Abstract
Background and aims
Due to the production of large amounts of tannins and phenolics by Rhizophora mangle, it was hypothesized that the invasion of this mangrove species in salt marshes due to global warming will result in changes in the cycling of carbon and nitrogen.
Methods
Leaf litter and/or seedlings of R. mangle were placed into 1-m2 experimental plots in a Distichlis spicata-dominated salt marsh on the Atlantic Coast of central Florida (USA). An additional litter decomposition experiment was conducted in all plots by adding litter bags containing 10 g of dried D. spicata shoot litter. Seedling growth was measured yearly. One and four years after the start of the experiment, soil samples were collected to determine physical and chemical soil conditions, potential nitrification and denitrification activities and abundances of genes that are related to microbial processes in the nitrogen cycle.
Results
Growth of R. mangle seedlings was stimulated in the presence of R. mangle litter, while decomposition rates of D. spicata litter were lower in plots with R. mangle litter and seedlings. The presence of R. mangle litter and/or seedlings had no significant effect on potential nitrification and denitrification activities and on the abundances of genes.
Conclusion
The colonization of R. mangle into D. spicata-dominated salt marshes will affect the carbon cycle, but not necessarily the nitrogen cycle, which is likely due to the pre-existing nitrogen-limited conditions in the salt marsh.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being at the fringe of terrestrial and marine ecosystems, mangrove-dominated wetlands have high biogeochemical, ecological and economical values (Valiela et al. 2001). The northern and southern boundaries of the distribution of coastal mangroves are directly related to the frequency of frost periods (Chen and Twilley 1998; Stuart et al. 2007). As a result of global warming, the range of mangroves is expected to expand north- and southward resulting in the encroachment of mangroves into salt marshes (Bianchi et al. 2013; Saintilan et al. 2009; Saintilan et al. 2014). The migration of mangroves into salt marshes has led to substantial increases in the aboveground biomass and belowground carbon storage (Kelleway et al. 2016). Shifts in the latitudinal distribution of mangroves will result in changes in patterns and rates of nutrient cycling (Doughty et al. 2016) even though the growth of herbaceous salt marsh species may be stimulated by global warming more than mangroves (Coldren et al. 2016).
Nitrogen is the major limiting nutrient in many mangrove forests (Feller et al. 2003). Hence, efficient nitrogen utilization is a key survival strategy. Several nutrient conservation strategies may be developed by mangroves, such as a high nitrogen resorption efficiency in senescent leaves and high tannin levels (Kandil et al. 2004; Lin et al. 2010). Mangroves, and in particular species from the Rhizophoraceae, contain large amounts of tannins (Basak et al. 1999; Hernes et al. 2001; Hernes and Hedges 2004) and the release of tannins has large ecological implications (Kraus et al. 2003). The binding of proteins (Maie et al. 2008) will be specifically important during the diagenesis of mangrove leaves when significant amounts of proteins are leached (Benner et al. 1990). The tannin-protein complexes are recalcitrant against microbial degradation and may therefore serve as a long-term source of nitrogen in mangrove-dominated wetlands (Maie et al. 2008). In this way, tannins will repress the loss of inorganic nitrogen from the ecosystem. For example, the rate of aerobic nitrification, which is the key process in the biogeochemical nitrogen cycle converting the immobile ammonium ion into the mobile nitrite and nitrate ions, is often more than one order of magnitude lower in mangroves than salt marsh soils (cf. (Purvaja et al. 2008). The formation of complexes between tannins and exo-enzymes, which are involved in the degradation of organic polymers, will slow down the cycling of these polymers in mangrove soils, which may lead to increased carbon sequestration rates (Bianchi et al. 2013). In contrast to mangroves, tannins in salt marsh grasses are unlikely to be a significant component of humic substances (Alberts et al. 1988). We hypothesized that the expansion of mangroves into salt marshes will impact the cycling of inorganic nitrogen and carbon and consequently the rates of primary production, decomposition, carbon storage in the soil, pore water chemistry and soil-based microbial activity.
The goal of our project was to study the effects of the invasion of mangroves, specifically Rhizophora mangle, on the dynamics of carbon and nitrogen in a Distichlis spicata-dominated salt marsh. We mimicked the migration of mangroves by transplanting seedlings of R. mangle into a D. spicata-dominated salt marsh at Merritt Island National Wildlife Refuge (Brevard County, Florida, USA) and measured growth-related variables each year for a period of four years. We also included senesced R. mangle leaves (i.e. litter) into the experimental design. We determined additionally the effects of R. mangle seedlings and litter on D. spicata litter decomposition, on potential rates of nitrification and denitrification, and on the abundance of selected genes that are known to be involved in nitrogen cycling (Regan et al. 2017): Archaeal and bacterial amoA (ammonia oxidation), nxrB (nitrite oxidation), nirK and nirS (nitrite reduction), norB (nitric oxide reduction), nosZ (nitrous oxide reduction) and nifH (nitrogen fixation). Changes in the microbial community involved in nitrogen cycling will be reflected by changes in the abundance of genes.
Material and methods
Description of the study site
In March 2011, we established 28 plots of 1 m2 each in a salt marsh at Merritt Island National Wildlife Refuge (28o42’31.58” N, 80o44’16.16” W, Brevard County, Florida, USA). The 2-ha site was dominated by an almost monospecific stand of Distichlis spicata. The ecotone between the salt marsh where the experiment was conducted and the open water consisted of a narrow mangrove fringe with Avicennia germinans and Laguncularia racemosa and an understory of predominantly Batis maritima. Rhizophora mangle was only present at the border between the ecotone and open water. The first plot was established in the salt marsh approximately 50 m from the mangrove-dominated ecotone. We established the remaining 27 plots at least 10 m from each other along a random line that proceeded toward the interior of the salt marsh. There were no elevation differences between the plots and the wetland was only tidally inundated during monthly high tides and storm tides. Four treatments (Control – C; Litter – L, Seedling – S, Litter plus seedlings – LS) were randomly distributed among the plots and there were seven replicates of each treatment. For C plots, we cut all the shoots of D. spicata at the soil surface and placed the material back onto the plot. Most of the stems and leaves were dead at the moment we installed the plots, but some living stems were present as well. For L treatment plots, we removed all above-ground D. spicata and replaced it with 300 g of dried R. mangle leaf litter, an amount of litter that is representative of natural stands of R. mangle in the region (Twilley et al. 1986). Senescing leaves were hand-picked from R. mangle trees and air dried before they were added to the plots. S plots were established by cutting and placing back above-ground D. spicata and then planting four equally spaced R. mangle seedlings in each plot. Prior to planting them in the S and SL plots, the R. mangle seedlings had been collected in the Indian River Lagoon near Ft. Pierce, Florida and grown for about one year in plastic tubes that had been filled with peat and placed in the water immediately adjacent to a natural stand of R. mangle near the Smithsonian’s Marine Station at Ft. Pierce. At the time of planting, the height of each seedling was measured along with the total length (cm) of each lateral branch. The number of leaves on each branch was also counted. LS plots were treated similar to the L plots except that four equally spaced R. mangle seedlings were planted in each plot. The treatments were repeated yearly in March. Representative pictures of the four treatments are shown in Supplementary Fig. 1.
Soil sampling
In March 2012 and 2015, corresponding to one and four years after the start of the experiment, soil samples were collected from each experimental plot. In 2012, three soil cores (3.9 cm diameter and 10 cm long) were taken from each plot using an aluminum sampling tube that was sharpened at one end. The cores were immediately stoppered, placed on ice and returned to the laboratory. In 2015, three larger soil cores (5.2 cm diameter and 15 cm long) were collected from each plot, stored in plastic bags on ice and transferred to the laboratory. Since the salt marsh had been burned (as described in more detail below) in March 2015, one week before our sampling campaign, we collected 3 additional soil samples from seven plots (hence 21 extra soil samples in total) in the salt marsh in an area that was located between our first study plot and the ecotone described above. This non-burned area was floristically similar to the area where the 28 experimental plot were located (i.e. an almost monoculture of D. spicata).
