Abstract
Background
Cowpea is a grain legume of major importance in sub-Saharan Africa where it is cultivated by smallholder farmers on poor soils and production is often constrained by the parasitic weed Striga gesnerioides.
Method
Experiments were conducted to assess the potential of rhizobium inoculation to mitigate Striga infection and increase cowpea productivity. We infested soils with S. gesnerioides and assessed the impact of treatments combining cowpea genotypes and bradyrhizobium inoculation on Striga dynamics and cowpea yield. In total, 20 cowpea genotypes were included, of which nine were resistant to Striga and 11 were susceptible. In the first experiment these were factorially combined with three inoculation options (two bradyrhizobium strains USDA3384 and IRJ2180A, and uninoculated control) in a screen-house using potted sterile soils. Second, the same trial was repeated in the field with basal phosphorus applied at sowing and a fourth treatment of fertilizer-N (urea) included testing whether N was limiting cowpea growth. The field trial also included a separate treatment with no input that served as a negative check.
Result
Significant genotype x treatment interactions were observed in nodule counts, Striga attachment, emergence, and cowpea shoot growth in the screen-house. There were few nodules across all cowpea lines. Striga counts were the lowest for resistant varieties with no emerged plants. Rhizobial inoculants depressed Striga counts with consistent differences across cowpea genotypes. Inoculation with IRJ2180A performed the best against Striga attachment in resistant genotypes, and against Striga emergence in susceptible genotypes. In the field trial, cowpea grown without inputs had the least number of nodules. The genotype x treatment interaction was significant: resistant cowpea genotypes were free of emerged Striga while there was much more Striga emergence without input addition with susceptible genotypes. A significant genotype x treatment interaction was observed on cowpea grain yield. Yield response to inoculation was clearest with resistant genotypes inoculated with the strain IRJ2180A.
Conclusion
The integrated use of Striga-resistant cowpea lines, basal phosphorus fertilizer and elite bradyrhizobium inoculants is a promising approach to mitigate Striga infection and increase cowpea productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cowpea (V. unguiculata (L.) Walp.) is a grain legume of high agronomic, nutritional and economic importance in the semi-arid tropics where it is mainly cultivated by resource poor farmers. It constitutes a valuable source of protein in the diets of millions of people (Boukar et al., 2016). About 7.4 million metric tons of cowpea is annually produced worldwide on about 12.6 million hectares (FAOSTAT, 2017). Yet the productivity of cowpea on farmers’ fields remains poor due to numerous abiotic and biotic constraints. Cowpea is often infested by parasitic weed Striga gesnerioides (Willd.) Vatke, which is a major biotic constraint to crop production in the Savannah and Sahel agroecologies of West Africa. The degree of infestation of Striga is greatest when soil fertility is poor, sometimes causing complete loss of yield and forcing farmers to abandon their cultivated lands (Kamara et al., 2014).
S. gesnerioides is an autogamous parasite with a life cycle typical of other agriculturally important Striga spp., and a wide host range including cowpea and groundnut (Arachis hypogaea L.), many other legumes (Alysicarpusspp., Euphorbia spp., Indigofera spp.and Tephrosiaspp.) as well as tobacco (Nicotiana tabacum L.) and Ipomea spp. (Berner and Williams, 1998). Adaptations to parasitism of Striga species include the ability to produce a large number of tiny seeds with prolonged viability and special germination requirements (Siame et al., 1993). Seeds germinate only after exposure to exogenous germination stimulants, strigolactones, which are derived from root exudates of host and often non-host plant species (referred to as trap crops) (Bouwmeester et al., 2007; Yoneyama et al., 2009; Cardoso et al., 2014). Strigolactones are plant hormones which regulate plant shoot and root architecture in response to the environment (Gomez-Roldan et al. 2008; Cardoso et al., 2014), which also function as host recognition signals for arbuscular mycorrhizal fungi (Akiyama et al., 2005) and rhizobia, and trigger seeds of parasitic weeds such as Striga spp.to germinate (Soto et al., 2010; Foo and Davies, 2011; Foo et al., 2013).
Farmer practices to combat Striga weeds range from hand-pulling emerged Striga plants, crop rotations / intercropping to improve soil fertility, the use of trap crops that stimulate suicidal germination of Striga seeds, to the selection of resistant crop varieties. Promising methods to manage Striga arising from research include the use of mineral fertilizers (Jamil et al., 2011), chemicals (Kanampiu et al., 2001, 2003; Kountche et al., 2019), intercropping (Rusinamhodzi et al., 2012), improved tolerant/resistant germplasm (Lane et al. 2003) and biological control (Mabrouk et al., 2007a). However, yield loss attributable to Striga is increasing because many promising Striga control methods suffer from limited adoption and utility (Silberg et al., 2021; Jamil et al., 2021). Low uptake of agricultural technologies is driven by multiple socioeconomic constraints at the farm or higher levels, resulting from lack of/or competing use of land, labour, cash, or organic resources, and reluctance of farmers to experiment with new methods (Oswald, 2005; Giller et al., 2011; Kanampiu et al., 2018).
An integrated approach is required using direct and indirect measures in a concerted manner to prevent damage of parasitism, and ultimately to eradicate the seed bank (Rubiales and Fernandez-Aparicio, 2012). Integrated soil fertility management (ISFM) (Vanlauwe et al., 2010), including incorporation of legumes into cropping systems (Franke et al., 2018; Vanlauwe et al., 2019; Kamara et al., 2020) can contribute to reducing Striga infection. Many leguminous crops such as cowpea can stimulate suicidal germination of seeds of Striga spp. that infect cereals as non-host species (Berner and Williams, 1998), and are used as trap crops to reduce Striga spp.in cereal-legume rotation and intercropping systems (Midega et al., 2014; Hooper et al., 2015).
Strains of rhizobia have been identified with potential for controlling the parasitic weed Orobanche crenata Forsk. in inoculated pea (Mabouk et al., 2007a, b). Inoculation of pea with compatible rhizobia was reported to affect O. crenata by reducing seed germination, and decreasing root infection with limited capacity for tubercle development and necrosis of attachments (Mabouk et al., 2007a, b). Considering that the key factors determining productivity/ or performance of legume technologies range from the genotypes of both the legume (GL) and the associated root nodule rhizobia (GR), the test climate and soil environments (E), to the agronomic management used (M), such relationship can be expressed as - (GL× GR) × E × M (Giller et al., 2013). For a given environment, understanding in the same way the relationship between the effectiveness of various combinations of symbiotic partners (GL× GR) and managements options (M), and the variability in legume infection by Striga spp. is of major interest in view of explaining the potential of matching legume variety and inoculant strain in controlling S. gesnerioides.
