Abstract
Background and aims
Plant growth affects soil moisture, mineral N and organic C availability in soil, all of which influence denitrification. With increasing plant growth, root exudation may stimulate denitrification, while N uptake restricts nitrate availability.
Methods
We conducted a double labeling pot experiment with either maize (Zea mays L.) or cup plant (Silphium perfoliatum L.) of the same age but differing in size of their shoot and root systems. The 15N gas flux method was applied to directly quantify N2O and N2 fluxes in situ. To link denitrification with available C in the rhizosphere, 13CO2 pulse labeling was used to trace C translocation from shoots to roots and its release by roots into the soil.
Results
Plant water and N uptake were the main factors controlling daily N2O + N2 fluxes, cumulative N emissions, and N2O production pathways. Accordingly, pool-derived N2O + N2 emissions were 30–40 times higher in the treatment with highest soil NO3− content and highest soil moisture. CO2 efflux from soil was positively correlated with root dry matter, but we could not detect any relationship between root-derived C and N2O + N2 emissions.
Conclusions
Root-derived C may stimulate denitrification under small plants, while N and water uptake become the controlling factors with increasing plant and root growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil conditions for denitrification have frequently been studied with the main prerequisites being availability of nitrate (NO3−) and easily decomposable organic substances, and oxygen deficiency (Burford and Bremner 1975; Firestone et al. 1979). Growing plants modify all these parameters, particularly the availability of the main substrates (NO3− and Corg) and soil moisture, and may thus play an important role in regulating denitrification in situ (von Rheinbaben and Trolldenier 1984).
Plant N uptake largely controls concentration and distribution of mineral N in soils. Amounts and rates of plant N uptake depend on plant species, age, physiological status, root size, and nutritional status. N uptake rates of maize and cereals remain low during the first two months of growth, then increase linearly with increasing biomass reaching a maximum around the time of flowering (Novák and Vidovič 2003, Malhi et al. 2011).
Plant roots contribute to organic C input to the soil through rhizodeposition and decaying roots and root hairs. Thus, total and available concentration of Corg is higher in the rhizosphere compared to bulk soil (Cheng et al. 1993). The amount of rhizodeposited C and its quality depend on plant species, age, and development (Gransee and Wittenmayer 2000; Vancura 1964; Vancura and Hovadik 1965), and plant nutrient status (Carvalhais et al. 2011). In general, younger plants translocate a higher share of assimilated C belowground than mature plants (Kuzyakov and Domanski 2000; Nguyen 2003), and perennial plants translocate a higher share of assimilated C belowground than annual plants (Husáková et al. 2018; Pausch and Kuzyakov 2018).
C and N availability are closely interrelated in the rhizosphere: Under low mineral N concentrations, root morphology is altered, and exudation related to root mass is increased (Paterson and Sim 1999). In addition, the composition of maize root exudates is altered under N deficiency (Carvalhais et al. 2011). On the other side, N fertilization decreases the portion of below-ground translocated C (Kuzyakov and Domanski 2000).
Several studies have tried to disentangle the effects of N and C availability on denitrification with contradictive results. Higher denitrification rates were measured from planted compared to bare soil (Senbayram et al. 2020; Vinther 1984). Some studies showed a strong influence of roots (Philippot et al. 2009), increasing denitrification rates with increasing root biomass (Klemedtsson et al. 1987), and higher potential denitrification activity in rhizosphere soil compared to bulk soil (Hamonts et al. 2013; Malique et al. 2019). Higher denitrification rates in planted soils have been associated with higher Corg availability in the rhizosphere (Bakken 1988; Philippot et al. 2009). In addition, denitrification rates correlated with soil NO3− content (Philippot et al. 2009; von Rheinbaben and Trolldenier 1984). In contrast, other studies found no differences between planted and unplanted soil (Haider et al. 1985). Denitrification was increased only with poorly growing plants (von Rheinbaben and Trolldenier 1984) or when root biomass started to decrease (Haider et al. 1987), and NO3− availability did not affect denitrification (Haider et al. 1987; Hamonts et al. 2013). The majority of these studies measured potential denitrification applying the acetylene inhibition method (Yoshinari and Knowles 1976), which is considered outdated due to a number of drawbacks such as inhibiting nitrification (Groffman et al. 2006).
Accordingly, it is still unclear whether growing plants stimulate denitrification through root exudation or restrict it through NO3− uptake. Reliable measurements of N2 fluxes and N2O/(N2O + N2) ratios in the presence of plants are scarce. Direct measurement of N2 fluxes is only possible in either artificial N2-free atmosphere (Scholefield et al. 1997, Senbayram et al. 2020) or by applying highly enriched 15N labeled NO3− (Hauck and Melsted 1956). The latter is used in the 15N gas flux technique which enables direct quantification of N2O and N2 produced from the labelled NO3− pool and estimation of processes contributing to N2O and N2 formation including denitrification, co-denitrification, or nitrification and nitrifier denitrification (Buchen et al. 2016, Laughlin and Stevens 2002).
This study aimed to directly quantify N2O and N2 fluxes from soil with plants of the same age but different size of shoot and root systems and to relate denitrification to C availability from root exudation. As plant water uptake may also affect denitrification (von Rheinbaben and Trolldenier 1984), we aimed to keep soil moisture constant by continuous irrigation. We hypothesized that (I) plant N uptake governs NO3− availability for denitrification. When plant N uptake is low due to smaller root system or root senescence, N2O and N2 emissions are increased. (II) Denitrification is stimulated by higher Corg availability from root exudation or decaying roots increasing total gaseous N emissions and decreasing their N2O/(N2O + N2) ratios.
Materials and methods
Experimental concept
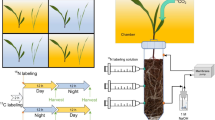
The experiment consisted of a pre-cultivation phase followed by the experimental phase. A schematic overview of both phases is presented in Fig. 1. In the pre-cultivation phase, plants were raised under controlled conditions. Maize plants (Zea mays L. cv. Ronaldinio) were grown under different N fertilization to obtain plants with different root and shoot biomass. As a second species, cup plant (Silphium perfoliatum L.) was included, a bioenergy plant that can produce similar aboveground biomass as maize (Gansberger et al. 2015) but has a higher root:shoot ratio. As cup plant is a perennial plant, it likely transfers more C belowground and exudes more organic substances than maize (Husáková et al. 2018; Pausch and Kuzyakov 2018). In all treatments, the N supply was scheduled to assure that at the end of the pre-cultivation phase, the soils were equally depleted in plant available N. With respect to background N supply, this permitted nearly equal starting conditions for the subsequent stable isotope labeling experiment.
To account for all necessary measurements, each treatment was replicated 19 times (Table 1). At the end of the pre-cultivation phase, the first set of replicates (1–6) was harvested to determine shoot and root biomass, N and C content, and 15N and 13C background concentrations. The second set of replicates (7–12) was labeled with 15NO3− and 13CO2, and gases evolving from soil were measured for the following 10 days. At the end of the experiment, replicates 7–12 were harvested. Replicate 13 was used to determine 13C uptake during 13CO2 pulse labeling and replicates 14–19 were used to determine 13C background values in soil-emitted CO2.