In the laboratory, one of the three cores was used for sampling pore water by transferring it into a plastic sandwich bag that was then sealed. A ceramic soil sipper (Rhizon soil moisture samplers: Model 12.01.SA, Eijkelkamp, Giesbeek, The Netherlands) was inserted through the plastic bag into the core and connected to a 50-ml syringe. Pulling and fixing the plunger of the syringe created a continuous negative pressure created to collect pore water. Collection of pore water using this technique occurred at room temperature in the laboratory and the process took from 1 to approximately 5 h to obtain a sample that was large enough for the analyses described below. The pore water was first analyzed for salinity with a hand-held salinity refractometer (VEE GEE Scientific, Inc. Kirkland, WA, USA) and pH with an Oakton 510 Series pH meter. Pore water was then refrigerated and taken to Utrecht University for further analysis of ammonium-N, nitrate-N, and phosphate-P on a Skalar AutoAnalyzer (Skalar, Breda, The Netherlands). Of the other two cores collected from each plot, only the top 5 cm was used for further analyses. The volume and wet weight of both 5-cm core segments were used to calculate bulk density. The material from this pair of cores from each plot was pooled, roots and coarse plant parts were removed, and the soil was thoroughly mixed and split into five subsamples (a-f) of 20 g fresh weight each. Subsample (a) was used to determine soil moisture and total nutrient content. After determining fresh weight, samples were dried at 105 °C for 24 h and re-weighed. The dried soil was stored and taken to Utrecht University for analysis of Total N, Total P, and Total K after digestion with a modified Kjeldahl approach (Bremner and Mulvaney 1982). Subsample (b) was extracted with 50 ml 1 M KCl for determining extractable ammonium, nitrate and phosphate. Extracts were refrigerated, transferred to Utrecht University and analyzed on a Skalar AutoAnalyzer (Skalar, Breda, The Netherlands). Subsamples c – f were used for determining the potential nitrification and denitrification activities (see below). In March 2015, an additional subsample of 15 ml was freeze-dried in plastic Greiner tubes and stored for analyses of tannin-like compounds and quantification of genes that are related to the nitrogen cycle.
Determination of total phenolics, total tannins and hydrolyzable tannins
To investigate the effects of the treatments four years after the start of the experiment, total phenolics and total tannins and hydrolyzable tannins were determined in soil samples collected in March 2015. Total phenolics (TP) were determined by using a small modification of a method described by (Asami et al. 2003; Morita 1980; Northup et al. 1995). The concentration of total phenolics was determined at 760 nm with tannic acid as a standard and the extraction solution without the chromogenic reagent as a blank. The total tannins content was determined according to a method described by (Adamczyk et al. 2008). Both total phenolics and total tannins are presented as milligrams of tannic acid equivalent (TAE) per gram soil. The amount of hydrolyzable tannins in each sample was determined by using a slightly modified version of the method described by (Adamczyk et al. 2008; Hartzfeld et al. 2002). Methyl gallate was used as standard. Results are presented as milligrams of methyl gallate equivalent (MGE) per gram soil.
Seedling growth
Plots were visited in the second week of March each year to measure the height of each seedling (cm), the total length (cm) of all branches on each seedling, and the number of leaves that had been produced on each branch. Plant height (cm) was measured as the distance between the soil surface at the base of the stem to the highest point on the main stem. Annual branch growth of each seedling was calculated as the total length of all branches in one year minus the total length of all branches the previous year. Annual leaf production was determined by counting the number of leaves that were produced on each plant annually. To obtain a measurement of total leaf production per plant, the number of new leaves of each branch was determined each year. At the time of each sampling, we used indelible ink to mark the abaxial surface of the two terminal leaves on each branch. A year later, the number of leaves that had developed beyond the two leaves that had been marked was counted. The process was repeated each year for each shoot on each plant. This method for measuring annual leaf production on mangrove branches has been previously described (Feller et al. 2003). We also recorded the number of individual prop roots that emerged from the stem of each seedling at the time of each visit.
When we returned to the site to make measurements in March 2015, we found that the area had been burned by staff of the US Fish and Wildlife Service that manages the site. All of the plants were dead but almost all of them were intact, except that they had lost their leaves. We examined each plant and took height and branch measurements on all plants that had not been damaged (i.e., lost branches or the terminal shoot had not been destroyed). As a result, growth data for the last year of the study only includes plant height, branch length, and prop root data. Interestingly, we later learned from a Smithsonian colleague (L. Simpson, personal observation) who had visited the site two weeks prior to our visit that there had also been a frost that had killed all of the leaves on the plants.
Decomposition of Distichlis spicata litter
In March 2011, D. spicata litter (mostly dead stems) was collected from the study site and dried at 40 °C for 48 h. We placed 5 g of dried litter into sausage-like, nylon litter bags (2 cm diameter, 15 cm long) of 2 mm mesh size. In March 2012, each of the 28 plots received 2 litter bags that were placed on the soil surface and held on place with two U-shaped metal pins. The litter bags were then covered with D. spicata (C and S treatments) or R. mangle litter (L and LS treatments). One litter bag was retrieved from each plot in June 2012 and the second in March 2014. In March 2013, we repeated the experiment with the original, dried D. spicata litter and placed another litter bag in each plot. Those litter bags were recovered in March 2014. After retrieving the litter bags, litter was removed from the bags, dried at 40 °C for 48 h and subsequently weighed. Litter weight losses were calculated after 12, 52 and 104 weeks of incubation in the plots.
Determination of potential nitrification activities
Potential nitrification activities (PNA) were determined at room temperature in the dark in slurries of 20 g fresh weight soil mixed with 50 ml of a commercial salt solution (Instant Ocean Blacksburg, VA) enriched with 1 mM ammonium, according to the protocol of Belser and Mays (Belser and Mays 1980), as modified by Verhagen and Laanbroek (Verhagen and Laanbroek 1991). Salinities of the mineral media were adapted to the pore water salinity of each individual sample. Measurements were performed within 16 h after collection of the soil samples and the activities were followed for 12 h with 2-h sampling intervals. At each time interval, 1.0 ml of slurry suspension was centrifuged for 10 min at 5000 rpm, the supernatant was stored at −20 °C and transferred frozen to Utrecht University, where the concentrations of nitrite plus nitrate were determined on a Skalar AutoAnalyzer (Skalar, Breda, The Netherlands). The linear production of nitrite plus nitrate over time was taken as a measure of the potential nitrification activity. In a parallel series, the potential activity measurements in slurries were performed in the presence of allylthiourea (ATU), a known inhibitor of mainly bacterial ammonia oxidation (Taylor et al. 2013; Taylor et al. 2010).
Determination of potential denitrification activities
Samples (20 g fresh weight) for determination of potential denitrification activities were added to 100-ml screw-cap bottles. Each bottle was flushed with N2 on ice and then capped with a screw cap that contained a rubber septum. Bottles meant for determining actual (i.e. non-amended) potential denitrification activities received then 20 ml of water with the same salinity as the soil pore water of the respective sampling plot. Bottles targeted to measure amended potential denitrification activities received 20 ml of saline water enriched with 22 mM glucose, 49 mM sodium acetate and 14 mM sodium nitrate. By adding 9 ml of acetylene to the capped bottles, denitrification was inhibited at the level of nitrous oxide reduction. Bottles were incubated at room temperature in the dark and gas samples were collected after 1 and 4 h of incubation and stored in 12-ml vacutainers (Labco, Buckinghamshire, UK). Changes in nitrous oxide concentrations were determined by gas chromatography (Agilent, Amstelveen, The Netherlands) at Utrecht University.
