Abstract
Purpose
Paludiculture (crop cultivation in wet peatlands) can prevent carbon and nutrient losses while enabling biomass production. As vegetation in rewetted peatlands is often nitrogen (N) limited, input of N-rich water may promote biomass production and nutrient removal. However, it is unclear how N loading and soil characteristics affect biomass yield, nutrient dynamics, and ecosystem service provisioning in paludiculture.
Methods
We studied the influence of N loading (0, 50, 150, and 450 kg N ha−1 yr−1) on biomass production and nutrient sequestration of Typha latifolia (broadleaf cattail) and Phragmites australis (common reed) in mesocosms containing rewetted agricultural peat soil (intensively managed, near-neutral (IN)). To assess the interaction with soil characteristics T. latifolia was also grown on an extensively managed, acid (EA) peat soil.
Results
N loading stimulated biomass production and nutrient uptake of both T. latifolia and P. australis, with T. latifolia showing the most pronounced response. Biomass yield of T. latifolia was higher on IN soil than on EA soil due to the higher pH, despite lower nutrient availability. N was largely taken up by the vegetation, whereas bare soils showed N accumulation in pore and surface water, and 80% loss through denitrification. Soil phosphorus was efficiently taken up by T. latifolia, especially at high N loads.
Conclusion
N loading in paludiculture with T. latifolia and P. australis boosts biomass production while kick-starting peatland ecosystem services including nutrient removal. Nutrient availability and pH appear to be decisive soil characteristics when it comes to crop selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pristine peatlands worldwide accommodate a unique set of ecosystem services, including carbon sequestration, water quality improvement, biodiversity preservation, water retention and flood control (Zedler and Kercher 2005; Keddy et al. 2009). Accumulation of organic material makes peatlands important sinks and stores for carbon (C) and nutrients. Furthermore, peat has the capacity to store large amounts of water and thus plays an important role in the hydrology and water retention of the landscape (Holden 2005).
Currently, 15 percent of peatlands around the world are drained to enable peat extraction and agriculture (Barthelmes 2016; Joosten 2016). Drainage leads to oxic conditions and accelerated organic matter decomposition, resulting in a loss of peat. Peat oxidation brings about a myriad of issues including land subsidence, loss of biodiversity, and the transition from C sink to source through enhanced carbon dioxide (CO2) emissions (Verhoeven and Setter 2009; Miettinen et al. 2017). Although drained peatlands cover only a small fraction of the earth’s surface (0.3%), they contribute 6% to total anthropogenic CO2 emissions (Tanneberger and Wichtmann 2011). In order to restore peatland ecosystems and associated services such as carbon sequestration and water storage, many rewetting projects have been carried out in the past (Zak et al. 2011; Lamers et al. 2015; Günther et al. 2020).
Rewetting of former agricultural land often brings challenges, such as eutrophication and loss of productive land. Former agricultural soil often contains many nutrients, which can be mobilized by inundation, leading to eutrophication of water bodies (Van de Riet et al. 2013; Cabezas et al. 2014). Especially in formerly fertilized soils, phosphorus (P) mobilization can lead to poor water quality (Zak et al. 2017). Additionally, rewetting agricultural soils results in loss of productive land, which has economic drawbacks.
A proposed solution to these challenges is paludiculture: the use of wet or rewetted peatlands for biomass production (Wichtmann et al. 2016). Paludiculture offers a means of rewetting without losing the land’s productivity, and thus combines agriculture with ecosystem services and functioning (Wichtmann and Wichmann 2011). By cultivating fast-growing perennial crops, excess nutrients from soil and surface water can be extracted, similar to constructed wetlands (Vymazal et al. 2006). By harvesting aboveground biomass, nutrients can be removed in order to prevent leaching and downstream eutrophication (Toet et al. 2005; Hille et al. 2018). Next to that, rewetting of peatlands can reduce climate warming by carbon sequestration despite increased methane (CH4) emissions (Günther et al. 2015; 2017; 2020), and counteract or even reverse soil subsidence by organic matter accumulation and peat formation (Miller et al. 2008).
A paludiculture system can become more nutrient-poor over time when nutrient output (biomass harvesting) exceeds nutrient input (e.g. in surface water or atmospheric). Nutrient deficiencies could hamper productivity and economic returns in the long run. Nitrogen (N) is generally the first nutrient to become limiting for plant growth in peat soils that turn anaerobic after inundation. Under anaerobic conditions, denitrification is stimulated, as facultative anaerobic bacteria use nitrate (NO3−) instead of oxygen (O2) as terminal electron acceptor (Cameron et al. 2013). Anammox bacteria can also directly convert ammonium (NH4+) into N2 or N2O (Kartal et al. 2007). On top of these processes, some plant species create aerobic zones in wet soils by radial oxygen loss (ROL) from their root system. This creates a mosaic of aerobic and anaerobic zones, leading to a coupled nitrification–denitrification (Reddy et al. 1989), which speeds up N depletion even further.
Next to N limitation, pH can be a determining factor for plant growth. Most commonly grown crops cannot grow well on very acidic soils (< 5). On highly organic soils, such as peat soils, liming is a frequently used strategy on agricultural soils to increase pH. Although wetland species can stand more acidic soils than most commercial crops, earlier studies indicate that Typha species grow best at pH of > 4.5. Although Typha species can grow at pH 3.5–4.5 for a short period, cation deficiencies and ammonium toxicity may occur which will hamper growth (Brix et al. 2002; Dyhr-Jensen and Brix 1996). As biomass yield is an important aim in paludiculture, soil pH may be an important influencing factor.
To ensure long-term biomass production, in particular of fast-growing paludiculture crops (“paludicrops"; e.g. Typha species, Phragmites australis, Arundo species; Giannini et al. 2017), adequate nutrient availability and stoichiometry, and pH need to be provided (Olde Venterink et al. 2003). Productive paludicrops need inlet of N-rich surface water or groundwater to warrant sustained biomass production and removal of P and other nutrients. In this way, paludicrop productivity can be ensured while offering the possibility of water purification and the improvement of other peatland ecosystem services. However, it is unclear 1) how N supply affects paludicrop biomass yields, 2) how N supply will affect the uptake and mobilization of nutrients (N, P, potassium (K)) in a paludiculture system, and (3) how this is influenced by soil pH. In this mesocosm study, we therefore investigate 1) the effect of N loading on biomass yield and nutrient dynamics in paludiculture with Typha latifolia and Phragmites australis, 2) the effect of land use history and soil pH in paludiculture with T. latifolia and 3) how these factors affect nutrient allocation.