Pre-experimental plant cultivation
The soil for the experiment was collected from a long-term experimental field site of the Höhere Landbauschule Rotthalmünster, Germany (latitude N48°21′, longitude E13°11′, elevation 360 m above sea level) in summer 2016. It was sieved to 10 mm, air dried, and stored at 4 °C until setup of the experiment. The soil was classified as a Haplic Luvisol with a silty loam texture (19% clay, 71% silt, 10% sand). Soil properties were: total C 1.23%, total N 0.14%, C:N 8.76, pH (CaCl2) 6.74.
Seven kg dry soil was mixed with fertilizers (0.14 g P kg− 1 as Ca(H2PO4)2, 0.2 g K kg− 1 as K2SO4 and 0.04 g Mg kg− 1 as MgSO4 * 7 H2O including 0.135 g S kg− 1) and filled in pots of 15 cm diameter and 35 cm height to a bulk density of 1.3 g cm− 3. Soil moisture was adjusted to 60% water holding capacity (WHC, 21.9% gravimetric water content) and watered regularly. TDR soil moisture sensors (Decagon Devices, Pullman, USA) were used to monitor soil water content during plant growth.
Maize seeds (Zea mays L. cv. Ronaldinio) were germinated on wet paper for 4 days. Cup plant (Silphium perfoliatum L.) had been pre-cultivated for 2 years in 5 cm-pots and had 2–4 leaves. Per pot, either one germinated maize seed or one cup plant seedling was transplanted. Plants were cultivated in a climate chamber (Weiss, Loughborough, UK) with a diurnal cycle of 16 h day (light intensity 300 µmol m− 2 s− 1, air temperature 25 °C, relative humidity 50%) and 8 h night (18 °C, 60%). Daytime included 4 h sunrise and 4 h sunset when light intensity, temperature, and relative humidity were gradually adjusted. All pots were fully randomized weekly to avoid microclimatic effects.
Maize N fertilization differed between treatments to achieve plants of different size: Maize S (no N fertilization, small plants), Maize M (0.05 g N kg− 1 (0.35 g N pot− 1, as NH4NO3 split in 7 doses), medium sized plants), and Maize L (0.086 g N kg− 1 (0.6 g N pot− 1, as NH4NO3 split in 7 doses), large plants). Cup plants were fertilized like Maize M (0.05 g N kg− 1 (0.35 g N pot− 1, as NH4NO3 split in 7 doses)).
Experimental 15NO3 − and 13CO2 pulse labeling
Replicates 7–12 of each treatment received 0.1 g N kg− 1 15N-labeled Ca(NO3)2 (~ 60 at% 15N2, Campro Scientific GmbH, Berlin, Germany) 45 days after transplanting. The tracer was dissolved in H2Odest and applied by injection with stainless steel needles as described by Buchen et al. (2016). Briefly, 15N fertilizer solution was injected via 12 needles to 6 depths (2.5, 7.5, 12.5, 17.5, 22.5 and 27.5 cm) aiming for optimal three-dimensional homogenous label distribution (Wu et al. 2011). Per injection point, 10 ml tracer solution were injected via a peristaltic pump (Ismatec, Wertheim, Germany) to simultaneously increase soil water content to 75% water-filled pore space (WFPS, equivalent to 80% WHC).
After injection of 15N tracer solution, all pots were closed with acrylic glass lids with a hole for the plant shoot, leaving a small headspace (2–3 cm) between soil surface and lid. Pots were then sealed with silicone paste (Tacosil 171, Thauer & Co. KG, Dresden, Germany). Plants were labeled with 13C in four separate chambers made from translucent greenhouse film (one for each treatment Maize S, Maize M, Maize L, and Cup plant). In each chamber, 15N labeled replicates 7–12 and the non-labeled replicate 13 were labeled with 13C. To enrich the chamber atmosphere with 13CO2, 60 ml of 5 M H2SO4 were added to 5 g Na213CO3 (99 at%) dissolved in H2Odest in each chamber. For internal chamber ventilation, two fans were installed. The plants were pulse labeled in the 13CO2 enriched atmosphere for 5 h. Before opening each chamber, an air sample was analyzed for CO2 concentration to ensure that maximum amounts of CO2 had been taken up by plants. Chambers were opened and CO2 evolving from the soil was trapped. Replicate 13 was harvested directly after chamber opening to estimate the amount of 13C assimilated during labeling.
To determine natural abundance background of 13C in CO2, replicates 14–19 were used. 0.1 g N kg− 1 was injected using Ca(NO3)2*6 H2O dissolved in H2Obidest, and pots were sealed using the same methods as described above.
Irrigation
After pots had been sealed with silicon paste, plants were irrigated by injecting water through a valve on the bottom of the pots. To irrigate pots without applying too much pressure, peristaltic pumps (Watson-Marlow, Zollikon, Switzerland) with a pumping rate of 1.5 ml min− 1 were used. Target soil moisture was 75% water-filled pore space (WFPS, equivalent to 80% WHC) during the gas sampling phase. To estimate irrigation demand, TDR soil moisture sensors (Decagon Devices, Pullman, USA) were used to monitor soil water content during the experiment in one replicate for each treatment. In addition, all pots were weighed one, three, and five days after 13C labeling to compare whether irrigation demand differed between pots. As pot weights were comparable within treatments, sensor data were used to compare soil moisture.
Gas sampling
One additional pot was filled with dry quartz sand, sealed with silicone paste as described before, and used as a reference to determine background gas concentrations. To flush the headspace of all pots with CO2-free air, pressurized air was first run through a glass column filled with soda lime (pellets made of NaOH and Ca(OH)2 mixture) to remove CO2. For trapping CO2 emitted from soils, the outlet tubes of the pots’ headspaces were connected to glass tubes containing 15 ml of 1 M NaOH solution. Starting one day after labeling, NaOH solution was changed in intervals of 6, 12, or 24 hours. To determine 13C background in CO2, replicates 14–19 were treated similarly: the headspaces were flushed for 6 hours and CO2 was trapped in glass tubes containing 15 ml of 1 M NaOH solution. To estimate the total CO2 efflux, the C concentration of the NaOH solutions was determined with a TIC-analyzer (multi N/C 2100S, Analytik Jena, Jena, Germany). For 13C measurements, CO2 trapped in NaOH was precipitated as SrCO3 with an excess of 1 M SrCl2 solution. The precipitants were centrifuged, washed with deionized water until the pH was neutral, the precipitate was frozen, and then freeze-dried with a rotation vacuum concentrator (RVC 2–25 CDplus, Martin Christ, Osterode am Harz, Germany) and a cooling trap (CT 02–50, Martin Christ, Osterode am Harz, Germany), both connected to a vacuum pump.