Striga-resistant varieties and promising lines of cowpea have already been identified (Lane et al., 2003; Boukar et al., 2016), which provide a key entry point for testing integrated Striga management options. Genotypes of cowpea (Lane et al., 2003) and many cereals such as rice (Rodenburg et al., 2017), sorghum (Rodenburg et al., 2006) and maize (Badu-Aprakuet al., 2020) are reported to combine improved degrees of resistance and tolerance to Striga. Resistance to Striga spp. refers to genotype ability to support fewer infections, whereas tolerance denotes its potential to show less damage for similar infection levels when compared with other varieties of the same species (Rodenburg et al., 2006). Resistant varieties can induce the germination of Striga seeds while preventing the parasite from attaching to host roots or killing the attached parasitic plants (Badu-Apraku et al., 2020).
In this paper, we present the results of an integrated evaluation of BNF technologies consisting of crop varieties, rhizobium inoculants, and the application of phosphorus (P) and nitrogen (N) fertilizers. The objectives are (i) to evaluate underscreen-house conditions the potential of rhizobia as biocontrol agents against S. gesnerioides in Striga-resistant and susceptible cowpea varieties, (ii) to assess the relative performance of rhizobial inoculants to control Striga infection of cowpea in the field as compared with standard mineral fertilisation, and (iii) to identify the most effective combination of rhizobia, cowpea genotype and fertilizer for optimal control of S. gesnerioides.
Materials and methods
Experimental site and soil description
Two sets of experiments were conducted in 2014 in Kano (Kano state, northern Nigeria) under screen-house and field conditions. A screen-house trial was carried out under artificial irrigation during the dry season at the Kano Station of International Institute of Tropical Agriculture (IITA). A subsequent field trial was conducted under natural rainfall during the following rainy season at the IITA research farm (12°10′42”N,8°32′39″Eat 500 m above sea level), located at Minjibir village 45 km north of Kano. Both locations fall under the Sudan savanna agro-ecological zone, where climate is dry with a unimodal distribution of 700–800 mm annual rainfall (Ronner et al., 2016) over a short growing season (about 120 days) from July to September. The mean monthly rainfall and temperature in 2014 at Minjibir are shown in Fig. 1.
Soil sampling was done before land preparation by taking soil cores from 0 to 15 cm depth at random points describing a “Z” pattern across the whole field. The cores were bulked and mixed thoroughly to provide a composite sample, and a sub-sample was analysed for physical and chemical properties according to standard procedures (IITA, 1989) in the analytical laboratory of the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. The abundance of soil bradyrhizobia was determined by the plant infection technique (Vincent, 1970) (Table 1). The soil was coarse textured and texture fell within the loamy sand and sandy loam class (Table 1). Soil organic carbon (9 g kg−1) was relatively low and total nitrogen (0.36 g kg−1) in the very low N fertility class. The concentration of available Cu (1.07 mg kg−1) was low and there was relatively high concentration of Mn (46 mg kg−1) and Fe (178 mg kg−1) due to the slightly acidic pH (5.6) of the soil (Table 1). Available soil P in the field (8.6 mg kg−1) was as low, as is common in sandy savanna soils with low organic matter content (Kwari et al., 2011).
Cowpea and Striga seed sources
Twenty cowpea genotypes (Table 2) were compared in both screen-house and field station experiments. These lines have earlier shown variable responses to Striga ranging from lack of infection even at high Striga infestation rates (resistant genotypes) to presence of parasitism even at low Striga infestation (susceptible genotypes) (Muranaka et al., 2011). Seeds of cowpea lines were obtained from the Genetic Resource Center (GRC) of IITA. The Striga seeds were harvested in 2010 from a research farm at Malam Madori in Jigawa State, a Striga hot-spot where cowpea is widely cultivated. Striga seeds were manually threshed and cleaned to remove chaff. Seeds were then surface-sterilized with a sodium hypochlorite solution (NaOCl, 3.0%) for 3 min (Berner and Williams, 1998). Thereafter, the seeds were thoroughly rinsed with tap water through a fine sieve and left to air-dry in the laboratory.
Bradyrhizobiumstrains and inocula preparation
Bradyrhizobium sp. strain USDA 3384 (National Rhizobium Culture Collection, Beltsville, Maryland, USA) and B. Japonicumstrain IRJ 2180A were obtained from the soil microbiology laboratory of IITA. These area reference broad host-range rhizobia strain (Foster et al., 1998; Hashem et al., 1997) and an elite soybean inoculant strain (Sanginga et al., 2002; Okogun and Sanginga, 2003), respectively. Each strain was grown in yeast extract mannitol medium for five days to reach 109 cell ml−1, which was used as liquid inoculant in screen-house studies. Bradyrhizobial broths were used to prepare peat-based inoculants to coat seed for field experiments. Standard peat carrier (APT, USA) was sterilized by gamma irradiation (Ghana Atomic Energy Commission, Accra, Ghana) in bags containing 50 g of peat each. Upon radiation, each bag was aseptically injected with 50 ml of the appropriate rhizobial broth, injection hole sealed, contain thoroughly mixed and then cured in the same bag at 28 °C for two weeks to obtain at least 109 cell g−1 inoculant (Hoben and Somasegaran, 1982).
