Abstract
Aims
The general aim of this meta-analysis is to synthesize and summarize the mean response of barley yield variables to elevated CO2 (eCO2) and how temperature and nitrogen (N) affect the CO2-induced yield responses of barley.
Methods
A meta-analysis procedure was used to analyze five yield variables of barley extracted from 22 studies to determine the effect size and the magnitude concerning eCO2 and its interaction with temperature and N.
Results
CO2 enrichment increased aboveground biomass (23.8%), grain number (24.8%), and grain yield (27.4%). The magnitude of the responses to eCO2 was affected by genotype, temperature, nitrogen, and CO2 exposure methods. Genotype “Anakin” shows the highest CO2 response of aboveground biomass (47.1%), while “Bambina” had the highest grain number (58.4%). Grain yield response was observed to be higher for genotypes “Alexis” (38.1%) and “Atem” (33.7%) under eCO2. The increase of aboveground biomass and grain yield was higher when plants were grown under eCO2 in combination with higher N (151–200 kg ha−1). The interaction between eCO2 and three different temperature levels was analyzed to identify the impacts on barley yield components. The results revealed that the CO2-induced increase in grain number and grain yield was higher in combination with a temperature level of 21–25 °C as compared to lower levels (< 15 and 16–20 ℃). The response of barley yield to eCO2 was higher in growth chambers than in other CO2 exposure methods. Moreover, a higher response of aboveground biomass and grain yield to eCO2 was observed for pot-grown plants compared to field-grown.
Conclusions
Overall, results suggest that the maximal barley production under eCO2 will be obtained in combination with high N fertilizer and temperature levels (21–25 °C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important challenges of the twenty-first century is to find solutions to the problems caused by global climate change. Alleviating future food security challenges will need to estimate crop production response to the ongoing increase of atmospheric carbon dioxide (CO2), together with rising temperature, and soil fertility. Evidence indicates that atmospheric CO2 concentration increased globally, from 280 ppm in the pre-industrial period to about 419 ppm in 2021 and it might increase to 550 ppm by 2050 (IPCC 2021). CO2 is the most important anthropogenic greenhouse gas (GHG) and it represented 74% of overall anthropogenic GHG emissions in 2018 (IPCC 2021). The changes in CO2 concentration and other GHG emissions are expected to increase air temperature by 2.5 to 4.8 °C at the end of the twenty-first century (IPCC 2021). These environmental changes will have a substantial effect on crop growth and food supply in the future. At the same time, the total global food production has to increase by 25 to 70% within the next 40 years, to meet the food demand for the projected increase in the global population (Fróna et al. 2019; United Nations 2011). As the raw material for plant photosynthesis, an increase in CO2 concentration will inevitably affect the growth and development of plants. An increase in atmospheric CO2 generally exerts beneficial effects on plant biomass by increasing net photosynthesis by 30 to 50% and reducing photorespiration (Drake et al. 1997; Poorter and Navas 2003; Schapendonk et al. 2000). This has been studied for cereals including barley, wheat, rice, oat, and rye (Conroyac et al. 1994; Kimball et al. 2002; Long et al. 2006). For instance, in a meta-analysis comprising 79 crops and wild species, Jablonski et al. (2002) documented an increase in yield of 28% averaged across crops and wild species due to elevated CO2 (eCO2). A climate chamber experiment with 700 ppm CO2 on barley reported an increment of grain yield by 54% compared to 400 ppm (Alemayehu et al. 2014), while 47% enhancement of grain yield averaged across two genotypes was reported by Schmid et al. (2016) under eCO2 level of 550 ppm. Moreover, Manderscheid and Weigel (2006) evaluated the effects of eCO2 on barley using Free-air CO2 enrichment (FACE) at 550 ppm, and obtained yield increases of 7 and 15% under the combination of eCO2 with low and high N supply respectively.
The projected increase in biomass and grain yield of C3 crops due to eCO2 is affected by certain environmental factors such as rising air temperature and nitrogen (N) (Jaggard et al. 2010; Weigel et al. 1994). Despite a good response of C3 crops production to eCO2 at near-optimal temperature (18–23 ℃), the impact may be countered by rising temperature by 2–4 ℃ (Ainsworth 2008; Peng et al. 2004; Lobell and Field 2007; Tao et al. 2008). Accordingly, Dieleman et al. (2012) and Wang et al. (2012) have highlighted the relevance of the interactive impacts of eCO2 and temperature on rice yield, but no particular meta-analysis addressing barley has been undertaken. In general, increased temperature is primarily linked with higher evapotranspiration, acceleration of plant development, and consequently shortening of developmental phases, leading to early maturation and decreased yields (Barnabás et al. 2008; Cox et al. 2000; Hansen et al. 2000; Högy et al. 2013; Mangelsen et al. 2011; Vara Prasad et al. 2002). Studies on six major crops including barley have indicated that increasing the seasonal average temperature by 1 °C results in a significant grain yield reduction by 4 to 10% (Barnabás et al. 2008; Hatfield et al. 2011). Clausen et al. (2011) found a grain yield reduction of barley by 14% under eCO2 and elevated temperature (+ 3 °C) in comparison with the same level of eCO2 and ambient temperature. A 53% reduction in grain yield of barley was recorded by another study due to elevated temperature in combination with eCO2 (Alemayehu et al. 2014).
Elevated CO2 typically leads to a marked increase of biomass in well-fertilized plants (Bowes 1996), this response is modified when the N fertilization is suboptimal. Among the various environmental factors, N availability can have a significant impact on crop biomass and yield formation in response to eCO2 (Stitt and Krapp 1999; Kimball et al. 2002). Several studies have revealed CO2 and N interactions, and it is widely assumed that N deficiency acts as a growth inhibitory factor, potentially decreasing the relative response to eCO2 (Ainsworth and Long 2005). In general, studies address biomass accumulation under eCO2 and variable N supply levels across cereal production (Reich et al. 2006). According to Ziska and Bunce (2007), there is now enough evidence to suggest that crop yield stimulation by eCO2 is dependent on N availability. For example, this has been shown for barley (Kleemola et al. 1994) and wheat (Wolf 1996) and in chamber studies. However, some recent studies with rice that examined crop responses under FACE conditions and low N availability reported similar yield stimulation by eCO2 as under sufficient N supply (Kim et al. 2003, Liu et al. 2008; Yang et al. 2009). There are still uncertainties whether this holds true also for other crops under field conditions.