13C enrichment in precipitated SrCO3 was analyzed: Natural abundance samples were measured on an elemental analyzer NA 11,100 (CE Instruments, Milano, Italy) linked to a Delta Plus gas-isotope ratio mass spectrometer (Finnigan MAT, Bremen, Germany) via a ConFlo III interface (Finnigan MAT, Bremen, Germany). For enriched samples, depending on capacity, one of the following combinations was used: (i) elemental analyzer Flash 2000 (Thermo Fisher Scientific, Cambridge, UK) linked to a Delta V Advantage gas-isotope ratio mass spectrometer (Thermo Electron, Bremen, Germany) via a ConFlo III interface (Thermo Electron, Bremen, Germany), or (ii) elemental analyzer NA1108 (Fisons-Instruments, Milan, Italy) linked to a Delta C gas-isotope ratio mass spectrometer (Finnigan MAT, Bremen, Germany) via a ConFlo III interface (Thermo Electron Cooperation, Bremen, Germany).
For N2O and N2 sampling, the airflow through the pots’ headspace was interrupted to accumulate gases in the headspace. After 1 h, duplicate samples were taken using a syringe and filled in pre-evacuated 12-ml Exetainer® glass vials (Labco, High Wycombe, UK). Samples were analyzed for N2O concentration using a gas chromatograph (GC 7890A, Agilent, Santa Clara, USA). The analytical precision of the GC was determined by repeated measurements of standard gases (300 ppb N2O) and was consistently < 3%. The second duplicate was analyzed for m/z 28 (14N14N), 29 (14N15N) and 30 (15N15N) of N2 using a modified GasBench II preparation system coupled to an isotope ratio mass spectrometer (MAT 253, Thermo Fisher Scientific, Bremen, Germany) according to Lewicka-Szczebak et al. (2013). This system allows a simultaneous determination of mass ratios 29R (29/28) and 30R (30/28) of three separated gas species (N2, N2 + N2O, and N2O), all measured as N2 gas after N2O reduction in a Cu oven. Typical repeatability of 29R and 30R (1 σ of 3 replicate measurements) was 5 × 10− 7 for both values. For each of the analyzed gas species, the fraction originating from the 15N-labeled pool with respect to total N in the gas sample (Fp) as well as the 15N enrichment of the active 15N-labeled N pool (ap) producing N2O (ap_N2O) or N2 + N2O (ap_N2+N2O) were calculated after Spott et al. (2006) as described in Lewicka-Szczebak et al. (2017).
Harvest and analyses of plant and soil material
Before labeling (44 days after transplanting), replicates 1–6 were harvested. Eleven days after 13C labeling (57 days after transplanting), all labeled plants (replicates 7–12) were harvested. At both harvests, plants were separated into shoots and roots including maize crown roots. As all pots were densely rooted, a separation of rhizosphere and bulk soil was not possible. Roots were shaken gently to separate them from soil and washed. From replicates 7–12, a subsample of root washing water was analyzed for water-extractable organic C (WEOC) and the amount of 13C lost during root washing was determined. To estimate their amount in soil, fine roots were picked by hand from a subsample of soil (~ 400 g soil) for a defined time. All plant material and a soil subsample were dried at 60 °C, milled in a ball mill and analyzed for total C, 13C, total N, and 15N content using an elemental analyzer coupled to a gas-isotope ratio mass spectrometer as described earlier. For determination of water-extractable organic C (WEOC) content, a subsample of fresh soil was analyzed according to Chantigny et al. (2007). Briefly, fresh soil was homogenized with deionized water (1:2 w/v). Samples were centrifuged and filtered with 0.45 µm polyether sulfone filters (Labsolute, Renningen, Germany), split in two subsamples and stored at -20 °C. The extracts were analyzed for total C, organic C, and total N content using a multi N/C® Analyzer (Analytik Jena, Jena, Germany).
For determination of soil mineral N content, a subsample of 50 g was frozen at -20 °C. Frozen samples were extracted with a 2 M KCl solution (1:5 w/v) for 60 min on an overhead shaker (85 rpm). The extracts were filtered with 615 ¼ filter paper (Macherey – Nagel GmbH & Co. KG, Düren, Germany), split in two subsamples, and stored at -20 °C. The extracts were analyzed colorimetrically for the concentrations of NO3− and NH4+ using a San++ continuous flow Analyzer (Skalar Analytical B.V., Breda, The Netherlands). 15N concentration in NH4+ and NO3− was analyzed using an automated sample preparation unit for inorganic nitrogen coupled to a membrane inlet quadrupole mass spectrometer (QMS, GAM 200, InProcess, Bremen, Germany) as described in detail by Eschenbach et al. (2017, 2018). In parallel subsamples, soil water content was determined by oven drying at 105 °C.
Calculations and statistics
Plant N uptake was calculated by multiplying dry mass with the respective tissue N content (root, shoot).
Cumulative CO2 emissions were calculated from CO2 trapped in NaOH, CO2 fluxes were calculated by dividing cumulative CO2 through the respective trapping time (6, 12, or 24 h). 13C recovery in CO2 (13Crecovery; CO2, mg) was calculated as the excess (above background) 13C concentration multiplied with the total CO2 trapped (CO2, mg CO2-C):
where 13CCO2 is the 13C enrichment of CO2 (at%) trapped after labeling and 13CNA; CO2 is the natural 13C background (at%) from unlabeled plants (replicates 14–19). 13C recovery in soil (13Crecovery; soil, mg) was calculated as follows:
where 13Csoil is the enrichment of 13C (at%) of the soil C pool after labeling, 13CNA; soil is the natural abundance of 13C in soil before labeling (at%), Csoil is the total content of C in soil (mg g− 1), and masssoil is the mass of soil per pot (g). Relative 13Crecovery (% of recovered 13C) of a particular pool (CO2, soil) was calculated by dividing the amount of 13C recovered in that pool (13Crecovery; pool) by the sum of the amount of 13C recovered in all pools (CO2, shoot, root, soil, root washing water).
Total N2O fluxes (ftot, µg N kg− 1 h− 1) were calculated from GC measurements:
where CH is the mass concentration in the headspace and CB is the background concentration in the reference pot (µg N m− 3) corrected by the chamber temperature according to the ideal gas law, t is the accumulation time (h), V is the volume of the headspace (m3), and m is the dry mass of soil per pot (kg).
We calculated the 15N enrichment of the active NO3− pool undergoing denitrification (ap_N2O, ap_N2O + N2) from the non-random distribution of N2 and/or N2O isotopologues using calculations by Spott et al. (2006) as described by Buchen et al. (2016) and Lewicka-Szczebak et al. (2017):
where ap is the 15N abundance of the 15N labeled NO3− pool undergoing denitrification, abgd is the measured 15N abundance of atmospheric background N2, am is the measured 15N abundance of N2 or N2O
and 30xm is the measured fraction of m/z 30 in N2 and converted N2O:
The fraction of N derived from the active NO3− pool (Fp) was calculated using Eq. (8) if 30R was significant and otherwise Eq. (9) was used. In the latter case, ap_N2O was assumed to be identical with ap_N2 and ap_N2O + N2 and was thus used when calculating Fp_N2 and Fp_N2O + N2 from Eq. 9. If ap_N2O of a sampling date was not available, the mean value from the other replicates from the same sampling date was used as best estimate. Fp calculated from Eq. (9) with a given 29R is relatively insensitive to changes in ap between 0.4 and 0.6 since the nominator yields values between 0.48 and 0.5. Hence, uncertainty in the estimation of ap within that range causes minor uncertainty in calculated Fp (Well and Myrold 1999). Because ap values in our study were typically between 0.4 and 0.6, we assume that uncertainty in Fp calculation from the missing of individual ap values was small.
where lower case sa and bgd denote sample and background (ambient air), respectively.