Treatments, experimental design and crop management
In a first experiment, test soil was artificially infested with Striga to assess responses of 20 cowpea genotypes to three inoculation options under screen-house conditions. Inoculation treatments comprised of the two bradyrhizobial inoculants used separately, and an uninoculated control. Sixty treatments were studied consisting of 20 × 3 factorial combinations of both factor levels. Treatments were established in three replications using a split plot arrangement where inoculation options were randomly assigned to main plots and cowpea genotypes to sub-plots as to minimize cross contamination. Cowpea plants were grown on potted-soil infested with Striga seeds using a modified protocol of Singh and Emechebe (1990). A mixture of top soil and river sand (2:1; v/v) was prepared and sterilized by autoclaving (120 °C for 1 h) prior to incorporating Striga seed. The soil used in screen-house trial was sampled from the field where the field trial was conducted. Growth units consisted of plastic pots (13 cm, height and width) containing ca. 2.5 kg pot- 1 soil mixture. Four seeds of cowpea were planted and inoculated with 1 ml of bradyrhizobial broth pot−1. The growth units were subsequently irrigated with tap water as necessary on alternate days to ensure adequate moisture and good germination. Pots were thinned to two seedlings at 2 weeks after planting then fertilized with 180 mg P as single super phosphate (SSP, 18% P). Seedling leaves were further sprayed with a combined insecticide (35 ml of Lamdacyhalothrine and 75 ml of Cypermethrin plus Dimethoate in 15 l sprayer) to control insect pests.
In the second experiment conducted in the field, responses of Striga infection and crop performance of the same cowpea varieties to five treatments were tested on land heavily infested with Striga (five plants m−2 on average). The treatments were a negative check with no input (inherent soil fertility), a control treatment which received only P fertilizer, the two inoculants applied together with P fertilizer, and a treatment which received urea-N and P fertilizer (Table 3). We refer to the inoculant treatments and the N fertilizer treatment jointly as “N sources”. P fertilizer was applied at the rate of 30 kg P ha−1as single-super phosphate and N applied as urea at a rate of 20 kg N ha−1. All fertilizer was applied at sowing in furrows and covered with soil to minimise losses. The experiment was set up as a split plot with 20 cowpea genotypes and 5 soil fertility treatments, replicated three times. The field was mown, harrowed and plant debris removed. The field was then ridged at 0.75minter-ridge spacing. Cowpea seeds were pre-treated with fungicide-insecticide Apron Star (20% w/w Thiamethoxam, 20% w/w Metalaxyl-M, 2% w/w Difenoconazole) to prevent soil-borne pests attack on seeds and seedlings. Plots (6 ridges × 4 m) were planted manually at the rate of three seeds per hill with 0.2 m intra-row spacing. Immediately before planting, cowpea seed was coatedwith peat-based inoculants (10 g kg−1 seed) using a solution of gum arabic (ca. 20 g l−1) as sticker. Pre-emergence herbicide Paraquat and Pendimethalin (3 l ha−1) was sprayed immediately after sowing to control weeds. Two weeks after planting, plots were thinned to two plants per hill and SSP fertilizer was applied at the rate of 30 kg P ha−1. Lamdacyhalothrine 25 EC (0.7 l ha−1) and Cypermethrin, plus Dimethoate (1 l ha−1) was applied at flowering stage to control insect pests. The field was maintained free of weeds except Striga plants by regular manual weeding at three, six and eight weeks after planting.
Data collection and statistical analyses
In the screen-house study, plants were harvested at 64 days after planting (DAP). Potted plants were cut at the soil level and pots were gently flushed with tap water to discard soil from the root systems for count assessment of nodules, Striga attached to roots and emerged Striga. All plant parts were oven-dried separately at 65 °C for 48 h for biomass. In the field trial data were collected from the flowering stage onward. Twenty plants were randomly uprooted from border rows of each plot, nodules recovered, counted and oven-dried as above for dry mass assessment. Number of emerged Striga plants was monitored three times from the two central rows of plots until 70 DAP and cumulative number calculated. At maturity, the same inner rows were harvested, shoots, pods and grains air-dried and weight recorded.
Biomass data (cowpea and Striga dry weights) from the screen-house trial, and grain yield from the field study were analysed using a linear mixed model where genotype, strain, and genotype x strain were considered as fixed effects, and replicate, genotype x replicate as random effects. Count data across screen-house and field experiments (number of nodules, Striga attachments and emerged plants) were analysed using a generalized mixed model (McCullagh and Nelder, 1989) assuming a Poisson distribution. Least-squares means and the associated standard errors derived from the mixed model were computed. Custom hypothesis tests were used to perform contrast comparisons between specific groups among cowpea genotypes and N-sources (Tables 4 and 5). All data were analysed using MIXED (linear mixed model) and GLIMMIX (generalized linear mixed model) procedures in SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute, 2019).
Results
Striga infection, nodulation and plant growth in the screen-house
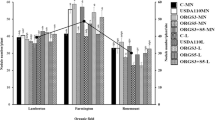
At harvest 64 DAP, the majority of inoculated cowpea plants were poorly nodulated as they had reached physiological maturity (Fig. 2). The occurrence of nodulation appeared to be inversely-related to infection of cowpea roots by Striga, with most plants exhibiting either nodules or Striga attachments (Fig.3B, C). In addition, the smallest values for cowpea dry weight were found on plants with the least number of nodules (Fig. 3A).
There was a differential response of cowpea genotypes to inoculation treatments in shoot dry weight (P = 0.0155, Table 4). Resistant varieties grew the best, yet were not responsive to inoculation in pots. By contrast, susceptible lines showed substantial responses to inoculation with an average of 63% increase in shoot dry weight (P ≤ 0.0001,Table 4; Fig.4A). Similarly, there were large differences between susceptible cowpea varieties which had significantly larger counts of emerged Striga plants (P = 0.0047) and number of Striga attachments to cowpea than resistant varieties (P ≤ 0.0001) (Fig. 3; Table 4; Fig. 4B-C). The number of emerged Striga plants from pots was the greatest (2.5 plant−1) for susceptible genotypes grown without inoculation (control treatment) (P = 0.0168; Fig. 4B). Moreover, inoculation with strain IRJ 2180A was the most effective totally in preventing emergence of Striga plants in the potted susceptible genotypes (P = 0.0246; Fig. 4C). On average, the smallest numbers of Striga attachments to cowpea roots were observed in resistant varieties (P = 0.0046), and in inoculated plants (P = 0.0065, Table 4; Fig. 4D-E). When compared with the uninoculated (control) treatments, addition of rhizobial inoculants was slightly more effective in reducing Striga attachments on resistant (90%) than susceptible (80%) varieties (P = 0.0168; Fig. 4D). Resistant genotypes performed best against Striga attachment to root with strain IRJ 2180A inoculant (P = 0.0246; Fig. 4E).
