Abstract
Purpose
To investigate whether soil clay content, cultivar and seasonal variation have any effect on soil CO2 emission rates and leaf CO2 assimilation rates in a drip-irrigated commercial Citrus sinensis orchard.
Methods
The study was carried out in the field as a randomised complete block design in a 2 × 2 factorial consisting of two soil types and two citrus cultivars on a drip-irrigated commercial Citrus sinensis orchards with 2-week interval measurements of soil CO2 emission and leaf gas exchanges for a year.
Results
Soil clay content did not influence plant CO2 assimilation rates and soil CO2 emission rates in irrigated citrus. However, seasonal variation significantly influenced both processes. Soil CO2 emission rates were highest in summer and were more than double the rates observed in winter while leaf CO2 assimilation rates were highest in autumn and four times higher than the winter season rates. Mean seasonal soil CO2 emission rates were strongly influenced by mean minimum seasonal temperatures while leaf CO2 assimilation rates only showed a relatively weak relationship with mean maximum seasonal temperatures.
Conclusions
Soil clay content did not influence soil CO2 emission and assimilation rates in drip irrigated citrus suggesting a non-significant effect of clay content for soils subjected to similar management practices. Citrus CO2 assimilation rate peaks in the autumn while soil CO2 emission rates peak in summer. A snapshot analysis of CO2 sequestration rates suggests that irrigated citrus orchards are net sinks of CO2 in summer, autumn and winter season.
Similar content being viewed by others
Introduction
Soil CO2 fluxes play a vital role in the terrestrial carbon cycle as they integrate the decomposition of organic matter and root respiration (Paudel et al. 2018; Wu 2020). It is believed that 25 to 60 % of CO2 assimilated through photosynthesis is lost through respiration and that soil respiration alone contributes up to 88 % (Paudel et al. 2018). Due to climate change, soil respiration is anticipated to contribute even more CO2 to the atmosphere due to its sensitivity to global warming (Davidson and Janssens 2006). However, the dynamics of soil respiration are still not well understood due to the high measured variability in terrestrial ecosystems (Bond-Lamberty and Thomson 2010). The high measured variability in soil respiration and the need to accurately predict the response of soil respiration to climate change as well as its feedback to global warming, have promoted the urgent need to determine soil respiration at higher temporal and spatial scales.
In agricultural systems, it is even more crucial to understand this spatiotemporal variation in soil respiration in combination with CO2 assimilation to better understand if systems are net sinks or net emitters of CO2. Soil CO2 emission rates and leaf CO2 assimilation rates respond strongly to environmental conditions such as temperature and water availability (Avola et al. 2008; Bond-Lamberty and Thomson 2010; Davidson and Janssens 2006; Qu et al. 2020; Tankari et al. 2019; Wu 2020). Some studies have found soil water content and temperature to have a confounding effect on soil respiration (Yuste et al. 2003; Wu 2020) while others such as Davidson et al. (1998), found the two to either act independently or together in controlling soil respiration. Nonetheless, there are still several studies that dispute the temperature dependence of organic matter decomposition (Fang et al. 2005; Giardina and Ryan 2000). Thus, there is no consensus on how global warming affects CO2 fluxes. Soil texture is also another factor that can influences soil CO2 fluxes as it influences gas movement in soils (Yang et al. 2018). Soils with high clay content present restrictions to gas movements due to the tortuosity of the micropores (Neira et al. 2015). In addition, soil with high clay content can physically protect organic carbon within aggregates from microbial decomposition thus also decreasing the amount of CO2 lost through decomposition (Hassink et al. 1993; Razafimbelo et al. 2008).
The CO2 from soil comes from the autotrophic respiration of plant roots and the decomposition of soil organic carbon by microbes (heterotrophic respiration). All the processes are regulated by soil organic carbon availability, root activity, microbes, soil and air temperature, and soil moisture content (Atarashi-Andoh et al. 2012; Wu 2020; Hu et al. 2018). Higher soil temperature and moisture content increase microbial activity (Qu et al. 2020) and thus higher CO2 emission rates. However, the impact of soil microbial community and function on CO2 emission still needs more research.
Similar to soil respiration, leaf CO2 assimilation is also controlled by both ambient temperature and soil moisture. However, unlike soil respiration which is predominantly affected by soil moisture and temperature, leaf CO2 assimilation is also strongly influenced by other factors such as light, nutrient availability and atmospheric CO2 concentration (Davidson and Janssens 2006). Several studies have shown that soil moisture controls CO2 assimilation through its impact on stomatal conductance (Tankari et al. 2019; Munjonji et al. 2017; Zhang et al. 2013; Carvalho et al. 2019; Rivas et al. 2016). Under unlimited soil moisture, leaf CO2 assimilation is high due to a higher stomatal conductance with the reverse being true for limited water supply. Likewise, temperature also controls CO2 assimilation by increasing vapour pressure deficit and the subsequent effect on stomatal conductance. A high vapour pressure deficit leads to lower leaf CO2 assimilation as the plant closes its stomata to limit water loss (Aliniaeifard et al. 2014).
Cultivar differences can also show differences in CO2 assimilation. Lu et al. (2012) reported differences between five cultivars of mangos. In a similar study with litchis (another tropical fruit) phenological differences in CO2 assimilation were reported whereby higher CO2 assimilation in leaves at the fruiting stage were observed compared to the de-fruiting stage (Chang and Lin 2007). In apples as well, higher CO2 assimilation rates were reported at fruiting compared to at the vegetative stage (Fujii and Kennedy 1985).
The main challenge of using leaf CO2 assimilation rates to estimate carbon capture is that they are mostly instantaneous and represent individual leaves. Over the years models have been developed and used to upscale leaf measurements to canopy level using leaf area index (LAI) (Friend 2001; Gara et al. 2019; Sprintsin et al. 2012; Luo et al. 2018). The most common methods of upscaling are the Big-Leaf model, Two-Big-Leaf model and Two-leaf model which are all well described by Luo et al. (2018). The Big-Leaf model assumes that canopy carbon fluxes have the same relative responses to the environment as any single unshaded leaf on a canopy top (Sprintsin et al. 2012) while the other two stratify the canopy into sunlit and shaded leaves (Luo et al. 2018). The Big-Leaf model is believed to under-estimate CO2 assimilation by approximately 20 % (Friend 2001) thus the Two-Big-Leaf model and the Two-leaf model are more accurate.
