Abstract
Background and aims
Leaf litter decomposition constitutes a valuable ecosystem service by recycling nutrients, transferring energy, and sequestrating carbon (C) in terrestrial ecosystems. However, variations in microclimate and decomposers among studies have impeded a mechanistic understanding of what controls litter decomposition. We conducted a “common plot” experiment leveling-off impacts of environmental and biotic factors to explore roles of the initial leaf litter traits in decomposition.
Methods
The experiment was carried out in an evergreen broadleaf forest in the Wuyi Mountains from May 2017 to August 2018. Leaf litter was collected from Populus deltoids in a nitrogen (N) addition experiment at Dongtai Forest Farm, Liriodendron chinense × tulipifera and Platanus acerifolia on campus Nanjing Forestry University, and Quercus variabilis and Pinus taeda at Xiashu Forest Farm.
Results
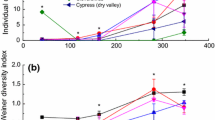
Our results showed that N addition significantly increased leaf litter decomposition of P. deltoids. This may result from N induced increases in litter quality, indicated by a high N content and a low C:N ratio of leaf litter. Across the five tree species (with no litter from P. deltoids under N addition treatment), we found litter decomposition of the coniferous tree (P. taeda) was much lower than that of the other four broadleaf trees due to its lower litter quality. Multiple regression analyses indicated that the initial C:N ratio and sodium (Na) content that was critical for decomposers dominated decomposition.
Conclusions
Our results confirmed the importance of the initial litter quality, such as C:N ratio, in regulating leaf litter decomposition. In addition, our results suggest the Na control of the decomposition of leaf litter in subtropical ecosystems through Na limiting the activity and abundance of decomposers in brown food webs.



Similar content being viewed by others
References
Abelho M (2016) Litter traits and decomposer complexity set the stage for a global decomposition model. Funct Ecol 30:674–675. https://doi.org/10.1111/1365-2435.12640
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Albariño RJ, Balseiro EG (2002) Leaf litter breakdown in Patagonian streams: native versus exotic trees and the effect of invertebrate size. Aquat Conserv: Mar Freshwat Ecosyst 12:181–192
Ardón M, Pringle CM, Eggert SL (2009) Does leaf chemistry differentially affect breakdown in tropical vs temperate streams? Importance of standardized analytical techniques to measure leaf chemistry. Freshwater Science 28:440–453
Bakker MA, Carreno-Rocabado G, Poorter L (2011) Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct Ecol 25:473–483. https://doi.org/10.1111/j.1365-2435.2010.01802.x
Barbe L, Jung V, Prinzing A, Bittebiere A-K, Butenschoen O, Mony C (2017) Functionally dissimilar neighbors accelerate litter decomposition in two grass species. New Phytol 214:1092–1102. https://doi.org/10.1111/nph.14473
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515:505–511. https://doi.org/10.1038/nature13855
Bauer GA, Bazzaz FA, Minocha R, Long S, Magill A, Aber J, Berntson GM (2004) Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in the NE United States. Forest Ecol Manag 196:173–186. https://doi.org/10.1016/j.foreco.2004.03.032
Berg B, Mcclaugherty C (2013) Initial litter chemical composition. In: Plant Litter: decomposition, humus formation, carbonsequestration. https://doi.org/10.1007/978-3-642-38821-7_4
Berg B, Steffen KT, McClaugherty C (2007) Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 82:29–39
Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA (2016) Understanding the dominant controls on litter decomposition. J Ecol 104:229–238
Bradford MA, Veen GF, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, Crowther TW, De Long JR, Freschet GT, Kardol P, Manrubia-Freixa M, Maynard DS, Newman GS, Logtestijn RSP, Viketoft M, Wardle DA, Wieder WR, Wood SA, van der Putten WH (2017) A test of the hierarchical model of litter decomposition. Nature Ecology & Evolution 1:1836–1845. https://doi.org/10.1038/s41559-017-0367-4