A-B Shoot dry weight, (C-D) emerged Striga count, and (E-F) Striga attachment responses to inoculation with bradyrhizobia of Striga-resistant and -susceptible cowpea genotypes in potted soils under screen-house conditions; the cowpea plants were inoculated with strains IRJ 2180A or USDA 3384 (inoculant) or not (control). (B): Variety x strain not significant. Errors bar represents the standard error of means
Striga infection, nodulation and plant growth in the field trial
At early cowpea flowering stage, the number of nodules was 14.9 nodules plant−1on average with significant differences among varieties (P = 0.0315), and between nutrient management options (P ≤ 0.0001; Table 5; Fig. 5A-D). The least nodule number (5.3 nodules plant−1) was found for cowpea managed without any input (P ≤ 0.0001; Fig. 5A). The application of Pfertilizer alone improved nodulation (13.1 nodulesplant−1), but to a lesser extent than when P and N sources were combined (P ≤ 0.0001; Fig. 5B). Addition of urea resulted in less nodules (13.3 nodules plant−1) than when rhizobial inoculants were applied (21.5 nodules plant−1) (P ≤ 0.0001; Fig. 5C). Among the inoculant treatments, the largest number of nodules was found in the presence of the strain IRJ 2180A (29.8 nodules plant−1;P ≤ 0.0001, Table 5; Fig. 5D).
Overall, strong positive relationships were found between nodule number and cowpea yield and negative relationships with Striga emergence (data not shown). Emergence of Striga plants decreased with increasing nodule number, which led to improved cowpea yield. The density of Striga plants was highest for the less well nodulated cowpea plants managed without any input (≤5 nodules plant−1) and lowest for the abundantly-nodulated plants in the P+ IRJ 2180A treatment (≥25 nodules plant−1). For most cowpea genotypes, grain yield had a significant negative correlation with Striga count.
Significant variety x management interactions was observed in the cumulative count of emerged Striga plants (P ≤ 0.0001, Table 5). Plots with resistant cowpea varieties were remarkably free of emerged Striga almost throughout the field experiment (Fig. 6A-D). Susceptible varieties had stronger infection with Striga count dependent upon the management option (Fig. 6A-D). Sprouting of Striga was densest without any amendment (no input) compared with nutrient addition (P ≤ 0.0001, Table 5; Fig. 6A). On average, managing susceptible cowpea varieties with various combinations of P and N-sources led tomore than 2.6-fold reduction in the number of Striga plants relative to plots without any input (Fig. 6A). Effectiveness in the control ranged from the least with P alone to the highest for P combined with N-sources (Fig. 6B). However, emerged Striga count when fertilized with urea was comparable with that for rhizobial inoculants (Fig. 6C-D).
The differential responses of the cowpea genotypes to management options resulted in large grain yield differences at harvest (P ≤ 0.0001, Table 5). Grain yield as well as its response to input additions was consistently stronger in resistant lines than susceptible ones (P ≤ 0.0001, Table 5; Fig. 7A-D). Overall, grain yield was more than doubled in response to nutrient supply compared with no input treatments, with increase being marginally larger for resistant (128%) than susceptible (119%) lines (Fig. 7A). On average in addition, grain yield in susceptible varieties was more responsive to P alone (+16%) than P + N treatments (P ≤ 0.01, Table 5; Fig. 7B). When P was applied the yield response of the cowpea varieties to inoculant was significantly greater (ca + 10%) than with urea (P = 0.0174; Fig. 7B). Overall, cowpea yielded substantially more grain (31–34% more) with inoculant strain IRJ 2180A than USDA 3384(P ≤ 0.0001; Fig. 7D). Also, resistant varieties outperformed susceptible lines in yield responses to inoculation, which was most obvious in the presence of inoculant strain IRJ 2180A (P = 0.0043; Fig. 7D).
Discussion
Our results demonstrate that integration of resistant varieties, fertilizer application and inoculation with Bradyrhzobium could effectively control Striga in cowpea. A pot experiment was used first to investigate genotypic variability in cowpea responses to co-inoculation with rhizobia and Striga in the screen-house. Although the cowpea plants were poorly-nodulated by time of harvest (< 1 nodule plant−1 on average; Fig. 2), plant infection by Striga was strongly reduced as compared to the control without inoculation. Poor nodulation in the screen-house could be partly due to the harvest of mature plants, as well as the soil sterilization which would have killed off arbuscular mycorrhizal (AM) fungi as well as native rhizobia. In addition to the well-established role of AM in plant uptake of phosphorus in P-deficient soils which supports good nodulation and nitrogen fixation in legumes (Hayman, 1986, de Novais et al., 2020), there is evidence that AM fungi can suppress Striga infection in cereals. While the precise mechanisms need to be elucidated, AM both suppress germination of Striga seeds and attachment to host roots, leading to reduced root colonisation and emergence of Striga plants (Gworgwor and Weber 2003; Lendzemo et al., 2005,2006), by altering the release of Striga seeds germination stimulants in host root exudates (Akiyama et al., 2005; Lendzemo et al., 2007). This suggests that further studies on the role of the tripartite interaction of the host legume, AM and rhizobia on Striga suppression are warranted. On average, the resistant cowpea varieties had the least number of Striga attachments to the roots as well as the number and weight of emerged Striga shoots (Fig. 3). Surprisingly, resistant varieties were also found to be the most responsive to rhizobial inoculants for the control of Striga: compared with the uninoculated control plants of these varieties, there was as high as a tenfold reduction in the number of Striga attachments which resulted in cowpea plants almost clean of the parasites (Fig. 4).
In comparison with some practices recommended against Striga such as hand or hoe-weeding and conventional biocontrol, which operate only when the parasite has already infected the crop and subsequently developed emerged shoots, eradicating the seed bank and preventing root colonization by Striga may be the ideal solution to its control (López-Ráez et al., 2008; Kountche et al., 2019). The use of crop varieties that combine resistance with high tolerance levels is presently suggested among the most promising and easy to adopt control options against the weed Striga, particularly when combined also with other management practices (Rodenburg et al., 2006, 2017; Badu-Apraku et al., 2020). Although much literature has emphasised the importance of integrated technologies involving improved crop germplasm, companion legumes, fertilizer and crop management option for control of Striga spp. (Tesfaye and Ejeta, 2011; Midega et al. 2014; Randrianjafizanaka et al., 2018; Kamara et al., 2020), little attention has so far been paid to the potential contribution of rhizobial inoculants against the weed. Our study shows the potential of using rhizobial inoculants as part of integrated management to aid Striga control. We provide insights into how resistant varieties, fertilizer and rhizobial inoculants can be combined to manage Striga in cowpea, although our results need to be further confirmed across more locations and seasons.