In many agroecosystems in semi-arid environments, soil water content appears to be the most studied due to it being the most limiting as well as the seasonality of crop production. Many annual crops are seasonal and hence are produced when ambient temperatures are relatively similar. However, the dynamics may differ in perennial fruit trees. Deciduous fruit trees such as apples and peaches lose their leaves in winter while evergreen fruit trees such as citrus maintain them. A perennial evergreen crop such as citrus is subjected to large seasonal variation of environmental conditions throughout the annual cycle (Ribeiro and Machado 2007) hence their ecophysiology is unique and probably less understood. As leaves are the main photosynthetic organs in citrus trees (Iglesias et al. 2013), their annual CO2 assimilation would be expected to be higher than for deciduous as reported in similar studies by Iglesias et al. (2013) and Luyssaert et al. (2007). However, soil respiration was found to vary similarly with the season between deciduous species and evergreen forest trees (Sun et al. 2020).
According to Paudel et al. (2018), carbon balance i.e. the net result of CO2 assimilation (sequestration) and emission may vary with season, management practices, irrigation method and water quality. Thus, it is important to understand the variation and the factors that control CO2 efflux and assimilation to accurately predict the impact of climate change and global warming on CO2 assimilation and loss from agroecosystems. Currently, not much work has been done on the variation of CO2 assimilation rates and emission rates in irrigated citrus orchards. The objectives of this current study were to investigate (1) whether soil clay content has any effect on soil CO2 emission rates and leaf CO2 assimilation rates in a drip-irrigated commercial Citrus sinensis orchard and (2) how soil CO2 emission rates and leaf CO2 assimilation rates vary with seasons in a drip-irrigated commercial Citrus sinensis orchard.
Methodology
Study site
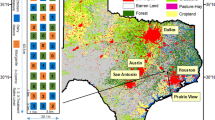
The study was conducted at Mahela Group, a commercial citrus orchard, in Letsitele area, located about 100 km east of Polokwane in the Limpopo province of South Africa (Fig. 1) from May 2018 to May 2019. The coordinates of the location are 23°52’5.10” S and 30°23’28.65” E. The general climate of the area is warm-temperate, dry winter and hot summer (Cwa) (Kottek et al. 2006). The area receives unimodal rainfall, most of which falls between December and April. A total of 485 mm of rainfall were received during the study period with the highest amount of 193 mm being received in February alone. The mean minimum temperatures ranged from 8 to 21 °C while mean maximum temperatures ranged from 24 to 32 °C. The weather data shown in Fig. 1 was obtained from an automatic weather station installed at the experimental site.
Experimental design and management
This experiment was conducted on a commercial farm and thus the orchards were already established. The design of the experiment was done after a survey on the soil types and cultivars grown on the farm. The experiment was then laid out in a randomised complete block design in a 2 × 2 factorial arrangement consisting of two soil types and two citrus cultivars. The treatments were replicated three times. The two soil types were rich clay content soil (RC) with more than 20 % clay and moderate clay content soil (MC) with 10–20 % clay. The two citrus cultivars studied were Alpha Valencia and Valencia late. All cultivars were at the fruit-bearing stage and more than 10 years old. The trees were spaced at 7 × 3 m resulting in 476 trees per hectare. The irrigation was by drip and the schedule differed with the season, weather and soil type. Generally, irrigation was applied for two hours a day and three times a week in autumn and winter (March to August) and three hours a day, three days a week in spring and summer (September to February). Fertilisation is done following soil and foliar analysis. Foliar and soil samples are normally collected in March and sent for analysis. Depending on the deficient nutrient fertilizers are then applied through fertigation, foliar sprays or as granules.
Installation of chambers and measurement of soil CO2
The CO2 chambers were installed in May 2018 at a distance of 1 m from the main stem. Three chambers were installed in three adjacent rows at the centre of each orchard. A total of 12 chambers were installed i.e. 3 chambers x 4 treatments (n = 12). The CO2 chambers were made and installed in the field according to the USDA-ARS GRACEnet Project Protocols (Parkin and Venterea 2010). The gas chambers were however modified and interfaced with an infrared CO2 sensor, GMP343 CO2 probe along with MI70 data logger (Vaisala, Vantaa, Finland) to allow for in-situ CO2 measurements. The chambers consisted of two separate PVC rings: 1) a PVC ring/collar (0.20 m diameter and 0.15 m in height and 2) another PVC ring (0.20 m diameter and 0.10 m height) sealed on one side with a PVC circle to make a chamber lid. The chamber lid had a small gas ball valve on it to discourage pressure build up in the chambers during measurements. The chamber lid was also perforated on top to fix the mounting flange that holds the GMP343 CO2 probe. The collars were hammered into the soil to a depth of 0.05 m leaving 0.10 m above the soil. These collars were left in the soil for a few days to settle before the measurements were taken (Fig. 2).
When taking measurements, the chamber lid with the fixed CO2 probe on it was attached to the collar and secured with a tube strip to make it airtight (Fig. 2). The CO2 probe GMP343 was set to record measurements every 30 s for 5 min. All measurements were taken between 10h00 and 14h00. CO2 fluxes, which is the emission rate of CO2, were measured for 12 months starting from June 2018 to May 2019 at 2 weeks intervals.
The emission rates were then calculated as described in Munjonji et al. (2020).
Calculation of CO2 fluxes
The CO2 probe GMP343 gives measurements of CO2 in parts per million (ppm). The measurements were first converted to mg m− 3 using the ideal gas law (Eq. 1):
Where P = pressure, V = volume, n = moles of gas, R = gas law constant (8.3145 J mol− 1 K− 1), and T = temperature in Kelvin. The molar volume of an ideal gas at 1 atm pressure (101.325 kPa) and 25 °C is 22.4 L mol− 1. Thus, at different pressures, the molar volume of the gas can be calculated as follows:
At different temperature and pressure the concentration of CO2 in mg m− 3 was calculated as follows:
Where CO2 ppm is the measured concentration of CO2 at any given time, T is the chamber temperature (Temperature in °C + 273.15 K) and P is the ambient pressure.