Even though the pattern of yield response to eCO2 and its interactions with temperature and N are similar within C3 crops, distinctions are evident across species, genotypes, and growing conditions (Connor 2002). The yield response of crops to eCO2 is widely affected by enclosure systems and rooting conditions. Open-top chambers (OTC) have been widely used in eCO2 field experiments but also questioned since they alter the micro-climate of the plants and thus may modify the magnitude of crop responses (Schimel 2006) . Comparison of conditions in OTCs to the open field show that temperatures and vapor pressure deficits are higher inside chambers and airflow is altered in the plant canopy (Ziska and Bunce 2007). The use of OTCs will also reduce transmission of solar radiation and shift the ratio between diffuse and total radiation (Rawson 1995). FACE systems have been developed to create a less artificial experimental setup compared to enclosure systems like OTCs. On the other hand, FACE systems have the drawback of not being able to reach strongly elevated concentrations for eCO2 treatments and possibly less stable concentration levels that may lead to underestimation of plant eCO2 responses (Leakey et al. 2009). However, eCO2 concentrations are often lower in FACE (e.g., 550 ppm) as compared to OTCs or climate chambers (> 600 ppm). It is not clear whether OTCs or FACE studies showed larger effects of elevated CO2 on crop biomass and yield than studies performed in greenhouses or growth chambers. Plants in both greenhouse and climate chambers should be subject to edge effects like those in OTCs (Long et al. 2004). Furthermore, it is questionable whether results from experiments with plants grown in pots can be comparable to field conditions since the response to eCO2 might be reduced due to the restricted rooting volume and the pot size (Loladze 2014; Högy and Fangmeier 2008). Field-grown wheat had similar or lower responses to eCO2 than plants grown in a pot (Wang et al. 2013). This contradicts the premise that restricted root development, nutrient, and water supply in pot studies leads to a decrease in photosynthesis and, as a result, a reduction in plant responsiveness to eCO2 (Arp 1991; Curtis and Wang 1998).
Barley (Hordeum vulgare L.) is one of the most important and extensively cultivated cereal crops worldwide for human nutrition and as animal feed. The production of barley in 2020 was about 150 × 106 tonnes and it has been cultivated in more than 100 countries worldwide (FAOSTAT 2020). However, despite its importance, the effect of eCO2 and its interaction with temperature and N fertilizer on barley production has not been quantitatively reviewed using meta-analysis techniques. Previous meta-analytic studies on C3 crops, such as wheat, rice, and soybean, have provided insights into the extent of the effects of eCO2 on yield variables such as aboveground biomass, grain yield, grain number, thousand-grain weight, and harvest index (Ainsworth 2008; Broberg et al. 2019; Feng et al. 2008). The rationale for a meta-analysis is that, by combining the samples of the individual studies, the overall sample size is increased, thereby improving the statistical power of the analysis as well as the precision of the estimates of treatment effects. Several studies have investigated the effects of eCO2 and their interactions with temperature and N on barley (e.g., Weigel and Manderscheid 2012; Manderscheid et al. 2009; Fangmeier et al. 2000). However, inconsistency in the findings and estimation of the effects in individual studies was noticed. For the first time to our knowledge, the response of barley to eCO2 and its interaction with temperature and N as well as the effect of growing condition (CO2 exposure methods, rooting volume) or genotype is quantitatively reviewed. The objectives of the present meta-analysis are therefore two-fold (1) to synthesize and summarize the mean response of barley yield variables (i.e. aboveground biomass, grain yield, grain number, thousand-grain weight, and harvest index) to eCO2, temperature, N fertilization, and their interactions, and (2) to determine whether different CO2 exposure methods, rooting conditions, or genotypes significantly alter the mean response of barley to eCO2.
Materials and Methods
Database development
Peer-reviewed primary literature focusing on barley yield responses to eCO2, temperature, and N were searched on Scopus, Science Direct, and Google Scholar. The search strings used to search the literature on the search engines are presented in Appendix 1. The search was intended to be comprehensive, including all relevant studies that were published between 1991 and 2020. The response of five yield variables (aboveground biomass, grain number, grain yield, thousand-grain weight, and harvest index) of barley were included in the database as well as in the search strings. The following four inclusion criteria were applied for including studies in the database: (1) the ambient CO2 (aCO2) level had to be ≤ 450 ppm (intended to represent the past and the near future concentration) and the eCO2 has to be ≥ 451 ppm (representing CO2 concentration in the future); (2) at least one of the selected yield variables is evaluated; (3) the response means, and sample sizes (n) are reported directly in the text, table or can be indirectly derived from figures, and (4) the CO2 exposure technique is specified. Publication bias was checked by looking at the symmetry in a funnel plot (Appendix 2, Fig. 9. The final database covered a total of 22 studies that included CO2, temperature, and N treatments over the entire experimental period (Fig. 1). Out of the 22 studies, 84 observations were extracted and analyzed. From these 84 observations, 42 was on the response of barley to eCO2 as a single factor, 18 on the interaction of eCO2 with temperature, and 34 on the interaction of eCO2 with N. Observations were considered as independent within studies, if measurements were taken on different CO2 concentration levels, genotypes, or combinations with N and temperature following previous meta-analysis studies (Ainsworth et al. 2002; Gurevitch and Hedges 1999).
To test the interaction effect of eCO2 with temperature and N, we extracted data only from studies that included CO2, temperature, and N treatments. For experiments involving additional environmental factors, such as O3 and drought, the mean of the controls group was used. For each response variable, means and sample size were recorded from the treatment and control groups for each observation. The response of barley yield variables to eCO2 might be affected by different moderators, thus the data were grouped into several groups, such as CO2 fumigation methods (growth chamber, GC; greenhouse, GH; open-top chamber, OTC; and Free-air CO2 enrichment, FACE), CO2 levels (451–550, 551–650, and 651–720 ppm), air temperature levels (< 15, 15–20, and 21–25 °C), N fertilizer levels (0–50, 51–100, 101–150, and 151-200 kg ha−1), growing conditions (pot-grown and field-grown), and genotypes. Sixteen genotypes were found in several studies, and they were used for the analysis of the CO2 effects on the response of barley yield variables (Table 1). However, in addition to these 16 genotypes, a group of spring cultivars and accessions were used for the overall non-genotype-based analysis. For the analysis of all categorical variables including the interaction of eCO2 with temperature and N, averaged eCO2 was used across all levels.
