Abstract
Aims
Gypsum soils are P-limited atypical soils that harbour a rich endemic flora. These singular soils are usually found in drylands, where plant activity and soil nutrient availability are seasonal. No previous studies have analysed the seasonality of P nutrition and its interaction with the arbuscular mycorrhiza fungi (AMF) colonisation in gypsum plants. Our aim was to evaluate the seasonal changes in plant nutrient status, AMF colonisation and rhizospheric soil nutrient availability in gypsum specialist and generalist species.
Methods
We evaluated seasonal variation in the proportion of root length colonised by AMF structures (hyphae, vesicules and arbuscules), plant nutrient status (leaf C, N and P and fine root C and N) and rhizospheric soil content (P, organic matter, nitrate and ammonium) of three gypsum specialists and two generalists throughout a year.
Results
All species showed arbuscules within roots, including species of Caryophyllaceae and Brassicaceae. Root colonisation by arbuscules (AC) was higher in spring than in other seasons, when plants showed high leaf P-requirements. Higher AC was decoupled from inorganic N and P availability in rhizospheric soil, and foliar nutrient content. Generalists showed higher AC than specialists, but only in spring.
Conclusions
Seasonality was found in AMF colonisation, rhizospheric soil content and plant nutrient status. The mutualism between plants and AMF was highest in spring, when P-requirements are higher for plants, especially in generalists. However, AMF decoupled from plant demands in autumn, when nutrient availability increases in rhizospheric soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) and phosphorus (P) are the most common limiting nutrients in a wide variety of terrestrial ecosystems (Vitousek et al. 2010). Nutrient availability underlies the nutritional strategy of plants (Chapin 1980). In the case of nutrient-poor environments as drylands, plants have frequently evolved a retention strategy versus a rapid growth strategy, affecting acquisition, use, storage and resorption of nutrients (Aerts and Chapin 1999). These nutritional strategies are reflected in plant nutrient concentration (Grime et al. 1997), which summarises the functioning of plants in relation to their environment (Peñuelas et al. 2019).
Plant nutrient concentrations vary throughout the year due to shifts in nutrient availability and plant activity imposed by climate seasonality (Chapin 1980). Plant phenology of perennial species in Mediterranean drylands is characterized by predominant shoot growth in spring, root growth mainly in autumn and flowering in spring and early summer (Orshan 1989; Palacio and Montserrat-Martí 2007). Shoot growth requires high N and P in leaves (Palacio et al. 2014), while flowering demands high P (Milla et al. 2005). However, nutrient availability in drylands strongly depends on soil moisture (Querejeta et al. 2021). The availability of inorganic P is high in late summer (Magid and Nielsen 1992), and inorganic N is high in autumn in soils from Mediterranean drylands (Delgado-Baquerizo et al. 2011). Consequently, peak plant demands for N and P may be decoupled from soil availability in Mediterranean drylands. Unfortunately, seasonal studies linking nutrient acquisition strategies, plant nutrient status and soil nutrient availability in Mediterranean drylands are scarce (but see Palacio et al. 2014).
Plant strategies for nutrient acquisition in soils vary depending on the structural and functional features of roots, and the association of roots with microorganisms (Richardson et al. 2009). The association of roots with microorganisms has been broadly explored as a strategy to enhance N and P acquisition in nutrient poor-environments (Aerts and Chapin 1999). Plants may be associated with symbionts to improve N uptake, as N-fixing bacteria o ectomycorrhizal fungi (Chalot and Brun 1998; Miller and Cramer 2005), and with arbuscular mycorrhiza fungi (AMF) to improve N and P acquisition (Vance et al. 2003). AMF symbiosis generally improves plant growth in P-limited soils (Johnson 2010), providing plants with access to low-mobility P inorganic forms, such as phosphates (Hawkesford et al. 2012), although there is variability in the benefit provided by different AMF species with some fungi even behaving as cheaters (Kiers and Denison 2008). Root colonisation by AMF is seasonal, as it relates to plant activity (Jakobsen et al. 2003) and soil nutrient availability (Hoeksema et al. 2010). However, few studies have demonstrated a relationship between seasonal AMF colonisation and soil P concentration in natural populations of wild plants (i.e. Mullen and Schmidt 1993). Consequently, shifts in AMF colonisation may be determined by the interaction of soil nutrient availability and plant demands, which ultimately define C supply by plants to the fungi.
The analysis of AMF structures within roots allows us to understand fungal activity in relation to plant activity (Jakobsen et al. 2003). Arbuscules appear when nutrient plant requirements and nutrient exchanges rates between fungi and plants are high, whereas at other times they may be absent (Allen 1983; Mullen and Schmidt 1993). Contrastingly, vesicles are storage structures, which appear in periods without high nutrient plant acquisition (Abbott et al. 1984). Seasonal shifts in AMF colonisation within roots have been described in drylands (Roldán and Albaladejo 1993; Varela-Cervero et al. 2016; Fakhech et al. 2019), and have been related to plant activity (López-Sánchez and Honrubia 1992). Previous studies found high AMF colonisation in spring (Roldán and Albaladejo 1993) generally when plants sprouted or flowered, and slightly high in autumn (López-Sánchez and Honrubia 1992). Most of the studies on AMF seasonality on drylands only provided hyphal colonisation (hereafter, HC). However, seasonal studies on arbuscular colonisation (hereafter, AC) and vesicular colonisation (hereafter, VC) are required to improve knowledge on AMF activity in nutrient acquisition (Jakobsen et al. 2003).