Cowpea lines grown in fields infested with S. esnerioides readily formed root nodules without inoculation, demonstrating the presence of adequate native populations of compatible rhizobia (Fig. 5). Unlike other legume crops such as soyabean which have a symbiotic requirement for specific rhizobial strains, cowpea is a promiscuous host, able to form root nodules with a broad range of highly diverse rhizobia (Giller, 2001). On the other hand, poor soil fertility is a major constraint to the proper establishment and function of legume symbiosis in sub-Saharan Africa (Vanlauwe et al., 2019). The improved nodulation of cowpea in response to addition of P is consistent with widespread reports of soil P limitations for growth and production of grain legumes in sub-Saharan Africa (Ronner et al., 2016; vanHeerwaarden et al., 2018; Belete et al., 2019; Rurangwa et al., 2018). It appears that cowpea generally needs to be fertilized with P, except in a few locations such as in Ghana where field-grown cowpea genotypes nodulated well without any nutrient addition (Adjei-Nsiah et al., 2008).
As expected from previous studies, our results confirmed a consistent varietal effect on Striga infection with a clear discrimination between resistance and susceptible cowpea lines in the field (Lane et al., 2003; Muranaka et al., 2011). Striga plants were not observed in the plots planted with resistant cowpea genotypes and significant interactions between the variety and management were evident. This confirms the importance of resistant varieties to control Striga (Rodenburg et al., 2006; Badu-Apraku et al., 2020). Alleviation of nutrient deficiency through addition of P fertilizer and either rhizobial inoculant or N fertilizer also suppressed Striga (Fig. 6.), as poor soil fertility is known to favour the spread of S. gesnerioides. In a similar vein, Jamil et al. (2011) showed N and P fertilizers reduced secretion of Striga seed germination stimulants in maize resulting in less Striga infection.
We further found a large difference in performance of the two rhizobial strains. Strain IRJ 2180A was much more effective in promoting cowpea nodulation and in suppressing Striga than strain USDA 3384. Our findings suggest that the improved nutritional status of cowpea is the most likely mechanism through rhizobial inoculants contributed to mitigate Striga infection. This could be mediated through reduced strigolactone production from inoculated cowpea plants leading to less Striga germination, and/or the ability of more vigorous plants to tolerate and escape the deleterious effects of Striga. It could also involve mechanisms of induced systemic resistance in the host roots by rhizobia, which leads to mechanical and chemical barriers against parasitic weeds (Mabrouk et al., 2007a, b, 2008, 2010). Rhizobium leguminosarum strain P.SOM reduces pea root infection by the parasitic weed O. crenata through inhibition of seed gemination (more than 3-fold less parasite seeds germinated, with a large proportion ofnecrotic sprouted seeds), and attachment (up to 7-times less tubercles per plant, Mabrouk et al., 2014). Histological studies have shown that lignification of host endodermal and pericycle cells may be an additional resistance mechanism that prevents parasite penetration at the initial stage of infection, and leads to necrosis and death of developed tubercles (Perezde Luque et al., 2005). Further research is needed to elucidate the mechanisms. The effective reduction in the number of Striga plants in the field consistently resulted in better cowpea grain yields, and differences were consistent with trends in the repression of the parasite (Figs. 6 and 7). The increase of cowpea yield obtained here with the combined use of improved germplasm, fertilizers and rhizobial inoculants is a clear application of the concept of integrated soil fertility management (ISFM) needed for the sustainable intensification of African agriculture (Vanlauwe et al., 2015).
The productivity of cowpea grown without any inputs was very poor (211–280 kg ha−1), the choice of cowpea genotype was clearly the most relevant starting point to increase yields. The use of improved cowpea seed alone led to the highest impacts both in the control of the weed (46 times less infection) and grain yield (+33%). As a second step, P fertilization was effective for controlling Striga in susceptible varieties (38% less emerged plants), though responses in grain yield remained strongest for resistant lines (2.6-compared with 2.1-fold increase for susceptible lines). Effective rhizobial inoculants formed a third level of intervention. For the susceptible lines, the most effective strain (IRJ 2180A) led to additional decrease (52%) in Striga emergence and enhancement in grain yield (18%) over and above the effects of P fertilization alone (442 kg ha−1). For the resistant varieties also, responses were almost similar with around 56% less Striga emergence and 15% increase in grain yield in comparison with P fertilization alone (715 kg ha−1). This suggests overall a sequence of improved germplasm, P fertilizer and rhizobial inoculant as steps towards implementation of ISFM.
Conclusion
We have demonstrated the potential of using bradyrhizobial inoculants as effective biocontrol agents as part of an integrated strategy against S. gesnerioides weed infection of cowpea. Field-grown cowpea was confirmed to perform well in terms of grain yield and in control of infection by Striga when improved germplasm was used together with P fertilization. The use of appropriate cowpea genotypes is therefore suggested to be a suitable entry point for ISFM application, followed by P fertilizer and rhizobium inoculants. P fertilization was found to be crucial to both cowpea productivity and its responsiveness to rhizobial inoculants. Further evidence of a strong genotypic host plant x rhizobia strain interaction highlights the possibility for further selection of combinations to counter Striga damage. Whilst these are promising results, further investigations are needed to confirm the robustness these control options across more agroecological environments and seasons.