Meta-analysis
Meta-analysis commonly describes the extent of an experimental treatment mean (ӯT) relative to the control treatment mean (ӯC) (Ainsworth et al. 2002). The log response ratio (mean yield of the experimental to the control group) was used as the effect size to calculate the magnitude of CO2, and its interaction with temperature and N treatment on the selected yield variables of barley. The log transformation can make the data better approximate to the normal distribution, reduce skewness, and make non-linear relationships linear. For each treatment (eCO2, eCO2 with temperature, and N), we calculated the natural logarithm of the ratio as, r = ӯT/ӯC and its percentage change from the control ([r-1] × 100). Thus, the expected mean percentage change is positive for r > 1 but negative for r < 1. Linear mixed models were fitted, assuming that differences among studies within a treatment combination are due to both sampling error and random variation. As variance or related parameters were not reported in several studies, unweighted analyses were performed for all the variables. However, resampling and bootstrapping techniques were used to obtain the confidence intervals of the mean effect size (Gurevitch and Hedges 1999). For each categorical variable, between-group heterogeneity (QB) was examined. The significance of the mean differences from categorical variables was tested (Gurevitch and Hedges 1999). All the analyses were performed in R statistical software (R Core Team 2019). The linear mixed model was fitted using the R library lme4.
Results
CO2 effect on barley yield variables
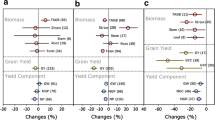
Across all the 42 observations out of 22 studies, a significant enhancement in most of the measured yield components was observed at eCO2 levels (i.e., 451–720 ppm) relative to aCO2 (≤ 450 ppm). Aboveground biomass of barley was increased by 23.8% [CI: 18.0–27.8%] under eCO2 compared to barley plants grown under aCO2. Grain yield was increased by 27.4% [CI: 18.5–36.2%], mainly due to higher grain number (24.8% [CI: 17.7–31.9%]) and thousand-grain weight (5.6% [CI: 3.5–8.1%]), however, the response of harvest index was not affected by eCO2 (Fig. 2). The response of barley yield components varied with eCO2 concentration levels (Fig. 3). For instance, the highest percent enhancement of aboveground biomass (28.7%) was observed under the eCO2 concentration level of 551–650 ppm (Fig. 3). Under 451–550 ppm, aboveground biomass was increased by x%. Due to data limitations, the response of aboveground biomass was not evaluated under the highest eCO2 level (651–720 ppm). Significantly higher positive responses of grain yield, grain number, harvest index, and thousand-grain weight were observed under the highest eCO2 concentration level (651–720 ppm) relative to all the lower levels (Table 2 and Fig. 3).
Relative percentage change in barley yield response to eCO2 analyzed out of 42 observations. The symbols represent the percentage change (± 95% CI) in response relative to the corresponding control. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
Relative percentage change in barley yield response to three levels of CO2 treatment (451–550, 551–650, 651–720 ppm) analyzed out of 42 observations on the response of yield variables/parameter to eCO2. Due to a lack of data, the response of aboveground biomass to the highest level of CO2 concentration was not evaluated. The symbols represent the percentage change (± 95% CI) in response relative to the corresponding control. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
Interaction of eCO2 with N fertilization and temperature treatments
The response of barley yield components to eCO2 was significantly affected by N fertilizer and temperature treatments (Table 2). The results from the interaction of eCO2 with N showed an increase of yield components with increasing N level except for thousand-grain weight and harvest index. The highest responses of aboveground biomass (57.4% [CI: 54–62%]) and grain yield (58.7% [CI: 55–63%]) to eCO2 were observed under the N level of 151–200 kg ha−1, relative to lower N levels (Fig. 4). Grain number was increased by 28.8% due to eCO2 under the application of 51–100 kg ha−1 N, which is not significantly different from the response under N level (151–200 kg ha−1) as shown in Fig. 4. The response of thousand-grain weight and harvest index to eCO2 were not significantly different between the N levels.
Relative percentage change in barley yield response to eCO2 under four different N fertilization treatments. The four levels of N were 0–50, 51–100, 101–150, 151–200 kg ha−1 analyzed out of 34 observations on the interaction between eCO2 and N. Due to lack of data the response of harvest index to eCO2 under N levels 0–50 and 101–150 kg ha−1 was not evaluated. The symbols represent the percentage change (± 95% CI) in eCO2 response relative to the corresponding control. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
In comparison, aboveground biomass was increased, under the combination of eCO2 with 15–20 °C (38.5% [CI: 34–43%]) compared to temperature levels < 15 and 21–25 °C (Fig. 5). On the other hand, we observed a mixed trend in the response of barley yield components to eCO2 in combination with different temperature levels. Grain number (36.4% [CI: 29–41.4%]), and grain yield (59.7% [CI: 54–63%]) were higher when eCO2 combined with the higher temperature level of 21–25 °C compared to the lower levels (Fig. 5). Due to lack of data, the interaction of temperature higher than 25 °C with eCO2 was not evaluated in the present study.
Relative percentage change in barley yield response to eCO2 under four different temperature treatments. The three temperature levels were < 15, 15–20, and 21–25 °C out of 18 observations. Temperature > 25 °C were not evaluated due to a lack of data. The symbols represent the percentage change (± 95% CI) in eCO2 response relative to the corresponding control. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
Genotypic variation
Barley genotypes had a significantly different response of yield components to eCO2 (Table 2 and Fig. 6). Comparing 13 genotypes on the response of aboveground biomass, the highest increase by 47.1% [CI: 43–51%] was observed for genotype “Anakin”, while the lowest was observed for “Harrington” (2.7% [CI: -1–6%]) under eCO2 (Fig. 6). The grain yield response was only available for 6 genotypes, out of them “Alexis” showed the highest grain yield by 38.1% [CI: 32–43%], while the lowest response was observed for genotype “Gairdner”. The response of grain number by 58.1% [CI: 32–43%] was the highest for the “Bambina”, while harvest index response was the highest for genotype “Golden_Promise” under eCO2 (29.2 [CI: 24–33%]) (Fig. 6).