Nutrient limitation increases in soils with minimal content of clay and organic matter, such as gypseous soils (Casby-Horton et al. 2015). Gypseous are special soils with high gypsum (calcium sulphate dehydrate) content (Herrero and Porta 2000), which frequently occur in drylands around the world (Verheye and Boyadgiev 1997). The high gypsum content of gypseous soils modifies the physical and chemical proprieties of soils (Herrero et al. 2009). For example, the high solubility of gypsum produces high Ca2+ activity in the soil solution (Casby-Horton et al. 2015), leading to a decrease in macronutrient availability and plant acquisition, particularly P (Stout et al. 1951). These features of gypseous soils severely limit plant life (FAO 1990). Despite these limitations, gypsum environments host a unique flora, identified as an international conservation priority (Escudero et al. 2015; Ochoterena et al. 2020).
Gypsum plants are adapted to a harsh substrate (Moore et al. 2014), where there is a strong seasonality in water and nutrient availability (Delgado-Baquerizo et al. 2011; Palacio et al. 2017). There are two types of gypsum plants according to their gypsum affinity (Meyer 1986): specialist species (also referred as gypsophiles), and generalist species (gypsovags). Gypsum specialist species are considered edaphic endemics with specific features related to gypseous soils (Duvigneaud and Denaeyer-De Smet 1968). Gypsum specialist species differ from generalist species in their foliar S, Ca and Mg concentrations (Palacio et al. 2007; Merlo et al. 2019), but not in their leaf P and N (Muller et al. 2017; Sánchez-Martín et al. 2021). In addition, plants growing on gypseous soils show low foliar P concentrations (Cera et al. 2021). Previous studies analysed the differences in AMF colonisation between gypsum specialist and generalist species. They found higher AMF colonisation and higher phylogenetic diversity of AMF in roots of gypsum generalist vs. specialist species (Palacio et al. 2012; Torrecillas et al. 2014). However, these studies were usually performed in spring, and no previous studies have evaluated the seasonality in AMF colonisation in gypsum plants, or the possible links with soil nutrient availability and plant activity and nutrient demands which are seasonal in these ecosystems (Palacio and Montserrat-Martí 2005; Delgado-Baquerizo et al. 2011).
The aim of this study was to evaluate the seasonal changes in plant nutrient status, AMF colonisation and rhizospheric soil nutrient availability and their interaction in five studied plant species, which included both gypsum specialists and generalists. Root colonisation by AMF (accounting for hyphae, vesicles and arbuscules separately), concentration of C, N and P in leaves and of C, N in fine roots and POlsen, organic matter content, and concentration of nitrate and ammonium in the rhizospheric soil were analysed four times throughout a year. We hypothesised that: 1) All species will display AMF structures (hyphae, vesicles and arbuscules) indicative of AMF colonisation/symbiosis throughout the year, because gypseous soils are remarkably P-improvished; 2) The degree of AMF colonisation will vary seasonally, according to previous studies in semiarid environments (Varela-Cervero et al. 2016); 3) The seasonality of AMF colonisation will follow plant nutrient content and rhizospheric soil nutrient concentration (especially P), displaying the highest HC and AC in autumn and spring, when nutrient plant concentration will be high, and the highest VC in summer, when both plants and fungi have to cope with the harshest environmental conditions, 4) Generalist gypsum species will show higher HC and AC than specialist gypsum species according to previous studies (Palacio et al. 2012).
Materials and methodology
Study site
This study was conducted at one locality in the Middle Ebro Basin (Villamayor, Zaragoza, NE Spain, 41°42′39.2"N 0°44′22.8"W; 295 m a.s.l), within a sampling area of approximately 3000 m2.The main lithology is an extensive area of massive gypsum deposits and gypseous soils with high contents of gypsum (Palacio et al. 2012) with a few thin inserted outcrops of marls and clays (Quirantes 1978; Table 1). The locality has a semi-arid Mediterranean climate, with an annual average rainfall of 322 mm and a mean annual temperature of 15.5 °C (data from the nearest weather station at Zaragoza 41°37′15''N, 0°56′6''W, between 1981–2010). Vegetation was composed predominantly of shrubs, forbs and grasses, like, Gypsophila struthium subsp. hispanica (Willk.) G. López, Helianthemum squamatum Pers., Helianthemum syriacum (Jacq.) Dum. Cours., Herniaria fruticosa L., Lepidium subulatum L., Rosmarinus officinalis L., Thymus vulgaris L., Plantago albicans L., Brachypodium retusum (Pers.) P. Beauv., Stipellula parviflora (Desf.) Röser & Hamasha.
Sampling design
Five plant species were selected for analysis. All of them were sub-shrubs, which are prevalent growth forms in gypsum outcrops (Parsons 1976; Martínez-Hernández et al. 2011). They included two Cistaceae: a specialist (Helianthemum squamatum Pers.) and its congener generalist (Helianthemum syriacum (Jacq.) Dum. Cours.); two Brassicaeae: a specialist (Lepidium subulatum L.) and a con-familial generalist (Matthiola fruticulosa (L.) Maire); and a Caryophyllaceae specialist (Gypsophila struthium Loefl.).
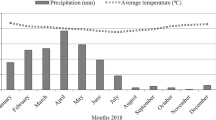
Five specimens of each species were collected in the same locality at four different times: late autumn (28th November 2017), spring (26th April 2018), summer (21st August 2018) and late autumn (13th December 2018). We chose isolated individuals located at least five meters apart from each other. Selected individuals were healthy adult plants with their foliage exposed to full sunlight. We selected spring as the main period of growth, summer as the period of arrested shoot growth (Palacio and Montserrat-Martí, 2005; Table 2), and autumn as the period with high soil nutrient availability (Delgado-Baquerizo et al. 2011). The autumn harvest in 2017 followed a dry summer (with 79.9 mm of rainfall) and a dry autumn (with only 14.3 mm precipitation; Fig. 1). Contrastingly, the autumn harvest in 2018 followed a wet summer (128.6 mm) and autumn (93.1 mm). We collected complete specimens, with rhizospheric soil attached, placed them individually in polyethylene bags and transported them to the laboratory, where plant tissues were separated from the soil and processed.