We hypothesize that N supply via the surface water will increase biomass production of both paludicrops, but that T. latifolia is more responsive to increased N loads and will remove more phosphorus (P) from the soil, as it normally has lower tissue N:P ratios than P. australis (Koerselman and Meuleman 1996; Vroom et al. 2018). Increasing N loads could lead to excess N and higher N:P ratios, which may induce P or K limitation (Ulrich and Burton 1988). Furthermore, we hypothesize that P. australis will suffer from NH4+ toxicity at high N loads (Tylová et al. 2008). We also expect that a more acid peat soil will hamper T. latifolia growth and nutrient removal capacity in general (Brix et al. 2002). Results will be discussed with regard to the N and P balance and allocation in rewetted peat soils used for paludiculture, potential nutrient removal ability of paludicrops, suitability of peat soils for paludiculture, and water management strategies in relation to N supply.
Materials and methods
Site characteristics and soil sampling
For the experiment that compares the response of T. latifolia and P. australis to different N loads (hereafter called “vegetation experiment”), 46 soil cores were collected from a drained agricultural peat meadow in Zegveld, the Netherlands (52°08'N, 4°50'E), at four randomly selected sub-sites. This peat meadow is an actively fertilized and limed grassland (soil pH 5.6) grazed by cattle with a mean peat depth of 6 m. This soil will be referred to as intensively managed, near-neutral (IN) soil from here on.
For the experiment that addresses the effect of soil pH on T. latifolia (hereafter called “pH experiment”), 16 additional soil cores were collected in Bûtefjild, the Netherlands (53°15'N, 5°57'E), at four sub-sites. Bûtefjild is a former agricultural peat meadow where artificial fertilization and liming ceased 20 years ago (soil pH 4.4). Since then it has been extensively managed (sheep grazing and occasional mowing). The remaining peat has a mean thickness of 1.5 m. This soil will be referred to as extensively managed, acid (EA) soil from here on.
Soil cores (total n = 62) were collected from the field using PVC tubes (called “mesocosms”; 15 cm diameter) that were carefully drilled into the soil down to a depth of 30 cm. From each mesocosm, 5 cm of topsoil (including grass and roots) was removed to mimic the most common soil preparation in paludiculture and to remove the soil layer with high labile carbon content. Soil samples were taken from the four sub-sites at each location in order to characterize chemical composition of the soil prior to the experiments. Both experiments were run alongside each other in the greenhouse of the Radboud University and partially overlapped, as the T. latifolia treatments on IN soil were the same for both experiments.
Experimental set-up
T. latifolia was grown from seeds collected in Bûtefjild alongside nutrient-rich ditches. For the vegetation experiment, five T. latifolia seedlings of 20 ± 5 cm were planted in 16 mesocosms with IN soil. In 16 other mesocosms with IN soil, five P. australis rhizomes with viable new shoots were planted. Control mesocosms with IN soil and without vegetation (n = 14) were covered by a black canvas to prevent growth of plants and algae. For the pH experiment, five T. latifolia seedlings were planted in 16 additional mesocosms with EA soil. Plants were cut down to a length of 5 cm to ensure comparable starting conditions. During the first two weeks of the experiments, non-viable plants were replaced by new individuals.
Rewetting of soils was simulated by adding demineralized water up to a level of 5 cm above the soil surface following the growth of the plants to prevent total submersion of the shoots. This was the maximum possible level after removing the topsoil. Each mesocosm was placed in a plastic bag (open at the top) to prevent water exchange with the cooling water bath. Mesocosms of both experiments were positioned in a water bath, kept at 14 °C by a cryostat. Light conditions (irradiance) were maintained at 186 W m−2 or higher for 16 h per day, using grow lights if sunlight was not sufficient. Average daytime air temperature in the greenhouse was 21.7 °C in March, 22.4 °C in April, and 23.8 °C in May. The average relative humidity (RH) at daytime was 42.3%. Water levels were kept constant by replacing evaporated water with demineralized water thrice a week. Mesocosms were kept at these conditions for five days prior to the initiation of nitrogen treatments in order to stabilize. Twice during the experiment, 5 mL 1 M KCl solution (11.1 g K m−2/111 kg K ha−1) was added to each mesocosm to prevent K limitation.
Nutrient addition
In both experiments, four different N treatments were applied to the mesocosms. N was added as a combination of ammonium nitrate (NH4NO3) and ammonium chloride (NH4Cl) in a molar ratio of 1:1.5 to obtain a NH4:NO3 ratio that simulates field conditions, resulting in four different total N loads: 0 kg N ha−1 (control), 50 kg N ha−1, 150 kg N ha−1 and 450 kg N ha−1. N was added to the surface water to simulate the inlet of N-rich water in a paludiculture system, such as ditch water or farm runoff, with different residence times. The N load of 50 kg ha−1 is based on realistic N concentrations of 5 mg l−1 as found in surface waters in the surroundings of Bûtefjild (Kros et al. 2011) and a three-month retention time. The N load of 150 kg ha−1 simulates a situation in which this surface water has a one-month retention time in the system, which is similar to previous field pilots with paludiculture (e.g. Geurts and Fritz 2018). A load of 450 kg N ha−1 was chosen as an extreme scenario in the range of constructed wetland loading and removal rates (Land et al. 2016). Four replicate mesocosms were used for each combination of soil, vegetation and N load, with exception of the unvegetated control (IN soil) that received 150 and 450 kg N ha−1 (n = 3). Total N loads were divided into twelve weekly additions of fertilizer solution using a small syringe, starting one week after planting and lasting until one week before harvest.
Water sampling and analysis
Surface water and pore water samples were taken five times throughout the experiment. Pore water was sampled from the upper 10 cm of the soil using Rhizon samplers (Rhizosphere Research Products, Wageningen, The Netherlands) attached to a syringe under vacuum. After pH and alkalinity were determined with a Ag/AgCl electrode (Orion Research, Beverly, MA, USA) and TIM 840 Titration Manager (Radiometer Analytical SAS, Villeurbanne, France), surface water and pore water samples were stored at 4 °C (after adding 0.1 mL of 65% HNO3 to a 10 mL subsample) and -20 °C until further analyses.
