Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol 187:485–493
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154:791–795
Högberg P, Högberg MN, Göttlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, Linder S, Näsholm T (2008) High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol 177:220–228
Högberg P, Johannisson C, Yarwood S, Callesen I, Näsholm T, Myrold DD, Högberg MN (2011) Recovery of ectomycorrhiza after 'nitrogen saturation' of a conifer forest. New Phytol 189:515–525
Horwath WR, Pregitzer KS, Paul EA (1994) 14C allocation in tree-soil systems. Tree Physiol 14:1163-1176
Huang G, Hayes PE, Ryan MH, Pang J, Lambers H (2017) Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration at very low phosphorus availability. Oecologia 185:387–400
Hurewitz J, Janes HW (1983) Effect of altering the root-zone temperature on growth, translocation, carbon exchange rate, and leaf starch accumulation in the tomato [Lycopersicon esculentum, relationship of growth response of seedlings to relative translocation and photosynthetic rates]. Plant Physiol 73:46–50
Ibrahim MH, Jaafar HZE (2012) Impact of elevated carbon dioxide on primary, secondary metabolites and antioxidant responses of Eleais guineensis Jacq. (oil palm) seedlings. Molecules 17:5195–5211
Iversen CM, Ledford J, Norby RJ (2008) CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol 179:837–847
Jackson RB, Cook CW, Pippen JS, Palmer SM (2009) Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90:3352–3366
Julkunen-Tiitto R, Tahvanainen J, Silvola J (1993) Increased CO2 and nutrient status changes affect phytomass and the production of plant defensive secondary chemicals in Salix myrsinifolia (Salisb.). Oecologia 95:495–498
Kagawa A, Sugimoto A, Maximov TC (2006) Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytol 171:793–804
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Bioch 41:1233–1244
Kaštovská E, Santruckova H (2007) Fate and dynamics of recently fixed C in pasture plant–soil system under field conditions. Plant Soil 300:61–69
Keeley JE (1988) Population variation in root grafting and a hypothesis. Oikos 52:364–366
Keith H, Raison R, Jacobsen K (1997) Allocation of carbon in a mature eucalypt forest and some effects of soil phosphorus availability. Plant Soil 196:81–99
Klein T, Siegwolf RTW, Körner C (2016b) Belowground carbon trade among tall trees in a temperate forest. Science 352:342–344
Klein T, Bader MK, Leuzinger S, Mildner M, Schleppi P, Siegwolf RTW, Körner C (2016a) Growth and carbon relations of mature Picea abies trees under 5 years of free-air CO2 enrichment. J Ecol 104:1720–1733
Koptur S (1992) Interactions between insects and plants mediated by extrafloral nectaries. In: Bernays E (ed) Insect plant interactions, Vol. 4. CRC Press, Boca Raton, Fla, pp 85–132
Koricheva J (1998) Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83:212–226
Körner C (2013) Growth controls photosynthesis – mostly. Nova Acta Leopoldina NF 114(391):273–283
Lambers H (1980) The physiological significance of cyanide-resistant respiration in higher-plants. Plant Cell Environ 3:293–302
Lambers H, Oliveira RS (2019) Plant Physiological Ecology. Springer International Publishing, Cham
Lambers H, Ahmedi I, Berkowitz O, Dunne C, Finnegan PM, Hardy GE, St J, Jost R, Laliberté E, Pearse SJ, Teste FP (2013) Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conserv Physiol 1:cot010
Lambers H, Brundrett MC, Raven JA, Hopper SD (2011) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Lambers H (1993) Rising CO2, secondary plant metabolism, Plant-herbivore interactions and litter decomposition: theoretical considerations. Vegetatio 104(105):263–271
Lavola A, Julkunen-Tiitto R (1994) The effect of elevated carbon dioxide and fertilization on primary and secondary metabolites in birch, Betula pendula (Roth). Oecologia 99:315–321