Soil analyses
Physical and chemical soil properties were analysed from the five replicates per species collected on every sampling date (N = 100). Rhizospheric soil, here considered as soil adhered to the root system, was gently separated from the fine roots using dissection forceps, and subsequently divided into two subsamples: one to be sieved at 2 mm and air dried for 2 months at room temperature prior to physical and chemical analyses, and another one to be stored at 4ºC prior to extraction with KCl for nitrate and ammonium analyses. Gravimetric soil water content was measured in all soil samples before drying and storage, weighing before and after drying in the oven at 40 ºC during five days, this temperature was selected to avoid gypsum de-hydration, which would alter soil water content estimates (Herrero et al. 2009). Dried soils were used to measure the following variables: gypsum content, measured according to Artieda et al. (2006); carbonate content, measured by Bernard calcimetry (Muller and Gatsner 1971); soil texture, determined with a particle laser analyser (Mastersizer 2000 Hydro G, Malvern, UK); soil pH and conductivity, measured with a pH/conductivity meter (Orio StarA215, Thermo Scientific, Waltham-MA, USA) by diluting samples with distilled water to 1:2.5 (w/v) and 1:5 (w/v), respectively; and available Olsen-P following standard methods (Anderson and Ingram 1989). A subsample of each dried and sieved soil was finely ground using a ball mill (Retsch MM200, Restch GmbH, Haan, Germany) and subsequently used to analyse organic matter following standard methods (Anderson and Ingram 1989). For nitrate and ammonium analyses, 10 g of fresh soil were extracted with 50 mL KCl (1 M). Extracts were shaken and filtered through a filter (7–9 µm pore, 0.160 mm thickness). Ammonium concentration in the extracts was estimated by colorimetry (salicylate method, Kempers and Zweers, 1986). Nitrate concentration was analysed according to Kaneko et al. (2010) as the difference between absorbance between 260 and 220 nm.
Plant analyses
Leaves and a subsample of fine roots were collected at each harvest, washed and dried to a constant weight at 50 ºC for 5 days and subsequently finely ground using a ball mill (Retsch MM200, Restch GmbH, Haan, Germany) to measure P, N and C concentrations. P concentration was determined by vanado-molybdate colorimetry (Becker 1961). N and C concentrations were measured with an elemental analyzer (TruSpec CN, LECO, St. Joseph-MI, USA). N, C analyses were performed by EEZ-CSIC Analytical Services, and P analyses by IPE-CSIC Analytical Services.
Mycorrhizal colonisation
A subsample of fine roots was separated from each plant, washed in distilled water to remove soil and stored in 50% ethanol at 4 ºC. Mycorrhizal colonisation was analysed by cutting the roots into approx.1 cm fragments and rinsing them in distilled water. Dead and old fine roots were removed under a stereo microscope. Root samples were cleared in 10% KOH for 20 min at 120 °C (5 min longer for some species with very dark roots) as in Brundrett et al. (1996) and stained with trypan blue in lactoglycerol as in Phillips and Hayman (1970). Later, the roots were mounted on glass slides with Hoyer’s medium (Cunningham 1972) for examination under the microscope. The proportion of root length containing arbuscules, vesicles and hyphae (i.e. arbuscular (AC), vesicle (VC) and hyphal colonisation (HC)) was calculated under an optical microscope following the magnified intersections method (McGonigle et al. 1990). The average number of total intersections observed ranged between 332 and 405 per individual plant per species and season, in order to obtain high statistical power, especially in the analysis of arbuscular colonization (Palacio et al. 2012).
Statistical analyses
All statistical analyses and graphics were performed using R version 4.0.2. The effect of season and gypsum affinity on mycorrhizal colonisation, plant nutrient concentrations and rhizospheric soil characteristics was evaluated using generalised linear mixed models (GLMMs) with season and gypsum affinity as fixed factors and family and species nested within family as random factors. We also analysed the effect of season within each species on mycorrhizal colonisation, plant nutrient concentrations and rhizospheric soil characteristics using generalised linear models (GLMs) with season as a fixed factor. Shapiro–Wilk and Bartlett's K-squared tests were performed to check for normality and homoscedasticity of residuals. Models were run with the glm or glmer functions (Bates et al. 2007). When residuals were normally distributed, models were fitted to a Gaussian distribution. While when not normally distributed, models were fitted to a: Gamma distribution if data were continuous, had a constant coefficient of variation and variances increased with means (McCullagh and Nelder 1989); Binomial distribution if dealing with mycorrhizal colonisation (Alvarez-Santiago et al. 1996); and Negative Binomial distribution if data were proportions (McCullagh and Nelder 1989). Dispersion of residuals for data without normal distribution was checked using simulateResiduals function in DHARMa package version 0.3.1 (Hartig 2017). If residuals were dispersed, we ran analyses with a Quasibinomial distribution or Binomial distribution weighted by total of intercepts for mycorrhizal colonisation data (Hartig 2017), and with glmmTMB (Magnusson et al. 2019) for other variables. When differences were statistically significant, multiple comparisons among levels of fixed factors were assessed with the glht function in multcomp package version 1.4–13 in R (Hothorn et al. 2009).
To analyse the relationships among soil features, we performed a Principal Component Analysis (PCA) with the rhizospheric soil features measured underneath each plant using the rda function in the vegan package version 2.4–6 (Oksanen et al. 2007).
Results
AMF colonisation
All species displayed arbuscular mycorrhizal fungi in their fine roots, showing typical structures of arbuscular mycorrhizas (hyphae, vesicles and arbuscules) in all samples throughout the year studied (Fig. 2). The main differences in AMF colonisation were between different families, while individuals from the same species showed similar colonisation (data not shown). A significant effect of sampling time (season) was found for hyphal, arbuscular and vesicular colonisation (Table 2, Fig. 3). Gypsum affinity was not a significant factor affecting AMF colonisation, although we found a significant interaction between gypsum affinity and season for arbuscular colonisation (Table 2, Fig. 3). The highest HC was observed in spring and the lowest in autumn 2018, while the highest VC was in summer. The highest AC was in spring, when gypsum generalists also showed higher AC than specialist species, and the lowest AC was in summer (Fig. 3).