Carreiro M, Sinsabaugh R, Repert D, Parkhurst D (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Cornelissen JH, Perez-Harguindeguy N, Díaz S, Grime JP, Marzano B, Cabido M, Vendramini F, Cerabolini B (1999) Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol 143:191–200
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cromack FJ, Sollins P, Todd RL, Crossley DAJ, Fender WM, Fogel R, Todd AW (1977) Soil microorganism–arthropod interactions: fungi as major calcium and sodium sources. Pages 78–84 in W. J. Mattson, editor. The role of arthropods in forest ecosystems. Springer Verlag, New York, New York, USA
Dong L, Sun T, Berg B, Zhang L, Zhang Q, Wang Z (2019) Effects of different forms of N deposition on leaf litter decomposition and extracellular enzyme activities in a temperate grassland. Soil Biol Biochem 134:78–80. https://doi.org/10.1016/j.soilbio.2019.03.016
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Freschet GT, Aerts R, Jhc C (2012) A plant economics spectrum of litter decomposability. Funct Ecol 26:56–65
García-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett 16:1045–1053
García-Palacios P, Mckie BG, Handa IT, Frainer A, Hättenschwiler S (2016) The importance of litter traits and decomposers for litter decomposition: a comparison of aquatic and terrestrial ecosystems within and across biomes. Funct Ecol 30:819–829
Goering HK, Van Soest PJ (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications). Pages 1–20 in U.S. Department of Agriculture Agricultural Handbook 379
Hattenschwiler S, Jorgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763. https://doi.org/10.1111/j.1365-2745.2010.01671.x
Henry HAL, Cleland EE, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland. Oecologia 142:465–473. https://doi.org/10.1007/s00442-004-1713-1
Hisabae M, Sone S, Inoue M (2011) Breakdown and macroinvertebrate colonization of needle and leaf litter in conifer plantation streams in Shikoku, southwestern Japan. J For Res 16:108–115
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–2644. https://doi.org/10.1890/07-1119.1
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–2297
Jia Y, Kong X, Weiser MD, Lv Y, Akbar S, Jia X, Tian K, He Z, Lin H, Bei Z, Tian X (2015) Sodium limits litter decomposition rates in a subtropical forest: additional tests of the sodium ecosystem respiration hypothesis. Appl Soil Ecol 93:98–104. https://doi.org/10.1016/j.apsoil.2015.04.012
Jones JA, Swan CM (2016) Community composition and diversity of riparian forests regulate decomposition of leaf litter in stream ecosystems. Restor Ecol 24:230–234
Kaspari M, Yanoviak SP, Dudley R, Yuan M, Clay NA (2009) Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proc Natl Acad Sci U S A 106:19405–19409. https://doi.org/10.1073/pnas.0906448106
Kaspari M, Clay NA, Donoso DA, Yanoviak SP (2014) Sodium fertilization increases termites and enhances decomposition in an Amazonian forest. Ecology 95:795–800. https://doi.org/10.1890/13-1274.1
Knops JMH, Naeemw S, Reich PB (2007) The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Glob Chang Biol 13:1960–1971. https://doi.org/10.1111/j.1365-2486.2007.01405.x
Lidman J, Jonsson M, Burrows RM, Bundschuh M, Sponseller RA (2017) Composition of riparian litter input regulates organic matter decomposition: implications for headwater stream functioning in a managed forest landscape. Ecol Evol 7:1068–1077
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041
Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S, Tscherko D (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40:688–698. https://doi.org/10.1016/j.soilbio.2007.08.023
Mao J, Mao Q, Zheng M, Mo J (2020) Responses of foliar nutrient status and stoichiometry to nitrogen addition in different ecosystems: a meata-analysis. Biogeosciences. https://doi.org/10.1029/2019JG005347
Norby RJ (1998) Nitrogen deposition: a component of global change analyses. New Phytol 139:189–200
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Pérez-Harguindeguy, N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in Central Argentina. Plant Soil 218:21–30
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149. https://doi.org/10.1007/s10533-010-9439-0
Prescott CE, Vesterdal L, Preston CM, Simard SW (2004) Influence of initial chemistry on decomposition of foliar litter in contrasting forest types in British Columbia. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 34:1714–1729. https://doi.org/10.1139/x04-040