Ledo A, Paul KI, Burslem, David FRP, Ewel JJ, Barton C, Battaglia M, Brooksbank K, Carter J, Eid TH, England JR, Fitzgerald A, Jonson J, Mencuccini M, Montagu KD, Montero G, Mugasha WA, Pinkard E, Roxburgh S et al (2018) Tree size and climatic water deficit control root to shoot ratio in individual trees globally. New Phytol 217:8–11
Li L, Liu C, Lian X (2010) Gene expression profiles in rice roots under low phosphorus stress. Plant Mol Biol 72:423–432
Loehle C, Jones RH (1990) Adaptive significance of root grafting in trees. Funct Ecol 4:268–271
McCormick AJ, Cramer MD, Watt DA (2006) Sink strength regulates photosynthesis in sugarcane. New Phytol 171:759–770
Mercier J, Lindow SE (2000) Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl Environ Microbiol 66(1):369–374
Millenaar FF, Lambers H (2003) The alternative oxidase: in vivo regulation and function. Plant Biol 5:2–15
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci U S A. 107:19368–19373
Norby RJ, Warren JM, Iversen CM, Childs J, Jawdy SS, Walker AP (2021) Forest stand and canopy development unaltered by 12 years of CO2 enrichment. Tree Physiol. https://doi.org/10.1093/treephys/tpab107
Nguyen C (2009) Rhizodeposition of organic C by plant: mechanisms and controls. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C (eds) Sustainable Agriculture. Springer, Dordrecht, pp 97–123
Ostle N, Whiteley AS, Bailey MJ, Sleep D, Ineson P, Manefield M (2003) Active microbial RNA turnover in a grassland soil estimated using a 13CO2 spike. Soil Biol Biochem 35:877–885
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol 24:1–12
Pieters AJ, Paul MJ, Lawlor DW (2001) Low sink demand limits photosynthesis under Pi deficiency. J Exp Bot 52:1083–1091
Pollierer MM, Langel R, Körner C, Maraun M, Scheu S (2007) The underestimated importance of belowground carbon input for forest soil animal food webs. Ecol Lett 10:729–736
Preece C, Peñuelas J (2016) Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 409:1–17
Prescott CE, Rui Y, Cotrufo MF, Grayston SJ (2021) Managing plant surplus carbon to generate soil organic matter in regenerative agriculture. J Soil Water Conserv 76:99–104A
Prescott CE, Grayston SJ, Helmisaari H, Kaštovská E, Körner C, Lambers H, Meier IC, Millard P, Ostonen I (2020) Surplus carbon drives allocation and plant–soil interactions. Trends Ecol Evol 35:1110–1118
Prescott CE, Nery V, van Niejenhuis A, Sajedi T, Marshall P (2013) Nutrition management of cedar and hemlock plantations in coastal British Columbia. New Forests 44:769–784
Radhika V, Kost C, Bartram S, Heil M, Boland W (2008) Testing the optimal defence hypothesis for two indirect defences: extrafloral nectar and volatile organic compounds. Planta 228:449–457
Rodger G, Blakeman JP (1984) Microbial colonization and uptake of 14C label on leaves of sycamore. Trans Br Mycol Soc 82:45–51
Ruiz-Vera UM, De Souza AP, Long SP, Ort DR (2017) The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front Plant Sci 8:998
Sakano K (2001) Metabolic regulation of pH in plant cells: Role of cytoplasmic pH in defense reaction and secondary metabolism. In: International Review of Cytology. Elsevier Science & Technology, United States, pp 1-44
Sanborn P, Pawluk S (1983) Process studies of a Chernozemic pedon, Alberta (Canada). Geoderma 31:205–237
Selinski J, Scheibe R, Day DA, Whelan J (2018) Alternative oxidase is positive for plant performance. Trends Plant Sci 23:588–597
Simard SW, Perry DA, Jones MD, Myrold DD, Durall DM, Molina R (1997) Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388:579–582
Smith LM, Reynolds HL (2015) Plant-soil feedbacks shift from negative to positive with decreasing light in forest understory species. Ecology 96:2523–2532