As for the differences in AMF colonisation between seasons for each plant species (Fig. 4), L. subulatum did not show seasonality in any AMF structures, whereas the rest of species showed seasonality in some of the structures (Supplementary Data, Tables A2 and A3). Significant differences in HC were found for G. struthium, showing higher values in spring. VC varied significantly among seasons in G. struthium and both Helianthemum species. Seasonal shifts in AC were significant in M. fruticulosa and H.squamatum. However, while the trend was to show an increase in AC in spring, H.squamatum showed also a peak in AC in autumn 2017.
Differences in leaf P, soil POlsen and mycorrhizal colonisation in different sampling dates for each study species. Bars are means with standard errors. Different letters indicate significant differences among seasons within each species (see GLM in Supplementary Tables) after multiple comparisons (Tukey test). Lines are means for all species. Soil POlsen values of M. fruticulosa were divided by 10. HC: Hyphal colonisation. AC: Arbuscular colonisation. VC: Vesicular colonisation
Plant nutrient content
Leaf C, N, P, N:P ratio and fine root N concentration showed significant seasonal variability (Table 3). Gypsum affinity was not a significant factor affecting plant nutrient content, although a significant interaction between gypsum affinity and season was found for leaf P and fine root N (Table 3). Overall, the highest leaf C and N concentrations were found in autumn, and the lowest in summer (Table 4, Fig. 4). Similarly, the highest P was observed in autumn and the lowest in summer, but specialist species showed higher leaf P than generalist species in autumn 2017 and spring (Table 4). The highest fine root N concentrations were observed in autumn and the lowest in summer (Table 4). Generalist species showed higher fine root N than specialist species in both autumns, and lower values in spring and summer. The highest leaf N:P ratio was in spring, and the lowest in autumn 2017 (Table 4).
When we analysed each species separately (Fig. 4, see GLMs and means with SE for each species in Supplementary Data, Tables A4 and A5), leaf N, C and P concentrations varied seasonally in all species (P < 0.05). Species showed similar patterns of seasonal variation for leaf C, N and P, except for H. syriacum, with highest leaf N and leaf P in spring, and Helianthemum species, with the highest leaf C in summer and the lowest in autumn 2017. In the case of fine roots, M. fruticulosa and L. subulatum showed significant seasonal differences for fine root C (P < 0.05), with the lowest C concentration in spring and the highest in autumn. M. fruticulosa also displayed seasonality for fine root N (P < 0.05), following general trends. Season was a significant effect for leaf N:P ratio only in Helianthemum species (P < 0.05).
Rhizospheric soil chemical characteristics
Season was a significant factor affecting all variables measured in the rhizospheric soil, except for organic matter (P = 0.312). Gypsum affinity had only a marginally significant effect (P = 0.072) for ammonium concentration (Table 4). In general, the highest soil water content was found in spring and autumn 2018, and the lowest in autumn 2017 and summer (Table 5). The highest soil nitrate and ammonium concentrations were in autumn 2018, whereas the lowest nitrate was in autumn 2017 and summer (Table 5). We recorded the highest Polsen in summer, while other seasons displayed similar concentrations (Table 5, Fig. 4). However, the rhizospheric soil collected underneath generalist species showed higher P content than that of specialist species in all seasons, except in autumn 2018 (Tables 4 and 5).
Rhizospheric soil underneath each species showed different ranges in gypsum content, conductivity, carbonate content and pH (Supplementary Data, Tables A6 and A7). Season was also a significant factor affecting the water content of the rhizospheric soil of all species (P < 0.05, see Supplementary Data, Tables A6 and A7). All species had different POlsen in their rhizosphere in different sampling dates following the general trend (Fig. 3), except for M. fruticulosa, with highest POlsen content in autumn 2017, since they were collected in very low gypsum content (Supplementary Data, Tables A6 and A7). The only species displaying significant seasonal changes for ammonium and nitrate were G.struthium and M. fruticulosa, respectively (Fig. 4).
Discussion
According to our first hypothesis, all gypsum species studied displayed AMF in their fine roots, showing typical structures of arbuscular mycorrhizae (hyphae, vesicles and arbuscules) in all samples throughout the year. In support to our second hypothesis, AMF colonisation was seasonal, since the highest VC was in summer and the highest AC was in spring. Contrary to our third hypothesis, the highest AMF root colonisation did not concur with the highest foliar or lowest rhizospheric soil P content, but with the time of maximum P demand for plant growth (i.e. the time when leaf N:P ratios were lowest). Finally, in partial support of our last hypothesis, gypsum generalist species showed higher AMF colonisation than specialist species, although only for AC in spring.
Gypsum species showed seasonal differences in AMF colonisation
All five gypsum plant species analysed displayed AMF, with the formation of arbuscules throughout the year. They included Brassicaceae and Caryophyllace species, which are usually cited as non-mycorrhizal families (Brundrett 2009). Colonisation by arbuscules had already been found in L. subutatum and G. struthium on gypsum (Palacio et al. 2012) and in other taxa of Lepidium, Matthiola and Gypsophila from other environments (Hempel et al. 2013), which calls for caution when assuming the inability of Brassicaceae and Caryophyllaceae to interact with AMF. Studied species of Cistaceae showed the highest hyphal colonisation and Brassicaceae showed the lowest, independently of their affinity to gypsum soils. Apart from AMF, we observed Hartig nets typical of ectomycorrhiza fungi in both Helianthemum species, although we did not quantify their root colonisation. Gypsum plants in our study also had colonisation of dark septate endophytes, such as those described by Porras-Alfaro et al. (2014) in plants growing on gypseous soils of the Chihuahuan Desert.