The CO2 concentration in mg m− 3 was then plotted against time (min) giving a slope in mg m− 3 min− 1. The slope of the resulting regression lines was then determined for each installed chamber. The slope was then multiplied by the volume of the chamber and divided by the area covered by the chamber giving the resultant flux in mg m− 2 min− 1, which describes the CO2 flux out of the soil. Cumulative CO2 was calculated by assuming that the rate of CO2 release was constant between two measurement points.
Leaf gas exchange
Leaf gas exchanges were measured on the trees that were next to the CO2 chambers. Leaf gas exchanges were measured using LCi-SD Ultra-Compact Photosynthesis System (BioScientific, UK). The measurements were taken between 10h00 and 14h00 on clear sunny days on fully grown illuminated leaves at a height of about 1.5 m. The leaves were located on the periphery of the crown. When conditions allowed, measurements were taken every 2 weeks. The measured parameters included CO2 assimilation rate (A), stomatal conductance (gs), transpiration rate (E) and intercellular CO2 concentration (Ci). Leaf gas exchanges were upscale to canopy level using the Big Leaf Model (Luo et al. 2018). The upscaling was done using the formula:
Where Ac is the total canopy photosynthesis rate, A0 is the photosynthetic rate of the fully illuminated leaves on the canopy, k is the extinction coefficient and LAItot is the total leaf area index of the canopy.
Intrinsic and Instantaneous water use efficiencies were calculated from the gas exchange measurements using the following formulas:
Determination of bulk density, infiltration rate and particle size
Bulk density was measured using the core ring method. Cores with a diameter of 5 cm and a height of 5 cm were used to collect undisturbed soils cores in the top 5 cm of the soils. A total of 12 soil cores were collected following the design of 2 × 2 × 3 replications. The soils were then oven-dried at 105 °C for 24 h before being weighed. The infiltration rate was measured using mini-disk infiltrometers (Decagon Devices, USA). The infiltration measurements were taken once in each experimental plot (n = 12). Soil samples for particle size were also collected from each experimental plot (n = 12) and determined using the pipette method (Day 1965). All three parameters (bulk density, infiltration rate and particle size) were measured about 1 m from the tree trunk and close to the installed chambers.
Soil chemical analyses
Soil samples were collected from two depths i.e. 0–15 cm and 15–30 cm using augers. Similar to the other parameters 12 soil auger samples were collected per depth (one composited sample per experimental plot). Three samples were collected in each experimental plot and composited to make one sample. The samples were then air-dried and sieved to pass through a 2 mm sieve. Soil pH was determined in both water and KCl. De-ionised water and 1 M KCl solution were used in a ratio of 1:2.5 and measured using a glass electrode. Calcium and Mg were determined by atomic absorption after being extracted using 1 M KCl, filtered and diluted with 20 ml of 0.0356 M SrCl2. Phosphorus, K, Zn, Cu, and Mn were extracted using Ambic-2 solution and determined using atomic absorption. Total C and N were analyzed by the Automated Dumas dry combustion method using a LECO CNS 2000 (Leco Corporation, Michigan).
Determination of soil carbon stocks
Soil carbon stocks refer to the amount of carbon in a soil layer of known bulk density. Soil carbon stocks are normally expressed as weight/mass per unit area and are commonly limited to the soil fraction of less than 2 mm in size. Soil C stocks were determined by the following formula (Batjes 1996):
Where Soil C stock is soil carbon stock (kg C m− 2), Ctot is the total carbon content (g C g− 1 soil), BD is bulk density (kg m− 3); d is soil depth (m). Carbon stocks were calculated from the top 5 cm since bulk density was only determined in that depth.
Data analysis
A two-way ANOVA was run using SPSS 25 (SPSS, USA) to determine the effect of soil type, cultivar and their interaction on the measured parameters. A further ANOVA was carried out to determine the impact of season on the measured parameters. Where differences were significant, Tukey HSD was used to separate means. Paired T-tests were also carried out to compare leaf-level gas exchanges and canopy level gas exchanges (upscaled values). Regression analyses were run to compare minimum, maximum and mean seasonal temperatures with mean seasonal CO2 flux rates and mean seasonal photosynthesis rates. Where cultivar by soil type interaction was non-significant, the impact of soil type was compared across cultivars, whereas that of cultivar was made across soil types. When running ANOVA for seasonal variation, data of the two cultivars and the two soil types were combined.
Results
Physical and chemical characteristics of the soils
The average clay content in the rich clay content (RC) soil was twice that of the moderate clay content (MC) soil (Table 1). A two-sample T-test showed that the potassium (K) was significantly higher in the RC soil compared to the MC soil (p = 0.004). The results showed that the RC soil had 66.81 to 297.57 mg kg− 1 more K than in the MC soil. The other macronutrients (Ca and Mg), did not differ between the two soils but tended to be higher in the RC soil compared to the MC soil. Phosphorus (P) on the other hand did not differ between the two soils but was relatively higher in the MC soil compared to the RC soil. The concentrations of micronutrients Mn and Zn in the RC soil were significantly higher compared to in the MC soil. The soil pH was 1 unit higher in MC soil but both soils were slightly acidic with pH values of 6.9 and 5.9 for MC and RC soil, respectively. The total carbon content was not different between the two soils only with only a 0.2 % difference between MC soils and the RC soil. Due to the 0.2 % difference observed between the two soils, carbon stocks were relatively higher in MC soil compared to the RC soil (Fig. 3a). Figure 3B also shows that bulk density was higher in MC soils when compared to RC soil. Moderate clay content soils had an average bulk density of about 1450 kg m− 3 while the RC soil had an average bulk density of 1310 kg m− 3.