Prieto I, Almagro M, Bastida F, Querejeta JI (2019) Altered leaf litter quality exacerbates the negative impact of climate change on decomposition. J Ecol 107:2364–2382
Salinas N, Malhi Y, Meir P, Silman M, Cuesta RR, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M, Farfan F (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977. https://doi.org/10.1111/j.1469-8137.2010.03521.x
Santiago LS (2007) Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology 88:1126–1131. https://doi.org/10.1890/06-1841
Sardans J, Grau O, Chen HYH, Janssens IA, Ciais P, Piao S, Peñuelas J (2016) Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob Chang Biol 23(9):3849–3856. https://doi.org/10.1111/gcb.13721
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. https://doi.org/10.1007/s004420100740
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in Ecology 5(14):2772–2774
Taylor BR, Parkinson D, Parsons WF (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Valachovic YS, Caldwell BA, Cromack K, Griffiths RP (2004) Leaf litter chemistry controls on decomposition of Pacific northwest trees and woody shrubs. Can J For Res 34:2131–2147. https://doi.org/10.1139/x04-089
Vivanco L, Austin AT (2019) The importance of macro- and micro-nutrients over climate for leaf litter decomposition and nutrient release in Patagonian temperate forests. For Ecol Manag 441:144–154. https://doi.org/10.1016/j.foreco.2019.03.019
Wang Q, Kwak JH, Choi WJ, Chang SX (2018) Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: effects of litter chemistry and forest floor microbial properties. For Ecol Manag 412:53–61. https://doi.org/10.1016/j.foreco.2018.01.042
Ward SE, Orwin KH, Ostle NJ, Briones MJI, Thomson BC, Griffiths RI, Oakley S, Quirk H, Bardgett RD (2015) Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology 96:113–123
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavenderbares J, Chapin T, Cornelissen JH, Diemer M (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Xia M, Talhelm AF, Pregitzer KS (2015) Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol 208:715–726
Xu X, Zhou Y, Ruan HH, Luo YQ, Wang JS (2010) Temperature sensitivity increases with soil organic carbon recalcitrance along an elevational gradient in the Wuyi Mountains, China. Soil Biol Biochem 42:1811–1815
Yu ZP, Huang ZQ, Wang MH, Liu RQ, Zheng LJ, Wan XH, Hu ZH, Davis MR, Lin TC (2015) Nitrogen addition enhances home-field advantage during litter decomposition in subtropical forest plantations. Soil Biol Biochem 90:188–196
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Zhang TA, Luo YQ, Chen HYH, Ruan HH (2018) Responses of litter decomposition and nutrient release to N addition: a meta-analysis of terrestrial ecosystems. Appl Soil Ecol 128:35–42. https://doi.org/10.1016/j.apsoil.2018.04.004
Zhang M, Cheng X, Geng Q, Shi Z, Luo Y, Xu X (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28:1469–1486. https://doi.org/10.1111/geb.12966
Acknowledgements
This study is financially supported by the National Science Foundation of China (31700376), the Natural Science Key Fund for Colleges and Universities of Jiangsu Province of China (17KJA180006), the Six Talent Peaks Program of Jiangsu Province (JY-041& TD-XYDXX-006), the “5151” Talent Program of Nanjing Forestry University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
All authors contributed intellectual input and assistance to this study and manuscript preparation. X.X. conceived the idea and designed the study. M.Z. and Q. L. collected and analyzed the data with help from X.X. Q. L. and M.Z. wrote the paper with input from all authors.
Corresponding author
Additional information
Responsible Editor: Amandine Erktan.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 95 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Zhang, M., Geng, Q. et al. The roles of initial litter traits in regulating litter decomposition: a “common plot” experiment in a subtropical evergreen broadleaf forest. Plant Soil 452, 207–216 (2020). https://doi.org/10.1007/s11104-020-04563-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04563-8