Soil analysis
Soil samples were taken prior to the experiment as well as after plants were harvested. Samples were collected along the entire depth of the mesocosm and homogenized. To determine bio-available NH4+ and NO3− concentrations, salt extractions were carried out using 17.5 g of fresh soil, incubated with 50 mL of 0.2 M sodium chloride (NaCl). After 120 min of incubation on a shaker at 105 RPM, pH was determined and fluid was extracted using Rhizon samplers (Rhizosphere Research Products B.V.) under vacuum conditions. In addition, subsamples of fresh soil were dried at 70 °C for 48 h to determine dry weight and bulk density. Bio-available P (Olsen-P) was measured by a 30 min incubation of 3 g dried soil in 60 mL 0.5 M sodium carbonate (NaHCO3) on a shaker at 105 RPM (Henriksen 1965) and extraction using the above method. After the extractions, samples were stored at -20 °C until further analysis. Total phosphorus (TP) and total potassium (TK) contents were determined by digesting 200 mg soil in 4 mL HNO3 (65%) and 1 mL H2O2 (35%) in Teflon vessels, heated in an Ethos D microwave (Milestone, Sorisole Lombardy, Italy). After digestion, samples were stored at 4 °C until further analysis.
Biomass growth and analysis
Plant length was recorded weekly. Maximum length of T. latifolia was measured for each individual shoot. For P. australis, maximum and average length were determined per mesocosm. All aboveground plant biomass was harvested after 91 days, dried at 70 °C for four days, weighed, and ground up. TP and TK were determined after digestion, whereas TN and TC was measured using a CNS analyser (methods described below). Belowground biomass yield was estimated along the mesocosm depth using a soil corer with a diameter of 1 cm. Five soil cores per mesocosm were taken. After washing off the soil from the belowground biomass, it was dried and weighed in the same way as the aboveground biomass. The weight of the belowground biomass in the five cores was then extrapolated to the whole mesocosm.
Chemical analysis
Concentrations of NH4+, NO3− and phosphate (PO43−) were determined by colorimetric methods (Auto Analyser III, Bran and Luebbe GmbH, Norderstedt, Germany) in the water samples that were stored at − 20 °C (Geurts et al. 2008). Subsequently, inductively coupled plasma emission spectrometry (ICP-OES) was used to measure concentrations of Fe, K, and P (IRIS Intrepid II, Thermo Electron corporation, Franklin, MA, USA) in the samples that were stored at 4 °C (water and destruction samples) and in the extraction samples (only P). Total carbon (TC) and total nitrogen (TN) were determined in dry soil and plant material by an elemental CNS analyser (NA 1500, Carlo Erba; Thermo Fisher Scientific, Franklin, USA).
Nutrient budget calculations
N and P budgets were created by determining absolute amounts of N and P in the soil, water and plant fractions of each mesocosm (mg mesocosm−1). Aboveground biomass was multiplied by its respective nutrient concentration. For T. latifolia, belowground biomass was multiplied by nutrient contents from a previous study following a similar design that focussed on different N sources (0.56% N, 0.19% P; Vroom et al. 2018). Nutrient concentrations in belowground biomass of P. australis were taken from a field study (1.2% N, 0.15%P; Behrends et al. 1996). The amounts of N and P in surface water were calculated by multiplying their concentration with the surface water volume. The amounts of N and P in pore water were calculated similarly, using soil volume and water content. NaCl extractable N was determined by using soil bulk density and moisture content, and subsequent subtraction of pore water N values. Net N and P loss were calculated by subtracting end values (above and belowground biomass, pore water, surface water and soil extractable for N, above and belowground biomass, pore water and surface water for P) from the start values (N load, soil extractable and pore water for N, pore water for P). N and P loss (in %) were calculated by dividing N or P loss by the sum of N or P in all start fractions. Positive loss values indicate removal from the system (e.g. through denitrification or binding to the soil adsorption complex), whereas negative loss values indicate mobilisation from the soil matrix.
Statistical analysis
Statistical analyses were performed using R v. 3.3.2 (R core team 2016). Figures were made using ggplot2 (Wickham, 2016). Student’s t tests were used to determine differences in soil characteristics between the two soils (Table S1). Biomass yield and nutrient removal were extrapolated from mesocosm size (g mesocosm−1) to values per hectare by multiplying with a factor 0.5659 (ton ha−1) or 565.9 (kg ha−1). Although this may lead to overestimation, the values per hectare seem realistic as they are in the same range as Geurts et al. (2020) found in young stands under field conditions. Nitrogen utilisation efficiency (NUtE) was calculated by dividing the dry biomass yield by the plant N content, both per mesocosm. Effects of N load and soil type on biomass yield, nutrient removal and plant nutrient content were tested using two-way ANOVA (with N load, soil type and their interaction) and one-way ANOVA (with N load) for T. latifolia and P. australis, respectively (Tables S2 and S3). Differences in surface water and pore water chemistry as well as nutrient budgets were tested using two-way ANOVA, using either plant type and N load (vegetation experiment) or soil type and N load (pH experiment) and their interactions as explanatory factors (Tables S4 and S5). We assessed individual differences between treatments with Tukey post-hoc tests. We used Q-Q plots, Shapiro–Wilk tests and residual plots to verify model assumptions.
Results
Site-specific peat soil characteristics
IN peat soil had a higher pH than EA soil, as was expected from its liming history (5.57 and 4.36 respectively, p = 0.002). Organic matter content and bulk density indicated that both soils were degraded peat soils (Liu and Lennartz 2019). EA soil also contained higher extractable NH4+ (p = 0.026), Fe (p < 0.001) and P (p = 0.033), and had a lower C:N ratio (p < 0.001) than IN peat soil, although EA soil had a lower total-K content (p < 0.001) (Table 1).
Biomass yield and nutrient removal
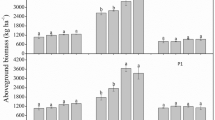
T. latifolia produced more biomass than P. australis in all treatments of the vegetation experiment: on average 12.7 g dry weight (DW) mesocosm−1 and 6.9 g DW mesocosm−1, respectively (p < 0.001; Fig. 1). Increasing N loads lead to higher biomass production of T. latifolia on IN soil (p = 0.001), increasing from 5.3 ton DW ha−1 in controls to 10 ton DW ha−1 at 450 kg N ha−1. Biomass production of P. australis on IN soil increased from 2.6 ton DW ha−1 to an optimum of 5.5 ton DW ha−1 at 150 kg N ha−1. However, biomass production did not increase any further at 450 kg N ha−1 (p < 0.05; Table 2), and algae were observed in the surface water.