DNA extraction
Total genomic DNA was extracted by using 0.2 g of freeze-dried sample that was homogenized in 1 ml cetyltrimethylammonium bromide (CTAB) buffer in MP Lysing Matrix tubes (MP Biomedicals, Santa Ana, California, USA), subjected twice to disruption by bead-beating at a 5.0 m/s rotation for 60 s, and incubated at 37 °C for 30 min in the presence of 5 μl proteinase K (20 mg/ml), while vortexing every 15 min. The samples were supplemented with 150 μl of 20% sodium dodecyl sulfate (SDS) solution and incubated at 65 °C for 1 h in a thermoblock and vortexed every 15 to 20 min. After centrifugation at 10,000 X g for 10 min., approximately 500 μl the supernatant was collected and directly added to the lysis buffer of the Maxwell®16 (Promega, Fitchburg, Wisconsin USA) DNA Tissue extraction kit. Quantity and quality of soil DNA was established by a Nanodrop ND-1000 Spectrophotometer (Thermo Scientific, Waltham, EUA) and stored at −80 °C for qPCR of functional genes.
Quantitative PCR assays for functional genes
Quantitative PCR (qPCR) of functional genes was performed using an QIAgility automated pipettor (Qiagen, California, USA) and a Rotor-Gene 6000 qPCR Detection System (QIAGEN, Hilden, Germany). The following primer sets were applied for the different genes: Archaeal amoA: Arch-amoAF (Francis et al. 2005)] /AOA_amoA_175Brev (Laanbroek et al. 2017); bacterial amoA: amoA-1F (Stephen et al. 1999)] /amoA-2R (Rotthauwe et al. 1997); nrxB: nxrB169f/ nxrB638r (Pester et al. 2014); nirK: F1aCu/R3Cu (Throback et al. 2004); nirS: cd3aF/R3cd (Throback et al. 2004); norB: norB2F/norB6R (Casciotti and Ward 2005); nosZ: nosZ2F/nosZ2R (Henry et al. 2006); and nifH: IGK3/DVV (Gaby and Buckley 2012). The PCR reactions were performed in 20 μl volumes containing 10.0 μl SYBR® Green Master Mix (Bio-Rad Laboratories, Hercules, CA, USA), 5 μl of a 10 times diluted extract of soil DNA, 0.5 μl of bovine serum albumin (10 mg·ml−1) and 1 μl of each primer (0.25 μM). The qPCR temperature profiles were as follows: 5 min at 95 °C, 40 cycles of 20 s at 95 °C, 20 s at the primer set-dependent annealing temperatures, 20 s at 72 °C, and 15 s at 82 °C. The primer set-dependent annealing temperatures of the archaeal amoA gene, the bacterial amoA gene, the nxrB gene, the nirK gene, the nirS gene, the norB gene, the nosZ gene, and the nifH gene were 58 °C, 58 °C, 56 °C, 57 °C, 57 °C, 56 °C, 60 °C, and 58 °C, respectively. Fluorescence was read during each cycle at 82 °C. All samples and genes were analyzed in duplicate.
High amplification efficiencies of 95–105% were obtained for all genes with R2 values 0.97–1.00 and slopes from −3.0 to −3.4. No-template DNA was involved in each qPCR assay as a negative control. Product specificity was confirmed by melting curve analysis and visualization in 1.0% agarose gels. No significant inhibition was found in the DNA extracts.
Statistical analyses
Statistical analyses on plant growth parameters, litter decomposition figures, and soil related data were performed with the IBM-SPSS software package version 23 (IBM Corp. Armonk, NY). Before the analyses started, outliers detected as extreme values (i.e. > 1.5 x Interquartile Range) in SPSS boxplots were removed from the data. The number of outliers was generally small. The distribution of the remaining data was tested for normality and homoscedasticity of residuals by Wilk-Shapiro and Levene’s tests, respectively. In case of normal distribution of the data significant differences between means were established by a univariate analysis of variance based on a general linear model. When the data, even after log10 transformation, did not meet the requirements for normality and homoscedasticity of residuals, the differences between groups were analyzed with the non-parametric Kruskal-Wallis and Mann-Whitney tests. Correlation between factors was tested with a Spearman’s rank order analysis.
To investigate how the abiotic soil factors affected the distribution of the genes, a single predictive model was fitted for each gene at all experimental plots, assuming a different environmental response for different genes. Next, ecological niche-modelling was performed, which consisted of a generalized linear model for the generalization of a multiple regression model that uses the so-called link function to accommodate response variables (Austin et al. 1990). As a variable selection criteria the stringent rule called LASSO (least absolute shrinkage and selection operator) penalty was adapted, which performs both variable selection and regularization to provide a more accurate and interpretable model (Osborne et al. 2000). This rule shrinks any coefficient to zero that did not increase the model fit (r2) nor decrease the Akaike Information Criteria (AIC), and therefore avoids over fitting. This procedure allowed standardized regression coefficients that determined the influence of the environmental variable on each gene. Finally, a cluster analysis with these coefficients was performed to investigate which genes responded similarly to the environmental factors. Our analysis was performed in R using the packages mvabund (Wang et al. 2012), pvclust (Suzuki and Shimodaira 2006) and ComplexHeatmap (Gu et al. 2016).
Results
Soil characteristics and nutrients
The treatments had little effect on most of the soil parameters (Table 1). On the contrary, the differences between the samples collected in 2012 and 2015 were generally more pronounced than the differences between treatments (Table 1). Significantly lower bulk densities, salinities, total soil nitrogen, and extractable nitrate were found in 2015, whereas significantly higher pore water pH values, soil C/N ratios, pore water ammonium, extractable ammonium, pore water phosphate and extractable phosphate were observed in 2015. Differences between years appear to be most likely due to between-year salinity values. It could well be that all these significant differences are associated with more heavy rainfall in the period before the sampling campaign in March 2015 that introduced fresh water in the salt marsh. Based on meteorological data from the nearby NOAA weather station on Merritt Island (NCEI; https://gis.ncdc.noaa.gov/maps/ncei/summaries/monthly), the total precipitation in the three winter months preceding the collection of soil samples in 2015 (i.e. December – February) was twice as much compared to the same period of time in March 2012 (172 vs. 87 mm, see also Supplementary Fig. 2).
The obvious effect of increased fresh water on most measured soil characteristics and nutrients became also apparent when we analyzed the data using the Spearman rank order correlation analysis. Based on that analysis, all soil factors except total soil carbon and nitrogen were significantly correlated with salinity (Supplementary Table 1). Salinity was positively correlated with dry bulk density, pore water nitrate, and extractable nitrate, and negatively with soil moisture content, pore water pH, soil C/N ratio, pore water ammonium and phosphate, and extractable ammonium and phosphate. Total soil carbon and nitrogen were significantly and positively correlated with soil moisture content. While total tannins and hydrolyzable tannins were only significantly correlated with total soil carbon and moisture content, total phenolics were significantly correlated with bulk density, soil moisture content, salinity, total soil carbon, soil C/N ratio, pore water nitrate and extractable phosphate (Supplementary Table 2).
Rhizophora mangle Seedlings growth
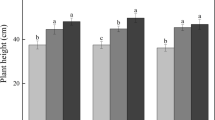
Annual increases in plant height, branch growth and numbers of leaves produced annually were significantly higher in plots that had both seedlings and R. mangle leaf litter (SL treatment) (Fig. 1, Supplementary Table 3a). Hence, the presence of R. mangle leaf litter stimulated the growth of the seedlings. The increase in mean plant height was significant (p < 0.05) for each period of 12 months (Supplementary Table 3b). The same was true for the average numbers of leaves, but for a shorter period (i.e. March 2012 – March 2014). The mean annual branch growth became only significant in 2014 (Supplementary Table 3b). Since few plants had produced prop roots during the first four years of the study, it was only possible to compare these prop roots in the S and SL treatments in 2015. By March 2015, the number of prop roots on the seedlings in the plots with seedlings and R. mangle leaf litter was significantly higher (i.e. 5.93 ± 1.08 versus 3.72 ± 1.66; mean and standard deviation).
