Abstract
Aims
The aim of this study was to examine the effect of plant species differing in functional and phylogenetic traits on the decomposition processes of leaf litter in a grassland of Japanese pampas grass (Miscanthus sinensis) and adjacent forests of Japanese red pine (Pinus densiflora) and Japanese oak (Quercus crispula), representing sequential stages of secondary succession.
Methods
The litterbag experiments were carried out for 3 years in a temperate region of central Japan.
Results
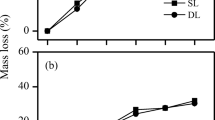
The decomposition constant (Olson’s k) was 0.49, 0.39, and 0.56/year for grass, pine, and oak, respectively. Nitrogen mass decreased in grass leaf litter during decomposition, whereas the absolute amount of nitrogen increased in leaf litter of pine and oak during the first year. Holocellulose in grass leaf litter decomposed selectively over acid-unhydrolyzable residues more markedly than in leaf litter of pine and oak. 13C nuclear magnetic resonance analysis also revealed a decrease in the relative area of O-alkyl-C in grass.
Conclusions
The different decomposition among the three litter species implied that the secondary succession from grassland to pine forest and from pine to oak forests could decrease and increase, respectively, the rate of accumulation and turnover of organic materials and N in soils.




Similar content being viewed by others
References
Aber JD, Mellilo JM (1982) Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin concentration. Can J Bot 60:2263–2269
Baldock JA, Oades JM, Nelson PN, Skene TM, Golchin A, Clarke P (1997) Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust J Soil Res 35:1061–1083
Bechtold JS, Naiman RJ (2009) A quantitative model of soil organic matter accumulation during floodplain primary succession. Ecosystems 12:1352–1368
Bending GD, Turner MK (1999) Interaction of biochemical quality and particle size of crop residues and its effect on the microbial biomass and nitrogen dynamics following incorporation into soil. Biol Fertil Soils 29:319–327
Berg B (1986) Nutrient release from litter and humus in coniferous forest soils - a mini review. Scand J For Res 1:359–369
Berg B, Laskowski R (2006) Litter decomposition: a guide to carbon and nutrient turnover. Academic Press, Amsterdam
Berg B, McClaugherty CA (1989) Nitrogen and phosphorus release from decomposing litter in relation to the disappearance of lignin. Can J Bot 67:1148–1156
Berg B, Söderström B (1979) Fungal biomass and nitrogen in decomposing Scots pine needle litter. Soil Biol Biochem 11:339–341
Berg B, Staaf H (1987) Release of nutrients from decomposing white birch leaves and Scots pine needle litter. Pedobiologia 30:55–63
Berg B, Wessén B (1984) Changes in organic-chemical components and ingrowth of fungal mycelium in decomposing birch leaf litter as compared to pine needles. Pedobiologia 26:285–298
Berg B, Ekbohm G, McClaugherty C (1984) Lignin and holocellulose relations during long-term decomposition of some forest litters. Long-term decomposition in a Scots pine forest. IV. Can J Bot 62:2540–2550
Berg B, McClaugherty C, Johansson MB (1997) Chemical changes in decomposing litter can be systemized with respect to the initial chemical composition of the litter. Swedish University of Agricultural Sciences report 74, Uppsala
Cortez J, Garnier E, Pérez-Harguindeguy N, Debussche M, Gillon D (2007) Plant traits, litter quality and decomposition in a Mediterranean old-field succession. Plant Soil 296:19–34
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gallardo A, Merino J (1993) Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influence of substrate quality. Ecology 74:152–161
Gallardo A, Merino J (1999) Control of leaf litter decomposition rate in a Mediterranean shrubland as indicated by N, P and lignin concentration. Pedobiologia 43:64–72
Geng X, Pastor J, Dewey B (1993) Decay and nitrogen dynamics of litter from disjunct, congeneric tree species in old-growth stands in northeastern China and Wisconsin. Can J Bot 71:693–699
Gilardi G, Abis L, Cass AEG (1995) Carbon-13 CP/MAS solid-state NMR and FT-IR spectroscopy of wood cell wall biodegradation. Enzym Microbiol Technol 17:268–275
Hayashi I (1967) Studies on the plant succession in Sugadaira, central Japan (1). Bull Sugadaira Biol Lab 1:1–18 (in Japanese with English abstract)
Hayashi I, Hishinuma Y, Yamasawa T (1981) Structure and functioning of Miscanthus sinensis grassland in Sugadaira, central Japan. Vegetatio 48:17–25
Hobara S, Osono T, Hirose D, Noro K, Hirota M, Benner R (2014) The roles of microorganisms in litter decomposition and soil formation. Biogeochemistry in press
Holden SR, Gutierrez A, Treseder KK (2013) Changes in soil fungal communities, extracellular enzyme activities, and litter decomposition across a fire chronosequence in Alaskan boreal forest. Ecosystems 16:34–46
Hossain MZ, Okubo A, Sugiyama SI (2010) Effects of grassland species on decomposition of litter and soil microbial communities. Ecol Res 25:255–261
Iimura Y, Fujimoto M, Hirota M, Tamura K, Higashi T, Yonebayashi K, Fujitake N (2010) Effects of ecological succession on surface mineral horizons in Japanese volcanic ash soil. Geoderma 159:122–130
Kato J, Hayashi I (2003) The determination and prediction of pine to oak forest succession in Sugadaira, central Japan. Korean J Ecol 26:155–163