In the pH experiment, average biomass production of T. latifolia was 40% higher on IN soil compared to EA soil (12.7 and 7.6 g DW mesocosm−1, respectively; p < 0.001). A higher N load also stimulated biomass production of T. latifolia was on EA soil (p < 0.001; Table 2), increasing from 2.5 ton DW ha−1 in controls to 5.9 ton DW ha−1 at 450 kg N ha−1. On both soils NUtE of T. latifolia was lowest in the treatments receiving 450 kg N ha−1 (p < 0.05).
Nutrient removal largely followed the same trends as the biomass results (Table 2). Plants stored 31 up to 205 kg N ha−1 in aboveground biomass, depending on N load, soil type and plant species. N removal was positively related to N load for both T. latifolia (on both soils; p < 0.001) and P. australis (p = 0.008). P removal (5 to 21 kg P ha−1) and K removal (27 to 141 kg K ha−1) by aboveground biomass did not significantly increase with N load. N, P, and K removal by P. australis was highest at an N load of 150 kg ha−1 compared to lower N loadings (p < 0.05). At all N loads of the vegetation experiment, P and K removal rates of T. latifolia were higher than P. australis on IN soil (p < 0.001 for both). In the pH experiment, P and K removal rates were also higher for T. latifolia on IN soil than on EA soil (p = 0.003 and p < 0.001, respectively).
Nutrient stoichiometry
N loading increased the N content of T. latifolia (p < 0.001) (Table 3) to more than 2% in aboveground biomass on both soils. The N content of P. australis ranged between 1.5% and 2.3%, but was not affected by N loading (p = 0.273). For T. latifolia, P content was not affected by N loading, but K content decreased in the 450 kg N ha−1 treatment compared to the 50 kg N ha−1 treatment (p = 0.042). N:P and N:K ratios also increased significantly in T. latifolia receiving 450 kg N ha−1 (p < 0.001), reaching average N:P ratios of 10 and N:K ratios of 1.7 to 2.2. P. australis showed a higher K content at 150 kg N ha−1 compared to 50 kg N ha−1 (p = 0.033), but N loading had no significant effect on P content (p = 0.392) and N:P (p = 0.255) or N:K (p = 0.567) ratios. C:N ratios strongly decreased in T. latifolia treatments receiving 450 kg N ha−1 (p < 0.001), with no differences between soil types (p = 0.242), from 38 to 22 on average. C:N ratios in P. australis did not differ significantly between different N loads (p = 0.134).
Pore and surface water nutrients
In the vegetation experiment, N (mainly NH4+) accumulated in the pore water in all unvegetated controls as opposed to vegetated treatments (p < 0.001; Fig. 2), with concentrations that became 5–40 times higher than at the start of the experiment. This led to surface water N concentrations of 142, 347, 1909, and 7933 µmol/l, respectively, for the increasing N loads, at the end of the experiment (Fig. 3). Pore water NO3− in control mesocosms increased after N application, but was depleted in all treatments towards the end of the experiment.
In vegetated mesocosms, NH4+ was depleted or strongly decreased in surface water and pore water towards the end of the experiment as the plants increased in biomass, leading to a 97–100% reduction in the T. latifolia mesocosms on IN soil, a 90–98% reduction in the T. latifolia mesocosms on EA soil, and a 52–100% reduction in the P. australis mesocosms on IN soil. Surface water NO3− was also depleted in all vegetated mesocosms at the end of the experiment, leading to a 97–100% reduction in all treatments.
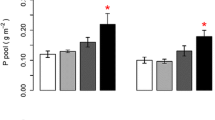
Pore water P concentrations decreased in T. latifolia treatments (81% on IN soil; 26% on EA soil; p < 0.001), but not in P. australis or controls (p < 0.001; Fig. 4). P concentrations in pore water were highest in EA soil, between 100 and 300 µmol/l at the start of the experiment, but no PO43− mobilization to the water column occurred (Figure S1). In IN soil, PO43− mobilization decreased with increasing N load, meaning that mobilization to the water layer occurred mostly in the low N treatments. Added K accumulated to concentrations of around 1500 µmol/l in surface water of unvegetated mesocosms, but was depleted in vegetated mesocosms in all treatments (Figure S2). K did not accumulate in the pore water.
P concentrations in in pore water over time for different N loads The first N load was applied on day 0. Left panels: IN soil, right panel: EA soil. Note: different Y axis scale for T. latifolia on EA soil. Markers indicate individual mesocosms (n = 4 per treatment) and can overlap. Lines indicate average trends
N budget
N budgets show the division of N over different compartments at the end of the experiment (Table 4). Net N loss, defined as the amount of N not covered in any compartment, increased with increasing N load. Net N losses were up to 62% for treatments receiving 450 kg N ha−1. Net N mineralization occurred at the lowest two N loads. Plants (aboveground and belowground) took up 73–389 mg N mesocosm−1 on average, positively related to N load. In unvegetated mesocosms, up to 98 mg N mesocosm−1 accumulated in surface water, and up to 135 mg N mesocosm−1 in pore water. Soil available N also increased in unvegetated mesocosms (up to 229 mg N mesocosm−1), and, less so, in P. australis mesocosms receiving 0 and 450 kg N ha−1 (up to 96 mg N mesocosm−1). In all other vegetated mesocosms, soil N stocks decreased slightly.
P budget
P budgets show the division of P over different compartments at the end of the experiment (Table 5). Net P mineralisation occurred in all vegetated mesocosms, and was higher at the highest N loads. In most control treatments net P loss occurred, which is defined as the amount of P not covered in any compartment. This net P loss was lower at higher N loads and negative at the highest N load, meaning that P mineralisation occurred in the latter case. Plants (aboveground and belowground) took up 15–52 mg P mesocosm−1, with higher uptake rates at the highest N loads. In unvegetated mesocosms, up to 0.25 mg P mesocosm−1 accumulated in surface water, and up to 2.67 mg P mesocosm−1 in pore water.