Fp values were multiplied with respective total sample N concentration (N2O, N2) to obtain pool-derived gas concentrations (in ppm). Then, pool-derived fluxes (fp) were calculated from concentrations similar to Eq. (4). The same calculations were used for N2O, N2, and N2O + N2, resulting in respective values for fractions of pool-derived N and for the respective 15N abundances of the active N pools (ap_N2O, ap_N2, ap_N2O + N2). Non-pool derived N2O fluxes were calculated by subtracting pool-derived N2O fluxes from total N2O fluxes.
The ratio of denitrification end products was calculated from pool-derived N2O (fp_N2O) and N2O + N2 (fp_N2O + N2):
Cumulative N2O, N2, and N2O + N2 emissions were calculated by linear interpolation of fluxes. The % of N2 + N2O emitted with respect to added N was estimated by dividing cumulative pool-derived N2O + N2 emission by the amount of N added with 15NO3− labeling.
All statistical analyses were performed using the statistical software R version 3.6.0 (R Core Team 2019). Means and standard deviations were calculated over all replicates. For harvest data, cumulative CO2, and 13C recovery a one-way ANOVA was calculated followed by Tukey’s HSD post-hoc test at p ≤ 0.05 to separate treatment effects. As cumulative N emissions were not normally distributed, the Kruskal-Wallis rank sum test was used followed by LSD post-hoc test at p ≤ 0.05 to separate treatment effects. To compare soil and plant samples between harvests, and to test whether soil 15a_NO3− contents at final harvest and ap_N2O or ap_ N2O + N2 at last sampling date differed, a t-test was used at p ≤ 0.05. Simple linear regression models were tested to analyze the effects of soil and plant parameters on CO2 and N fluxes and cumulative emissions.
Results
Plant growth after labeling
Shoot dry matter increased significantly in all treatments between the first and the second harvest, but differences between treatments did not change (Table 2, results of 1st harvest are displayed in supplementary table S1). Root dry matter significantly decreased in Maize S and M until the end of the experiment. Increases in root dry matter in Maize L and Cup plant were not significant. Root:shoot ratio decreased in all treatments but remained twice as high in cup plant compared to maize, which is typical for perennial plants compared to annual plants (Husáková et al. 2018). Nitrogen content increased in previously unfertilized Maize S plants and was similar in all maize treatments at the final harvest. Nitrogen content in cup plant shoots and roots was significantly greater than in all maize treatments and total N uptake corresponded with N fertilization Maize L > Maize M = Cup plant > Maize S. Soil NO3− content analyzed at the end of experiment was on average still twice as high in Maize S compared to all other treatments.
Total N2O and pool-derived N2O + N2 fluxes and cumulative emissions
Total and pool-derived N fluxes were highest in Maize S but followed a similar pattern in all treatments (Fig. 2a + c). Total N2O fluxes strongly increased in Maize S reaching highest values on day 3 (11.3 µg N2O-N kg− 1 h− 1, Fig. 2a) and a second smaller peak on day 6 (5.9 µg N2O-N kg− 1 h− 1). Pool-derived N2O + N2 fluxes followed a similar general pattern as total N2O fluxes (Fig. 2c) and peaks were detected at similar times as N2O peaks, in Maize S on day 3 (48 µg N2-N kg− 1 h− 1) and larger peaks on day 6.5 (67 µg N2-N kg− 1 h− 1) and day 9.5 (61 µg N2-N kg− 1 h− 1). Total N2O and pool-derived N2O + N2 fluxes in all other treatments followed a similar pattern but on a lower scale. The product ratio (N2O/(N2O + N2), Fig. 2d) of pool-derived fluxes followed a similar pattern in all treatments. The product ratio decreased for the first days after onset of incubation reaching values between 0.2 and 0.5 as N2 became the dominant end product of denitrification. It shortly increased and peaked on day 3.5, then decreased again until day 6 to values between 0 and 0.2. After day 6.5, the product ratio ranged between 0 and 0.5 until the end.
Total and pool-derived cumulative N emissions were 20–43 times higher in Maize S compared to all other treatments (Table 3). No significant differences were detected between the other treatments. Similarly, recovery of added NO3− in N2O + N2 was highest in Maize S and not significantly different in the other treatments. The mean N2O/(N2O + N2) ratio ranged from 0.14 to 0.16 in maize treatments and was 0.24 in cup plant.
15N enrichment of N pools and pool-derived fraction of N2O
Treatments did not exhibit continuous patterns of ap and Fp values throughout the experiment. The fraction of N2O derived from the active labeled NO3− pool (Fp_N2O) decreased during the experiment showing that the contribution of N2O from sources other than the labeled NO3− pool increased with time (Fig. 2b). Fp_N2O was close to 1.0 in Maize S for three days after labeling, then decreased to 0.4 on day 5, and ranged between 0.5 and 0.8 until the end of the experiment. For the other treatments, Fp_N2O continuously decreased until day 5/6. After day 6.5, Fp_N2O increased in Maize M and L, fluctuating between 0.1 and 0.6. At the last day, Fp_N2O was < 0.07 in all treatments.
The time course of 15N enrichment of the active NO3− pool producing N2O and N2 (ap_N2O, ap_N2O + N2) was different in Maize S than in the other treatments. During the first days after labeling, 15N enrichment of the active NO3− pool producing N2O and N2 (ap_N2O, ap_N2O + N2) was close to 60 at% in all treatments (Fig. 3). In Maize S, ap_N2O and ap_N2O + N2 were higher than 50 at% during the whole experiment and only decreased on the last day. In all other treatments, ap_N2O and ap_N2O + N2 continuously decreased until day 6.5. On day 7, ap-values were higher than 50 at% and decreased again until the end of the experiment. 15N enrichment of the total soil NO3− pool (15a_NO3−) was measured at final harvest and was mostly significantly lower (p < 0.05) than 15N enrichment of the active NO3− pool producing N2O and N2 (ap_N2O and ap_N2O + N2) from the last gas measurement (Fig. 3, Supplementary table S2).
Soil moisture and its effect on N fluxes
Data from soil moisture sensors showed that soil moisture content was higher in Maize S than all other treatments for the first days after labeling (Fig. 4). However, it was lower than the targeted value of 75%WFPS. As all plants respired large amounts of water, it took a few days to adjust irrigation amounts to plant water demand, and soil moisture could not be kept constant throughout the experiment.