Relative percentage change in yield response to eCO2 for 16 different barley genotypes analyzed out of 84 observations. The symbols represent the percentage change (± 95% CI) in response relative to the corresponding control. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
CO2 exposure methods and rooting conditions
The responses of barley yield to eCO2 were significantly affected by the four CO2 exposure methods except for thousand-grain weight (Table 2). The percent of aboveground biomass enhancement under eCO2 was higher when plants were grown in GH (34.9% [CI: 30–39%]) followed by GC (29.3% [CI: 24–33%]) as shown in Fig. 7. On the other hand, the highest increase in barley grain number (41.8% [CI: 37–45%]) and grain yield (31.8% [CI: 26–37.3%]) under eCO2 were observed for the plants grown in the GC. In contrast, barley plants that were grown under FACE had a significantly negative response of grain number, harvest index, and thousand-grain weight (Fig. 7). On the other hand, comparing yield variable response of barley plants grown in pots and on-field conditions, higher responses were observed for plants grown in pots except for harvest index. Higher responses of aboveground biomass (29.8% [CI: 25–34%]), grain number (38.3% [CI: 35–42%]), and grain yield (25.3% [CI: 21.1–29.2%]) under eCO2 were obtained for the plants grown in pots. However, the response of harvest index of barley was significantly higher for plants grown on field conditions compared to pot-grown plants (Table 2 and Fig. 8).
Relative percentage change in barley yield response to eCO2 under four different CO2 exposure methods. The four exposure methods were free-air CO2 enrichment (FACE), growth chambers (GC), greenhouses (GH), open-top chambers (OTC). The symbols represent the percentage change (± 95% CI) in response relative to the corresponding control. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
Relative percentage change in barley yield response to eCO2 under two different rooting conditions (field-grown and pot-grown). The symbols represent the percentage changes (± 95% CI) in response relative to the corresponding controls. TGW: thousand-grain weight; HI: harvest index; GY: grain yield; GN: grain number; AGB: aboveground biomass
Discussion
Responses of barley yield to eCO2 and the interaction with N and temperature
A meta-analysis technique was used to quantitatively review and synthesize the literature on barley yield as a function of eCO2, and its interaction with temperature, and N fertilizer treatments. The rise in atmospheric CO2 causes mostly an increase of the total biomass of C3 plants such as barley, by stimulating net photosynthesis and reducing photorespiration (Drake et al. 1997; Mitterbauer et al. 2017; Schapendonk et al. 2000). The average increase in aboveground biomass by 23.8% under eCO2 in this study is in line with previous meta-analysis studies on C3 crops (Ainsworth 2008; Wang et al. 2013). Similarly, a meta-analytic study of 79 crop and wild species also documented an average enhancement of biomass by 28.2% across all species due to eCO2 (Jablonski et al. 2002). In the present study, the aboveground biomass and grain yield showed similar patterns of increase with increasing levels of eCO2. Plants grown under an eCO2 level of 551–650 ppm showed the highest response in aboveground biomass compared to lower eCO2 concentrations (450–550 ppm). In the present study, grain yield and grain number were significantly increased under eCO2 (651–720 ppm). Consequently, harvest index response was significantly increased under the highest eCO2 (651–720 ppm), however, no significant variation was observed for lower eCO2 levels. A higher harvest index under eCO2 implies that a relatively higher proportion of assimilated carbon is allocated to the grains. Ainsworth et al. (2002) reported a higher percentage of stimulation on aboveground biomass and grain yield at the highest eCO2 level (600–699 ppm) in rice. Also, Kimball et al. (2001) reported an increase in the grain yield of wheat under eCO2. The present study’s findings also revealed an association with an increase in grain yield due to a larger increase in grain number rather than thousand-grain weight. As a result, the response of barley yield to eCO2 has largely been driven by an increase in grain number. This result is similar to findings reported in other studies on C3 plants (Wilcox and Makowski 2013; Knox et al. 2016), Furthermore, increased grain yields have been linked to a higher number of tillers and grains per spike rather than an increase in the number of spikes or grain size in wheat and barley (Bourgault et al. 2013; Pleijel and Högy 2015; Amthor 2001; Wang et al. 2013). The additional carbon assimilates produced by eCO2 levels may ensure the development of flowers and grains (Deng and Woodward 1998). However, the effect of eCO2 on individual grain weight varied, with increases (Van Oijen et al. 1999; Li et al. 2001), decreases (Rawson 1995; Batts et al. 1997; Van Oijen et al. 1999; Heagle et al. 2000), and no change (Heagle et al. 2000; Pleijel et al. 2000).
The CO2-derived "fertilization" effect may differ/may be different under different growth conditions such as nitrogen and temperature levels (Aranjuelo et al. 2011). It has been shown that the eCO2 effect on total biomass and grain yield of barley decreases if the N availability is reduced (Wang et al. 2015). In line with a previous meta-analysis study on rice (Wang et al. 2015), we observed a reduction of grain yield response to eCO2 under limited N fertilizer (0–50 kg ha−1). In the present study, the highest CO2-induced increase of aboveground biomass and grain yield was observed under the higher N level (151–200 kg ha−1). In comparison, the response grain number showed a larger response to eCO2 with 101–150 kg ha−1 N. In addition, the percentage increase in grain yield at eCO2 with a combination of higher N level was related to the percentage increase in grain number, demonstrating a positive relationship. Low N fertilization limited N concentration in vegetative plant parts, limiting any increase in grain number and, consequently, grain yield response to eCO2 is reduced (Kim et al. 2003). Limitation in N fertilizer may also cause more pronounced acclimation of photosynthesis to eCO2, which can limit total biomass increases at eCO2 (Suter et al. 2001; Ainsworth et al. 2003). Because eCO2 has a significant effect on crop N uptake and concentration in biomass, crop production’s response to elevated CO2 is highly dependent on the availability of nutrient resources (Leakey et al. 2012). In our meta-analysis, low N input constrained barley yield in response to eCO2 as compared to high N input. This decrease is most likely due to the direct relationship between N availability and growth parameters such as grain number throughout the growing season (Mitchell et al. 1993; Kim et al. 2001, 2003).
On the other hand, the temperature is also one of the most determinant factors of crops development rates and yield (Wang et al. 2015). Higher temperatures result in accelerated crop development, and thus a shorter growing period, resulting in lower grain yield (Hatfield and Prueger 2015). We found a significant interaction between eCO2 and temperature on barley yield variables. In comparison, better enhancement of aboveground biomass was observed under the combination of eCO2 and temperature level (15–20 °C). Moreover, grain yield and grain number were also increased under the interaction of eCO2 and temperature level (21–25 °C)), which disagrees with previous studies (Wang et al. 2015; Hatfield and Prueger 2015). Due to lack of data, the interactive effect of eCO2 with a temperature higher than 25 °C is missing in the present study. Nevertheless, the significantly higher number of grains observed in the present study might be responsible for higher grain yield under the combination of eCO2 and higher temperature (21–25 °C). In contrast to our study, Wang et al. (2015) reported that under eCO2 an increase of temperature by 1 °C may lead to a decrease in rice yield by 9.4% at temperature levels of 24–26 °C. In addition, Amthor (2001) reviewed the effects of eCO2 on wheat and found that increasing temperatures by 5 °C from the ambient level (12.7 °C) may offset the positive effects of eCO2. However, he mentioned also that eCO2 can counteract the negative effects of higher temperatures, which may partially explain the increase in grain yield and grain number due to eCO2 in combination with higher temperature levels in the present study.