References
Adjei-Nsiah S, Kuyper TW, Leeuwis C, Abekoe MK, Cobbinah J, Sakyi-Dawson O, Giller KE (2008) Farmers’ agronomic and social evaluation of productivity, yield and N2-fixation in different cowpea varieties and their subsequent residual N effects on a succeeding maize crop. Nutr CyclAgroecosyst 80:199–209. https://doi.org/10.1007/s10705-007-9133-3
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435(7043):824–827. https://doi.org/10.1038/nature03608
Badu-Apraku B, Adewale S, Paterne A, Gedil M, Asiedu R (2020) Identification of QTLs controlling resistance/tolerance to Striga hermonthica in an extra-early maturing yellow maize population. Agronomy 2020(10):1168. https://doi.org/10.3390/agronomy10081168
Belete S, Bezabih M, Abdulkadir B, Tolera A, Mekonnen K, Wolde-meskel E (2019) Inoculation and phosphorus fertilizer improve food-feed traits of grain legumes in mixed crop livestock systems of Ethiopia. Agric. Ecosyst Environ 279:58–64. https://doi.org/10.1016/j.agee.2019.04.014
Berner DK, Williams OA (1998) Germination stimulation of Striga gesnerioidesseeds by hosts and nonhosts. Plant Dis 82:1242–1247. https://doi.org/10.1094/pdis.1998.82.11.1242
Boukar O, Fatokun CA, Huynh B-L, Roberts PA, Close TJ (2016) Genomic tools in cowpea breeding programs: status and perspectives. Front Plant Sci 7:757. https://doi.org/10.3389/fpls.2016.00757
Bouwmeester HJ, Roux C, Lopez-Raez JA, Becard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12:224–230. https://doi.org/10.1016/j.tplants.2007.03.009
Cardoso C, Zhang Y, Jamil M, Hepworth J, Charnikhova T, Dimkpa SON, Meharg C, Wright MH, Liu J, Meng X, Wang Y, Li J, McCouch SR, Leyser O, Price AH, Bouwmeester HJ, Ruyter-Spira C (2014) Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc Natl Acad Sci U S A 111:2379–2384. https://doi.org/10.1073/pnas.1317360111
de Novais CB, Sbrana C, da ConceiçãoJesus E, Rouws LFM, Giovannetti M, Avio L, Siqueira JO, SagginJúnior OJ, da Silva EMR, de Faria SM (2020) Mycorrhizal networks facilitate the colonization of legume roots by a symbiotic nitrogen-fixing bacterium. Mycorrhiza 30:389–396. https://doi.org/10.1007/s00572-020-00948-w
FAOStat (2017). FAOSTAT, Statistical data base. Food and Agricultural Organizations of the United Nations, Rome. http://www.fao.org/faostat/ 2017
Foo E, Davies NW (2011) Strigolactones promote nodulation in pea. Planta 234:1073–1081. https://doi.org/10.1007/s00425-011-1516-7
Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB (2013) Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant 6:76–87. https://doi.org/10.1093/mp/sss115
Foster CM, Horner HT, Graves WR (1998) Nodulation response of woody Papilionoid species after inoculation with rhizobia and soil from Hawaii, Asia and North America. Plant Soil 205:103–111. https://doi.org/10.1023/A:1004309414388
Franke AC, van den Brand GJ, Vanlauwe B, Giller KE (2018) Sustainable intensification through rotations with grain legumes in sub-Saharan Africa: A review. Agric Ecosyst Environ 261:172–185. https://doi.org/10.1016/j.agee.2017.09.029
Giller KE (2001) Nitrogen fixation in tropical cropping systems, 2nd edn. CAB International, Wallingford
Giller, K.E., Franke, A.C., Abaidoo, R., Baijukya, F., Bala, A., Boahen, S., Dashiell, K., Kantengwa, S., Sanginga, J.-M., Sanginga, N., Simmons, A.J., Turner, A., de Wolf, J., Woomer, P., and Vanlauwe, B.(2013). “N2Africa: putting nitrogen fixation to work for smallholder farmers in Africa,” In Agro-ecological Intensification of Agricultural Systems in the African Highlands, eds. B. Vanlauwe, P.J.A. van Asten, and G. Blomme (Routledge, London), 156–174
Giller KE, Tittonell P, Rufino MC, van Wijk MT, Zingore S, Mapfumo P, Adjei-Nsiah S, Herrero M, Chikowo R, Corbeels M, Rowe EC, Baijukya F, Mwijage A, Smith J, Yeboah E, van der Burg WJ, Sanogo OM, Misiko M, de Ridder N et al (2011) Communicating complexity: integrated assessment of trade-offs within African farming systems to support development policy. Agric Syst 104:191–203. https://doi.org/10.1016/j.agsy.2010.07.002
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194. https://doi.org/10.1038/nature07271
Gworgwor NA, Weber HC (2003) Arbuscular mycorrhizal fungi-parasite-host interaction for the control of Striga hermonthica (Del.) Benth. In sorghum [ sorghum bicolor (L.) Moench]. Mycorrhiza 13:277–281. https://doi.org/10.1007/s00572-003-0238-5
Hashem FM, Saleh SA, van Berkum P, Voll M (1997) Survival of Bradyrhizobium sp. (Arachis) on fungicide-treated peanut seed in relationship to plant growth and yield. World J Microbiol Biotechnol 13:335–340. https://doi.org/10.1023/a:1018595310239
Hayman DS (1986) Mycorrhizae of nitrogen-fixing legumes. MIRCEN J Appl Microbiol Biotechnol 2:121–145. https://doi.org/10.1007/BF00937189
Hoben HJ, Somasegaran P (1982) Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl Environ Microbiol 44:1246–1247. https://doi.org/10.1128/aem.44.5.1246-1247.1982
Hooper AM, Caulfield JC, Hao B, Pickett JA, Midega CAO, Khan ZR (2015) Isolation and identification of Desmodium root exudates from drought tolerant species used as intercrops against Striga hermonthica. Phytochem. 117:380–387. https://doi.org/10.1016/j.phytochem.2015.06.026
IITA (1989). Automated and Semi-automated Methods for Soil and Plant Analysis. Manual Series No. 7. IITA, Ibadan, Nigeria
Jamil M, Charnikhova T, Cardoso C, Jamil T, Ueno K, Verstappen F, Asami T, Bouwmeester HJ (2011) Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Res 51:373–385. https://doi.org/10.1111/j.1365-3180.2011.00847.x