Previous studies had reported AMF colonisation in plants from gypseous soils (Alguacil et al. 2009; Palacio et al. 2012; Torrecillas et al. 2014; Hernández y Hernández et al. 2020, but seasonality was neglected and most of these studies were conducted only in spring, when plants show high growth activity (Alguacil et al. 2009). Our results confirm that arbuscular mycorrhizal colonisation in gypsum species varies seasonally, similar to previous studies in other drylands (Roldán and Albaladejo 1993; Varela-Cervero et al. 2016; Fakhech et al. 2019). Most of these previous studies measured the highest hyphal colonisation in spring, but did not account for vesicular or arbuscular colonisation. Our results for arbuscular colonisation agree with those for hyphal colonisation of previous studies. However, these results are not fully comparable, since arbuscules and hyphae differ in functionality. Arbuscules are the unique structures involved directly in nutrient transfer to the plant (Allen 1983; Mullen and Schmidt 1993), whereas hyphae are the vegetative structures of fungi (Brundrett 2009), and vesicules are storage structures (Jakobsen et al. 2003). We observed seasonality in arbuscular (AC) and vesicular colonisation (VC), but not in hyphal colonisation (HC). AC was high in spring, when the highest AM fungal activity is expected in the Mediterranean climate (Alguacil et al. 2009), and low in summer, when plants showed reduced growth activity in our study system (Palacio and Montserrat-Martí 2005). In addition, VC was high in summer, since vesicles appear at later stages of fungal colonisation (Jakobsen et al. 2003) and during arbuscule senescence (Brundrett 2009). AM fungi are not the unique root-associated fungi with seasonal colonisation (Mandyam and Jumpponen 2008), and consequently we also found seasonal colonisation of dark septate endophytes (DSE) between autumn 2017 and spring (data not shown). While the beneficial role of arbuscules formed by AM fungi on plant nutrition is well-established (Johnson 2010), the structures of DSE (hyphae and microsclerotia) cannot be interpreted as interfaces for nutrient exchange between fungi and their hosts (Newsham 2011).
Both gypsum specialist and generalist species showed increased root colonisation by arbuscules during high P-requirements in spring
All plants analysed showed the highest foliar P and N concentrations in autumn, after the peak of POlsen rhizospheric soil concentration in summer, and concurring with maximum nitrate and ammonium concentrations in the soil. Such increased nutrient foliar concentrations were decoupled from arbuscular colonisation, since we observed low root colonisation by arbuscules in summer and autumn. We expected a high arbuscular colonisation when plants demanded P, either autumn or spring, since gypsum are very P-impoverished soils (FAO 1990). For example, gypseous soils led to lower plant growth and lesser P accumulation on leaves than other similar calcareous soils (Cera et al. 2021). Hernández y Hernández et al. (2020) also found a negative correlation between AMF root colonisation, dissolved organic nutrients in soil and microbial N and P in gypseous soils from the Chihuahua Desert. These results may indicate that, despite the low N and P concentration in gypseous soils, gypsum plants use other acquisition strategies, different to AMF, to uptake P and N, especially when nutrient availability in the soil is high (for example in autumn with high water content). Symbiosis with AM fungi may benefit plants when P demand by the plant exceeds the capacity of the root system to uptake nutrients independently of AMF (Fitter 1991).
In the seasonal environment analysed, most studied species arrested growth in summer and some species, like Lepidium subulatum and Matthiola fruticulosa are summer deciduous. Gypsum plants restart their growth at the end of summer (Palacio and Montserrat-Martí 2005), probably remobilising nutrients from storage organs (Milla et al. 2005; Palacio et al. 2014) and absorbing nutrients with acquisition strategies not only related to AMF symbiosis, but to phosphatase and organic acid exudation, or enhanced expression of Pi transporters (Vance et al. 2003; Lambers et al. 2018). All study species but G. struthium have shallow roots (Guerrero-Campo et al. 2006), without specialised root architecture to enhance P-mining (Palacio et al. 2012). However, the main root growth in these plants is in autumn (Palacio and Montserrat-Martí 2007), which can favour nutrient uptake. For example, Lepidium subulatum shows an opportunistic growth to exploit sporadic N pulses in autumn (Palacio et al. 2014), probably with rapid root proliferation to enhance nutrient acquisition in seasonal environments (Jackson and Caldwell 1989; Palacio and Montserrat-Martí 2007). A decrease in AMF colonisation may occur when P supply by roots is high and plants limit the symbiosis with fungi to reduce associated carbon costs (Lambers et al. 2008). Accordingly, we found that N content in fine roots was high in autumn, indicating high fine root activity (Roumet et al. 2016). In addition, during the wet autumn (2018), species showed higher vesicular colonisation than in autumn 2017 (dry), probably because AMF may not be providing nutrients to the host plants, but keeping them to support growth or storage (Johnson 1993; Koyama et al. 2017). In addition, the inter-annual climate variability observed in these two autumns indicates the importance of studying AMF colonization over different seasons and years.
In spring, the studied species showed high leaf N:P ratio, which indicates high P requirements in leaves and P limitation to primary productivity (Güsewell 2004). At this time of the year, most study species showed a peak in shoot growth rate (Palacio and Montserrat-Martí 2005), flowering in spring and early summer (data not shown), with increased demand for P (Milla et al. 2005). However, such increased demand concurred with decreased P-inorganic (measured as POlsen) availability in rhizospheric soils. It is, hence, not surprising that the highest arbuscular colonisation (AC) was recorded in spring, when plants can benefit from AMF getting extra P than that available to their roots alone.
The gypsum generalist species studied displayed higher arbuscular colonisation than specialist species, although only in spring. This result was similar to another previous study on gypsum outcrops (Palacio et al. 2012), indicating that spring is the most discriminating season to analyse responses in AMF between gypsum generalist and specialist species. According to Palacio et al. (2012) and Torrecillas et al. (2014), specialist species seem to be more specialised to gypseous soils, and to its P cycle and seasonal availability, likely displaying other mechanisms of nutrient acquisition, because they displayed reduced AMF-symbiosis. On the other hand, the dependence of generalist species on AMF symbiosis would indicate a stress-tolerant strategy to cope with the limiting conditions in gypsum environments (Palacio et al. 2012).