Discussion
This study shows that crop choice, N loading and soil characteristics (e.g. liming legacy) are important controls on ecosystem service provisioning in paludiculture by driving carbon sequestration (growth), nutrient dynamics and potential nutrient removal by biomass harvesting. Growing paludicrops can prevent excessive nutrient export to the surface water following rewetting. We found that intermediate N loads (50 to 150 kg ha−1) can stimulate P removal without accumulation of N in the surface water. In contrast, high N loads (450 kg ha−1) or the absence of paludicrops could result in nutrient accumulation in the surface water in the long term. Furthermore, biomass production of T. latifolia may become hampered in low pH soil, following the absence of liming, which requires additional management such as different crop choice or water table regulation. In the long run, K availability needs to stay in balance with N loads and K removal to optimize nutrient removal rates.
N loading stimulates biomass production and nutrient removal
As expected, N loading had a positive effect on biomass production of both T. latifolia and P. australis (Geurts et al. 2020), although the highest N load did not increase P. australis biomass anymore, whereas more algae growth was observed. This is probably caused by NH4+ accumulation and toxicity (Tylová et al. 2008). Of the two paludicrops we expected that T. latifolia would be more responsive to increased N input, because of its lower N:P ratios in plant tissue (Mason and Bryant 1975) and higher nitrogen use efficiency (Hirtreiter and Potts 2012; Ren et al. 2019) compared to P. australis. This was also shown in our experiment: N:P ratios of 6 in T. latifolia and 10 in P. australis in the 0 N treatment. Vroom et al. (2018) found even lower N:P ratios of 3.4 in a T. latifolia experiment without N addition. At the highest N load, N:P ratios increased to 10 in T. latifolia and 11 in P. australis. All these N:P ratios, however, still suggest N limitation according to the threshold of 14 found by Koerselman and Meuleman (1996) for fen vegetation dominated by meadow species (e.g. grasses, sedges). In a similar mesocosm experiment, where N was also supplied to the surface water, Ren et al. (2019) found higher N:P ratios, which were around 30 in T. latifolia and 12–20 in different P. australis genotypes. Both species had a lower NUtE (< 55 g dry weight per g N) at the highest N treatment (T. latifolia) or the two highest N treatments (P. australis) compared to the lower N treatments (> 60 g dry weight per g N). This effect of increasing N availability on NUtE was also shown for T. angustifolia by Steinbachová-Vojtíšková et al. (2006) and can be attributed to luxury consumption of N at higher N loads, or even toxicity.
By harvesting the aboveground biomass, nutrients can be removed from the system, which prevents possible nutrient leaching and downstream eutrophication after rewetting (Toet et al. 2005; Hille et al. 2018). Average P and K removal by T. latifolia on the intensively managed, near-neutral peat soil was two times higher than that of P. australis (17 and 8 kg P/ha, and 124 and 62 kg K/ha, respectively). Differences in P and K removal between the two species were also shown by Geurts et al. (2020). P removal was comparable with other studies on Typha (Mason and Bryant 1975; Vroom et al. 2018), but lower than in constructed wetlands with a mixed vegetation (Mitsch et al. 2000; Land et al. 2016; Giannini et al. 2019). On the other hand, N uptake of P. australis was relatively high, and at 150 kg N ha−1 N removal of P. australis was even > 30% higher than that of T. latifolia on the intensively managed, near-neutral soil (122 and 93 kg N/ha respectively). N removal was high compared with other mesocosm studies (Vroom et al. 2018), but low compared to annual N removal in constructed wetlands (Mitsch et al. 2000; Land et al. 2016; Giannini et al. 2019). At the highest N load, T. latifolia had an almost two times higher N removal than P. australis (202 and 105 kg N/ha respectively). Of the two crops studied, T. latifolia is therefore the preferred crop to produce biomass and purify water at very high N loads.
Soil pH determines biomass production and nutrient removal of T. latifolia
Despite higher nutrient availability (i.e. N and P), T. latifolia growth was hampered on the EA former agricultural peat soil with a low pH (4.4), resulting in a 27–52% lower biomass, a 25–49% lower N removal, a 28–50% lower P removal, and a 46–74% lower K removal than on the IN agricultural peat soil, which had a higher pH due to liming. Nutrient uptake (i.e. nutrient content in plant tissue) was only affected for K, which was significantly lower on the EA soil (17–45%), whereas N and P uptake did not differ on the two investigated soil types. We cannot rule out that the first K dose was applied too late, hampering growth in the first 4–5 weeks of the experiment. It is known from other studies that nutrient uptake and cation supply become restricted at a pH < 4 (Dyhr-Jensen and Brix 1996; Brix et al. 2002). In our experiment, however, reduced uptake of K at low pH was probably the main factor limiting plant growth, because uptake of Ca and Mg was even higher on the acid soil than on the near-neutral soil. On both investigated soil types, T. latifolia biomass significantly increased with increasing N input. Biomass yield of T. latifolia in the 0 N treatment on the EA soil (2.5 ton dm/ha) was comparable with the same treatment in the study of Vroom et al. (2018), whereas yield of the 0 N treatment on the IN soil (5.3 ton dm/ha) was more comparable with the same treatment in the study of Ren et al. (2019). The T. latifolia biomass yield of the 150 N treatment on both soils (4.8 and 8.0 ton dm/ha for EA and IN soil respectively) was in the same range as Vroom et al. (2018) found for this treatment, but lower than Ren et al. (2019) reported. This could be a result of a higher K loading throughout their experiment.
Despite the low N:P ratios and expected N limitation in T. latifolia, (co)limitation of other nutrients is also possible, especially when N loads increase (Ulrich and Burton 1988). Because of very low K availability in the EA soil halfway the experiment, and therefore possible K limitation, extra K was added to all mesocosms twice. This finally resulted in K percentages of 1.1 to 1.6 in T. latifolia on EA soil, which is still low, and therefore dilution of K in plant tissue and a high N:K ratio of 2.1 was observed at the highest N load. This is comparable with the N:K ratios Ren et al. (2019) found, although they harvested during plant senescence, meaning that K allocation to the roots had already started. The N:K ratio is on the high end of the range in K limitation thresholds for wetland plants of 1.2 to 2.1 found in literature (Pegtel et al. 1996; Olde Venterink et al. 2003; Lawniczak et al. 2009), and therefore K limitation cannot be excluded at this high N load. We expect that cation competition between NH4+ and K+ also plays a role at increasing N loads (Ten Hoopen et al. 2010). N:K ratios in the treatments with lower N loads were significantly lower, and therefore clearly not K limited. Besides differences in pH and K availability, there were also clear differences in P dynamics between the two soil types. Whereas total P concentrations were higher in the IN soil, pore water P and Olsen-P were higher in the EA soil. This is probably due to the lower pH in the EA soil, as acid soil types tend to have higher P mineralization rates than more neutral soil types (Bridgham et al. 1998). Higher pore water P concentrations in this soil did not lead to a higher P mobilization to the water layer, as Typha took up most of the available P. Furthermore, dissolved iron concentrations in this soil were higher than in the IN soil, and this iron can effectively bind P at the water-peat interface (Zak et al. 2004; Geurts et al. 2010).