Jamil M, Kountche BA, Al-Babili S (2021) Current progress in Striga management. Plant Physiol 185:1339–1352. https://doi.org/10.1093/plphys/kiab040
Kamara AY, Ekeleme F, Jibrin JM, Tarawali G, Tofa I (2014) Assessment of level, extent and factors influencing Striga infestation of cereals and cowpea in a Sudan savanna ecology of northern Nigeria. Agric Ecosyst and Environ 188:111–121. https://doi.org/10.1016/j.agee.2014.02.027
Kamara AY, Menkir A, Chikoye D, Tofa AI, Fagge AA, Dahiru R, al. (2020) Mitigating Striga hermonthica parasitism and damage in maize using soybean rotation, nitrogen application, and Striga-resistant varieties in the Nigerian savannas. Exp Agric 56:620–632. https://doi.org/10.1017/S0014479720000198
Kanampiu FK, Ransom JK, Gressel J (2001) Imazapyr seed dressings for Striga control on acetolactate synthase target-site resistant maize. Crop Prot 20:885–895. https://doi.org/10.1016/S0261-2194(01)00038-2
Kanampiu FK, Kabambe V, Massawe C, Jasi L, Friesen D, Ransom JK, Gressel J (2003) Multi-site, multi-season field tests demonstrate that herbicide seedcoating herbicide-resistance maize controls Striga spp. and increases yields in several African countries. Crop Prot 22:697–706. https://doi.org/10.1016/S0261-2194(03)00007-3
Kanampiu FK, Makumbi D, Mageto E, Omanya G, Waruingi S, Musyoka P, Ransom J (2018) Assessment of management options on Striga infestation and maize grain yield in Kenya. Weed Sci 66:516–524. https://doi.org/10.1017/wsc.2018.4
Kountche BA, Jamil M, Yonli D, Nikiema MP, Blanco-Ania D, Asami T, Zwanenburg B, Al-Babili S (2019) Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants, People, Planet 1:107–118. https://doi.org/10.1002/ppp3.32
Kwari, J.D., Kamara, A.Y., Ekeleme, F., and Omoigui, L. (2011). Soil fertility variability inrelation to the yields of maize and soybean under intensifying cropping systemsin the tropical savannas of northeastern Nigeria. Innovations as Key to theGreen Revolution in Africa. Springer, Netherlands, pp. 457–464
Lane JA, Bailey JA, Butler RC, Terry PJ (2003) Resistance of cowpea [(Vigna unguiculata (L.) Walp.)] to Strigagesnerioides (Willd.) Vatke, a parasitic angiosperm. New Phytol 125:405–412. https://doi.org/10.1111/j.1469-8137.1993.tb03893.x
Lendzemo VW, Kuyper TW, Kropff MJ, van Ast A (2005) Field inoculation with arbuscular mycorrhizal fungi reduces Striga hermonthica performance on cereal crops and has the potential to contribute to integrated Striga management. Field Crop Res 91:51–61. https://doi.org/10.1016/j.fcr.2004.05.003
Lendzemo VW, van Ast A, Kuyper TW (2006) Can arbuscular mycorrhizal fungi contribute to Striga management on cereals in Africa? Outlook Agric 35:307–311. https://doi.org/10.5367/000000006779398236
Lendzemo VW, Kuyper TW, Matusova R, Bouwmeester HJ, andvan Ast, A. (2007) Colonization by arbuscular mycorrhizal fungi of sorghum leads to reduced germination and subsequent attachment and emergence of Striga hermonthica. Plant SignalingBehav 2:58–62. https://doi.org/10.4161/psb.2.1.3884
López-Ráez JA, Matusova R, Cardoso C, Jamil M, Charnikhova T, Kohlen W, Ruyter-Spira C, Verstappen F, Bouwmeester H (2008) Strigolactones: ecological significance and use as a target for parasitic plant control. Pest Manag Sci 65:471–477. https://doi.org/10.1002/ps.1692
Mabrouk Y, Zourgui L, Sifi B, Delavault P, Simier P, Belhadj O (2007a) Some compatible Rhizobium leguminosarum strains in peas decrease infections when parasitized by Orobanche crenata. Weed Res 47:44–53. https://doi.org/10.1111/j.1365-3180.2007.00548.x
Mabrouk Y, Zourgui L, Sifi B, Belhadj O (2007b) The potential of Rhizobium strains for biological control of Orobanche crenata. Biologia 62:139–143
Mabrouk Y, Zourgui L, Sifi B, Belhadj O (2008) Mechanism of induced resistance in pea by rhizobium against Orobanche crenata. Rev Arid Areas 3:1234–1240
Mabrouk Y, Mejri S, Hemissi I, Simier P, Delavault P, Saidi M, Belhadj O (2010) Bioprotection mechanisms of pea plant by Rhizobium leguminosarum against Orobanche crenata. Afr J Microbiol Res 4:2570–2575
Mabrouk Y, Mejri S, Delavault P, Simier P, Delavault P, Saidi M, Belhadj O (2014) Lipopolysaccharide isolated from Rhizobium leguminosarum strain P.SOM induces resistance in pea roots against Orobanche crenata. Afr J Microbiol Res 8:2624–2630. https://doi.org/10.5897/AJMR2013.5762
McCullagh, P., and Nelder, J.A. (1989). Generalized linear models. London, UK, Chapman & Hall
Midega CAO, Salifu D, Bruce TJ, Pittchar J, Pickett JA, Khan ZR (2014) Cumulative effects and economic benefits of intercropping maize with food legumes on Strigahermonthica infestation. Field Crop Res 155:144–152
Muranaka S, Fatokun C, Boukar O (2011) Stability of Striga gesnerioidesresistance mechanism in cowpea under high- infestation level, low soil fertility and drought stresses. J Food Agric Environ 9:313–318
Okogun JA, Sanginga N (2003) Can introduced and indigenous rhizobial strains compete for nodule formation by promiscuous soybean in the moist savanna agroecological zone of Nigeria? Biol Fertil Soils 38:26–31. https://doi.org/10.1007/s00374-003-0611-8
Oswald, A. (2005). Striga control: technologies and their dissemination. Crop Prot 24, 333–342. https://doi.org/10.1016/j.cropro.2004.09.003