CO2 flux rates over the year and seasons
The CO2 flux rates fluctuated throughout the year as shown in Fig. 4a. Figure 4a shows averaged values for the two cultivars under each soil type. The CO2 flux rates did not significantly differ between the RC soil and the MC soil (p = 0.845). However, the average CO2 flux rate for the MC soil was slightly higher (0.109 ± 0.037 tons ha− 1 day− 1) as compared to 0.107 ± 0.036 tons ha− 1 day− 1 of the RC soil. Cumulative CO2 over the whole experimental period did not significantly differ between the two soils (Fig. 4b). However, at the end of the experimental period, an MC soil had cumulatively emitted 36.29 tons ha− 1 which was 7.56 % higher than that of the RC soil with 33.74 tons ha− 1. CO2 flux rates differed in response to the season. The flux rates were lowest in the late autumn and winter with an average of 0.065 tons ha− 1 day− 1 and highest in summer at 0.136 tons ha− 1 day− 1 (Fig. 5a). Spring and autumn seasons did not differ in their CO2 flux rates. The results showed that CO2 flux rates in summer were more than double the rates observed in winter. Similarly, the cumulative CO2 emitted per season was also different (Fig. 5b). As observed with the flux rates, the winter season emitted the least amount of CO2 with an average cumulative amount of 3.16 tons ha− 1 which was more than three times lower than emitted in summer of 9.982 tons ha− 1.
Annual and seasonal leaf gas exchanges
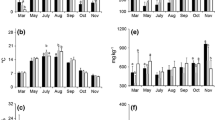
Leaf gas exchanges of the two citrus cultivars were measured throughout the year. Stomatal conductance (gs), photosynthesis rate (A), transpiration rate (E) and intercellular CO2 concentration (Ci) did not differ between the two cultivars (Fig. 6a-d). Both cultivars peaked in gs, A and E in March. On the other hand, Ci remained relatively constant throughout the year with values ranging from 200 to 400 ppm. A similar trend was also observed with the two soils (results not shown) and no differences were also observed. Similar to CO2 flux rates, only season had a significant influence on the leaf gas exchanges (Fig. 7). Seasonal transpiration rate was highest in autumn (3.77 mmol m− 2 s− 1) followed by the summer season (2.68 mmol m− 2 s− 1) and then winter and spring (Fig. 7b). No differences in seasonal transpiration rate were observed between the winter and the spring season. Seasonal CO2 assimilation rates responded similarly to transpiration rates with the rates following the order of autumn (9.75 µmol m− 2 s− 1) > summer (4.64 µmol m− 2 s− 1) > winter (2.13 µmol m− 2 s− 1) = spring (2.20 µmol m− 2 s− 1) (Fig. 7d). Seasonal stomatal conductance was not different between the summer and autumn seasons but was higher than the winter and the spring seasons. As expected intercellular CO2 concentration did not vary over the seasons (Fig. 7a).
Leaf area index and canopy leaf gas exchanges
Leaf area index varied with the season (Fig. 8a). Leaf area index was higher in autumn compared to summer and winter. The LAI in spring did not differ from the other seasons. The average LAI in spring was 6.81, summer 5.27, winter 4.91 and spring 5.89. Leaf area index was used to upscale leaf gas exchange parameters such as gs, E and A to canopy level using Eq. 1. The results of the upscaling are presented in Fig. 8. Differences between seasons at canopy level gas exchanges were the same as those observed with leaf-level gas exchanges. The only difference was that canopy values were relatively higher than leaf-level gas exchanges. Paired samples tests showed significant differences between canopy and leaf-level for transpiration rate, stomatal conductivity and photosynthesis rate. The canopy level transpiration rate was 1.05 to 1.52 mmol m− 2 s− 1 higher than the leaf-level transpiration rate. Stomatal conductance at canopy level was 0.41 to 0.62 mol m− 2 s− 1 higher than leaf-level gs. Likewise, the canopy photosynthesis rate was 2.06 to 3.26 µmol m− 2 s− 1 higher than leaf-level A. There were also differences in the upscaling factors for the different seasons. The highest percentage increase from leaf to canopy level of 79 % occurred in spring followed by summer 61 %, autumn 56 % and winter 26 %.
Intrinsic (IntrWUE) did not differ between autumn, winter and spring seasons. No differences in IntrWUE were also observed between summer, winter and spring. IntrWUE was however significantly higher in autumn compared to summer (Fig. 9a). On the other hand, instantaneous (InstWUE) water use efficiency was higher in autumn compared to the other seasons (Fig. 9b). No variation in both IntrWUE and InstWUE were observed between summer, winter and the spring seasons. Also, due to similar scaling up factors, no differences were observed between the leaf level and canopy level. The values shown in Fig. 9 are therefore for both leaf level and canopy level.
Relationships between temperature, CO2 flux rates and CO2 assimilation
A strong linear relationship (P < 0.05, r2 = 0.99) between mean seasonal minimum temperatures and CO2 flux rate was observed (Fig. 10a). The results showed that 99 % of the variation observed in CO2 flux rates could be explained by mean seasonal minimum temperatures. Mean seasonal maximum temperatures did not show a significant relationship with CO2 flux rate. CO2 assimilation rate was also not related to either mean seasonal minimum or maximum temperatures during the measurement period (Fig. 10b).
Comparison between CO2 assimilation and CO2 emission rates
Canopy CO2 assimilation rates and soil CO2 emission rates were compared per season (Fig. 11). Significant differences in the rates were observed for the summer, autumn and winter seasons while the spring season showed no significant variation between the two. In the autumn season, the citrus trees assimilated CO2 at rates (578.4 kg ha− 1 day− 1) that were almost six times higher than the soil CO2 emission rates (96.8 kg ha− 1 day− 1). In the summer season, the assimilation rate was almost double the emission rate. Overall, canopy CO2 assimilation rates were higher than soil CO2 emission rates. There was a balance between CO2 assimilation and CO2 emission rates in the spring season.
