Abstract
Aims
Eutrophication of coastal waters can have consequences for the growth, function and soil processes of coastal wetlands. Our aims were to assess how nutrient enrichment affects growth, biomass allocation and decomposition of plant tissues of a common and widespread mangrove, Avicennia marina, and how eutrophication drives changes in below-ground carbon sequestration.
Methods
We assessed this through the measurement of above- and belowground growth and decomposition rates of plants and plant tissue in unenriched or nutrient enriched treatments.
Results
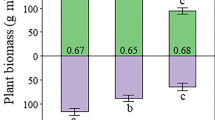
Nutrient enrichment increased biomass allocation above-ground compared to below-ground in seedlings but not in fully developed, mature trees where we observed the opposite pattern. Experiments to assess root decomposition found that 40–50% of biomass was lost within six months with little change between 12 and 18 months, indicating a high potential for accumulation of organic matter over time. We estimate root-derived carbon sequestration rates of 53, 250 and 94 g C m−2 year−1 for unenriched control, N and P enriched treatments, respectively.
Conclusions
These results show coastal eutrophication can be beneficial and detrimental to ecosystem function of coastal plants. Eutrophication stimulates root growth in fully developed trees, increasing organic matter input to soils. Our data suggests that organic matter accumulation will increase in areas with high nutrient availability where root growth is increased and rates of decomposition are low.




Similar content being viewed by others
References
Abal EG, Dennison WC, O'Donohue MJH (1998) Seagrasses and mangroves in Moreton Bay. In: Tibbets IR, Hall NJ, Dennison WC (eds) Moreton Bay and catchment. The University of Queensland, Brisbane, Schhol of Marine Science, pp 269–278
Adame M, Lovelock C (2011) Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 663:23–50
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6:195–219
Bala Krishna Prasad M (2012) Nutrient stoichiometry and eutrophication in Indian mangroves. Environmental Earth Sciences 67:293–299
Ball M (1988) Salinity tolerance in the mangroves Aegiceras corniculatumand Avicennia marina. I. Water use in relation to growth, carbon partitioning, and salt balance. Funct Plant Biol 15:447–464
Ball MC (2002) Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees 16:126–139
Ball MC, Pidsley SM (1995) Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata, in northern Australia. Funct Ecol 9:77–85
Bompy F, Lequeue G, Imbert D, Dulormne M (2014) Increasing fluctuations of soil salinity affect seedling growth performances and physiology in three Neotropical mangrove species. Plant Soil 380:399–413
Brewer JS, Rand T, Levine JM, Bertness MD (1998) Biomass allocation, clonal dispersal, and competitive success in three salt marsh plants. Oikos 82:347–353
Castañeda-Moya E, Twilley RR, Rivera-Monroy VH, Marx BD, Coronado-Molina C, Ewe SML (2011) Patterns of root dynamics in mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. Ecosystems 14:1178–1195
Chapin FS (1991) Integrated responses of plants to stress. Bioscience 41:29–36
Cloern J (1999) The relative importance of light and nutrient limitation of phytoplankton growth: a simple index of coastal ecosystem sensitivity to nutrient enrichment. Aquat Ecol 33:3–15
Clough BF (1992) Primary productivity and growth of mangrove forests. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. Coastal and estuarine studies No 41. American Geophysical Union, Washington, pp 225–249
Development Core Team R (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Downing JA, McClain M, Twilley R, Melack JM, Elser J, Rabalais NN, Lewis WM Jr, Turner RE, Corredor J, Soto D, Yanez-Arancibia A, Kopaska JA, Howarth RW (1999) The impact of accelerating land-use change on the N-cycle of tropical aquatic ecosystems: current conditions and projected changes. Biogeochemistry 46:109–148
Duke N, Ball M, Ellison J (1998) Factors influencing biodiversity and distributional gradients in mangroves. Global Ecology & Biogeography Letters 7:27–47
Feller IC (1995) Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecol Monogr 65:477–505
Feller IC, Whigham DF, O’Neill JP, McKee KL (1999) Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 80:2193–2205
Feller IC, Lovelock CE, Mckee KL (2007) Nutrient addition differentially affects ecological processes of Avicennia germinans in nitrogen versus phosphorus limited mangrove ecosystems. Ecosystems 10:347–359
Ferreira V, Castagneyrol B, Koricheva J, Gulis V, Chauvet E, Graça MAS (2015) A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol Rev 90:669–688
Fujii S, Mori AS, Koide D, Makoto K, Matsuoka S, Osono T, Isbell F (2017) Disentangling relationships between plant diversity and decomposition processes under forest restoration. J Appl Ecol 54:80–90
Gleeson SK, Tilman D (1992) Plant allocation and the multiple limitation hypothesis. Am Nat 139:1322–1343