N loading flips N mineralization to N loss in paludiculture systems
Results show that both paludicrops can mitigate the N accumulation observed in the soil, pore water, and surface water of the unvegetated control treatments by taking up the excess of N. Grace (1988) also found a large reduction (90–95%) in sediment NH4+ concentrations by Typha growth. It seems unlikely that this mitigation effect is caused by enhanced denitrification in the treatments with plants, because increased oxygen concentrations by ROL would normally decrease denitrification rates (Veraart et al. 2011). Microbial processes in rewetted peat are known to remove NO3− from NO3− enriched surface water and this removal rate increases with N load (Cabezas et al. 2012), although the fraction removed may decrease at higher loads (Land et al. 2016). In our experiment, net N loss to the atmosphere is also highest at the highest N load, irrespective of plant presence. Vroom et al. (2018) only found a slightly higher N loss at a higher N load.
Microbial activity in the soil, and therefore mineralization, including denitrification, and coupled nitrification–denitrification are enhanced by extra N input (Reddy et al. 1989).
Management options
The choice for a certain paludicrop and the potential nutrient removal ability depends on the nutrient availability and pH of the soil. We found that a higher nutrient load cannot compensate the negative effects of a low pH and therefore acid soils with bog peat are less suitable for T. latifolia, and more suitable for Sphagnum farming (Temmink et al. 2017; Gaudig et al. 2017; Vroom et al. 2020). More neutral soils with a higher nutrient availability are suitable for fast-growing species like Typha species, P. australis, and Arundo species (Tho et al. 2017; Ren et al. 2019). Cropping Carex species on rewetted soils warrant further investigation given Carex’s plasiticity to a wide pH range and their high carbon sequestration potential (Hinzke et al. 2021). Rewetting using alkaline ditch water and restoring groundwater flow, often high in bicarbonate, may prevent low pH conditions that hamper plant growth excessively.
Water management is very important in a paludiculture. First of all, the water level should stay within the optimal range for a specific paludicrop to avoid loss in biomass production and vitality. Secondly, nutrients should be supplied through the inlet of water (Temmink et al. 2017; Geurts and Fritz 2018; Ren et al. 2019; Vroom et al. 2020). As we showed, N supply is of particular importance, because N partly disappears from the soil after rewetting by denitrification (Cameron et al. 2013) and anaerobic ammonium oxidation (Kartal et al. 2007). The retention time of the water and the hydraulic load is also crucial in that respective, which is already known for constructed wetlands (Vymazal et al. 2006; Wu et al. 2015), but not studied specifically for a paludiculture situation yet. A high retention time and a low hydraulic load can encourage nutrient limitation, whereas a low retention time and high hydraulic load can lead to nutrient leaching downstream from the paludiculture site. For the same reason, the use of artificial fertilizers is not desirable, but instead N-rich ditch water originating from agricultural run-off can be used. Care should also be taken when high labile carbon concentrations (i.e. DOC) are present in the inlet water, because these can induce methanogenesis and subsequently increase CH4 emissions (Vroom et al. 2018).
We have shown that paludiculture can combine biomass production with effective water purification, and nutrient removal, next to other ecosystem services and functions that peatland rewetting can provide, i.e. water retention, peat preservation, CO2 emission reduction, and soil subsidence reversal. Therefore, paludiculture is especially promising in buffer zones between agricultural areas and nature areas. The effectiveness of combining biomass production and water purification increases by intermediate to high (50–150 kg N) N loads. Very high N loads may maximize yields, but are not desirable because of potential crop damage, nutrient leakage after the growing season and higher costs.
Availability of data and material
The datasets generated during and/or analysed during the current study are available in the Radboud Data Repository.
Code availability (software application or custom code)
Not applicable.
References
Barthelmes A (2016) The global potential and perspectives for paludiculture. In: Wichtmann W, Schröder C, Joosten H (eds) Paludiculture - Productive use of wet peatlands. Schweizerbart Science Publishers, Stuttgart, pp 200–203
Behrends LL, Bailey E, Bulls MJ, Coonrod, Sikora FJ (1996) Seasonal trends in growth and biomass accumulation of selected nutrients and metals in six species of emergent aquatic macrophytes. Tennessee Valley Authority, Muscle Shoals, AL, USA. https://www.osti.gov/servlets/purl/236254
Bridgham SD, Updegraff K, Pastor J (1998) Carbon, nitrogen, and phosphorus mineralization in northern wetlands. Ecology 79(5):1545–1561
Brix H, Dyhr‐Jensen K, Lorenzen B (2002) Root‐zone acidity and nitrogen source affects Typha latifolia L. growth and uptake kinetics of ammonium and nitrate. Journal of Experimental Botany 53(379):2441–2450
Cabezas A, Gelbrecht J, Zwirnmann E, Barth M, Zak D (2012) Effects of degree of peat decomposition, loading rate and temperature on dissolved nitrogen turnover in rewetted fens. Soil Biol Biochem 48:182–191
Cabezas A, Pallasch M, Schönfelder I, Gelbrecht J, Zak D (2014) Carbon, nitrogen, and phosphorus accumulation in novel ecosystems: Shallow lakes in degraded fen areas. Ecol Eng 66:63–71
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Annals of Applied Biology 162(2):145–173
Dyhr-Jensen K, Brix H (1996) Effects of pH on ammonium uptake by Typha latifolia L. Plant, Cell & Environment 19:1431-1436
Gaudig G, Krebs M, Joosten H (2017) Sphagnum farming on cut-over bog in NW Germany: Long-term studies on Sphagnum growth. Mires and Peat 20:1–19