Variation in the response of barley yield to eCO2
Genotypic variation
The identification of consistent genotypic variability in the response to eCO2 is a prerequisite to using this information in breeding programs. Barley yield variable responses to eCO2 were different among barley genotypes, which might be related to the varietal character of genotypes. In the present study modern spring barley genotype “Anakin” had more aboveground biomass under eCO2, followed by the old landrace “Gammel_Dansk”, “Bambina” and “Aura”, while “Iranis” showed the lowest response. The genotype “Bambina”, a mid-maturing spring cultivar, had the highest grain number followed by early maturing and high yielding genotype “Golden Promise” at eCO2. However, grain yield and harvest index response were higher for “Alexis”, a heat-tolerant, two-rowed German genotype, and “Atem”, a drought-tolerant European modern spring genotype. Genetic variability on the response of total biomass and grain yield to eCO2 was reported in previous studies on wheat (Ziska et al. 2004) and rice (Wang et al. 2015). Modern genotypes do not necessarily always perform better than old ones at higher CO2 levels. For example, the aboveground biomass of an older wheat genotype can increase more than that of a modern genotype in response to increasing CO2 (Hay and Gilbert 2001). In contrast to the present findings, previous studies in soybean (Bishop et al. 2015) and common bean (Bunce 2008) could not detect differences between the cultivars tested in their response to eCO2 for grain yield and other yield variables evaluated. The response of thousand-grain weight to eCO2 did not differ among the barley genotypes in the present study. However, previous studies have found a variety of genotypes response of thousand-grain weight under eCO2 (Weigel et al. 1994). The findings from the present meta-analysis suggest breeding for the exploitation of eCO2 might enhance future crop production. Previous studies have suggested that there is very little evidence that breeders have inadvertently selected for increased CO2 responsiveness, and indeed several studies have suggested the opposite, that older genotypes are more responsive to eCO2 than modern genotypes (Ainsworth et al. 2008; Leakey and Lau 2012; Ziska et al. 2012).
Experimental conditions
The estimation of the response of barley yield to eCO2 can be significantly affected by CO2 exposure methods. Previous studies reported an enhancement of aboveground biomass and grain yield at eCO2 is lower under FACE experiments than other enclosure methods (Long et al. 2006; Tubiello et al. 2007). In addition, a meta-analysis study by Broberg et al. (2019) recorded a higher wheat yield for plants grown in OTCs than with FACE. The present meta-analysis shows that plants grown in GC had relatively higher aboveground biomass and grain yield due to eCO2 than those plants grown under FACE or OTC, which is in line with an earlier study (Long et al. 2006). Similarly, when plants were grown in GC, higher grain yield due to eCO2 has been reported in meta-analyses of rice (Wang et al. 2015) and wheat (Wang et al. 2013). The findings from the present meta-analysis and previous studies showed that the rapid fluctuation of CO2 concentration has lowered plant photosynthesis in FACE experiments, which resulted in a lower accumulation of biomass and grain yield (Holtum and Winter, 2003). However, another meta-analysis of wheat noted no significant difference between FACE and OTC experiments concerning the response of grain yield to eCO2 (Feng et al. 2008). Nonetheless, no study seems to have directly compared the response of barley yield variables to different CO2 exposure methods of the same genotype grown under identical soil, environmental condition, and cultivation practice.
The effect of growth conditions on the response of barley yield to eCO2 varies between yield variables (Wang et al. 2015; Broberg et al. 2019). In the present study, the response of aboveground biomass, grain number, and grain yield were significantly higher for pot-grown barley rather than under field grown. This disagrees with the hypothesis that restricted root growth in pot experiments leads to a down-regulation of photosynthesis and consequently diminishes the response of plants to eCO2 (Arp 1991; Curtis and Wang 1998). In the present meta-analysis, the restricted rooting volume for pot-grown plants did not have a major impact on the eCO2 stimulation of aboveground biomass and the number of grains in barley. One possible reason for the apparent discrepancy may be that all the results from pot-grown plants in the present meta-analysis derived from GC studies, which showed much larger responses to eCO2 than FACE conditions. In agreement with our findings, previous meta-analyses have also reported higher aboveground biomass and grain yield responses of pot-grown wheat plants under eCO2 as compared to field-grown plants (Taub and Wang 2008; Wang et al. 2013). In contrast, other studies have reported non-significant variation in responses of grain yield to eCO2 for pot-grown and field-grown wheat plants (Feng et al. 2008). However, the responses of harvest index to eCO2 were higher for field-grown plants in the present study, which disagrees with the previous study on rice (Ziska and Bunce 2000).
Conclusions
This meta-analysis quantified the effect of eCO2 as a single factor and its interaction with N and temperature on barley production. A strong positive effect of eCO2 was observed for aboveground biomass, grain yield, and grain number. However, the responses of aboveground biomass, grain number, and grain yield to eCO2 were lower under limited N fertilizer (< 50 kg ha−1). In general, the magnitude of the CO2-induced effect on barley grain yield will depend on the future atmospheric CO2 concentration and agronomic practices such as genotype choice, and growing conditions. The existence of genetic variation in barley response to eCO2 is needed to breed barley to the future atmospheric environment. Modern barley genotype “Anakin” had higher aboveground biomass under eCO2 than older ones, whereas “Alexis” and “Atem” showed higher grain yield and harvest index. Grain number was relatively higher due to eCO2 for genotype “Bambina”. Uncertainties remain, however, regarding the responses to environmental conditions (temperature, N) of barley yield parameters, mainly aboveground biomass, grain number, and grain yield were significantly affected. The positive effect of eCO2 was observed to be higher in combination with high N (150–200 kg ha−1) and temperature levels (21–25 ℃). In the present meta-analysis, some other important interactions, which potentially affect crop production such as the interaction of eCO2 with drought and O3, were not quantified. In addition, there is a lack of data that compares the effect of different exposure methods and rooting conditions side by side on barley yield response to eCO2. Field experiments that better characterize the responses of barley and its interaction with additional factors to eCO2 can help reduce uncertainties due to climate change in estimating future food production. Such studies might be used for summarizing and drawing conclusions on estimating food production in the future.