Hartman WH, Richardson CJ (2013) Differential nutrient limitation of soil microbial biomass and metabolic quotients: is there a biological stoichiometry of soil microbes? PLoS One 8:e57127
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405
Huxham M, Langat J, Tamooh F, Kennedy H, Mencuccini M, Skov MW, Kairo J (2010) Decomposition of mangrove roots: effects of location, nutrients, species identity and mix in a Kenyan forest. Estuar Coast Shelf Sci 88:135–142
James JJ, Tiller RL, Richards JH (2005) Multiple resources limit plant growth and function in a saline-alkaline desert community. J Ecol 93:113–126
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nature Geosci 3:315–322
Kauffman JB, Donato D (2012) Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stocks in mangrove forests. In: Center for International Forestry Research (CIFOR). Indonesia, Bogor
Keuskamp JA, Dingemans BJJ, Lehtinen T, Sarneel JM, Hefting MM (2013) Tea bag index: a novel approach to collect uniform decomposition data across ecosystems. Methods Ecol Evol 4:1070–1075
Keuskamp JA, Hefting MM, Dingemans BJ, Verhoeven JT, Feller IC (2015) Effects of nutrient enrichment on mangrove leaf litter decomposition. Sci Total Environ 508:402–410
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Krauss KW, Keeland BD, Allen JA, Ewel KC, Johnson DJ (2007) Effects of season, rainfall, and Hydrogeomorphic setting on mangrove tree growth in Micronesia. Biotropica 39:161–170
Lovelock, C. E., Ball, M. C., Martin, K. C., and C. Feller, I. 2009. Nutrient enrichment increases mortality of mangroves. PLoS One 4:e5600
Lovelock CE, Adame MF, Bennion V, Hayes M, O’Mara J, Reef R, Santini N a (2014) Contemporary rates of carbon sequestration through vertical accretion of sediments in mangrove forests and saltmarshes of south East Queensland, Australia. Estuar Coasts 37:763–771
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Syst 5:39–64
McGraw JB, Garbutt K (1990) Demographic growth analysis. Ecology 71:1199–1204
McKee KL (1995) Interspecific variation in growth, biomass partitioning, and defensive characteristics of Neotropical mangrove seedlings: response to light and nutrient availability. Am J Bot 82:299–307
McKee KL (2011) Biophysical controls on accretion and elevation change in Caribbean mangrove ecosystems. Estuar Coast Shelf Sci 91:475–483
McKee KL, Faulkner PL (2000) Restoration of biogeochemical function in mangrove forests. Restor Ecol 8:247–259
Mckee KL, Cahoon DR, Feller IC (2007) Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob Ecol Biogeogr 16:545–556
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
Middleton BA, McKee KL (2001) Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J Ecol 89:818–828
Naidoo G (1987) Effects of salinity and nitrogen on growth and water relations in the mangrove, Avicennia marina (Forsk). New Phytol 107:317–325
Paerl HW (1997) Coastal eutrophication and harmful algal blooms: importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol Oceanogr 42:1154–1165
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Funct Plant Biol 27:595–607
Poret N, Twilley R, Rivera-Monroy V, Coronado-Molina C (2007) Belowground decomposition of mangrove roots in Florida coastal everglades. Estuar Coasts 30:491–496
Romero LM, Smith TJ, Fourqurean JW (2005) Changes in mass and nutrient content of wood during decomposition in a South Florida mangrove forest. J Ecol 93:618–631
Saintilan N, Rogers K, Mazumder D, Woodroffe C (2013) Allochthonous and autochthonous contributions to carbon accumulation and carbon store in southeastern Australian coastal wetlands. Estuar Coast Shelf Sci 128:84–92
Santini NS, Reef R, Lockington DA, Lovelock CE (2015) The use of fresh and saline water sources by the mangrove Avicennia marina. Hydrobiologia 745:59–68
Sherman RE, Fahey TJ, Martinez P (2003) Spatial patterns of biomass and aboveground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosystems 6:384–398
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ Sci Pollut Res 10:126–139
Twilley RR, Pozo M, Garcia VH, Rivera-Monroy VH, Zambrano R, Bodero A (1997) Litter dynamics in riverine mangrove forests in the Guayas River estuary, Ecuador. Oecologia 111:109–122
Viechtbauer, W. 2010. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 36(3):1–48
Acknowledgements
This work was partially supported by the CSIRO Marine and Coastal Carbon Biogeochemistry Cluster (Coastal Carbon Cluster) and The National Center for Groundwater Research and Training.
Author contributions
MH and CEL conceived and designed the experiments. MH, AJ, BT, and RR performed the experiments. MH, JK and CEL analysed the data. MH wrote the manuscript; with other authors providing editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeffrey Walck .
Rights and permissions
About this article
Cite this article
Hayes, M.A., Jesse, A., Tabet, B. et al. The contrasting effects of nutrient enrichment on growth, biomass allocation and decomposition of plant tissue in coastal wetlands. Plant Soil 416, 193–204 (2017). https://doi.org/10.1007/s11104-017-3206-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3206-0