Plant height (a), annual branch growth (b), number of leaves (c), and number of prop roots (d) of Rhizophora mangle seedlings in experimental Distichlis spicata plots in the absence (broken lines) and in the presence (solid lines) of senescent Rhizophora mangle leaves. Error bars present standard errors
Distichlis spicata Litter decomposition
Weight loss of D. spicata litter averaged 13.3, 24.1 and 48.8% after 12, 52 and 104 weeks, respectively. Decomposition rates differed between treatments (Fig. 2, Supplementary Table 4) with litter bags losing less weight in plots with R. mangle leaf litter plus seedling. Litter weight loss did not differ significantly from each other for the other treatments. In all treatments, D. spicata litter declined significantly with time. No significant interactive effect of treatment and time was detected.
Weight loss of Distichlis spicata litter kept in litter bags in control plots (white bars), and in experimental plots with senescent Rhizophora mangle leaves (light grey bars), with R. mangle seedlings (dark grey bars) and with R. mangle leaves and seedlings (black bars). Error bars represent standard errors
HierarchicalFig gene clustering based on a generalized linear model (GLM) of environmental factors, in which any coefficient that did not increase the model (R2) nor decreases the Akaike Information Criteria (AIC) has been shrunk to zero following the least absolute shrinkage and selection operator (LASSO) penalty rule. Brightness of squares denotes the strength of the positive (blue) and negative (red) associations.
Potential nitrification activities
Whereas there were no significant effects of treatments on potential nitrification activities after one year (i.e. in 2012), there were significant treatment effects after four years (Table 2). There were also significant effects between years, with the lowest rates in 2015 (Table 2).
Irrespective of the absence or presence of the nitrification inhibitor allylthiourea (ATU), the potential nitrification rates in the plots with R. mangle leaf litter were significantly (p < 0.05) higher compared to plots with R. mangle seedlings. In 2015, there was no significant difference in potential nitrification rates in soil samples collected from the burned control plots compared to the non-burned edge of the salt marsh (Table 2).
Potential nitrification activities in the absence and presence of ATU were significantly and positively correlated with soil moisture content and total soil nitrogen, but negatively with pore water pH, pore water and extractable ammonium and with pore water phosphate (Supplementary Table 3). In the absence but not in the presence of ATU, potential nitrification activities were significantly and positively correlated with salinity and negatively with soil C/N ratio. In 2015, i.e. the only year in which polyphenolic compounds had been measured, no significant correlations were observed between potential nitrification activities and total amounts of tannins and phenolics.
Potential denitrification activities
Significant differences in potential denitrification rates were measured one year after the start of the treatments, but the nature of the effect was dependent on the absence or presence of added carbon and nitrate (Table 2). In 2012, non-amended soil samples from the plots with only R. mangle seedlings had significantly higher potential denitrification activities than the soil samples from the control plots. Amended soil samples from the plots with only R. mangle leaf litter had significantly higher potential denitrification than samples from the plots with R. mangle leaf litter plus seedlings. In 2015, non-amended soil samples from the plots with R. mangle leaf litter had higher potential denitrification rates than the samples from the control plots, whereas no significant differences between treatments were observed in the amended samples. Again, potential denitrification rates in samples collected from the burned control plots in 2015 were not significantly different from the potential activities in samples from the non-burned area (Table 2). No significant differences in potential denitrification activities were observed between 2012 and 2015.
Potential denitrification activities in non-amended samples were not significantly correlated with any of the soil characteristics and nutrients, while the potential denitrification rates in amended samples were significantly and negatively correlated with pore water pH and with pore water ammonium (Supplementary Table 1). No significant correlations were found between potential nitrification and denitrification rates (Supplementary Table 1).
Abundances of nitrogen cycle-related genes
Abundances of genes that related to the nitrogen cycle were determined in soil samples collected in 2015 from the experimental plots and plots in the non-burned area. The abundance of the bacterial amoA gene (Table 3) was significantly higher in the soil samples from the plots with only seedlings compared to the samples from the plots with R. mangle leaf litter and seedlings. There was a lower abundance of the archaeal amoA and nosZ genes in soils collected in unburned plots compared to the control plots of the experimental series.
Several significant correlations were observed between abundances of genes and abiotic soil characteristics (Supplementary Table 2). The archaeal amoA gene was negatively correlated with dry bulk density and with total soil nitrogen. The bacterial amoA gene was positively correlated with soil moisture content, total soil carbon, total soil nitrogen and with total phenolics. The nirK gene was negatively correlated with soil moisture content, total soil carbon, total soil nitrogen, extractable ammonium, total tannins, and with hydrolyzable tannins. The nirS gene was negatively correlated with salinity, whereas the norB gene was positively correlated with soil moisture content, total soil carbon, total soil nitrogen, extractable phosphate, total tannins, and with total phenolics, but negatively with salinity. The nosZ gene was positively correlated with pore water nitrate, but negatively with soil moisture content, extractable ammonium, and with hydrolyzable tannins. The nxrB gene was negatively correlated with dry bulk density, while the number of the nifH gene was positively correlated with total soil carbon, extractable ammonium and phosphate, and with hydrolyzable tannins.
There were several significant correlations between the abundances of genes and the potential nitrification and denitrification rates (Supplementary Table 5). The archaeal amoA genes was positively correlated with the rates of potential nitrification in the absence and presence of the inhibitor ATU, and with the amended potential denitrification activity. The abundance of the nirK gene correlated negatively with the amended potential denitrification activity, while the nirS gene correlated positively with the potential nitrification activity in the absence and presence of the inhibitor ATU. The norB gene was positively correlated with the potential nitrification activity in the presence of the inhibitor ATU, but not in the absence of ATU. The nxrB gene correlated positively with the potential nitrification activity in the absence and presence of ATU, and with the amended potential denitrification activity. The bacterial amoA and the nifH genes did not correlate with any of the measured activities.
To unravel the set of environmental factors that govern the distribution of the nitrogen cycle-related genes, a cluster analysis was performed in a standardized coefficient table obtained after the generalized modeling of genes distribution. Fig. 3 shows that two clusters of genes were separated by two soil factors, i.e. total soil carbon and total soil nitrogen.
The abundance of the nirS gene did not cluster with one of these gene clusters. The genes of the clusters were differently related to these soil characteristics. The gene abundances within cluster C1, containing the archaeal amoA, the bacterial amoA, the norB, and the nifH genes, were positively affected by total soil nitrogen and negatively by total soil carbon. The genes within cluster C2, i.e. nirK, nosZ and nxrB, had the opposite relationship to the same soil factors. Although less clear than with total soil carbon and nitrogen, the clustering of genes also relates to their response to changes in soil moisture. Whereas the genes of cluster C1 were negatively affected by this environmental variable, the genes of cluster C2 positively associated with changes in the moisture fraction.
Discussion
Our four-year experiment with additions of Rhizophora mangle leaf litter and seedlings in a Distichlis spicata dominated salt marsh showed only a few significant effects on general soil variables and on processes related to the carbon and nitrogen cycle. The decomposition of D. spicata litter was inhibited by the combined addition of mangrove litter and seedlings and there were several significant effects on the potential nitrification and on the ammonium concentrations after 4 years. The treatments also had no effect on the abundance of genes associated with nitrogen cycling. There were, however, significant differences between years for soil-related variables and these differences were likely due to differences in precipitation in the months preceding sampling. Since the experimental plots had been burned two weeks before we sampled the plots in March 2015, we also sampled soils from non-burned areas within the D. spicata-dominated vegetation to look for possible effects of burning on the soil characteristics. With the exceptions of pore water nitrate and phosphate, and of total tannins and hydrolyzable tannins, which were all higher in the unburned area, we found no significant effects of burning. With respect to the abundance of genes, only the archaeal amoA and the nosZ gene were significantly different between burned and unburned area. So, it is likely that burning two weeks prior for sampling did not significantly affect the soil physical, chemical and microbial characteristics in the experimental plots.