Conclusions
Studied gypsum species showed seasonal AMF colonisation, decoupled from seasonal shifts in foliar N and P content and from shifts in N and P rhizospheric soil availability. Arbuscular colonisation was higher in spring, when P demand by the plant may exceed the capacity of the root system to uptake sufficient nutrients due to low soil availability. These trends were particularly marked in studied generalist species. Our results exemplify the need to study seasonal changes in plant-AMF-soil interactions to gain insight into P-acquisition strategies in plants growing in nutrient-limited environments.
Change history
22 February 2022
Springer Nature’s version of this paper was updated to reflect the Funding information: Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
References
Abbott LK, Robson AD, Boer GD (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol 97:437–446. https://doi.org/10.1111/j.1469-8137.1984.tb03609.x
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. In: Fitter AH, Raffaelli DG (eds) Advances in Ecological Research. Academic Press, pp 1–67
Alguacil MM, Roldán A, Torres MP (2009) Assessing the diversity of AM fungi in arid gypsophilous plant communities. Environ Microbiol 11:2649–2659. https://doi.org/10.1111/j.1462-2920.2009.01990.x
Allen MF (1983) Formation of vesicular-arbuscular mycorrhizae in Atriplex gardneri (Chenopodiaceae): Seasonal response in a cold desert. Mycologia 75:773–776. https://doi.org/10.1080/00275514.1983.12023753
Alvarez-Santiago SA, García-Oliva F, Varela L (1996) Analysis of vesicular-arbuscular mycorrhizal colonization data with a logistic regression model. Mycorrhiza 6:197–200. https://doi.org/10.1007/s005720050126
Anderson JM, Ingram JSI (1989) Tropical soil biology and fertility: a handbook of methods. Trop Soil Biol Fertil Handb Methods
Artieda O, Herrero J, Drohan PJ (2006) Refinement of the Differential Water Loss Method for Gypsum Determination in Soils. Soil Sci Soc Am J 70:1932–1935. https://doi.org/10.2136/sssaj2006.0043N
Bates D, Sarkar D, Bates MD, Matrix L (2007) The lme4 package. R Package Version 2:74
Becker M (1961) Analisis y valoración de piensos y forrajes. Acribia, Zaragoza
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Brundrett MC, Bougher N, Dell B, et al. (1996) Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research
Casby-Horton S, Herrero J, Rolong NA (2015) Chapter four - Gypsum soils—Their morphology, classification, function, and landscapes. In: Sparks DL (ed) Advances in Agronomy. Academic Press, pp 231–290
Cera A, Montserrat-Martí G, Ferrio JP et al (2021) Gypsum-exclusive plants accumulate more leaf S than non-exclusive species both in and off gypsum. Environ Exp Bot 182:104294. https://doi.org/10.1016/j.envexpbot.2020.104294
Chalot M, Brun A (1998) Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiol Rev 22:21–44. https://doi.org/10.1111/j.1574-6976.1998.tb00359.x
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260. https://doi.org/10.1146/annurev.es.11.110180.001313
Cunningham JL (1972) A miracle mounting fluid for permanent whole-mounts of microfungi. Mycologia 64:906–911. https://doi.org/10.2307/3757946
Delgado-Baquerizo M, Covelo F, Gallardo A (2011) Dissolved organic nitrogen in Mediterranean rcosystems. Pedosphere 21:309–318. https://doi.org/10.1016/S1002-0160(11)60131-8
Duvigneaud P, Denaeyer-De Smet SM (1968) Essai de classification chimique (éléments minéraux) des plantes gypsicoles du bassin de l’Èbre. Bull Société R Bot Belg Bull Van K Belg Bot Ver 101:279–291
Escudero A, Palacio S, Maestre FT, Luzuriaga AL (2015) Plant life on gypsum: a review of its multiple facets. Biol Rev 90:1–18. https://doi.org/10.1111/brv.12092
Fakhech A, Ouahmane L, Hafidi M (2019) Seasonality of mycorrhizal attributes, soil phosphorus and nitrogen of Juniperus phoenicea and Retama monosperma boiss. in an Atlantic sand dunes forest. J Sustain for 38:1–17. https://doi.org/10.1080/10549811.2018.1490653
FAO (1990) Management of Gypsiferous Soils. In: FAO Soils Bulletin 62. Food and Agriculture Organitzation of the United Nations, Rome, pp 96
Fitter AH (1991) Costs and benefits of mycorrhizas: Implications for functioning under natural conditions. Experientia 47:350–355. https://doi.org/10.1007/BF01972076
Grime JP, Thompson K, Hunt R et al (1997) Integrated screening validates primary axes of specialisation in plants. Oikos 79:259–281. https://doi.org/10.2307/3546011
Guerrero-Campo J, Palacio S, Pérez-Rontomé C, Montserrat-Martí G (2006) Effect of root system morphology on root-sprouting and shoot-rooting abilities in 123 plant species from eroded lands in north-east Spain. Ann Bot 98:439–447. https://doi.org/10.1093/aob/mcl122
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x
Hartig F (2017) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R Package Version 5:
Hawkesford M, Horst W, Kichey T et al (2012) Chapter 6 - Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 135–189
Hempel S, Götzenberger L, Kühn I et al (2013) Mycorrhizas in the Central European flora: relationships with plant life history traits and ecology. Ecology 94:1389–1399. https://doi.org/10.1890/12-1700.1
Hernández y Hernández D, Larsen J, González-Rodríguez A, et al (2020) Cooperation between Sporobolus airoides and associated arbuscular mycorrhizal fungi for phosphorus acquisition under drought conditions in an oligotrophic desert ecosystem. Rhizosphere 15:100225. https://doi.org/10.1016/j.rhisph.2020.100225