In Maize S and Cup plant, soil moisture increased with irrigation three days after labeling reaching values around 70%WFPS. In Maize M and L, soil moisture increased five days after labeling reaching values around 55%WFPS. In Cup plant, soil moisture stayed on a similar level around 70%WFPS with fluctuations due to water uptake and irrigation. Although soil moisture was in a similar range in Maize S and Cup plant from day 4 to 6 and in all maize treatments after day 7, total N2O and pool-derived N2O + N2 fluxes were always highest in Maize S. Thus, we did not find significant relationships between N fluxes and soil moisture during the experiment indicating that soil moisture was not the only factor controlling gaseous N losses (Table 5, supplementary table S3, supplementary figure S1). However, the N2O/(N2O + N2) ratio of pool-derived fluxes decreased with increasing soil moisture (%WFPS, adj. R²=0.14, p < 0.05) indicating that increasing soil moisture stimulated N2O reduction.
CO2 and 13CO2 efflux
The time course of cumulative CO2 efflux and 13C enrichment in CO2 was similar in all treatments (Fig. 5a + b). CO2 efflux was similar in Maize M and Maize L where it increased almost linearly during the whole experiment, and total cumulative CO2 was only slightly higher in Maize L than in Maize M (Table 4). Lowest cumulative CO2 efflux was measured under Maize S plants where efflux decreased considerably after 1.5 days. Cumulative CO2 efflux under cup plants was significantly lower than from Maize L and not statistically different from the other two maize treatments (Table 4). Overall, cumulative CO2 efflux was positively correlated with root dry matter at final harvest (adj. R²=0.36, p < 0.01, Table 5).
13C enrichment in CO2 strongly decreased in all treatments two days after labeling until the end of CO2 sampling (Fig. 5b). Highest 13C enrichment of soil emitted CO2 was measured under Cup plant and lowest in Maize L. It strongly decreased two days after labeling until the end of CO2 sampling. No statistically significant differences (p < 0.05) were found in relative 13C recovery in CO2, soil, or soil + CO2 (Table 4), but overall, mean relative 13C recovery in soil increased with root dry matter, indicating that root-derived C recovered in soil increased with root biomass.
Interactions between N emissions, soil NO3 − content, and Corg availability
Simple linear regression models were tested to identify effects of plant growth, N uptake, and Corg availability on cumulative N2O and N2 emissions (Table 5, supplementary table S3). Total cumulative N2O and pool-derived cumulative N2O + N2 emissions were significantly (p < 0.01) negatively correlated with root dry matter (adj. R²=0.41 and adj. R2 = 0.32, respectively) and plant N uptake (adj. R²=0.49 and adj. R2 = 0.33, respectively) indicating that gaseous N losses were highest under plants with small root system and low N uptake. In addition, total cumulative N2O emissions and soil NO3− content at final harvest were positively correlated (adj. R²=0.10, p < 0.05). As cumulative CO2 emissions were positively correlated with root dry matter and cumulative N emissions negatively, total cumulative N2O and pool-derived cumulative N2O + N2 emissions were negatively correlated with cumulative CO2 efflux (adj. R²=0.36, p < 0.01). No correlations were found between total or pool-derived cumulative N emissions or N2O/(N2O + N2) ratio and 13C recovery in soil and/or CO2. However, we identified a weak but significant positive relationship between N2O/(N2O + N2) of pool-derived fluxes and CO2 efflux (adj. R²=0.11, p < 0.01).
Discussion
Effect of soil moisture and plant N uptake on N2O and N2 emissions
Cumulative N emissions were 20–40 times higher in Maize S compared to all other treatments. Plant transpiration strongly affected soil moisture which was highest in Maize S during the first 4 days after 13C labeling. Soil moisture is an important control of denitrification as it regulates O2 concentration and diffusion in soil (Schlüter et al. 2018, Rohe et al. 2020). Plant roots constantly alter soil moisture and its distribution in soil by root water uptake. Accordingly, previous studies reported that plant growth controlled soil moisture and denitrification rates (Bakken 1988; von Rheinbaben and Trolldenier 1984). In our study, when soil moisture increased with irrigation, N2O and, especially, N2 fluxes increased shortly thereafter. Furthermore, the N2O/(N2O + N2) ratio of pool-derived fluxes decreased with increasing soil moisture which is consistent with N2 being the dominant end product of denitrification under high WFPS (Davidson 1991). Although soil moisture was highest in Cup plant from day 4 to 8, and similar in Maize treatments from day 6 to 9, N fluxes were highest in Maize S throughout the experiment. Thus, differences in soil moisture alone cannot explain the vast differences in N fluxes between Maize S and the other treatments in our study.
Maize S plants were characterized by lowest root dry matter of all treatments and lower shoot dry matter compared to the other maize treatments. Furthermore, soil NO3− content at final harvest was more than twice as high in Maize S compared to all other treatments. The relationship between soil NO3− content at the end of the experiment and cumulative N2O emissions was weak (0.10, p < 0.05). However, N uptake was negatively correlated with total cumulative N2O emissions and pool-derived cumulative N2O + N2 emissions (adj. R²=0.49, p < 0.001 and adj. R2 = 0.33, p < 0.01, respectively) indicating that NO3− availability played an important role in regulating denitrification. Our results show clearly that an increase in soil moisture led to increasing N2O + N2 fluxes, but N fluxes remained on a low level when NO3− availability was low due to rapid plant N uptake. Only when both N and water uptake were low, high NO3− availability and high soil moisture led to strongly increased N losses.
Effect of nitrate availability and soil moisture on pools and processes contributing to N2O formation
Different NO3− pools and N turnover processes contributed to N2O formation throughout the experiment. The 15N enrichment of the total soil NO3− pool (15a_NO3−) decreased from 60 at% after labeling to 10–30 at% until the end of the experiment as unlabeled organic N was mineralized and diluted the labeled NO3− pool (Buchen et al. 2016; Deppe et al. 2017). Thus, denitrification of unlabeled NO3−, as well as nitrification, nitrifier denitrification, and coupled nitrification-denitrification may have contributed to N2O formation (van Groenigen et al. 2015; Wrage-Mönnig et al. 2018; Wrage et al. 2001).
In Maize S, 15a_NO3− at final harvest was significantly higher than in all other treatments, indicating that nitrification was less relevant in this treatment. Accordingly, the fraction of N2O derived from the active labeled NO3− pool (Fp_N2O) was > 0.5 throughout the experiment indicating that most N2O was lost through denitrification from labeled NO3−. The 15N enrichment of the active NO3− pool producing N2O and N2O + N2 (ap_N2O and ap_N2O + N2) stayed close to its initial value of 60 at% highlighting that N2O and N2 were mainly lost from anoxic microsites where labeled NO3− had not been diluted by nitrification (Buchen et al. 2016).