An unanticipated but strongly significant effect was the higher growth rate of R. mangle seedlings in experimental plots where D. spicata litter was annually replaced by senesced R. mangle leaves. There are at least two logical explanations for this seedling response. First, even though mangroves are known to resorb nutrients prior to leaf abscission (Killingbeck 1996; Lin et al. 2010), recently senesced mangrove leaves leach soluble organic compounds (e.g. sugars, organic acids and proteins) and inorganic nutrients (nitrogen and phosphorus) during the initial stages of decomposition (Cundell et al. 1979; Hossain et al. 2014). Before the soluble forms of nutrients were lost from the system or immobilized, the mangrove seedlings could have taken them up. Mangrove seedlings have been shown to have a positive response to nutrient additions (Naidoo 1990) and our results suggest that under field conditions, the seedlings were able to compete with microbes for the available nutrients. Hence, the significantly higher growth rate of seedlings in plots with mangrove leaf litter compared to seedlings in plots with D. spicata litter (compare c and d in Supplemental Fig. 1) provides evidence that the senesced leaves were the source of nutrients that supported seedling growth. However, a relative shortage of available nutrients as a cause of lower growth rates of the seedlings in the plots with D. spicata litter may be less likely considering the amount of biomass present in these plots. Although we did not measure annual litter production in the D. spicata-dominated wetland where the experiment was conducted, similar wetlands in the south-eastern coast of the USA produced 485–1162 g m−2 dry weight of litter (median value 893) (Howard et al. 2016; Lonard et al. 2013), thereby exceeding the amount of 300 g m−2 dry weight of senescent R. mangle leaves that we added yearly to the experimental plots.
A second factor that could have influenced seedling growth was a change in microclimatic conditions at the soil surface caused by the replacement of D. spicata litter by R. mangle leaf litter (compare c and d and a and b in Supplementary Fig. 1), although moisture content and salinity did not significantly differ between the treatments with the different litter types. Experimentally thinning of an Avicennia germinans-dominated forest led to rapid changes in the microclimate of a coastal wetland (Guo et al. 2017), but this outcome is hard to compare with ours since the design of the experiments was different. Instead of planting mangrove seedlings into a salt marsh, Guo and colleagues (Guo et al. 2017) thinned an existing forest with mature trees of 1–2.5 m high.
There was significantly less weight loss of D. spicata litter in plots with R. mangle leaf litter plus seedlings compared to the control treatment. The smaller negative effects of R. mangle leaf litter or seedlings alone on the decomposition of D. spicata were not significant. It seems that both plant components reinforced each other (Fig. 2). Leaves and roots of R. mangle contain high amounts of tannins (Kraus et al. 2003), which are released during the decomposition (Hernes et al. 2001; Lin et al. 2006). Tannins also actively repress exo-enzymes that are involved in the decomposition of organic matter such as D. spicata litter (Kraus et al. 2003; Maie et al. 2008) and hence may have been responsible for the observed lower decomposition rates.
The fact that significant responses of the soil variables to the experimental conditions were virtually absent may have been the result of the sampling regime that we designed for the experiment. While treatments (i.e. addition of leaf litter) started in March 2011 and were repeated annually in March until 2014, soil samples were only collected in March 2012 and March 2015. Changes in soil conditions resulting from the addition of fresh litter each year may have occurred, as shown by the results of the seedling growth data but the effects of litter addition in the soil might have been short-lived and were not observable in most soil and pore water variables a year after litter addition. Lee and colleagues (Lee et al. 2008) showed large seasonal changes in pore water biogeochemistry and microbial soil metabolisms in a Belizean dwarf R. mangle habitat, which was attributed to seasonal changes in rainfall. Gonzalez-Acosta and colleagues (Gonzalez-Acosta et al. 2006) observed seasonal changes in the microbial community of an Avicennia germinans forest soil at Baja California Sur, Mexico, which was mostly driven by changes in seawater temperature. These latter studies show the existence of high temporal dynamics in the biogeochemistry and microbial communities in mangrove soils. Hence, changes in soil characteristics and microbial pathways due to treatments may have been missed during our once per year sampling regime. However, it remains surprising that we did not detect changes in the abundance of genes that are related to the nitrogen cycle. As changes in DNA composition are known to last for a longer period of time in soils, the microbial community involved in nitrogen-cycling did apparently not respond to the treatments.
We found few significant effects of the addition of R. mangle leaf litter and seedlings on total tannins and phenolics in the soil, even though plants in the Rhizophoraceae have been shown to contain large quantities of both compounds (Basak et al. 1999; Hernes et al. 2001; Hernes and Hedges 2004). The absence of significant effects in total tannins and phenolics was most likely due to the large variation in concentrations observed between replicates, but may also imply that tannins and phenolics from the treatments were rapidly degraded when entering the soil environment or that they disappeared immediately by self-aggregation or by co-precipitation with sediment particles (Maie et al. 2008). During a leaching experiment with submerged, senescent R. mangle leaves in a tropical estuary, a 30% loss of measurable tannins was observed in a period of less than 4 weeks (Hernes et al. 2001). Although self-aggregation of tannins seems to be limited at salinities above 15 PSU and co-precipitation of tannins with sediment particles is repressed by the presence of peat (Maie et al. 2008), phenolics and tannins leaked from R. mangle leave litter may have easily disappeared within an annual sampling regime.
Since inhibition of the microbial process of nitrification by tannins has often been reported (Baldwin et al. 1983; Lodhi and Killingbeck 1980; Olson and Reiners 1983; Rice and Pancholy 1973) we expected that the addition of tannin-rich R. mangle leaf litter and seedlings (including roots) would affect the rate of nitrification in the experimental plots. The lack of almost any effect of the presence of leaf litter and seedlings on nitrification may have been due to a fast disappearance of the tannins and phenolics. However, the role of tannins in the inhibition of nitrification has been questioned before (Kraus et al. 2004) as inhibition of nitrification could also be due to immobilization of ammonium, for example when low molecular weight tannins serve as carbon source (Fierer et al. 2001).
Another remarkable observation with respect to the nitrification rates that were measured, is the effect of the presence of allylthiourea (ATU). At the level of 100 μM, ATU is known to inhibit bacterial ammonia oxidizers (Taylor et al. 2013; Taylor et al. 2010). We observed on average inhibition percentages of 51 and 3% in March 2012 and 2015, respectively, meaning that the process of nitrification in D. spicata salt marsh soils was performed for a large part by archaeal ammonia oxidizers. In general, archaeal ammonia oxidizers are better adapted to conditions of low oxygen availability, low pH and lower amounts of ammonium than bacterial ammonia oxidizers (Hatzenpichler 2012; Prosser and Nicol 2012). In line with this, the potential nitrification rates without or with ATU correlated significantly and positively with the soil moisture content (Supplementary Table 1), which may reflect higher rates at lower oxygen availabilities. The same potential nitrification rates correlated significantly, but negatively with soil pH and with ammonium availability, meaning higher rates at lower pH values and lower ammonium concentrations. Finally, the potential nitrification rates in the absence and presence of ATU correlated significantly and positively with the log10 abundance of the archaeal amoA gene, but not with the bacterial amoA gene (Supplementary Table 5). In contrast, the potential nitrification rates in tidal Spartina-dominated salt marshes correlated positively with the abundance of the archaeal amoA gene and of the bacterial amoA gene (Moin et al. 2009). Moin and colleagues concluded that pH and salinity, but not pore water nitrogen, regulated the abundance of archaeal but not the bacterial ammonia oxidizers in Spartina salt marshes, while the abundance of the bacterial amoA gene did not correlate with any of the measured soil factors.