Herrero J, Artieda O, Hudnall WH (2009) Gypsum, a tricky material. Soil Sci Soc Am J 73:1757–1763. https://doi.org/10.2136/sssaj2008.0224
Herrero J, Porta J (2000) The terminology and the concepts of gypsum-rich soils. Geoderma 96:47–61. https://doi.org/10.1016/S0016-7061(00)00003-3
Hoeksema JD, Chaudhary VB, Gehring CA et al (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Hothorn T, Bretz F, Hothorn MT (2009) The multcomp package. R Package Version
Jackson RB, Caldwell MM (1989) The timing and degree of root proliferation in fertile-soil microsites for three cold-desert perennials. Oecologia 81:149–153. https://doi.org/10.1007/BF00379798
Jakobsen I, Smith SE, Smith FA (2003) Function and diversity of arbuscular mycorrhizae in carbon and mineral nutrition. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal Ecology. Springer, Berlin, Heidelberg, pp 75–92
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. https://doi.org/10.1111/j.1469-8137.2009.03110.x
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757. https://doi.org/10.2307/1942106
Kaneko S, Inagaki M, Morishita T (2010) A simple method for the determination of nitrate in potassium chloride extracts from forest soils. Proc 19th World Congr Soil Sci Soil Solut Chang World Brisb Aust 1–6 August 2010 Work Group 32 For Soil Process Change 4–7
Kempers AJ, Zweers A (1986) Ammonium determination in soil extracts by the salicylate method. Commun Soil Sci Plant Anal 17:715–723. https://doi.org/10.1080/00103628609367745
Kiers ET, Denison RF (2008) Sanctions, cooperation, and the stability of plant-rhizosphere Mutualisms. Annu Rev Ecol Evol Syst 39:215–236. https://doi.org/10.1146/annurev.ecolsys.39.110707.173423
Koyama A, Pietrangelo O, Sanderson L, Antunes PM (2017) An empirical investigation of the possibility of adaptability of arbuscular mycorrhizal fungi to new hosts. Mycorrhiza 27:553–563. https://doi.org/10.1007/s00572-017-0776-x
Lambers H, Albornoz F, Kotula L et al (2018) How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 424:11–33. https://doi.org/10.1007/s11104-017-3427-2
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
López-Sánchez ME, Honrubia M (1992) Seasonal variation of vesicular-arbuscular mycorrhizae in eroded soils from southern Spain. Mycorrhiza 2:33–39. https://doi.org/10.1007/BF00206281
Magid J, Nielsen NE (1992) Seasonal variation in organic and inorganic phosphorus fractions of temperate-climate sandy soils. Plant Soil 144:155–165. https://doi.org/10.1007/BF00012872
Magnusson A, Skaug H, Nielsen A, et al. (2019) Package ‘glmmTMB’. R Package Version
Mandyam K, Jumpponen A (2008) Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza 18:145–155. https://doi.org/10.1007/s00572-008-0165-6
Martínez-Hernández F, Pérez-García FJ, Garrido-Becerra JA et al (2011) The distribution of Iberian gypsophilous flora as a criterion for conservation policy. Biodivers Conserv 20:1353–1364. https://doi.org/10.1007/s10531-011-0031-2
McCullagh P, Nelder JA (1989) Generalized linear models: Chapman & Hall, London
McGonigle TP, Miller MH, Evans DG et al (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Merlo ME, Garrido-Becerra JA, Mota JF, Salmerón-Sánchez E, Martínez-Hernández F, Mendoza-Fernández A, Pérez-García FJ (2019) Threshold ionic contents for defining the nutritional strategies of gypsophile flora. Ecol Indic 97:247–259. https://doi.org/10.1016/j.ecolind.2018.10.001
Meyer SE (1986) The ecology of gypsophile endemism in the Eastern Mojave Desert. Ecology 67:1303–1313. https://doi.org/10.2307/1938686
Milla R, Castro-Díez P, Maestro-Martínez M, Montserrat-Martí G (2005) Relationships between phenology and the remobilization of nitrogen, phosphorus and potassium in branches of eight Mediterranean evergreens. New Phytol 168:167–178. https://doi.org/10.1111/j.1469-8137.2005.01477.x
Miller AJ, Cramer MD (2005) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36. https://doi.org/10.1007/s11104-004-0965-1
Moore M, Mota J, Douglas N et al (2014) The ecology, assembly, and evolution of gypsophile floras. In: Rajakaruna N, Boyd R, Harris T (eds) Plant Ecology and Evolution in Harsh Environments. Nova Science Publishers, Hauppauge, NY, pp 97–128
Mullen RB, Schmidt SK (1993) Mycorrhizal infection, phosphorus uptake, and phenology in Ranunculus adoneus: implications for the functioning of mycorrhizae in alpine systems. Oecologia 94:229–234. https://doi.org/10.1007/BF00341321
Muller CT, Moore MJ, Feder Z et al (2017) Phylogenetic patterns of foliar mineral nutrient accumulation among gypsophiles and their relatives in the Chihuahuan Desert. Am J Bot 104:1442–1450. https://doi.org/10.3732/ajb.1700245
Muller G, Gatsner M (1971) Chemical analysis. Neues Jahrb Für Mineral Monatshefte 10:466–469
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793. https://doi.org/10.1111/j.1469-8137.2010.03611.x
Ochoterena H, Flores-Olvera H, Gómez-Hinostrosa C, Moore MJ (2020) Gypsum and plant species: A marvel of Cuatro Ciénegas and the Chihuahuan Desert. In: Mandujano MC, Pisanty I, Eguiarte LE (eds) Plant Diversity and Ecology in the Chihuahuan Desert: Emphasis on the Cuatro Ciénegas Basin. Springer International Publishing, Cham, pp 129–165
Oksanen J, Kindt R, Legendre P et al (2007) The Vegan Package Community Ecol Package 10:719