Discussion
Annual and seasonal soil CO2 flux rates
Studies, such as that of Neira et al. (2015) and Yang et al. (2018) have shown that soil clay content influences the rate at which gases move in the soil through its influence on soil porosity and gaseous movement. Thus, soils with high clay content would be expected to restrict gas movements due to the tortuosity of the micropores (Neira et al. 2015). In this study, though not statistically significant, the moderate clay content (MC) soil cumulatively released about 7.56 % more CO2 compared to the rich clay content (RC) soil. This variation could be attributed to the differences in clay content. The MC soil had an average of 11 % clay while the RC soil had 22 %. The difference might have also resulted from the differences in soil carbon stocks which were observed to be higher in MC soil (1.47 kg m− 2) compared to RC soil (1.16 kg m− 2).
Seasonal variations of soil CO2 emission showed that both the rate and the cumulative amount were highest in the summer season and lowest in the winter season (Fig. 5). The average soil CO2 emission rate in the summer season was 0.136 tons ha− 1 day− 1 which was more than double the rate observed in the winter season (0.065 tons ha− 1 day− 1). The differences were mainly due to the variation in temperatures between these two seasons as also observed by Yuste et al. (2003). The influence of temperature on soil respiration has also been reported in other studies (Burton and Pregitzer 2003; Davidson and Janssens 2006). Several studies have shown that soil moisture and temperature are the main drivers of soil CO2 emission rates (Burton and Pregitzer 2003; Li et al. 2018; Wu 2020). However, in this study moisture was well controlled and kept at near-optimal thus only temperature influenced that variation in the CO2 release. Due to the differences observed in the rates of soil CO2 release, the cumulative amounts of CO2 emitted per season also differed and was congruent to the emission rates. Thus, the summer season also cumulatively released more CO2 to the atmosphere compared to the other seasons. In the summer season alone, the citrus orchard released 9.98 tons of CO2 per hectare compared to the winter season where 3.16 tons ha− 1 were emitted. The high CO2 released in summer could be attributed to an increase in both root and microbial respiration resulting from a temperature increase which promotes enzymatic and microbial activity (Li et al. 2021; Hu et al. 2018; Wu 2020). The significantly higher amounts of CO2 emitted in warmer months of the year suggest future increases in CO2 emission even in cooler months due to global warming. Climate change projections suggest that Southern Africa should expect temperature increases of up to 2˚C if society continues to use fossil fuels at a moderate growth rate, while drastic increases of up to 7˚C are expected under high emission scenarios (Davis et al. 2017; DEA 2013).
It is interesting to note that Yuste et al. (2003) reported that in the absence of severe drought, soil temperature becomes a more reliable predictor of soil respiration. In this current study, the citrus orchards were well-watered throughout the year. Thus, the differences can therefore be attributed to the differences in mean seasonal temperatures as observed in Fig. 10. The results showed that 99 % of the variation observed in the soil CO2 emission rate could be explained by the minimum ambient temperatures while maximum temperatures could only explain 66 % of the variation. These results show that minimum ambient temperatures have a stronger influence on soil CO2 emission rates compared to maximum temperatures. Mean maximum seasonal temperatures were less influential on soil CO2 emission rates probably because the temperatures are close to optimal. Though variable, depending on substrate and moisture content, the optimal temperature for soil respiration is believed to be around 35 °C (Richardson et al. 2012). Temperature sensitivity of respiration is also reported to decreases as temperature increases (Yuste et al. 2003). Even though no actual soil temperature measurements were conducted in this study, Islam et al. (2015) reported a strong relationship between atmospheric and soil temperature thus the recorded ambient temperatures could be indicative of the soil temperatures. Others studies such as Brown et al. (2000) and Zheng et al. (1993) have also shown positive relationships between soil and air temperature and that soil temperature can be predicted from air temperature. Yilmaz (2012) found both soil and air temperature to be significant and positively related to CO2 emission rates.
The findings of this study suggest that if temperatures get warmer as predicted by the IPCC (2014), more CO2 would be released in the citrus orchards in the winter and spring seasons. The reason being that increasing global temperatures could potentially cause large increases in root and associated soil respiration (Burton and Pregitzer 2003). The increase in CO2 emission could result in potentially strong feedback to global warming. However, according to Burton and Pregitzer (2003), if root respiration acclimates to higher temperatures, increases in CO2 emission could be minimal.
Annual and seasonal leaf gas exchanges
As observed with soil type, citrus cultivars did not vary in the CO2 assimilation rates and other leaf gas exchange parameters such as gs, E and Ci but fluctuated throughout the experimental period. Seasonal variation had a strong influence on leaf gas exchanges. The highest rates of CO2 assimilation were observed in autumn as compared to the other seasons for CO2 emission rates. These findings are similar to those reported by Dovis et al. (2014) from a greenhouse experiment carried out in Brazil but are contrary to the review findings of Ribeiro and Machado (2007) who reported higher CO2 assimilation rates in spring followed by summer and lowest in winter. However, all studies report the lowest rates of CO2 assimilation rates in winter.
In this study, CO2 assimilation rates in the winter season were four times lower than those reported in the autumn season. Ribeiro et al. (2009) who reported similar findings attributed the differences to decreased root permeability and plant hydraulic conductance due to low temperatures. Poor root permeability is thought to result from increased viscosity of water which then affects water supply to the leaves (Angelocci et al. 2004). The low plant hydraulic conductivity due to poor root permeability results in lower stomatal conductance leading to lower CO2 assimilation rates (Ribeiro and Machado 2007; Ribeiro et al. 2009). Accordingly, gs in winter was about 75 % lower when compared to mean gs in autumn. In a lime orchard, Angelocci et al. (2004) found gs to decrease with lower temperatures and vapour pressure deficit. Lower CO2 assimilation rates in winter could also be attributed to the reduction in the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) an enzyme that catalyses the fixation of CO2 in C3 plants (Hendrickson et al. 2004; Sage 2002). Also, according to Ribeiro et al. (2018), citrus plants have a low-temperature threshold of around 13 °C. Thus citrus plant metabolism is severely reduced at lower temperatures.