In the other treatments, ap_N2O, ap_N2, and Fp_N2O did not exhibit continuous trends. While values first decreased due to dilution with NO3− from nitrification, values increased after day 6.5 (Fig. 3), presumably due to the slight increase in WFPS after irrigation on day 6.5 (Fig. 4). Increasing soil moisture increased denitrification rates in anoxic hotspots, which corresponds with increasing N2O and especially N2 fluxes in Maize M and L. At the same time, it restricted nitrification and thus decreased the share of nitrification-dependent processes contributing to N2O formation (reflected in increasing Fp_N2O). Simultaneously increasing ap_N2O and ap_N2O + N2 in Maize M and L on Day 7 indicate that 15N enrichment of the active labeled NO3− pool was still close to 60 at% in microsites where denitrification took place. The rise of actual ap-values back towards initial values is in line with a change in the anaerobic volume where denitrification occurs (Bergstermann et al. 2011). A recent study conducted with the same soil but without plants showed that O2 concentration in repacked soil cores was highly variable and average O2 saturation decreased with increasing soil moisture while the anaerobic soil volume fraction increased with increasing soil moisture and soil depth (Rohe et al. 2020). After day 6.5, N2O + N2 were predominately lost from domains that had been continuously anoxic or were most distant from oxic domains and thus were less diluted with unlabeled NO3−. We anticipate that in these microsites O2 concentrations had been low enough to prevent nitrification during the first days so that the labeled pool was not diluted by unlabeled NO3− from nitrification.
While fungal co-denitrification has been reported as the dominant N2O source in a planted soil with high NO3− content (Laughlin and Stevens 2002), our data provide no indications for co-denitrification, because ap_N2O and ap_N2O + N2 were always higher than 15a_NO3−, but co-denitrification would lead to ap lower than 15a_NO3− due to hybrid formation of N2O or N2 (Spott and Stange 2007).
Thus, in our study, N2O and N2 fluxes mainly derived from denitrification of labeled 15NO3− in anoxic microsites, while nitrification simultaneously occurred in more oxic parts of the soil, potentially contributing to formation of unlabeled N2O.
Effect of root-derived C on N emissions
One of the core hypotheses of this study was that availability of root-derived C is a key driver of denitrification in planted soils. It was based on a number of studies reporting higher denitrification activity in rhizosphere compared to bulk soil which was explained by higher soil C (Hamonts et al. 2013; Klemedtsson et al. 1987; Malique et al. 2019 ; Smith and Tiedje 1979). Detectable rhizodeposition and C flow into belowground respiration result from C translocation from shoots to roots (Remus and Augustin 2016). Thus, we used 13CO2 pulse labeling to trace C translocation from shoots to roots, its release by roots into the soil, and 13CO2 emitted from soil.
We found a positive correlation between root dry matter at final harvest and cumulative CO2 efflux (adj. R²=0.36, p < 0.01) and, on average, relative 13C recovery in soil increased with increasing root dry matter indicating that root exudation increased with root dry matter. However, we could not detect any relationship between total or pool-derived N fluxes and total CO2 efflux or root-derived C and cumulative N emissions or the ratio of gaseous products.
Most previous studies investigating plant effects on denitrification did not measure N2O and N2 emissions under growing plants, but either potential denitrification (Klemedtsson et al. 1987; Malique et al. 2019; Smith and Tiedje 1979) or denitrification capacity (Hamonts et al. 2013) from soil samples taken from rhizosphere and/or bulk soil. In those studies, conducted under anoxic conditions with unlimited NO3− supply, higher C availability in rhizosphere soil samples led to higher emissions of N2O and N2. However, when separation of bulk soil and rhizosphere was not well-defined due to densely rooted soil in pots, no differences in potential denitrification were found (Malique et al. 2019). In the few studies with growing plants, higher denitrification rates were measured during the first weeks after emergence (Senbayram et al. 2020), with poorly growing plants (von Rheinbaben and Trolldenier 1984), or when root biomass was decreasing (Haider et al. 1987). No stimulation of denitrification was found when actively growing maize plants were compared to unplanted soil (Haider et al. 1985). Accordingly, root-derived C may stimulate denitrification when soil NO3− is not limited. We were not able to estimate the effect of C availability on denitrification as NO3− limitation and O2 inhibition were the factors controlling denitrification in our study.
Plant root effects on denitrification
The activity of denitrifying microorganisms in soil is primarily controlled by availability of O2, NO3−, and Corg (proximal factors, Groffman et al. 1988). Plant roots affect these through rhizodeposition, root respiration, N and water uptake (distal factors, Groffman et al. 1988). Figure 6 shows how proximal and distal factors change during plant and root growth and how these affect denitrification in planted soil. The presented conceptual drawing is based on two assumptions: (i) NO3−-based fertilizer is only added before plant growth and (ii) root water uptake is the main regulator of soil moisture.
Conceptual drawing of plant root effects (distal factors, green/grey) on drivers of denitrification (proximal factors, light grey) and potential N2O + N2 losses (orange/dark grey). Based on two assumptions: (i) NO3−-based fertilizer is only added before plant growth and (ii) root water uptake is the main regulator of soil moisture
In most agricultural soils, available Corg is low. With increasing root growth, rhizodeposition and root turnover increase Corg availability. At the same time, root respiration and microbial activity increase, decreasing O2 concentrations in the rhizosphere. This offers favorable conditions for denitrifying microorganisms as long as sufficient NO3− is available (Klemedtsson et al., 1987; Senbayram et al., 2020; Stefanson, 1972). As N uptake increases with plant and root growth, NO3− becomes limited for denitrifiers (Haider et al., 1985). Furthermore, increasing water uptake decreases soil moisture and restricts formation of anoxic microsites for denitrification (Bakken 1988, von Rheinbaben and Trolldenier, 1984). Accordingly, our study showed that with increasing plant and root growth, plant water and N uptake became the most important controls of denitrification. Similarly, soil moisture can vary strongly under field conditions depending on precipitation and plant water uptake. When NO3− is available (i.e. after fertilization), increasing soil moisture after rainfall can lead to strongly increased N2O + N2 emissions (Buchen et al. 2017, Ruser et al. 2017).
Overall, plants continuously change boundary conditions and substrate availability for denitrification in soil, and it requires high technical input and equipment to keep experimental conditions stable and controlled. However, as plants do control these conditions so strongly, it is very exciting and very important to further investigate these processes to understand and predict N cycling, denitrification, and gaseous N losses on the field scale. Further research is thus indispensable.
Conclusions
We aimed to investigate how plants control the main substrates for denitrification (NO3− and Corg) through N uptake and root exudation. To our knowledge, this is the first study combining in situ measurements of N2O + N2 fluxes with estimations of root-derived C availability.
Plant water uptake was a main factor controlling soil moisture and, thus, daily N2O + N2 fluxes, cumulative N emissions, and N2O production pathways. However, N fluxes remained on a low level when NO3− availability was low due to rapid plant N uptake. Only when both N and water uptake were low, high NO3− availability and high soil moisture led to strongly increased N losses. Our study provides evidence that most N losses originated from denitrification in small anoxic hotspots where NO3− was not diluted by nitrification. Simultaneously occurring nitrification in oxic parts of the soil potentially contributed to formation of unlabeled N2O.