With an average archaeal to bacterial amoA ratio of 0.31 over all treatments including the controls, the bacterial ammonia oxidizer slightly outnumbered the archaeal ammonia oxidizers. In the unburned areas that were sampled in 2015, the ratio was smaller (0.11) mostly due to lower numbers of archaeal ammonia oxidizers. In Spartina salt marshes, the abundance of archaeal ammonia oxidizers was generally larger than of their bacterial counterparts. Archaeal amoA genes outnumbered the bacterial amoA genes in tidal Spartina-dominated salt marshes in southeastern Connecticut by a median factor of 35 (Moin et al. 2009) or 7 (Bernhard et al. 2015). The ratio of archaeal and bacterial amoA genes in the top 5 cm of an S. alterniflora-invaded coastal wetland in the Jiulong River estuary in the Fujian Province, China, amounted to 2 (Zhang et al. 2013). However, the number of observations on archaeal to bacterial amoA ratios in salt marsh soils is low and in addition too close to one to draw conclusions on differences between Distichlis- and Spartina-dominated salt marshes with respect to the dominance of either ammonia oxidizer.
Like the abundance of the archaeal amoA gene, the abundance of the nxrB gene, which is a key gene of nitrite-oxidizing bacteria that codes for the nitrite oxidoreductase (Pester et al. 2014; Vanparys et al. 2007), was significantly and positively correlated with potential nitrification rates, irrespective of the absence or presence of the inhibitor allylthiourea. This suggests a potential chain of microorganisms involved in the process of nitrification: nitrite-producing archaea and nitrite-consuming bacteria. However, the abundance of the nxrB gene was also significantly and positively correlated with the amended potential denitrification activities. The abundances of the nirS and the norB gene, which are commonly known as being involved in the process of denitrification, were significantly and positively correlated with the potential nitrification activities, but not with the potential denitrification activities. While the presence of the nirS gene has not been reported in genomes of ammonia-oxidizing microorganisms, the norB gene is also present in some ammonia-oxidizing bacteria (Junier et al. 2010). Hence, it is not possible to connect each gene to solely the process of nitrification or denitrification.
The abundances of different nitrogen cycle-related genes are also mutually correlated. A cluster analysis of gene abundances superimposed on a clustering of soil characteristics and potential activities showed a clustering of the archaeal and bacterial amoA genes together with the nifH and norB genes on one side, and the nxrB, the nirK and the nosZ gene on the other side, and this clustering seems to be governed by the total amounts of soil carbon and soil nitrogen. If the amoA genes and the nxrB gene are supposed to be coupled with the process of nitrification, then the opposite behavior of the amoA and nxrB genes is hard to explain. The same holds for the “denitrification” genes nirK, norB, and nosZ. One of the obvious reasons is that genes within a cluster do not necessarily belong to one cluster only (Schreiber et al. 2012). The nirK and the norB genes for example are not only involved in the process of denitrification, but also in the process of nitrification. Another reason may be that processes become uncoupled. The process of nitrification for instance may be interrupted under conditions of oxygen limitation when nitrite-oxidizing bacteria may be repressed while ammonia oxidation is still going on (Laanbroek and Gerards 1993). Under such conditions nitrite-reducing microbes such as denitrifiers could use the nitrite produced. In all this, however, it should be kept in mind that the presence of certain genes does not have to correlate with the measured prevailing conditions, it may just be a legacy of conditions occurring in the past.
Conclusions
The hypothesis that a northward invasion of salt marshes mangroves, a process that is occurring due to warming climatic conditions (Bianchi et al. 2013; Comeaux et al. 2012; Gabler et al. 2017), will affect the carbon and nitrogen cycling should at least partly be rejected as we found no effects of treatments on nitrogen-converting pathways. Neither did we find any changes in the abundance of nitrogen cycle-related genes in response to the experimental treatments. However, growth of R. mangle seedlings was stimulated by leaf litter, demonstrating an increased availability of nutrients in R. mangle leaf litter compared to mangrove-free D. spicata litter. In addition, decomposition of that litter was lower in the presence of R. mangle leaf litter and seedlings, which is likely due to the release of tannins from the leaf litter and roots of R. mangle. Hence, the invasion of R. mangle in D. spicata-dominated salt marshes could lead to accumulation of carbon in the soil. The reason for the absence of an effect of R. mangle leaf litter and seedlings additions on the nitrogen-converting activities and related genes may be due to the fact that salt marshes are generally already nitrogen-limited (Valiela and Teal 1979). Due to differences in tissue chemistry between mangroves and graminoids with a notably higher C:N ratio in the first group, expansion of mangroves into salt marshes will result in an increase of the soil nitrogen content (Henry and Twilley 2013). In addition, differences between the nutrient content of soils in salt marshes and in sites that are being invaded by mangroves will likely initially be small and concealed by the high input of allochthonous deposition of sediments from both natural and anthropogenic disturbances (Henry and Twilley 2013; Perry and Mendelssohn 2009). These confounding factors make it difficult to clearly explain the absence of a treatment effect on the nitrogen cycle-related genes as observed in our study. In addition, whereas the measurements of plant growth and litter decomposition in litter bags reflect time-integrated rates over a period of at least 12 months, the measurements of nitrogen processes and their related genes show only snapshots in time that do not necessarily exhibit actual activities over a longer period. In a follow-up study, it may be better to determine the expression of genes and not merely their presence, and when possible more frequently in time.
A non-intended outcome from the gene analyses were the various results that suggest the major role of ammonia-oxidizing archaea in the process of nitrification in the D. spicata salt marsh soil: (1) Only the archaeal amoA genes and not the bacterial amoA genes correlated significantly with the potential nitrification activities, (2) the potential nitrification activities were significantly correlated with environmental factors that promote archaeal ammonia oxidizers, i.e. high soil moisture content, low pH and low amounts of ammonium, and (3) the presence of the bacterial nitrification inhibitor allylthiourea had only a marginal effect on potential nitrification activities. The dominant role of archaeal ammonia oxidizers in the D. spicata salt seems to confirm the nitrogen-limited nature of this marsh.
References
Adamczyk B, Kitunen V, Smolander A (2008) Protein precipitation by tannins in soil organic horizon and vegetation in relation to tree species. Biol Fertil Soils 45:55–64. https://doi.org/10.1007/s00374-008-0308-0
Alberts JJ, Filip Z, Price MT, Williams DJ, Williams MC (1988) Elemental composition, stable carbon isotope ratios and spectrophotometric properties of humic substances occurring in a salt-marsh estuary. Org Geochem 12:455–467
Asami DK, Hong YJ, Barrett DM, Mitchell AE (2003) Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J Agric Food Chem 51:1237–1241. https://doi.org/10.1021/jf020635c
Austin MP, Nicholls AO, Margules CR (1990) Measurement of the realized qualitative niche: environmental niches of five eucalyptus species. Ecol Monogr 60:161–177. https://doi.org/10.2307/1943043
Baldwin IT, Olson RK, Reiners WA (1983) Protein-binding phenolics and the inhibition of nitrification in subalpine balsam fir soils. Soil Biol Biochem 15:419–423. https://doi.org/10.1016/0038-0717(83)90006-8
Basak UC, Das AB, Das P (1999) Organic constituents in leaves of 9 mangrove species of Orissa coast, India. Pak J Bot 31:55–62
Belser LW, Mays EL (1980) Specific-inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol 39:505–510
Benner R, Hatcher PG, Hedges JI (1990) Early diagenesis of mangrove leaves in a tropical estuary - bulk chemical characterization using solid-state c-13 nmr and elemental analyses. Geochim Cosmochim Acta 54:2003–2013
Bernhard AE, Dwyer C, Idrizi A, Bender G, Zwick R (2015) Long-term impacts of disturbance on nitrogen-cycling bacteria in a New England salt marsh. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00046
Bianchi TS, Allison MA, Zhao J, Li XX, Comeaux RS, Feagin RA, Kulawardhana RW (2013) Historical reconstruction of mangrove expansion in the Gulf of Mexico: linking climate change with carbon sequestration in coastal wetlands. Estuar Coast Shelf Sci 119:7–16. https://doi.org/10.1016/j.ecss.2012.12.007
Bremner JM, Mulvaney CS (1982) Salicylic acid-thiosulphate modification of Kjeldahl metod to include nitrate and nitrite. In: AC Page, RH Miller, DR Keeney (eds) Methods of Soil Chemical Analysis,Part2: Chemical and Microbiological Properties. Soil Science Society of America, Madison WI.