Intercellular CO2 concentration (Ci) is one other parameter that is reported to influence CO2 assimilation in plants. In this current study, Ci did not vary with season and hence could not have affected CO2 assimilation. In other studies with other evergreen tree crops such as macadamia, CO2 assimilation rate responded to Ci where higher Ci resulted in increased CO2 assimilation (Smit et al. 2020). However, CO2 assimilation rates were as expected strongly related to E and gs (results not shown). However, mean minimum seasonal temperatures did not show any significant relationship with CO2 assimilation rate despite the obvious influence of temperature on CO2 assimilation rates. Mean maximum seasonal temperatures had a modest positive relationship (r2 = 0.41; Fig. 10b) with CO2 assimilation. The observed weak relationship between CO2 assimilation and temperature could have been due to the influence of temperature on the O2/CO2 ratio and consequently photorespiration. Studies have shown that as temperature increases the O2/CO2 ratio increases leading to higher photorespiration and lower net photosynthesis rate (Ku and Edwards 1978; Brooks and Farquhar 1985). This could also be the reason why CO2 assimilation rates were lower in summer compared to autumn. Another plausible reason for the poor temperature and CO2 relationship could be that other plant intrinsic factors are contributing to that.
In many seasonal crops, the CO2 assimilation rate normally peaks just before the flowering and fruiting stages probably due to higher sink activity (Lewis et al. 2002). And indeed some studies have suggested an association between sink demand and photosynthesis rate (Dovis et al. 2014; Nebauer et al. 2013; Ribeiro et al. 2012). As evergreen species, citrus trees assimilate and consume carbon throughout the year (Dovis et al. 2014). Citrus flowers in the spring but their CO2 assimilation rates were not highest in spring and this could be because the flowering was driven by stored assimilates. Dovis et al. (2014) showed that reserve assimilate consumption from the roots was high during citrus flowering.
Canopy CO2 assimilation vs. soil CO2 emission rate
Due to the limitation of studies on the role of orchards in sequestering atmospheric CO2 this study attempted to provide a snapshot of whether a citrus orchard is a net sink or emitter of CO2. As such seasonal canopy CO2 assimilation rates were compared to seasonal soil CO2 emission rate (Fig. 11). The results showed that in most seasons (summer, autumn and winter) CO2 assimilation rates were significantly higher than CO2 emission rates suggesting that citrus orchard could be net sinks of CO2. Even though this snapshot method applied in this study is not as accurate and common as the eddy covariance methods (Aubinet et al. 2012), it is however very applicable and acceptable in that it provides a relatively high spatial and temporal capture of the influence of management practices that are known to strongly influence both plant physiology and soil condition which consequently affect carbon exchange (Nardino et al. 2013). One drawback of this snapshot is that it does not capture CO2 losses from plant respiration (Huntingford et al. 2017) but it is known that plant assimilates more CO2 than they emit. Other studies use carbon stocks methods to estimate CO2 sequestration in tree-based systems (Dube et al. 2018; Ryan et al. 2011).
It was also observed that while leaf and canopy CO2 assimilation rates peaked in autumn, soil CO2 emission rates peaked in the summer season. These differences could just be attributed to the mechanisms and the optimal operational conditions for each of the process. As discussed earlier, soil respiration rates increase with temperature (Burton and Pregitzer 2003) and since soil CO2 emission rates were measured under a tree canopy, it is possible that maximum warming of the soil occurred in summer when temperatures were higher (Fig. 1). On the other hand, CO2 assimilation rates might have peaked in autumn due to a combination of factors that include optimal environmental conditions and an increase in assimilate demand by the plant. Interestingly, no significant difference in canopy CO2 assimilation and soil emission rates were observed in the spring season.
Conclusions
This study is one of the few studies to present empirically measured CO2 flux rates and CO2 assimilation rates in a commercial drip-irrigated citrus orchard. This information is important in designing policies to mitigate CO2 emission in agroecosystem thus help meet sustainable development goal 13 (SDG13) on climate action. The amount of CO2 emitted in summer, the warmest season, was three times higher than in winter, which is an indication of the potential CO2 that could be emitted from the soil in cooler seasons should temperature continue to increase due to global warming. The study showed that under near-optimal growth conditions, soil clay content did not influence the rate at which CO2 was released in the soil neither did it have any effect on the rate at which the citrus assimilated atmospheric CO2. These findings suggest that under similar management practices, the clay content of the soil does not affect the CO2 emission rate. Despite the near-optimal growth conditions, both soil CO2 emission rates and leaf CO2 assimilates rates responded strongly to seasonal variations confirming the influence of environmental conditions particularly of temperature on the release and capture of carbon in agroecosystems. The findings showed that the citrus CO2 assimilation rate peaked in the autumn and was six times higher than the CO2 emission rates but the two rates balanced up in the spring season. A snapshot analysis of the CO2 sequestration rates suggests that citrus orchards are net sinks of CO2 in summer, autumn and winter season.
Data availability
Some data can be made available through request.
Code availability
No custom codes were used.