Geurts JJM, Smolders AJP, Verhoeven JTA, Roelofs JGM, Lamers LPM (2008) Sediment Fe:PO4 ratio as a diagnostic and prognostic tool for the restoration of macrophyte biodiversity in fen waters. Freshw Biol 53(10):2101–2116
Geurts JJM, Fritz C (2018) Paludiculture pilots and experiments with focus on cattail and reed in the Netherlands-Technical report-CINDERELLA project FACCE-JPI ERA-NET Plus on Climate Smart Agriculture. https://repository.ubn.ru.nl/bitstream/handle/2066/192628/192628pub.pdf
Geurts JJM, Oehmke C, Lambertini C, Eller F, Sorrell BK, Mandiola SR, Grootjans AP, Brix H, Wichtmann W, Lamers LPM, Fritz C (2020) Nutrient removal potential and biomass production by Phragmites australis and Typha latifolia on European rewetted peat and mineral soils. Science of the Total Environment 747:141102
Geurts JJ, Smolders AJ, Banach AM, van de Graaf JP, Roelofs JG, Lamers LP (2010) The interaction between decomposition, net N and P mineralization and their mobilization to the surface water in fens. Water Res 44(11):3487–3495
Giannini V, Silvestri N, Dragoni F, Pistocchi C, Sabbatini T, Bonari E (2017) Growth and nutrient uptake of perennial crops in a paludicultural approach in a drained Mediterranean peatland. Ecol Eng 103:478–487
Giannini V, Bertacchi A, Bonari E, Silvestri N (2019) Recolonisation by spontaneous vegetation of a rewetted peatland after topsoil removal: a focus on biomass production and nutrient uptake. Wetlands 39:1079–1087
Grace JB (1988) The effects of nutrient additions on mixtures of Typha latifolia L. and Typha domingensis Pers. along a water-depth gradient. Aquatic Botany 31(1–2):83–92
Günther A, Huth V, Jurasinski G, Glatzel S (2015) The effect of biomass harvesting on greenhouse gas emissions from a rewetted temperate fen. GCB Bioenergy 7(5):1092–1106
Günther A, Jurasinski G, Albrecht K, Gaudig G, Krebs M, Glatzel S (2017) Greenhouse gas balance of an establishing Sphagnum culture on a former bog grassland in Germany. Mires and Peat 20:1–16
Henriksen A (1965) An automatic method for determining nitrate and nitrite in fresh and saline waters. Analyst 90:83–88
Günther A, Barthelmes A, Huth V, Joosten H, Jurasinski G, Koebsch F, Couwenberg J (2020) Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat Commun 11(1):1–5
Hille S, Graeber D, Kronvang B, Rubæk GH, Onnen N, Molina-Navarro E, Baattrup-Pedersen A, Heckrath GJ, Stutter MI (2018) Management options to reduce phosphorus leaching from vegetated buffer strips. J Environ Qual 48(2):322–329
Hinzke T, Li G, Tanneberger F, Seeber E, Aggenbach C, Lange J, Kozub L, Knorr KH, Kreyling J, Kotowski W (2021) Potentially peat-forming biomass of fen sedges increases with increasing nutrient levels. Funct Ecol 35(7):1579–1595
Hirtreiter JN, Potts DL (2012) Canopy structure, photosynthetic capacity and nitrogen distribution in adjacent mixed and monospecific stands of Phragmites australis and Typha latifolia. Plant Ecol 213(5):821–829
Holden J (2005) Peatland hydrology and carbon release: why small-scale process matters. Philosophical Transactions of the Royal Society a: Mathematical, Physical and Engineering Sciences 363(1837):2891–2913
Joosten H (2016) Peatlands across the globe. In: Bonn A, Allott T, Evans M, Joosten H, Stoneman R (eds) Peatland restoration and ecosystem services: science, policy and practice. Cambridge University Press, Cambridge, pp 19–43
Kartal B, Kuypers MMM, Lavik G, Schalk J, Op den Camp HJM, Jetten MSM, Strous M (2007) Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol 9(3):635–642
Keddy PA, Fraser LH, Solomeshch AI, Junk WJ, Campbell DR, Arroyo MTK, Alho CJR (2009) Wet and wonderful: the world’s largest wetlands are conservation priorities. Bioscience 59(1):39–51
Koerselman W, Meuleman AF (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33(6):1441–1450
Kros J, Frumau KFA, Hensen A, De Vries W (2011) Integrated analysis of the effects of agricultural management on nitrogen fluxes at landscape scale. Environ Pollut 159(11):3171–3182
Lamers LPM, Vile MA, Grootjans AP, Acreman MC, Van Diggelen R, Evans MG, Richardson CJ, Rochefort L, Kooijman AM, Roelofs JGM, Smolders AJP (2015) Ecological restoration of rich fens in Europe and North America: from trial and error to an evidence-based approach. Biol Rev 90(1):182–203
Land M, Granéli W, Grimvall A, Hoffmann CC, Mitsch WJ, Tonderski KS, Verhoeven JTA (2016) How effective are created or restored freshwater wetlands for nitrogen and phosphorus removal? A Systematic Review Environmental Evidence 5(1):9
Lawniczak AE, Güsewell S, Verhoeven JTA (2009) Effect of N: K supply ratios on the performance of three grass species from herbaceous wetlands. Basic Appl Ecol 10(8):715–725
Liu H, Lennartz B (2019) Hydraulic properties of peat soils along a bulk density gradient—A meta study. Hydrol Process 33(1):101–114
Mason C, Bryant RJ (1975) Production, nutrient content and decomposition of Phragmites communis Trin. and Typha angustifolia L. Journal of Ecology 63(1):71–95
Miettinen J, Hooijer A, Vernimmen R, Liew SC, Page SE (2017) From carbon sink to carbon source: extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett. 12(2):024014
Miller RL, Fram M, Fujii R, Wheeler G (2008) Subsidence reversal in a re-established wetland in the Sacramento-San Joaquin Delta, California, USA. San Francisco Estuary and Watershed Science 6(3):1–20. https://doi.org/10.15447/sfews.2008v6iss3art1
Mitsch WJ, Horne AJ, Nairn RW (2000) Nitrogen and phosphorus retention in wetlands-ecological approaches to solving excess nutrient problems. Ecol Eng 14(1/2):1–7
Olde Venterink H, Wassen MJ, Verkroost AWM, De Ruiter PC (2003) Species richness–productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84(8):2191–2199
Pegtel DM, Bakker JP, Verweij GL, Fresco LF (1996) N, K and P deficiency in chronosequential cut summer-dry grasslands on gley podzol after the cessation of fertilizer application. Plant Soil 178(1):121–131