Casciotti KL, Ward BB (2005) Phylogenetic analysis of nitric oxide reductase gene homologues from aerobic ammonia-oxidizing bacteria. FEMS Microbiol Ecol 52:197–205
Chen RG, Twilley RR (1998) A gap dynamic model of mangrove forest development along gradients of soil salinity and nutrient resources. J Ecol 86:37–51. https://doi.org/10.1046/j.1365-2745.1998.00233.x
Coldren GA, Barreto CR, Wykoff DD, Morrissey EM, Langley JA, Feller IC, Chapman SK (2016) Chronic warming stimulates growth of marsh grasses more than mangroves in a coastal wetland ecotone. Ecology 97:3167–3175. https://doi.org/10.1002/ecy.1539
Comeaux RS, Allison MA, Bianchi TS (2012) Mangrove expansion in the Gulf of Mexico with climate change: implications for wetland health and resistance to rising sea levels. Estuar Coast Shelf Sci 96:81–95. https://doi.org/10.1016/j.ecss.2011.10.003
Cundell AM, Brown MS, Stanford R, Mitchell R (1979) Microbial-degradation of Rhizophora mangle leaves immersed in the sea. Estuar Coast Mar Sci 9:281–286. https://doi.org/10.1016/0302-3524(79)90041-0
Doughty CL, Langley JA, Walker WS, Feller IC, Schaub R, Chapman SK (2016) Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuar Coasts 39:385–396. https://doi.org/10.1007/s12237-015-9993-8
Feller IC, McKee KL, Whigham DF, O'Neill JP (2003) Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 62:145–175
Fierer N, Schimel JP, Cates RG, Zou JP (2001) Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem 33:1827–1839. https://doi.org/10.1016/s0038-0717(01)00111-0
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Gabler CA, Osland MJ, Grace JB, Stagg CL, Day RH, Hartley SB, Enwright NM, From AS, McCoy ML, McLeod JL (2017) Macroclimatic change expected to transform coastal wetland ecosystems this century (vol 7, pg 142, 2017). Nat Clim Chang 7:227–227
Gaby JC, Buckley DH (2012) A comprehensive evaluation of PCR primers to amplify the nifH gene of Nitrogenase. PLoS One 7:e42149. https://doi.org/10.1371/journal.pone.0042149
Gonzalez-Acosta B, Bashan Y, Hernandez-Saavedra NY, Ascencio F, De la Cruz-Aguero G (2006) Seasonal seawater temperature as the major determinant for populations of culturable bacteria in the sediments of an intact mangrove in an arid region. FEMS Microbiol Ecol 55: 311–321. doi: https://doi.org/10.1111/j.1574-6941.2005.00019.x
Gu Z, Eils R, Schlesner M (2016) Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32:2847–2849. https://doi.org/10.1093/bioinformatics/btw313
Guo H, Weaver C, Charles SP, Whitt A, Dastidar S, D'Odorico P, Fuentes JD, Kominoski JS, Armitage AR, Pennings SC (2017) Coastal regime shifts: rapid responses of coastal wetlands to changes in mangrove cover. Ecology 98:762–772. https://doi.org/10.1002/ecy.1698
Hartzfeld PW, Forkner R, Hunter MD, Hagerman AE (2002) Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J Agric Food Chem 50:1785–1790. https://doi.org/10.1021/jf0111155
Hatzenpichler R (2012) Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl Environ Microbiol 78:7501–7510. https://doi.org/10.1128/aem.01960-12
Henry KM, Twilley RR (2013) Soil development in a coastal Louisiana wetland during a climate-induced vegetation shift from salt marsh to mangrove. J Coast Res 29:1273–1283. https://doi.org/10.2112/jcoastres-d-12-00184.1
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189. https://doi.org/10.1128/aem.00231-06
Hernes PJ, Hedges JI (2004) Tannin signatures of barks, needles, leaves, cones, and wood at the molecular level. Geochim Cosmochim Acta 68:1293–1307
Hernes PJ, Benner R, Cowie GL, Goni MA, Bergamaschi BA, Hedges JI (2001) Tannin diagenesis in mangrove leaves from a tropical estuary: a novel molecular approach. Geochim Cosmochim Acta 65:3109–3122
Hossain M, Siddique MRH, Abdullah SMR, Saha S, Ghosh DC, Rahman MS, Limon SH (2014) Nutrient dynamics associated with leaching and microbial decomposition of four abundant mangrove species leaf litter of the Sundarbans, Bangladesh. Wetlands 34:439–448. https://doi.org/10.1007/s13157-013-0510-1
Howard RJ, Biagas J, Allain L (2016) Growth of common brackish marsh Macrophytes under altered hydrologic and salinity regimes. Wetlands 36:11–20. https://doi.org/10.1007/s13157-015-0711-x
Junier P, Molina V, Dorador C, Hadas O, Kim OS, Junier T, Witzel KP, Imhoff JF (2010) Phylogenetic and functional marker genes to study ammonia-oxidizing microorganisms (AOM) in the environment. Appl Microbiol Biotechnol 85:425–440. https://doi.org/10.1007/s00253-009-2228-9
Kandil FE, Grace MH, Seigler DS, Cheeseman JM (2004) Polyphenolics in Rhizophora mangle L. leaves and their changes during leaf development and senescence. Trees-Structure and Function 18:518–528. https://doi.org/10.1007/s00468-004-0337-8
Kelleway JJ, Saintilan N, Macreadie PI, Skilbeck CG, Zawadzki A, Ralph PJ (2016) Seventy years of continuous encroachment substantially increases "blue carbon" capacity as mangroves replace intertidal salt marshes. Glob Chang Biol 22:1097–1109. https://doi.org/10.1111/gcb.13158
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727. https://doi.org/10.2307/2265777
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems - a review. Plant Soil 256:41–66
Kraus TEC, Zasoski RJ, Dahlgren RA (2004) Fertility and pH effects on polyphenol and condensed tannin concentrations in foliage and roots. Plant Soil 262:95–109. https://doi.org/10.1023/b:plso.0000037021.41066.79
Laanbroek HJ, Gerards S (1993) Competition for limiting amounts of oxygen between nitrosomonas-europaea and nitrobacter-winogradskyi grown in mixed continuous cultures. Arch Microbiol 159:453–459
Laanbroek HJ, Veenhuizen PTM, Keijzer RM, Hefting MM (2017) Numerical relationships between archaeal and bacterial amoA genes vary by Icelandic andosol classes. Microb Ecol:1–12. https://doi.org/10.1007/s00248-017-1032-9
Lee RY, Porubsky WP, Feller IC, McKee KL, Joye SB (2008) Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (twin cays, Belize). Biogeochemistry 87:181–198. https://doi.org/10.1007/s10533-008-9176-9
Lin YM, Liu JW, Xiang P, Lin P, Ye GF, Sternberg L (2006) Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River estuary, Fujian, China. Biogeochemistry 78:343–359. https://doi.org/10.1007/s10533-005-4427-5
Lin YM, Liu XW, Zhang H, Fan HQ, Lin GH (2010) Nutrient conservation strategies of a mangrove species Rhizophora stylosa under nutrient limitation. Plant Soil 326:469–479. https://doi.org/10.1007/s11104-009-0026-x
Lodhi MAK, Killingbeck KT (1980) Allelopathic inhibition of nitrification and nitrifying bacteria in a ponderosa pine (Pinus ponderosa dougl) community. Am J Bot 67:1423–1429. https://doi.org/10.2307/2442870