References
Aliniaeifard S, Malcolm Matamoros P, Van Meeteren U (2014) Stomatal malfunctioning under low VPD conditions: induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling? Physiol Plant 152:688–699
Angelocci LR, Marin FR, Oliveira RFD, Righi EZ (2004) Transpiration, leaf diffusive conductance, and atmospheric water demand relationship in an irrigated acid lime orchard. Braz J Plant Physiol 16:53–64
Atarashi-Andoh M, Koarashi J, Ishizuka S, Hirai K (2012) Seasonal patterns and control factors of CO2 effluxes from surface litter, soil organic carbon, and root-derived carbon estimated using radiocarbon signatures. Agric For Meteorol 152:149–158
Aubinet M, Vesala T, Papale D (2012) Eddy covariance: a practical guide to measurement and data analysis. Springer Science & Business Media, Berlin
Avola G, Cavallaro V, Patanè C, Ratanè E (2008) Gas exchange and photosynthetic water use efficiency in response to light, CO2 concentration and temperature in Vicia faba. J Plant Physiol 165:796–804
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582
Brooks A, Farquhar G (1985) Effect of temperature on the CO 2/O 2 specificity of ribulose-1, 5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165:397–406
Brown SE, Pregitzer KS, Reed DD, Burton AJ (2000) Predicting daily mean soil temperature from daily mean air temperature in four northern hardwood forest stands. For Sci 46:297–301
Burton AJ, Pregitzer KS (2003) Field measurements of root respiration indicate little to no seasonal temperature acclimation for sugar maple and red pine. Tree Physiol 23:273–280
Carvalho M, Castro I, Moutinho-Poutinho J, Correia C, Egea-Cortines M, Matos M, Rosa M, Carnide E, Lino-Neto T (2019) Evaluating stress responses in cowpea under drought stress. J Plant Physiol 241:153001
Chang J-C, Lin T-S (2007) Gas exchange in litchi under controlled and field conditions. Sci Hortic 114:268–274
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Chang Biol 4:217–227
Davis CL, Tadross M, Engelbrecht FA, Wolski MP, Archer ER (2017) Future climate change over Southern Africa. African Sun Media, Stellenbosch
Day PR (1965) Particle fractionation and particle-size analysis. In: Black CA (ed) Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling, 9. Wiley, New York, pp 545–567
DEA (Department of Environmental Affairs) (2013) Long-Term Adaptation Scenarios Flagship Research Programme (LTAS) for South Africa. Climate Trends and Scenarios for South Africa. Pretoria, South Africa
Dovis VL, Machado EC, Ribeiro RV, Magalhães Filho JR, Marchiori PE, Sales CRG (2014) Roots are important sources of carbohydrates during flowering and fruiting in ‘Valencia’ sweet orange trees with varying fruit load. Sci Hortic 174:87–95
Dube T, Muchena R, Masocha M, Shoko C (2018) Estimating soil organic and aboveground woody carbon stock in a protected dry Miombo ecosystem, Zimbabwe: Landsat 8 OLI data applications. Phys Chem Earth Parts A/B/C 105:154–160
Fang C, Smith P, Moncrieff JB, Smith JU (2005) Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433:57–59
Friend A (2001) Modelling canopy CO2 fluxes: are ‘big-leaf’simplifications justified? Glob Ecol Biogeogr 10:603–619
Fujii JA, Kennedy RA (1985) Seasonal changes in the photosynthetic rate in apple trees: A comparison between fruiting and nonfruiting trees. Plant Physiol 78:519–524
Gara TW, Skidmore AK, Darvishzadeh R, Wang T (2019) Leaf to canopy upscaling approach affects the estimation of canopy traits. GISci Remote Sens 56:554–575
Giardina CP, Ryan MG (2000) Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404:858–861
Hassink J, Bouwman L, Zwart K, Bloem J, Brussaard L (1993) Relationships between soil texture, physical protection of organic matter, soil biota, and C and N mineralization in grassland soils. Soil Structure/Soil Biota Interrelationships. Elsevier, Amsterdam
Hendrickson L, Ball M, Wood J, Chow W, Furbank RT (2004) Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ 27:795–809
Hu S, Li Y, Chang SX, Li Y, Yang W, Fu W, Liu J, Jiang P, Lin Z (2018) Soil autotrophic and heterotrophic respiration respond differently to land-use change and variations in environmental factors. Agric For Meteorol 250–251:290–298
Huntingford C, Atkin OK, Martinez-dela Torre A, Mercado IM, Heskel MA, Harper AB, Bloomfield KJ, O’Sullivan OS, Reich PB, Wythers KR, Butler EE, Chen M, Griffin KL, Meir P, Tjoelker MG, Turnbull MH, Sitch S, Wiltshire A, Malhi Y (2017) Implications of improved representations of plant respiration in a changing climate. Nat Commun 8:1602
Iglesias DJ, Quiñones A, Font A, Martínez-Alcántara B, Forner-Giner M, Legaz F, Primo-Millo E (2013) Carbon balance of citrus plantations in Eastern Spain. Agr Ecosyst Environ 171:103–111
IPCC (2014) Climate change 2013: The physical science basis. Cambridge University Press, Cambridge
Islam KI, Khan A, Islam T (2015) Correlation between atmospheric temperature and soil temperature: a case study for Dhaka, Bangladesh. Atmos Clim Sci 5:200
Kottek M, Grieser M, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263
Ku S-B, Edwards GE (1978) Oxygen inhibition of photosynthesis. Planta 140:1–6
Lewis JD, Wewis X, Griffin KL, Tissue DT (2002) Effects of age and ontogeny on photosynthetic responses of a determinate annual plant to elevated CO2 concentrations. Plant Cell Environ 25:359–368
Li C, Peng Y, Nie X, Yang Y, Yang L, Li F, Zhou G (2018) Differential responses of heterotrophic and autotrophic respiration to nitrogen addition and precipitation changes in a Tibetan alpine steppe. Sci Rep 8:16546
Li Q, Leroy F, Zocatelli R, Gogo S, Jacotot A, Guimbaud C, Laggoun-Défarge F (2021) Abiotic and biotic drivers of microbial respiration in peat and its sensitivity to temperature change. Soil Biol Biochem 153:108077
Lu P, Chacko E, Bithell S, Schaper H, Wiebel J, Cole S, Müller W (2012) Photosynthesis and stomatal conductance of five mango cultivars in the seasonally wet–dry tropics of northern Australia. Sci Hort 138:108–119
Luo X, Chen JM, Liu J, Black TA, Croft H, Staebler R, He I, Arain MA, Chen B, Mo G (2018) Comparison of big-leaf, two‐big‐leaf, and two‐leaf upscaling schemes for evapotranspiration estimation using coupled carbon‐water modeling. J Geophys Res Biogeosci 123:207–225