Data availability
Data obtained for the present study is available.
Code availability
Software application code is available.
References
Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Ra HSY, Zhu XG, Curtis PS, Long SP (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max.) physiology, growth, and yield. Glob Chang Biol 8:695–709. https://doi.org/10.1046/j.1365-2486.2002.00498.x
Ainsworth EA, Davey PA, Hymus GJ (2003) Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant Cell Environ 26:705–714. https://doi.org/10.1046/j.1365-3040.2003.01007.x
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–371. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob Chang Biol 14:1642–1650. https://doi.org/10.1111/j.1365-2486.2008.01594.x
Ainsworth EA, Leakey ADB, Ort DR, Long SP (2008) FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated CO2 impacts on crop yield and food supply. New Phytol 179:5–9
Alemayehu FR, Frenck G, van der Linden L, Mikkelsen TN, Jørgensen RB (2014) Can barely (Hordeum vulgare L.) adapt to fast climate changes? A controlled selection experiments. Genet Resour Crop Evol 61:151–161. https://doi.org/10.1007/s10722-013-0021-1
Amthor JS (2001) Effects of atmospheric CO2 concentration on wheat yield: a review of results from experiments using various approaches to control CO2 concentration. Field Crops Res 73:1–34. https://doi.org/10.1016/S0378-4290(01)00179-4
Aranjuelo I, Cabrera-Bosquet L, Morcuende R, Avice JC, Nogues S, Araus J.l., Martinez-Carrasco R, Perez P, (2011) Does ear C sink strength contribute to overcoimg photosyhthetic acclimation of wheat plants exposed to elevated CO2? J Exp Bot 62:3957–3969
Arp WJ (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875. https://doi.org/10.1111/j.1365-3040.1991.tb01450.x
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38. https://doi.org/10.1111/j.1365-3040.2007.01727.x
Batts GR, Morison JIL, Ellis RH, Hadley P, Wheeler TR (1997) Effects of CO2 and temperature on growth and yield of crops of winter wheat over four seasons. Eur J Agron 7:43–52
Bishop KR, Betzelberger AM, Long SP, Ainsworth EA (2015) Is there potential to adapt soybean (Glycine max Merr.) to future [CO2]? An analysis of the yield response of 18 genotypes in free- air CO2 enrichment. Plant Cell Environ 38:1765–1774. https://doi.org/10.1111/pce.12443
Bowes G (1996) Photosynthetic responses to changing atmospheric carbon dioxide concentration. In: Baker NR (ed) Photosynthesis and the environment. Kluwer Publishing, Dordrecht, pp 387–407 (ISBN 03978-3-540-27997-6)
Broberg MC, Högy P, Feng Z, Pleijel H (2019) Effects of elevated CO2 on wheat yield: Non-linear response and relation to site productivity. Agron 9:1–18. https://doi.org/10.3390/agronomy9050243
Bunce JA (2008) Contrasting responses of seed yield to elevated carbon dioxide under field conditions within Phaseolus vulgaris. Agric Ecosyst Environ 128:219–224. https://doi.org/10.1016/j.agee.2008.06.003
Clausen SK, Frenck G, Linden LG, Mikkelsen TN, Lunde C, Jørgensen RB (2011) Effects of single and multifactor treatments with elevated temperature, CO2 and ozone on oilseed rape and barley. J Agron Crop Sci 197:442–453. https://doi.org/10.1111/j.1439-037X.2011.00478.x
Connor D (2002) Climate change and global crop productivity. Crop Sci 42:978. https://doi.org/10.2135/cropsci2002.9780
Conroyac JP, Seneweeraa S, Basraa AS, Rogersa G, Woollerb BN (1994) Influence of Rising Atmospheric CO2 concentrations and temperature on growth, yield and grain quality of cereal Crops. Aust J Plant Physiol 21:741–758. https://doi.org/10.1071/PP9940741
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187. https://doi.org/10.1038/35041539
Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313. https://doi.org/10.1007/s004420050381
Dieleman WIJ, Vicca S, Dijkstra FA (2012) Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob Chang Biol 18:2681–2693. https://doi.org/10.1111/j.1365-2486.2012.02745.x
Deng X, Woodward FI (1998) The growth and yield responses of Fragaria ananassa to elevated CO2 and N supply. Ann Bot 81:67–71
Drake B, Gonzalez-Meler M, Long S (1997) More efficient plants: A consequence of rising atmospheric CO2. Plant Mol Biol 48:609–639. https://doi.org/10.1146/annurev.arplant.48.1.609
Fangmeier A, Chrost B, Högy P, Krupinska K (2000) CO2 enrichment enhances flag leaf senescence in barley due to greater grain nitrogen sink capacity. Environ Exp Bot 44:151–164. https://doi.org/10.1016/S0098-8472(00)00067-8
Food and Agriculture Organization of the United Nations (2020) FAOSTAT database is available at http://www.fao.org/faostat/en/#data/QC. Accessed on 19th December 2020
Feng Z, Kobayashi K, Ainsworth EA (2008) Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob Chang Biol 14:2696–2708. https://doi.org/10.1111/j.1365-2486.2008.01673.x
Fróna D, Szenderák J, Harangi-Rákos M (2019) The challenge of Feeding the World. Sustainability 11:5816. https://doi.org/10.3390/su11205816
Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analyses. Ecology 80:1142–1149. https://doi.org/10.1890/0012-9658(1999)080
Hansen J, Sato M, Ruedy R, Lacis A, Oinas V (2000) Global warming in the twenty-first century: An alternative scenario. Proc Natl Acad Sci U S A 97:9875–9880. https://doi.org/10.1073/pnas.170278997
Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Thomson AM, Wolfe D (2011) Climate impacts on agriculture: Implications for crop production. Agron J 103:351–370. https://doi.org/10.2134/agronj2010.0303
Hatfield JL, Prueger JH (2015) Temperature extremes: Effect on plant growth and development. Weather. Clim Extremes 10:4–10
Hay RKM, Gilbert RA (2001) Variation in the harvest index of tropical maize: Evaluation of recent evidence from Mexico and Malawi. Ann Appl Biol 138:103–109. https://doi.org/10.1111/j.1744-7348.2001.tb00090.x
Heagle AS, Miller JE, Pirsley WA (2000) Growth and yield response of winter wheat to kitures of ozone and carbon dioxide. Crop Sci 40:1656–1664
Högy P, Fangmeier A (2008) Effects of elevated atmospheric CO2 on grain quality of wheat. J Cereal Sci 48:580–591. https://doi.org/10.1016/j.jcs.2008.01.006
Högy P, Poll C, Marhan S, Kandeler E, Fangmeier A (2013) Impacts of temperature increase and change in precipitation pattern on crop yield and yield quality of barley. Food Chem 136:1470–1477. https://doi.org/10.1016/j.foodchem.2012.09.056
Holtum JAM, Winter K (2003) Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta 218:152–158. https://doi.org/10.1007/s00425-003-1089-1
IPCC (2021) Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. In Press
Jablonski LM, Wang X, Curtis PS (2002) Plant reproduction under elevated CO2 conditions: A meta-analysis of reports on 79 crop and wild species. New Phytol 156:9–26. https://doi.org/10.1046/j.1469-8137.2002.00494.x
Jaggard KW, Qi A, Ober S (2010) Possible changes to arable crop yields by 2050. Philos Trans r Soc B Biol Sci 365:2835–2851. https://doi.org/10.1098/rstb.2010.0153
Kim HY, Lieffering M, Kobayashi K, Okada M, Miura S (2003) Seasonal changes in the effects of elevated CO2 on rice at three levels of nitrogen supply: A free-air CO2 enrichment (FACE) experiment. Glob Chang Biol 9:826–837. https://doi.org/10.1046/j.1365-2486.2003.00641.x
Kim HY, Lieffering M, Miura S (2001) Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytol 150:223–229
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Adv Agron 77:293–368. https://doi.org/10.1016/S0065-2113(02)77017-X
Kimball B, Morris C, Pinter P (2001) Elevated CO2 drought and soil nitrogen effects on wheat grain quality. New Phytol 150:295–303
Kleemola J, Peltonen J, Peltonensainio P (1994) Apical development and growth of barley under different CO2 and Nitrogen regimes. J Agron Crop Sci 173:79–92
Knox J, Daccache A, Hess T, Haro D (2016) Meta-analysis of climate impacts and uncertainty on crop yields in Europe. Environ Res Lett 11:113004
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from face. J Exp Bot 60:2859–2876. https://doi.org/10.1093/jxb/erp096
Leakey ADB, Lau JA (2012) Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2]. Phil Trans R Soc B Biol Sci 367:613–629
Li A, Hou Y, Tren A (2001) Effects of elevated atmospheric CO2 and drought stress on individual grain filling rates and durations of the main stem in spring wheat. Agric Forest Meteorol 106:289–301
Liu J, Fritz S, Van Wesenbeeck C, Fuchs M, You L, Obersteiner M, Yang H (2008) A spatially explicit assessment of current and future hotspots of hunger in Sub-Saharan Africa in the context of global change. Global and Planetary Change 64:222–235
Lobell DB, Field CB (2007) Global scale climate–crop yield relationships and the impacts of recent warming. Environ Res Lett 2:014002. https://doi.org/10.1088/1748-9326/2/1/014002
Loladze I (2014) Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife 7:165. https://doi.org/10.7554/eLife.02245.001
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising Atmospheric Carbon Dioxide: Plants FACE the future. Annual Review of Plant Biology 55(1):591–628
Long SP, Ainsworth EA, Leakey ADB, Nösbsrger J, Ort DR (2006) Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312:1918–1921. https://doi.org/10.1126/science.1114722
Manderscheid R, Weigel J (2006) Responses of cereals and sugar beet in a crop rotation to free air carbon dioxide enrichment. Bibliotheca Fragm Agron 11:309–310
Manderscheid R, Pacholski A, Frühauf C, Weigel HJ (2009) Effects of free air carbon dioxide enrichment and nitrogen supply on growth and yield of winter barley cultivated in a crop rotation. Field Crops Res 110:185–196. https://doi.org/10.1016/j.fcr.2008.08.002
Mangelsen E, Kilian J, Harter K, Jansson C, Wanke D, Sundberg E (2011) Transcriptome analysis of high-temperature stress in developing barley caryopses: Early stress responses and effects on storage compound biosynthesis. Mol Plant 4:97–115. https://doi.org/10.1093/mp/ssq058
Mitchell RAC, Mitchell VJ, Driscoll SP (1993) Effects of increased CO2 concentration and temperature on growth and yield of winter wheat at two levels of nitrogen application. Plant Cell Environ 16:521–529
Mitterbauer E, Enders M, Bender J, Erbs M, Habekuß A, Kilian B, Ordon F, Weigel HJ (2017) Growth response of 98 barley (Hordeum vulgare L.) genotypes to elevated CO2 and identification of related quantitative trait loci using genome-wide association studies. Plant Breed 136:483–497. https://doi.org/10.1111/pbr.12501
Pleijel H, Högy, P (2015) CO2 dose-response functions for wheat grain, protein and mineral yield based on FACE and open-top chamber experiments. Environ. Pollut. 198:70–77.
