Abstract
Background and aims
The feather moss Pleurozium schreberi (Brid.) Mitt. is colonized by cyanobacteria, which fix substantial amounts of atmospheric nitrogen (N) in pristine and N-poor ecosystems. Cyanobacterial N2 fixation is inhibited by N deposition. However, the threshold of N input that leads to the inhibition of N2 fixation has not been adequately investigated. Further, the ability of N2 fixation to recover in mosses from high N deposition areas has not been studied to date.
Methods
We conducted two laboratory studies in which we (1) applied a range of concentrations of N as NH4NO3 to mosses from low N-deposition areas, and (2) we deprived mosses from a high N-deposition area of N to test their ability to recover N2 fixation.
Results
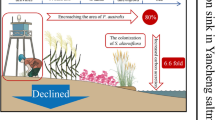
Higher addition rates (up to 10 kg N ha−1) did not systematically inhibit N2 fixation in P. schreberi. Conversely, upon weeks of N deprivation of mosses from a high N environment, N2 fixation rates increased.
Conclusions
The threshold of total N deposition above which N2 fixation in P. schreberi is inhibited is likely to be > 10 kg N ha−1. Further, cyanobacteria are able to recover from high N inputs and are able to fix atmospheric N2 after a period of N deprivation.




Similar content being viewed by others
References
Achermann B, Bobbink R (2003) Empirical critical loads for nitrogen. Environmental documentation No. 164. Swiss Agency for the Environment, Forests and Landscape, Berne
Ackermann K, Zackrisson O, Rousk J, Jones DL, DeLuca TH (2012) N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems 15:986–998
Bay G, Nahar N, Oubre M, Whitehouse MJ, Wardle DA, Zackrisson O, Nilsson MC, Rasmussen U (2013) Boreal feather mosses secrete chemical signals to gain nitrogen. New Phytol. doi:10.1111/nph.12403
Cleveland CC, Townsend AR, Schimel DS et al (1999) Global patterns of terrestrial biological nitrogen (N2) fixation. Glob Biogeochem Cycles 13:623–645
DeLuca TH, Zackrisson O, Nilsson MC, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917–920
DeLuca TH, Zackrisson O, Gentili F, Sellstedt A, Nilsson MC (2007) Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 152:121–130
DeLuca TH, Zackrisson O, Gundale MJ, Nilsson MC (2008) Ecosystem feedbacks and nitrogen fixation in boreal forests. Science 320:1181
Galloway JN, Cowling EB (2002) Reactive nitrogen and the world: 200 years of change. Ambio 31:64–71
Gundale MJ, DeLuca TH, Nordin A (2011) Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob Chang Biol 17:2743–2753
Gundale MJ, Wardle DA, Nilsson MC (2012) The effect of altered macroclimate on N-fixation by boreal feather mosses. Biol Lett 8:805–808
Hornung M, Sutton MA, Wilson RB (1995) Mapping and modelling of critical loads for nitrogen – a workshop report. Institute of Terrestrial Ecology, Edinburgh
Jackson BG, Martin P, Nilsson MC, Wardle DA (2011) Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency. Oikos 120:570–581
Jones MLM, Wallace HL, Norris D et al (2004) Changes in vegetation and soil characteristics in coastal sand dunes along a gradient of atmospheric nitrogen deposition. Plant Biol 6:598–605
Kaplan-Levy RN, Hadas O, Summers ML, Rücker J, Sukenik A (2010) Akinetes: Dormant Cells of cyanobacteria. In: Lubzens E et al. (ed) Dormancy and resistance in harsh environments. Top Curr Genet 21:5–27
Lindo Z, Gonzalez A (2010) The bryosphere: an integral and influential component of the earth’s biosphere. Ecosystems 13:612–627
Meeks JC, Elhai J (2002) Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant associated symbiotic growth states. Microbiol Mol Biol Rev 65:94–121
Nordin A, Strengbom J, Ericson L (2006) Responses to ammonium and nitrate additions by boreal plants and their natural enemies. Environ Pollut 141:167–174
Phil-Karlsson G, Akselsson C, Hellsten S, Karlsson PE, Malm G (2009) Vol. IVL rapport B 1851. IVL Svenska Miljöinstitutet, Gothenburg
Potts M (1994) Dessication tolerance of prokaryotes. Microbiol Rev 58:755–805
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, ISBN 3-900051-07-0. http://www.R-project.org
Rai AN, Söderbäck E, Bergman B (2000) Cyanobacterium-plant symbioses. New Phytol 147:449–481
Rousk K, Rousk J, Jones DL, Zackrisson O, DeLuca TH (2013) Feather moss nitrogen acquisition across natural fertility gradients in boreal forests. Soil Biol Biochem 61:86–95
Schöllhorn R, Burris RH (1967) Acetylene as a competitive inhibitor of nitrogen fixation. Proc Natl Acad Sci 58:213–218
Shaver GR, Chapin FS (1980) Response to fertilization by various plant growth forms in an Alaskan tundra: nutrient accumulation and growth. Ecology 61:662–675
Smith VR (1984) Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a Subantarctic island. Appl Environ Microbiol 48:594–600
Solga A, Burkhardt J, Zechmeister HG, Frahm JP (2005) Nitrogen content, 15N natural abundance and biomass of the two pleurocarpous mosses Pleurozium schreberi (Brid.) Mitt. and Scleropodium purum (Hedw.) Limpr. in relation to atmospheric nitrogen deposition. Environ Pollut 134:465–473
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Stewart KJ, Lamb EG, Coxson DS, Siciliano SD (2011) Bryophyte-cyanobacterial associations as a key factor in N2-fixation across the Canadian Arctic. Plant Soil 344:335–346
Tamm CO (1991) Nitrogen in terrestrial ecosystems. Springer, Berlin
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409
Zackrisson O, DeLuca TH, Nilsson MC, Sellstedt A, Berglund LM (2004) Nitrogen fixation increases with successional age in boreal forests. Ecology 85:3327–3334
Acknowledgments
Funding was provided by Bangor University. We thank Dr. J. Rousk for helpful comments on an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Relationship between numbers of cyanobacteria on P. schreberi leaves and acetylene reduction (μmol m−2 d−1) in the moss. Numbers of cyanobacteria were counted after 24 weeks of N deprivation. Shown are total cyanobacterial counts in moss from all sampling sites (n = 12). Regression equation, regression line and r2 values are given. (DOC 69 kb)
Rights and permissions
About this article
Cite this article
Rousk, K., Jones, D.L. & DeLuca, T.H. Exposure to nitrogen does not eliminate N2 fixation in the feather moss Pleurozium schreberi (Brid.) Mitt.. Plant Soil 374, 513–521 (2014). https://doi.org/10.1007/s11104-013-1908-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1908-5