Perezde Luque A, Rubiales D, Cubero JI, Press MC, Scholes J, Yoneyama K, Takeuchi Y, Plakhine D, Joel M (2005) Interaction between Orobanche crenata and its host legumes: Unsuccessfulhaustorialpenetration and necrosis of the developing parasite. Ann Bot 95:935–942. https://doi.org/10.1093/aob/mci105
Randrianjafizanaka MT, Autfray P, Andrianaivo AP, Ramonta IR, Rodenburg J (2018) Combined effects of cover crops, mulch, zero-tillage and resistant varieties on Striga asiatica (L.) Kuntze in rice-maize rotation systems. Agric. Ecosyst and Environ 256:23–33. https://doi.org/10.1016/j.agee.2017.12.005
Rodenburg J, Bastiaans L, Kropff MJ (2006) Characterization of host tolerance to Striga hermonthica. Euphytica 147:353–365. https://doi.org/10.1007/s10681-005-9030-2
Rodenburg J, Cissoko M, Kayongo N, Dieng I, Bisikwa J, Irakiza R, Masoka I, Midega CAO, Scholes JD (2017) Genetic variation and host–parasite specificity of striga resistance and tolerance in rice: the need for predictive breeding. New Phytol 214:1267–1280. https://doi.org/10.1111/nph.14451
Ronner E, Franke AC, Vanlauwe B, Dianda M, Edeh E, Ukem B, Bala A, van Heerwaarden J, Giller KE (2016) Understanding variability in soybean yield and response to P-fertilizer and rhizobium inoculants on farmers' fields in northern Nigeria. Field Crop Res 186:133–145. https://doi.org/10.1016/j.fcr.2015.10.023
Rubiales D, Fernandez-Aparicio M (2012) Innovations in parasitic weeds management in legume crops. A review Agron Sust Dev 32:433–449. https://doi.org/10.1007/s13593-011-0045-x.hal-00930517
Rurangwa E, Vanlauwe B, Giller KE (2018) Benefits of inoculation, P fertilizer and manure on yields of common bean and soybean also increase yield of subsequent maize. Agric Ecosyst Environ 261:219–229. https://doi.org/10.1016/j.agee.2017.08.015
Rusinamhodzi L, Corbeels M, Nyamangara J, Giller KE (2012) Maize–grain legume intercropping is an attractive option for ecological intensification that reduces climatic risk for smallholder farmers in Central Mozambique. Field Crop Res 136:12–22. https://doi.org/10.1016/j.fcr.2012.07.014
Sanginga N, Okogun J, Vanlauwe B, Dashiell K (2002) The contribution of nitrogen by promiscuous soybeans to maize based cropping the moist savanna of Nigeria. Plant Soil 241:223–231. https://doi.org/10.1023/A:1016192514568
SAS Institute (2019). The SAS System for Windows, version 9.4. Cary, NC, USA, SAS Institute Inc.
Siame BA, Weerasuriya Y, Wood K, Ejeta G, Butler LG (1993) Isolation of Strigol, a germination stimulant for Striga asiatica, from host plants. J Agric Food Chem 41:1486–1491. https://doi.org/10.1021/jf00033a025
Silberg TR, Renner K, Schmitt Olabisi L, Richardson RB, Chimonyo VGP, Uriona-Maldonado M, al (2021) Modeling smallholder agricultural systems to manage Striga in the semi-arid tropics. Agric Syst 187:103008
Singh BB, Emechebe AM (1990) Inheritance of Striga resistance in cowpea genotype B301. Crop Sci 30:879–881. https://doi.org/10.2135/cropsci1990.0011183X003000040023x
Soto MJ, Fernandez-Aparicio MN, Castellanos-Morales V, Garcia- Garrido JM, Ocampo JA, Delgado MJ, Vierheilig H (2010) First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol Biochem 42:383–385. https://doi.org/10.1016/j.soilbio.2009.11.007
Tesfaye TT, Ejeta G (2011) Integrating multiple control options enhances Striga management and sorghum yield on heavily infested soils. Agron J 103:1464. https://doi.org/10.2134/agronj2011.0059
vanHeerwaarden J, Baijukya F, Kyei-Boahen S, Adjei-Nsiah S, Ebanyat P, Kamai N, Wolde-Meskel E, Kanampiu F, Vanlauwe B, Giller KE (2018) Soyabean response to rhizobium inoculation across sub-Saharan Africa: patterns of variation and the role of promiscuity. Agric. Ecosyst. Environ. 261:211–218. https://doi.org/10.1016/j.agee.2017.08.016
Vanlauwe B, Bationo A, Chianu J, Giller KE, Merckx R, Mokwunye U, Ohiokpehai O, Pypers P, Tabo R, Shepherd K, Smaling EMA, Woomer PL (2010) Integrated soil fertility management operational definition and consequences for implementation and dissemination. Outlook Agric. 39:17–24. https://doi.org/10.5367/000000010791169998
Vanlauwe B, Descheemaeker K, Giller KE, Huising J, Merckx R, Nziguheba G, Wendt J, Zingore S (2015) Integrated soil fertility management in sub-Saharan Africa: unravelling local adaptation. Soil 1:491–508. https://doi.org/10.5194/soild-1-1239-2014
Vanlauwe B, Hungria M, Kanampiu F, Giller KE (2019) The role of legumes in the sustainable intensification of African smallholder agriculture: lessons learnt and challenges for the future. Agric Ecosyst Environ 284:106583. https://doi.org/10.1016/j.agee.2019.106583
Vincent, J.M. (1970). A manual for the practical study of root-nodule bacteria, (IBP handbook no 15) Blackwell, Oxford
Yoneyama K, Xie X, Yoneyama K, Takeuchi Y (2009) Strigolactones: structures and biological activities. Pest Manag Sci 65:467–470. https://doi.org/10.1002/ps.1726
Acknowledgements
The authors thank the Bill & Melinda Gates Foundation for funding this research through the N2Africa: Putting nitrogen fixation to work for smallholder farmers in Africa grant to Wageningen University. We are grateful to IITA staff at Kano Station and Mijibir farm for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Katharina Pawlowski
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdullahi, W.M., Dianda, M., Boukar, O. et al. Integrated management of Striga gesnerioides in cowpea using resistant varieties, improved crop nutrition and rhizobium inoculants. Plant Soil 473, 197–213 (2022). https://doi.org/10.1007/s11104-022-05295-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05295-7