Kato J, Hayasi I (2006) Quantitative analysis of a stand of Pinus densiflora undergoing succession to Quercus mongolica ssp. crispula. 1. A 31-year record of growth and population dynamics of the canopy trees. Ecol Res 21:503–509
King HGC, Heath GW (1967) The chemical analysis of small samples of leaf material and the relationship between the disappearance and composition of leaves. Pedobiologia 7:192–197
Koukoura Z, Mamolos AP, Kalburtji KL (2003) Decomposition of dominant plant species litter in a semi-arid grassland. Appl Soil Ecol 23:13–23
McClaugherty CA, Berg B (1987) Cellulose, lignin and nitrogen concentrations as rate regulating factors in late stages of forest litter decomposition. Pedobiologia 30:101–112
Mellilo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Moretto AS, Distel RA, Didoné NG (2001) Decomposition and nutrient dynamics of leaf litter and roots from palatable and unpalatable grasses in a semi-arid grassland. Appl Soil Ecol 18:31–37
Murphy KL, Klopatek JM, Klopatek CC (1998) The effects of litter quality and climate on decomposition along an elevational gradient. Ecol Appl:1061-1071
Novo-Uzal E, Pomar F, Ros LVG, Espiñeira JM, Barceló AR (2012) Evolutionary history of lignins. Adv Bot Res 61:311–350
Olson J (1963) Energy storage and the balance of produces and decomposers in ecological systems. Ecology 44:322–331
Osono T (2007) Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 22:955–974
Osono T (2010) Decomposition of grass leaves by ligninolytic litter-decomposing fungi. Grassl Sci 56:31–36
Osono T, Hirose D (2009) Ascomycete fungi associated with the bleaching of Quercus crispula leaf litter in Rishiri Island. Rishiri Res 28:55–60
Osono T, Hirose D (2011) Colonization and lignin decomposition of pine needle litter by Lophodermium pinastri. For Path 41:156–162
Osono T, Takeda H (2001) Organic chemical and nutrient dynamics in decomposing beech leaf litter in relation to fungal ingrowth and succession during 3-year decomposition processes in a cool temperate deciduous forest in Japan. Ecol Res 16:649–670
Osono T, Takeda H (2004) Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res 19:593–602
Osono T, Takeda H (2005) Decomposition of organic chemical components in relation to nitrogen dynamics in leaf litter of 14 tree species in a cool temperate forest. Ecol Res 20:41–49
Osono T, Trofymow JA (2012) Microfungal diversity associated with Kindbergia oregana in successional forests of British Columbia. Ecol Res 27:35–41
Osono T, Takeda H, Azuma JI (2008a) Carbon isotope dynamics during leaf litter decomposition with reference to lignin fractions. Ecol Res 23:51–55
Osono T, Iwamoto S, Trofymow JA (2008b) Colonization and decomposition of salal (Gaultheria shallon) leaf litter by saprobic fungi in successional forests on coastal British Columbia. Can J Microbiol 54:427–434
Osono T, Ishii Y, Takeda H, Seramethakun T, Khamyong S, To-Anun C, Hirose D, Tokumasu S, Kakishima M (2009) Fungal succession and lignin decomposition on Shorea obtusa leaves in a tropical seasonal forest in northern Thailand. Fungal Divers 36:101–119
Ostertag R, Marín-Spiotta E, Silver WL, Schulten J (2008) Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico. Ecosystems 11:701–714
Preston CM, Trofymow JA, CIDET Working Group (2000) Variability in litter quality and its relationship to litter decay in Canadian forests. Can J Bot 78:1269–1287
Shanker U (1994) Carbon and nutrient release from decomposing litter of four species in an excessively rainfed subtropical grassland. Acta Oecol 15:325–335
Sharma G, Sharma R, Sharma E (2008) Influence of stand age on nutrient and energy release through decomposition in alder-cardamom agroforestry systems of the eastern Himalayas. Ecol Res 23:99–106
Smith JH, Peckenpaugh RE (1986) Straw decomposition in irrigated soil: comparison of twenty-three cereal straws. Soil Sci Soc Am J 50:928–932
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman, New York
Stump LM, Binkley D (1993) Relationships between litter quality and nitrogen availability in Rocky Mountain forests. Can J For Res 23:492–502
Thomas RJ, Asakawa NM (1993) Decomposition of leaf litter from tropica forage grasses and legumes. Soil Biol Biochem 25:1351–1361
Trofymow JA, Addison J, Blackwell BA, He F, Preston CA, Marshall VG (2003) Attributes and indicators of old-growth and successional Douglas-fir forests on Vancouver Island. Environ Rev 11:S187–S204
Viereck LA, Dyrness CT, Foote MJ (1993) An overview of the vegetation and soils of the floodplain ecosystems of the Tanana River, interior Alaska. Can J For Res 23:889–898
Wessén B, Berg B (1986) Long-term decomposition of barley straw: chemical changes and ingrowth of fungal mycelium. Soil Biol Biochem 18:53–59
Acknowledgments
We thank Dr. S. Hobara and Mr. T. Hishinuma for his help in chemical analyses; Dr. M. Hirota for his useful discussions; and Dr. E. Nakajima for her critical reading of the manuscript. This work has received partial financial support from Grants for Excellent Graduate Schools, MEXT, Japan to Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim Moore.
Rights and permissions
About this article
Cite this article
Osono, T., Azuma, Ji. & Hirose, D. Plant species effect on the decomposition and chemical changes of leaf litter in grassland and pine and oak forest soils. Plant Soil 376, 411–421 (2014). https://doi.org/10.1007/s11104-013-1993-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1993-5