Stape JL, Binkley D, Ryan MG (2008) Production and carbon allocation in a clonal Eucalyptus plantation with water and nutrient manipulations. For Ecol Manage. 225:920–930
Sugiura D, Betsuyaku E, Terashima I (2019) Interspecific differences in how sink-source imbalance causes photosynthetic downregulation among three legume species. Ann Bot 123:715–726
Sugiura D, Terashima I, Evans JR (2020) A decrease in mesophyll conductance by cell-wall thickening contributes to photosynthetic downregulation. Plant Physiol 183:1600–1611
Tawaraya K, Horie R, Shinano T, Wagatsuma T, Saito K, Oikawa A (2014) Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci Plant Nutr 60:679–694
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL (2009) Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology 90:2808–2822
Trelease W (1881) The foliar nectar glands of Populus. Bot Gaz 6:284–290
Treonis AM, Ostle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P (2004) Identification of groups of metabolically-active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biol Biochem 36:533–537
Tukey HB, Wittwer SH, Tukey HB (1957) Leaching of carbohydrates from plant foliage as related to light intensity. Science 126:120–121
Vicca S, Luyssaert S, Peñuelas J, Campioli M, Chapin FS III, Ciais P, Heinemeyer A, Högberg P, Kutsch WL, Law BE, Malhi Y, Papale D, Piao SL, Reichstein M, Schulze ED, Janssens IA (2012) Fertile forests produce biomass more efficiently. Ecol Lett 15:520–526
Wang Y, Lambers H (2020) Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 447:135–156
Wasaki J, Yonetani R, Kuroda S, Shinano T, Yazaki J, Fujii F, Shimbo K, Yamamoto K, Sakata K, Sasaki T, Kishimoto N, Kikuchi S, Yamagishi M, Osaki M (2003) Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant Cell Environ. 26:1515–1523
Weber MG, Keeler KH (2013) The phylogenetic distribution of extrafloral nectaries in plants. Ann Bot 111:1251–1261
White JB (2001) Studies of western hemlock nutrition. PhD Thesis, University of British Columbia
Wildman HG, Parkinson D (1981) Seasonal changes in water-soluble carbohydrates of Populus tremuloides leaves. Can J Bot 59:862–869
Wright DP, Read DJ, Scholes JD (1998) Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ 21:881–891
Wu G, Chen FJ, Ge F, Xiao N (2011) Impacts of elevated CO2 on expression of plant defensive compounds in Bt-transgenic cotton in response to infestation by cotton bollworm. Agric For Entomol 13:77–82
Yarwood SA, Myrold DD, Högberg MN (2009) Termination of belowground C allocation by trees alters soil fungal and bacterial communities in a boreal forest. FEMS Microbiol Ecol 70:151–162
Young B, Wagner D, Doak P, Clausen T (2010) Within-plant distribution of phenolic glycosides and extrafloral nectaries in trembling aspen (Populus tremuloides; Salicaceae). Am J Bot 97:601–610
Zhu S, Vivanco JM, Manter DK (2016) Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl Soil Ecol 28:19021–19033
Acknowledgements
The article celebrates the curiosity, analytical rigor, and intellectual nimbleness to adapt one’s thinking when presented with new evidence that are hallmarks of Professor Hans Lambers. I gratefully acknowledge pre-submission reviews by Drs. Tim Philpott and Hans Lambers, and helpful suggestions from three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Honghua He.
Supplementary Information
Fig. S1.
Chloroplast of 8-year-old western hemlock trees growing under severe N and P deficiency (left) and in plots fertilized with N and P (right). In unfertilized needles, a single large starch grain occupied 80% of the cross-sectional area of the chloroplasts. (JPG 241 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prescott, C.E. Sinks for plant surplus carbon explain several ecological phenomena. Plant Soil 476, 689–698 (2022). https://doi.org/10.1007/s11104-022-05390-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05390-9