Total CO2 efflux was positively correlated with root dry matter, but there was no indication of any relationship between recovered 13C from root exudation and cumulative N emissions. We anticipate that higher Corg availability in pots with large root systems did not lead to higher denitrification rates, as NO3− was limiting denitrification due to plant N uptake. Overall, we conclude that root-derived C stimulates denitrification only when soil NO3− is not limited and low O2 concentrations enable denitrification.
References
Bakken LR (1988) Denitrification under different cultivated plants: effects of soil moisture tension, nitrate concentration, and photosynthetic activity. Biol Fertil Soils 6:271–278
Bergstermann A, Cárdenas L, Bol R, Gilliam L, Goulding K, Meijide A, Scholefield D, Vellejo A, Well R (2011) Effect of antecedent soil moisture conditions on emissions and isotopologue distribution of N2O during denitrification. Soil Biol Biochem 43:240–250. https://doi.org/10.1016/j.soilbio.2010.10.003
Buchen C, Lewicka-Szczebak D, Fuß R, Helrich M, Flessa H, Well R (2016) Fluxes of N2 and N2O and contributing processes in summer after grassland renewal and grassland conversion to maize cropping on a Plaggic Anthrosol and a Histic Gleysol. Soil Biol Biochem 101:6–19. https://doi.org/10.1016/j.soilbio.2016.06.028
Buchen C, Well R, Helfrich M, Fuß R, Kayser M, Gensior A, Benke M, Flessa H (2017) Soil mineral N dynamics and N2O emissions following grassland renewal. Agric Ecosyst Environ 246:325–342. https://doi.org/10.1016/j.agee.2017.06.013
Burford JR, Bremner JM (1975) Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biol Biochem 7:389–394. https://doi.org/10.1016/0038-0717(75)90055-3
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174:3–11. https://doi.org/10.1002/jpln.201000085
Chantigny MH, Angers DA, Kaiser K, Kalbitz K (2007) Extraction and characterization of dissolved organic matter. In: Carter MR, Gregorich DG (ed) Soil sampling and methods of analysis, 2nd edn, Taylor & Francis Group, Abingdon
Cheng W, Coleman DC, Carroll CR, Hoffman CA (1993) In situ measurement of root respiration and soluble C concentrations in the rhizosphere. Soil Biol Biochem 25:1189–1196. https://doi.org/10.1016/0038-0717(93)90214-V
Davidson EA (1991) Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In: Rogers JE, Whitman WB (eds) Microbial production and consumption of greenhouse gases: methane, nitrogen oxides and halomethanes. American Society for Microbiology, Washington (DC), pp 219–235
Deppe M, Well R, Giesemann A, Spott O, Flessa H (2017) Soil N2O fluxes and related processes in laboratory incubations simulating ammonium fertilizer depots. Soil Biol Biochem 104:68–80. https://doi.org/10.1016/j.soilbio.2016.10.005
Eschenbach W, Lewicka-Szczebak D, Stange CF, Dyckmans J, Well R (2017) Measuring 15N abundance and concentration of aqueous nitrate, nitrite, and ammonium by membrane inlet quadrupole mass spectrometry. Anal Chem 89:6076–6081. https://doi.org/10.1021/acs.analchem.7b00724
Eschenbach W, Well R, Dyckmans J (2018) NO reduction to N2O improves nitrate 15N abundance analysis by membrane inlet quadrupole mass spectrometry. Anal Chem 90:11216–11218. https://doi.org/10.1021/acs.analchem.8b02956
Firestone MK, Smith MS, Firestone RB, Tiedje JM (1979) The influence of nitrate, nitrite, and oxygen on the composition of the gaseous products of denitrification in soil. Soil Sci Soc Am J 43:1140–1144
Gansberger P, Montgomery LFR, Liebhard P (2015) Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind Crop Prod 63:362–372
Gransee A, Wittenmayer L (2000) Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J Plant Nutr Soil Sci 163:381–385
Groffman PM, Tiedje JM, Robertson GP, Christensen S (1988) Denitrification at different temporal and geographical scales: proximal and distal controls. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems. CAB International, Wallingford, pp 174–192
Groffman PM, Altabet MA, Böhlke JK, Butterbach-Bahl K, David MB, Firestone MK, Giblin AE, Kana TM, Nielsen LP, Voytek MA (2006) Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol Appl 16:2091–2122. https://doi.org/10.1890/1051-0761(2006)016[2091:MFMDDA]2.0.CO;2
Haider K, Mosier A, Heinemeyer O (1985) Phytotron experiments to evaluate the effect of growing plants on denitrification. Soil Sci Soc Am J 49:636–641
Haider K, Mosier A, Heinemeyer O (1987) The effect of growing plants on dentrification at high soil nitrate concentrations. Soil Sci Soc Am J 51:97–102
Hamonts K, Clough TJ, Stewart A, Clinton PW, Richardson AE, Wakelin AE, O’Callaghan M, Condron LM (2013) Effect of nitrogen and waterlogging on denitrifier gene abundance, community structure and activity in the rhizosphere of wheat. FEMS Microbiol Ecol 83:568–584. https://doi.org/10.1111/1574-6941.12015
Hauck RD, Melsted SW (1956) Some aspects of the problem of evaluating denitrification in soils. Soil Sci Soc Am J 20:361–364. https://doi.org/10.2136/sssaj1956.03615995002000030017x
Husáková I, Weiner J, Münzbergová Z (2018) Species traits and shoot-root biomass allocation in 20 dry-grassland species. J Plant Ecol 11:273–285. https://doi.org/10.1093/jpe/rtw143
Klemedtsson L, Svensson BH, Rosswall T (1987) Dinitrogen and nitrous oxide produced by denitrification and nitrification in soil with and without barley plants. Plant Soil 99:303–319. https://doi.org/10.1007/BF02370877
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. J Plant Nutr Soil Sci 163:421–431. https://doi.org/10.1002/1522-2624(200008)163:4<421::AID-JPLN421>3.0.CO;2-R
Laughlin RJ, Stevens RJ (2002) Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Sci Soc Am J 66:1540–1548. https://doi.org/10.2136/sssaj2002.1540
Lewicka-Szczebak D, Well R, Giesemann A, Rohe L, Wolf U (2013) An enhanced technique for automated determination of 15N signatures of N2, (N2 + N2O) and N2O in gas samples. Rapid Commun Mass Spectrom 27:1548–1558. https://doi.org/10.1002/rcm.6605
Lewicka-Szczebak D, Augustin J, Giesemann A, Well R (2017) Quantifying N2O reduction to N2 based on N2O isotopocules-validation with independent methods (helium incubation and 15N gas flux method). Biogeosciences 14:711–732. https://doi.org/10.5194/bg-14-711-2017
Malhi SS, Johnston AM, Schoenau JJ, Wang ZH, Vera CL (2011) Seasonal biomass accumulation and nutrient uptake of wheat, barley and oat on a Black Chernozem Soil in Saskatchewan. Can J Plant Sci 86:1005–1014. https://doi.org/10.4141/p05-116
Malique F, Ke P, Böttcher J, Dannenman M, Butterbach-Bahl K (2019) Interactive plant and soil effects on denitrification potential in agricultural soils. Plant Soil 20:6603