Orshan G (1989) Plant pheno-morphological studies in Mediterranean type ecosystems. Kluwer Academic Publishers, Dordrecht
Palacio S, Escudero A, Montserrat-Martí G et al (2007) Plants living on gypsum: Beyond the specialist model. Ann Bot 99:333–343. https://doi.org/10.1093/aob/mcl263
Palacio S, Johnson D, Escudero A, Montserrat-Martí G (2012) Root colonisation by AM fungi differs between gypsum specialist and non-specialist plants: Links to the gypsophile behaviour. J Arid Environ 76:128–132. https://doi.org/10.1016/j.jaridenv.2011.08.019
Palacio S, Maestro M, Montserrat-Martí G (2014) Differential nitrogen cycling in semiarid sub-shrubs with contrasting leaf habit. PLoS ONE 9(3):e93184. https://doi.org/10.1371/journal.pone.0093184
Palacio S, Montserrat-Martí G (2007) Above and belowground phenology of four Mediterranean sub-shrubs. Preliminary results on root–shoot competition. J Arid Environ 68:522–533. https://doi.org/10.1016/j.jaridenv.2006.07.008
Palacio S, Montserrat-Martí G (2005) Bud morphology and shoot growth dynamics in two species of Mediterranean sub-shrubs co-existing in gypsum outcrops. Ann Bot 95:949–958. https://doi.org/10.1093/aob/mci110
Palacio S, Montserrat-Martí G, Ferrio JP (2017) Water use segregation among plants with contrasting root depth and distribution along gypsum hills. J Veg Sci 28:1107–1117. https://doi.org/10.1111/jvs.12570
Parsons RF (1976) Gypsophily in plants-a review. Am Midl Nat 96:1–20. https://doi.org/10.2307/2424564
Peñuelas J, Fernández-Martínez M, Ciais P et al (2019) The bioelements, the elementome, and the biogeochemical niche. Ecology 100:e02652. https://doi.org/10.1002/ecy.2652
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Porras-Alfaro A, Raghavan S, Garcia M et al (2014) Endophytic fungal symbionts associated with gypsophilous plants. Botany 92:295–301. https://doi.org/10.1139/cjb-2013-0178
Querejeta JI, Ren W, Prieto I (2021) Vertical decoupling of soil nutrients and water under climate warming reduces plant cumulative nutrient uptake, water-use efficiency and productivity. New Phytol 230:1378–1393. https://doi.org/10.1111/nph.17258
Quirantes J (1978) Estudio sedimentológico y estratigráfico del Terciario continental de los Monegros. Instituto Fernando El Católico CSIC Diputación Provincial, Zaragoza
Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104-009-9895-2
Roldán A, Albaladejo J (1993) Vesicular-Arbuscular Mycorrhiza (VAM) fungal populations in a xeric torriorthent receiving urban refuse. Soil Biol Biochem 25:451–456. https://doi.org/10.1016/0038-0717(93)90070-R
Roumet C, Birouste M, Picon-Cochard C et al (2016) Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytol 210:815–826. https://doi.org/10.1111/nph.13828
Sánchez-Martín R, Querejeta JI, Voltas J et al (2021) Plant’s gypsum affinity shapes responses to specific edaphic constraints without limiting responses to other general constraints. Plant Soil. https://doi.org/10.1007/s11104-021-04866-4
Stout PR, Meagher WR, Pearson GA, Johnson CM (1951) Molybdenum nutrition of crop plants. Plant Soil 3:51–87. https://doi.org/10.1007/BF01343398
Torrecillas E, del Alguacil M, M, Roldán A, et al (2014) Modularity reveals the tendency of arbuscular mycorrhizal fungi to interact differently with generalist and specialist plant species in gypsum soils. Appl Environ Microbiol 80:5457–5466. https://doi.org/10.1128/AEM.01358-14
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Varela-Cervero S, López-García Á, Barea JM, Azcón-Aguilar C (2016) Spring to autumn changes in the arbuscular mycorrhizal fungal community composition in the different propagule types associated to a Mediterranean shrubland. Plant Soil 408:107–120. https://doi.org/10.1007/s11104-016-2912-3
Verheye WH, Boyadgiev TG (1997) Evaluating the land use potential of gypsiferous soils from field pedogenic characteristics. Soil Use Manag 13:97–103. https://doi.org/10.1111/j.1475-2743.1997.tb00565.x
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Acknowledgements
We are grateful to Laura de la Puente, Beste Özbey, Ebru Ozbeny, Esteban Gandalf Schroder, Juan Pedro Ferrio and Nate Heiden for help with sampling, to Elena Lahoz, Mercedes García, José Azorín, María Pérez-Serrano Serrano and Sara Alberdi for help with plant and soil analyses. Pablo Tejero, Cristina Nabais, Jorge Duran, Alexandra Rodríguez, Marta Correia, Maria José Fernández-Alonso, Núria Garcia and Paula Lorenzo provided useful comments on the results. Alicia Montesinos provided valuable comments on earlier versions of this manuscript. This work was supported by Gobierno de España [MINECO, CGL2015-71360-P and PID2019-111159GB-C31], by European Union’s Horizon 2020 [H2020-MSCA-RISE-777803] and by Consejo Superior de Investigaciones Científicas [COOPB20231]. AC and SP were funded by a FPI fellowship [MINECO, BES-2016-076455] and a Ramón y Cajal Fellowship [MINECO, RYC-2013-14164], respectively. Two anonymous referees and the Editor provided valuable comments on earlier versions of this manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: François Teste
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cera, A., Duplat, E., Montserrat-Martí, G. et al. Seasonal variation in AMF colonisation, soil and plant nutrient content in gypsum specialist and generalist species growing in P-poor soils. Plant Soil 468, 509–524 (2021). https://doi.org/10.1007/s11104-021-05140-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05140-3