Luyssaert S, Inglima I, Jung M, Richardson AD, Reichstein M, Papale D, Piao S, Schulze ED, Wingate I, Matteucci G (2007) CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob Chang Biol 13:2509–2537
Munjonji L, Ayisi KK, Boeckx P, Haesaert G (2017) Stomatal behavior of cowpea genotypes grown under varying moisture levels. Sustainability 10:12
Munjonji L, Ayisi KK, Mudongo EI, Mafeo T, Bafeo K, Mokoka M, Linstädter A (2020) Disentangling drought and grazing effects on soil carbon stocks and CO2 fluxes in a semi-arid African savanna. Front Environ Sci 8:207
Nardino M, Pernice F, Rossi F, Georgiadis T, Facini O, Motisi A, Drago A (2013) Annual and monthly carbon balance in an intensively managed Mediterranean olive orchard. Photosynthetica 51:63–74
Nebauer SG, Arenas C, Rodríguez-Gamir J, Bordón Y, Fortunato-Almeida A, Monerri C, Guardiola JL, Molina RV (2013) Crop load does not increase the photosynthetic rate in Citrus leaves under regular cropping conditions. A study throughout the year. Sci Hortic 160:358–365
Neira J, Ortiz M, Morales L, Acevedo E (2015) Oxygen diffusion in soils: Understanding the factors and processes needed for modeling. Chilean J Agric Res 75:35–44
Parkin T, Venterea RT (2010) Sampling protocols. Chapter 3. Chamber-based trace gas flux measurements. In: RF F (ed) Sampling protocols. USDA, Mankato
Paudel I, Bar-Tal A, Rotbart N, Ephrath J, Cohen S (2018) Water quality changes seasonal variations in root respiration, xylem CO2, and sap pH in citrus orchards. Agric Water Manag 197:147–157
Qu H, Zhao X, Lian J, Tang X, Wang X, Medina-Roldán E (2020) Increasing precipitation interval has more impacts on litter mass loss than decreasing precipitation amount in desert steppe. Front Environ Sci
Razafimbelo TM, Albrecht A, Oliver R, Chevallier T, Chapuis-Lardy L, Feller C (2008) Aggregate associated-C and physical protection in a tropical clayey soil under Malagasy conventional and no-tillage systems. Soil Tillage Res 98:140–149
Ribeiro RV, Machado EC (2007) Some aspects of citrus ecophysiology in subtropical climates: re-visiting photosynthesis under natural conditions. Braz J Plant Physiol 19:393–411
Ribeiro RV, Machado EC, Santos MG, Oliveira RF (2009) Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 47:215–222
Ribeiro RV, Machado EC, Habermann G, Santos MG, Oliveira RF (2012) Seasonal effects on the relationship between photosynthesis and leaf carbohydrates in orange trees. Funct Plant Biol 39:471–480
Ribeiro JEDS, Barbosa AJS, Lopes SDF, Pereira WE, Albuquerque MBD (2018) Seasonal variation in gas exchange by plants of Erythroxylum simonis Plowman. Acta Bot Bras (AHEAD) 32(2):287–296
Richardson J, Chatterjee A, Darrel Jenerette G (2012) Optimum temperatures for soil respiration along a semi-arid elevation gradient in southern California. Soil Biol Biochem 46:89–95
Rivas R, Falcão HM, Ribeiro RV, Machado EC, Pimentel C, Santos MG (2016) Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S Afr J Bot 103:101–107
Ryan CM, Williams M, Grace J (2011) Above-and belowground carbon stocks in a miombo woodland landscape of Mozambique. Biotropica 43:423–432
Sage RF (2002) Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53:609–620
Smit TG, Taylor NJ, Midgley SJ (2020) The seasonal regulation of gas exchange and water relations of field grown macadamia. Sci Hortic 267:109346
Sprintsin M, Chen JM, Desai A, Gough CM (2012) Evaluation of leaf-to‐canopy upscaling methodologies against carbon flux data in North America. J Geophys Res Biogeosci 117(G1)
Sun L, Hirano T, Yazaki T, Teramoto M, Liang N (2020) Fine root dynamics and partitioning of root respiration into growth and maintenance components in cool temperate deciduous and evergreen forests. Plant Soil 446:471–486
Tankari M, Wang C, Zhang X, Li I, Soothar RK, Ma H, Xing H, Yan C, Zhang Y, Liu F, Wang Y (2019) Leaf gas exchange, plant water relations and water use efficiency of Vigna Unguiculata L. Walp. Inoculated with Rhizobia under Different Soil Water Regimes. Water 11:498
Wu J (2020) Temporal variations in soil CO2 efflux in an alpine meadow site on the Qinghai–Tibetan Plateau. Grassl Sci 66:3–15
Yang X, Fan J, Jones SB (2018) Effect of soil texture on estimates of soil-column carbon dioxide flux comparing chamber and gradient methods. Vadose Zone J 17:1–9
Yilmaz G (2012) Seasonal variation of cumulative CO2 emission from a vertisol under apricot orchard in semi-arid Southeast Turkey. Pedosphere 22:322–332
Yuste JC, Janssens IA, Carrara A, Meiresonne L, Ceulemans R (2003) Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiol 23:1263–1270
Zhang S, Zhang G, Liu X, Xia J (2013) The responses of photosynthetic rate and stomatal conductance of Fraxinus rhynchophylla to differences in CO2 concentration and soil moisture. Photosynthetica 51:359–369
Zheng D, Hheng ER Jr, Running SW (1993) A daily soil temperature model based on air temperature and precipitation for continental applications. Clim Res 2:183–191
Acknowledgements
The authors would like to acknowledge the Risk and Vulnerability Science Centre, University of Limpopo for funding this research. The authors also acknowledge Mahela Group staff members Karla and Carl.
Funding
This study was supported by the Risk and VulnerabilityScience Centre.
Author information
Authors and Affiliations
Contributions
Conceptualization, LM, KKA and TPM; Data curation, LM; Formal analysis LM; Funding acquisition; Investigation, LM, KEM, TM; Methodology, LM, KKA and TPM ; Project administration; Resources; Visualization, LM, KEM, TM; Writing LM, KKA, TPM, KEM and TM.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors declare no conflict of interests.
Additional information
Responsible Editor: Elizabeth M Baggs.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munjonji, L., Ayisi, K.K., Mafeo, T.P. et al. Seasonal variation in soil CO2 emission and leaf gas exchange of well‐managed commercial Citrus sinensis (L.) orchards. Plant Soil 465, 65–81 (2021). https://doi.org/10.1007/s11104-021-04986-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04986-x