Lonard RI, Judd FW, Stalter R (2013) The biological Flora of coastal dunes and wetlands: Distichlis spicata (C. Linnaeus) E. Greene. J Coast Res 29:105–117. https://doi.org/10.2112/jcoastres-d-12-00018.1
Maie N, Pisani O, Jaffe R (2008) Mangrove tannins in aquatic ecosystems: their fate and possible influence on dissolved organic carbon and nitrogen cycling. Limnol Oceanogr 53:160–171
Moin NS, Nelson KA, Bush A, Bernhard AE (2009) Distribution and diversity of archaeal and bacterial ammonia oxidizers in salt marsh sediments. Appl Environ Microbiol 75:7461–7468. https://doi.org/10.1128/aem.01001-09
Morita H (1980) Total phenolic content in the pyrophosphate extracts of 2 peat soil profiles. Can J Soil Sci 60:291–297. https://doi.org/10.4141/cjss80-032
Naidoo G (1990) Effects of nitrate, ammonium and salinity on growth of the mangrove Bruguiera-Gymnorrhiza (L) lam. Aquat Bot 38:209–219. https://doi.org/10.1016/0304-3770(90)90006-7
Northup RR, Yu ZS, Dahlgren RA, Vogt KA (1995) Polyphenol control of nitrogen release from pine litter. Nature 377:227–229. https://doi.org/10.1038/377227a0
Olson RK, Reiners WA (1983) Nitrification in subalpine balsam fir soils - tests for inhibitory factors. Soil Biol Biochem 15:413–418. https://doi.org/10.1016/0038-0717(83)90005-6
Osborne MR, Presnell B, Turlach BA (2000) On the LASSO and its dual. J Comput Graph Stat 9:319–337. https://doi.org/10.1080/10618600.2000.10474883
Perry CL, Mendelssohn IA (2009) Ecosystem effects of expanding populations of Avicennia germinans in a Louisiana salt marsh. Wetlands 29:396–406. https://doi.org/10.1672/08-100.1
Pester M, Maixner F, Berry D, Rattei T, Koch H, Lucker S, Nowka B, Richter A, Spieck E, Lebedeva E, Loy A, Wagner M, Daims H (2014) NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ Microbiol 16:3055–3071. https://doi.org/10.1111/1462-2920.12300
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531
Purvaja R, Ramesh R, Ray AK, Rixen T (2008) Nitrogen cycling: a review of the processes, transformations and fluxes in coastal ecosystems. Curr Sci 94:1419–1438
Regan K, Stempfhuber B, Schloter M, Rasche F, Prati D, Philippot L, Boeddinghaus RS, Kandeler E, Marhan S (2017) Spatial and temporal dynamics of nitrogen fixing, nitrifying and denitrifying microbes in an unfertilized grassland soil. Soil Biol Biochem 109:214–226. https://doi.org/10.1016/j.soilbio.2016.11.011
Rice EL, Pancholy SK (1973) Inhibition of nitrification by climax ecosystems .2. Additional evidence and possible role of tannins. Am J Bot 60:691–702. https://doi.org/10.2307/2441448
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoa as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Saintilan N, Rogers K, McKee K (2009) Salt-marsh-mangrove interactions in Australia and the Americas. In: GME Perillo, E Wolanski, DR Cahoon, MM Brinson (eds) Coastal wetlands: an integrated ecosystem approach. Elsevier, Amsterdam.
Saintilan N, Wilson NC, Rogers K, Rajkaran A, Krauss KW (2014) Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob Chang Biol 20:147–157. https://doi.org/10.1111/gcb.12341
Schreiber F, Wunderlin P, Udert KM and Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbio 3:372. https://doi.org/10.3389/fmicb.2012.00372
Stephen JR, Chang YJ, Macnaughton SJ, Kowalchuk GA, Leung KT, Flemming CA, White DC (1999) Effect of toxic metals on indigenous soil p-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microbiol 65:95–101
Stuart SA, Choat B, Martin KC, Holbrook NM, Ball MC (2007) The role of freezing in setting the latitudinal limits of mangrove forests. New Phytol 173:576–583. https://doi.org/10.1111/j.1469-8137.2006.01938.x
Suzuki R, Shimodaira H (2006) Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22:1540–1542. https://doi.org/10.1093/bioinformatics/btl117
Taylor AE, Zeglin LH, Dooley S, Myrold DD, Bottomley PJ (2010) Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Appl Environ Microbiol 76:7691–7698. https://doi.org/10.1128/aem.01324-10
Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD, Sayavedra-Soto L, Bottomley PJ (2013) Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing Thaumarchaea and bacteria. Appl Environ Microbiol 79:6544–6551. https://doi.org/10.1128/aem.01928-13
Throback IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417. https://doi.org/10.1016/j.femsec.2004.04.011
Valiela I, Teal JM (1979) Nitrogen budget of a salt-marsh ecosystem. Nature 280:652–656. https://doi.org/10.1038/280652a0
Valiela I, Bowen JL, York JK (2001) Mangrove forests: one of the world's threatened major tropical environments. Bioscience 51:807–815
Vanparys B, Spieck E, Heylen K, Wittebolle L, Geets J, Boon N, De Vos P (2007) The phylogeny of the genus Nitrobacter based on comparative rep-PCR, 16S rRNA and nitrite oxidoreductase gene sequence analysis. Syst Appl Microbiol 30:297–308
Verhagen FJM, Laanbroek HJ (1991) Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited Chemostats. Appl Environ Microbiol 57:3255–3263
Wang Y, Naumann U, Wright ST, Warton DI (2012) Mvabund– an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Zhang QF, Peng JJ, Chen Q, Yang XR, Hong YW, Su JQ (2013) Abundance and composition of denitrifiers in response to Spartina alterniflora invasion in estuarine sediment. Can J Microbiol 59:825–836. https://doi.org/10.1139/cjm-2013-0516
Acknowledgements
We like to acknowledge the support obtained from the staff of the Smithsonian Marine Station at Fort Pierce, Florida, during our sampling weeks. We also like to thank our colleagues Mr. Gerrit Rouwenhorst for the chemical analyses and Dr. Boudewijn Beltman for critically reading the manuscript. Finally, we like to acknowledge two anonymous reviewers for their positive criticism on our manuscript, which help to improve its content. The study was supported by a grant of the Smithsonian Marine Science Network to H.J.L., J.T.A.V. and D.F.W. and by a grant from the National Natural Science Foundation of China to Q.-F.Z. (grant no. 41571252). This is publication number 6493 of the Netherlands Institute of Ecology and publication number 1082 of the Smithsonian Marine Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zucong Cai
Electronic supplementary material
ESM 1
(PDF 1174 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Laanbroek, H.J., Zhang, QF., Leite, M. et al. Effects of Rhizophora mangle leaf litter and seedlings on carbon and nitrogen cycling in salt marshes – potential consequences of climate-induced mangrove migration. Plant Soil 426, 383–400 (2018). https://doi.org/10.1007/s11104-018-3611-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3611-z