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396. https://doi.org/10.1051/agro:2003011
Novák V, Vidovič J (2003) Transpiration and nutrient uptake dynamics in maize (Zea mays L.). Ecol Modell 166:99–107. https://doi.org/10.1016/S0304-3800(03)00102-9
Paterson E, Sim A (1999) Rhizodeposition and C-partitioning of Lolium perenne in axenic culture affected by nitrogen supply and defoliation. Plant Soil 216:155–164. https://doi.org/10.1023/A:1004789407065
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol 24:1–12. https://doi.org/10.1111/gcb.13850
Philippot L, Hallin S, Börjesson G, Baggs EM (2009) Biochemical cycling in the rhizosphere having an impact on global change. Plant Soil 321:61–81. https://doi.org/10.1007/s11104-008-9796-9
R Core Team (2019) R: A Language and Environment for Statistical Computing
Remus R, Augustin J (2016) Dynamic linking of 14C partitioning with shoot growth allows a precise determination of plant-derived C input to soil. Plant Soil 408:493–513. https://doi.org/10.1007/s11104-016-3006-y
Rohe L, Apelt B, Vogel HJ, Well R, Wu GM, Schlüter S (2020) Denitrification in soil as a function of oxygen supply and demand at the microscale. Biogeosci Discuss. https://doi.org/10.5194/bg-2020-221 (in review)
Ruser R, Fuß R, Andres M, Hegewald H, Kesenheimer K, Köbke S, Räbiger T, Quinones TS, Augustin J, Christen O, Dittert K, Kage H, Lewandowski I, Prochnow A, Stichnothe H, Flessa H (2017) Nitrous oxide emissions from winter oilseed rape cultivation. Agric Ecosyst Environ 249:57–69. https://doi.org/10.1016/j.agee.2017.07.039(2017)
Schlüter S, Henjes S, Zawallich J, Bergaust L, Horn M, Ippisch O, Vogel HJ, Dörsch P (2018) Denitrification in soil aggregate analogues-effect of aggregate size and oxygen diffusion. Front Environ Sci 6:1–10. https://doi.org/10.3389/fenvs.2018.00017
Scholefield D, Hawkins JMBB, Jackson SM (1997) Development of a helium atmosphere soil incubation technique for direct measurement of nitrous oxide and dinitrogen fluxes during denitrification. Soil Biol Biochem 29:1345–1352. https://doi.org/10.1016/S0038-0717(97)00021-7
Senbayram M, Well R, Shan J, Bol R, Burkart S, Jones DL, Wu D (2020) Rhizosphere processes in nitrate-rich barley soil tripled both N2O and N2 losses due to enhanced bacterial and fungal denitrification. Plant Soil. https://doi.org/10.1007/s11104-020-04457-9
Smith MS, Tiedje JM (1979) The effect of roots on soil denitrification. Soil Sci Soc Am J 43:951–955
Spott O, Stange CF (2007) A new mathematical approach for calculation the contribution of anammox, denitrification and atmosphere to an N2 mixture based on a 15N tracer technique. Rapid Commun Mass Spectrom 21:2398–2406. https://doi.org/10.1002/rcm
Spott O, Russow R, Apelt B, Stange CF (2006) A 15N-aided artificial atmosphere gas flow technique for online determination of soil N2 release using the zeolite Köstrohlith SX6. Rapid Commun Mass Spectrom 20:3267–3274. https://doi.org/10.1002/rcm
Stefanson RC (1972) Soil denitrification in sealed soil-plant systems I. Effect of plants, soil water content and soil organic matter content. Plant Soil 127:113–127
van Groenigen JW, Huygens D, Boeckx P, Kuyper ThW, Lubbers IM, Rütting T, Groffman PM (2015) The soil N cycle: New insights and key challenges. Soil 1:235–256. https://doi.org/10.5194/soil-1-235-2015
Vancura V (1964) Root exudates of plants I. Analysis of root exudates of barley and wheat in their initial phases of growth. Plant Soil 21:231–248. https://doi.org/10.1007/BF02139643
Vancura V, Hovadik A (1965) Root exudates of plants II. Composition of root exudates of some vegetables. Plant Soil 22:21–32
Vinther FP (1984) Total denitrification and the ratio between N2O and N2 during the growth of spring barley. Plant Soil 76:227–232. https://doi.org/10.1007/BF02205582
von Rheinbaben W, Trolldenier G (1984) Influence of plant growth on denitrification in relation to soil moisture and potassium nutrition. Zeitschrift für Pflanzenernärung Bodenkd 147:730–738. https://doi.org/10.1002/jpln.19841470610
Well R, Myrold DD (1999) Laboratory evaluation of a new method for in situ measurement of denitrification in water-saturated soils. Soil Biol Biochem 31:1109–1119. https://doi.org/10.1016/S0038-0717(99)00029-2
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) The role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732. https://doi.org/10.1016/S0038-0717(01)00096-7
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O (2018) The role of nitrifier denitrication in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3–A16. https://doi.org/10.1016/j.soilbio.2018.03.020
Wu H, Dannenmann M, Fanselow N, Wolf B, Yao Z, Wu X, Brüggemann N, Zheng X, Han X, Dittert K, Butterbach-Bahl K (2011) Feedback of grazing on gross rates of N mineralization and inorganic N partitioning in steppe soils of Inner Mongolia. Plant Soil 340:127–139. https://doi.org/10.1007/s11104-010-0575-z
Yoshinari T, Knowles R (1976) Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun 69:705–710. https://doi.org/10.1016/0006-291X(76)90932-3
Acknowledgements
The authors thank Simone Urstadt, Finn Malinowski, Bridith Angulo Schipper, Ilse Thaufelder, and Jakob Streuber for help during experimental and laboratory work. We are thankful to Tomor Krasniqi for weighing endless numbers of C:N samples and to the Centre for Stable Isotope Research and Analysis of the University of Göttingen for analysis of C and N isotopes of soil and plant samples. Further, we thank Dr. Daniel Ziehe and Kerstin Gilke for GC analyses, Dr. Anette Giesemann and Martina Heuer for IRMS analyses of gas samples, and Dr. Wolfram Eschenbach for analysis of 15N in NO3− and NH4+. We thank Prof. Dr. Jürgen Böttcher for soil classification and Prof. Kenneth Albrecht and Dr. Pedro Gerstberger for supplying cup plant seedlings. We thank Dr. Bernd Steingrobe for his feedback on the manuscript. Furthermore, we acknowledge two anonymous reviewers for their advice to improve the quality of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through the research unit DFG-FOR 2337 (DI 546/4 − 1, We 1904/10 − 2): Denitrification in Agricultural Soils: Integrated Control and Modelling at Various Scales (DASIM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jorge Durán.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 33.7 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rummel, P.S., Well, R., Pfeiffer, B. et al. Nitrate uptake and carbon exudation – do plant roots stimulate or inhibit denitrification?. Plant Soil 459, 217–233 (2021). https://doi.org/10.1007/s11104-020-04750-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04750-7