Pleijel H, Gelang J, Sild E, Danielsson H, Younis S, Karlsson PE, Wallin G, Skärby L, Sellden G (2000) Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiol. Plantarum 108:61–70
Peng SB, Huang JL, Sheehy JE (2004) Rice yields decline with higher night temperature from global warming. Proc Natl Acad Sci U S A 101:9971–9975. https://doi.org/10.1073/pnas.0403720101
Poorter H, Navas ML (2003) Plant growth and competition at elevated CO2: On winners, losers, and functional groups. New Phytol 157:175–198. https://doi.org/10.1046/j.1469-8137.2003.00680.x
Rawson HM (1995) Yield responses of two wheat genotypes to carbon dioxide and temperature in field studies using temperature gradient tunnels. Aust J Plant Physiol 22(1):23. https://doi.org/10.1071/PP9950023
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tiulman D, Knops JMH, Naem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Schapendonk AHCM, Van Oijen M, Dijkstra P, Pot CS, Jordi WJRM, Stoopen GM (2000) Effects of elevated CO2 concentration on photosynthetic acclimation and productivity of two potato cultivars grown in open-top chambers. Aust J Plant Physiol 27:1119–1130. https://doi.org/10.1071/PP99205
Schimel D (2006) Climate change and crop yields: Beyond cassandra. Science 312:1889–1890. https://doi.org/10.1126/science.1129913
Schmid I, Franzaring J, Müller M, Brohon N, Calvo OC, Högy P, Fangmeier A (2016) Effects of CO2 enrichment and drought on photosynthesis, growth, and yield of an old and a modern barley cultivar. J Agron Crop Sci 202:81–95. https://doi.org/10.1111/jac.12127
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22:583–621
Suter D, Nösberger J, Lüscher A (2001) Response of perennial ryegrass to free-air CO2 enrichment (FACE) is related to the dynamics of sward structure during regrowth. Crop Science 41:810–817
Tao FL, Yokozawa M, Liu JY, Zhang Z (2008) Climate-crop yield relationships at provincial scales in China and the impacts of recent climate trends. Clim Res 38:83–94. https://doi.org/10.3354/cr00771
Taub DR, Wang X (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50:1365–1374. https://doi.org/10.1111/j.1744-7909.2008.00754.x
Tubiello F, Soussana J-F, Howden S (2007) Crop and Pasture Response to climate change. Proc Narl Acad Sci USA 104:19686–19690. https://doi.org/10.1073/pnas.0701728104
United Nations, Department of Economic and Social Affairs, Population Division (2011). World Population Prospects: The 2010 Revision, Volume I: Comprehensive Tables. ST/ESA/SER.A/313
Van Oijen M, Schapendonk AHCM, Jansen MJH, Pot C, Maciorwski R (1999) Do open-top chambers overestimate the effects of rising CO2 on plants? An analysis spring wheat. Global Change Biol 5:411–421
Vara Prasad PV, Boote KJ, Hartwell Allen L, Thomas JMG (2002) Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Glob Chang Biol 8:710–721. https://doi.org/10.1046/j.1365-2486.2002.00508.x
Wang J, Wang C, Chen N, Xiong Z, Wolfe D, Zou J (2015) Response of rice production to elevated [CO2] and its interaction with rising temperature or nitrogen supply: a meta-analysis. Clim Change 130:529–543. https://doi.org/10.1007/s10584-015-1374-6
Wang D, Heckathorn SA, Wang XZ, Philpott SM (2012) A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169:1–13. https://doi.org/10.1007/s00442-011-2172-0
Wang L, Feng Z, Schjoerring JK (2013) Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of present hypotheses. Agric Ecosyst Environ 178:57–63. https://doi.org/10.1016/j.agee.2013.06.013
Weigel HJ, Manderscheid R (2012) Crop growth responses to free-air CO2 enrichment and nitrogen fertilization: Rotating barley, ryegrass, sugar beet, and wheat. Eur J Agron 43:97–107. https://doi.org/10.1016/j.eja.2012.05.011
Weigel HJ, Manderscheid R, Jäger HJ, Mejer GJ (1994) Effects of season-long CO2 enrichment on cereals. I. Growth performance and yield. Agric Ecosyst Environ 48:231–240. https://doi.org/10.1016/0167-8809(94)90105-8
Wilcox J, Makowski D (2013) A meta-analysis of the predicted effects of climate change on wheat yields using simulation studies. Field Crops Res156:180–190
Wolf J. (1996) Effects of nutrient supply (NPK) on spring wheat response to elevated atmosperic CO2. Plant and Soil 185(1):113–123. https://doi.org/10.1007/BF02257568
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Ziska LH, Bunce JA (2000) Sensitivity of field-grown soybean to future atmospheric CO2: selection for improved productivity in the 21st century. Australian Journal of Plant Physiology 27:979–984
Ziska LH, Morris CF, Goins EW (2004) Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: can yield sensitivity to carbon dioxide be a factor in wheat performance. Glob Chang Biol 10:1810–1819. https://doi.org/10.1111/J.1365-2486.2004.00840.X
Ziska LH, Bunce JA (2007) Predicting the impact of changing CO2 on crop yields: Some thoughts on food. New Phytol 175:607–617. https://doi.org/10.1111/j.1469-8137.2007.02180.x
Ziska LH, Bunce JA, Shimono H, Gealy DR, Baker JT, Newton PCD, Wilson LT (2012) Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc R Soc B Biol Sci 279:4097–4105. https://doi.org/10.1098/rspb.2012.1005
Acknowledgements
The authors are grateful to Andrea Giovanna Nino Savala, Ireen Drebestedt, and Philipp Neher for their critical reading and valuable comments. Also, a special thank goes to CLIFOOD team members and coordinators. We also like to acknowledge DFG grant PI 377/24-1 for supporting Waqas Ahmed Malik.
Funding
Open Access funding enabled and organized by Projekt DEAL. This publication is an output of a Ph.D. scholarship at the University of Hohenheim in the framework of the project “German-Ethiopian SDG Graduate School: Climate Change Effects on Food Security (CLIFOOD)” between the University of Hohenheim (Germany) and the Hawassa University (Ethiopia), supported by the DAAD with funds from the Federal Ministry for Economic Cooperation and Development (BMZ).
Author information
Authors and Affiliations
Contributions
M.G. and P.H. conceived and designed the study; data collection was made by M.G. M.G and W.A.M participated in the analysis of the data; M.G. wrote the paper with substantial input from P.H. B.H and W.A.M.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Responsible Editor: Lucas Silva.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1
Literature search strings for the meta-analysis review were used in various databases.
Scopus, Science Direct, and Google Scholar were used to search the literature using the below search strings.
“climate change” [title] [tiab] [key] AND (“barley yield” [title] OR “yield variables” [title] OR “yield components” [title] OR “elevated CO2” [title], OR “carbon dioxide and temperature” [title] OR “carbon dioxide and nitrogen” [title] OR “elevated CO2” [title] OR (“temperature” OR “nitrogen” AND (“aboveground biomass” OR “grain yield” OR “grain number” OR “thousand-grain weight” OR “harvest index”)) [title] OR meta-analysis [title] OR "meta analysis" [title] OR "systematic review" [title] OR "systematic-review" [tiab] OR "quantitative review" [tiab]).
Appendix 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gardi, M., Haussmann, B.I.G., Malik, W.A. et al. Effects of elevated atmospheric CO2 and its interaction with temperature and nitrogen on yield of barley (Hordeum vulgare L.): a meta-analysis. Plant Soil 475, 535–550 (2022). https://doi.org/10.1007/s11104-022-05386-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05386-5