Reddy KR, Patrick WH, Lindau CW (1989) Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol Oceanogr 34(6):1004–1013
Ren L, Eller F, Lambertini C, Guo WY, Brix H, Sorrell BK (2019) Assessing nutrient responses and biomass quality for selection of appropriate paludiculture crops. Sci Total Environ 664:1150–1161
R Core Team (2016) R: A Language and Environment for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/
Steinbachová-Vojtíšková L, Tylová E, Soukup A, Novická H, Votrubová O, Lipavská H, Čížková H (2006) Influence of nutrient supply on growth, carbohydrate, and nitrogen metabolic relations in Typha angustifolia. Environ Exp Bot 57(3):246–257
Tanneberger F, Wichtmann W (2011) Carbon credits from peatland rewetting. Climate – biodiversity – land use. Science, policy, implementation and recommendations of a pilot project in Belarus. Schweizerbart Science Publishers, Stuttgart
Temmink RJM, Fritz C, Van Dijk G, Hensgens G, Lamers LPM, Krebs M, Gaudig G, Joosten H (2017) Sphagnum farming in a eutrophic world: The importance of optimal nutrient stoichiometry. Ecol Eng 98:196–205
Ten Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP (2010) Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J Exp Bot 61(9):2303–2315
Tho BT, Lambertini C, Eller F, Brix H, Sorrell BK (2017) Ammonium and nitrate are both suitable inorganic nitrogen forms for the highly productive wetland grass Arundo donax, a candidate species for wetland paludiculture. Ecol Eng 105:379–386
Toet S, Bouwman M, Cevaal A, Verhoeven JTA (2005) Nutrient removal through autumn harvest of Phragmites australis and Thypha latifolia shoots in relation to nutrient loading in a wetland system used for polishing sewage treatment plant effluent. J Environ Sci Health 40(6–7):1133–1156
Tylová E, Steinbachová L, Votrubová O, Lorenzen B, Brix H (2008) Different sensitivity of Phragmites australis and Glyceria maxima to high availability of ammonium-N. Aquat Bot 88(2):93–98
Ulrich KE, Burton TM (1988) An experimental comparison of the dry matter and nutrient distribution patterns of Typha latifolia L., Typha angustifolia L., Sparganium eurycarpum Engelm. and Phragmites australis (Cav.) Trin. ex Steudel. Aquatic Botany 32(1–2):129–139
Van de Riet BP, Hefting MM, Verhoeven JTA (2013) Rewetting drained peat meadows: risks and benefits in terms of nutrient release and greenhouse gas exchange. Water Air Soil Pollut 224(4):1440
Veraart AJ, De Bruijne WJJ, De Klein JJM, Peeters ETHM, Scheffer M (2011) Effects of aquatic vegetation type on denitrification. Biogeochemistry 104(1–3):267–274
Verhoeven JTA, Setter TL (2009) Agricultural use of wetlands: opportunities and limitations. Ann Bot 105(1):155–163
Vroom RJE, Xie F, Geurts JJM, Chojnowska A, Smolders AJP, Lamers LPM, Fritz C (2018) Typha latifolia paludiculture effectively improves water quality and reduces greenhouse gas emissions in rewetted peatlands. Ecol Eng 124:88–98
Vroom RJE, Temmink RJM, Van Dijk G, Joosten H, Lamers LPM, Smolders AJP, Krebs M, Gaudig G, Fritz C (2020) Nutrient dynamics of Sphagnum farming on rewetted bog grassland in NW Germany. Science of the Total Environment 726:138470
Vymazal J, Greenway M, Tonderski K, Brix H, Mander Ü (2006) Constructed wetlands for wastewater treatment. In: Verhoeven JTA, Beltman B, Bobbink R, Whigham DF (eds) Wetlands and natural resource management. Springer, Berlin/Heidelberg, pp 69–96
Wichtmann W, Wichmann S (2011) Environmental, social and economic aspects of a sustainable biomass production. Journal of Sustainable Energy & Environment Special Issue 77–81
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York
Wichtmann W, Schröder C, Joosten H (2016) Paludiculture – productive use of wet peatlands. Schweizerbart Science Publishers, Stuttgart
Wu H, Zhang J, Ngo HH, Guo W, Hu Z, Liang S, Fan J, Liu H (2015) A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Biores Technol 175:594–601
Zak D, Gelbrecht J, Steinberg CEW (2004) Phosphorus retention at the redox interface of peatlands adjacent to surface waters in northeast Germany. Biogeochemistry 70(3):357–368
Zak D, McInnes R, Gelbrecht J (2011) Preface: restoration, biogeochemistry and ecological services of wetlands. Hydrobiologia 674(1):1–4
Zak D, Meyer N, Cabezas A, Gelbrecht J, Mauersberger R, Tiemeyer B, Wagner C, McInnes R (2017) Topsoil removal to minimize internal eutrophication in rewetted peatlands and to protect downstream systems against phosphorus pollution: a case study from NE Germany. Ecol Eng 103:488–496
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annual Review of Environmental Resources 30:39–74
Acknowledgements
The authors thank Germa Verheggen, Roy Peters, Peter Cruijsen, Paul van der Ven, Sebastian Krosse, Raoul Luijten, Guus Middelbeek, Wout Hendriks (Radboud University) and the staff of the Experimental Garden of the Radboud University for assistance in practical work and analyses. We also thank Romke Kinderman (Bûtefjild) and Karel van Houwelingen (KTC Zegveld) for the permission to take soil cores on their land. This study was funded by FACCE ERA Net+ ‘Climate Smart Agriculture’ (EU) (CINDERELLA) and was part of the project “Veen, Voer en Verder”, financed by Utrecht-West and the province of Zuid-Holland. This study was also funded by the Interreg NWE project Carbon Connects and the ERA-Gas project Peatwise.
Funding
This study was funded by FACCE ERA Net + ‘Climate Smart Agriculture’ (EU) (CINDERELLA) and was part of the project “Veen, Voer en Verder”, financed by Utrecht-West and the province of Zuid-Holland. This study was also funded by the Interreg NWE project Carbon Connects and the ERA-Gas project Peatwise.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Luca Bragazza.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vroom, R.J.E., Geurts, J.J.M., Nouta, R. et al. Paludiculture crops and nitrogen kick-start ecosystem service provisioning in rewetted peat soils. Plant Soil 474, 337–354 (2022). https://doi.org/10.1007/s11104-022-05339-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05339-y