Abstract
Background and aims
Litter decomposition is a major process in the carbon (C) flow and nutrient cycling of terrestrial ecosystems, but the effects of litter type, microsite, and root diameter on decomposition are poorly understood.
Methods
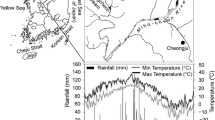
Litterbags were used to examine the decomposition rate of leaf litter and roots at three soil depths (5, 10 and 20 cm) over a 470-day period in Pinus sylvestris plantations in northern China.
Results
Leaves and the finest roots decomposed more quickly at 5 cm depth and coarser roots (>1-mm) decomposed more quickly at 10 and 20 cm depth. Roots generally decomposed more quickly than leaf litter, except at 5 cm deep; leaves decomposed more quickly than the coarsest roots (>5-mm). Root decomposition was strongly influenced by root diameter. Leaves experienced net nitrogen (N) immobilization and coarse roots (>2-mm) experienced more N release than fine roots. Significant heterogeneity was seen in N release for fine-roots (<2-mm) with N immobilization occurring in smaller (0.5–2-mm) roots and N release in the finest roots (<0.5-mm).
Conclusions
Soil depth of litter placement significantly influenced the relative contribution of the decomposition of leaves and roots of different diameters to carbon and nutrient cycling.








Similar content being viewed by others
References
Abiven S, Recous S, Reyes V, Oliver R (2005) Mineralisation of C and N from root, stem and leaf residues in soil and role and their biochemical quality. Biol Fertil Soils 42:119–128
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Chang Biol 14:2636–2660
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Austin AT, Araujo PI, Leva PE (2009) Interaction of position, litter type, and water pulses on decomposition of grasses from the semiarid Patagonian steppe. Ecology 90:2642–2647
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610
Berg B, McClaugherty C (2003) Plant litter: decomposition, humus formation, carbon sequestration. Springer, Berlin
Bird JA, Kleber M, Torn MS (2008) 13C and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org Geochem 39:465–477
Birouste M, Kazakou E, Blanchard A, Roumet C (2012) Plant traits and decomposition: are the relationships for roots comparable to those for leaves? Ann Bot 109:463–472
Bremner JM (1996) Nitrogen-total. In: Sparks DL (ed) Methods of soil analysis. SSSA Book Ser, Madison, pp 1085–1122
Burton AJ, Jarvey JC, Jarvi MP, Zak DR, Pregitzer KS (2012) Chronic N deposition alters root respiration–tissue N relationship in northern hardwood forests. Glob Chang Biol 18:258–266
Camiré C, Côté B, Brulotte S (1991) Decomposition of roots of black alder and hybrid poplar in short-rotation plantings: nitrogen and lignin control. Plant Soil 138:123–132
Chen H, Harmon ME, Griffiths RP (2001) Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: a chronosequence approach. Can J For Res 31:246–260
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Glob Chang Biol 15:1339–1355
Fahey TJ, Arthur MA (1994) Further studies of root decomposition following harvest of a northern hardwoods forest. Forest Sci 40:618–629
Fan P, Guo D (2010) Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163:509–515
Frey S, Elliott E, Paustian K, Peterson G (2000) Fungal translocation as a mechanism for soil nitrogen inputs to surface residue decomposition in a no-tillage agroecosystem. Soil Biol Biochem 32:689–698
Gholz HL, Wedin D, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Chang Biol 6:751–765
Gill RA, Burke IC (2002) Influence of soil depth on the decomposition of Bouteloua gracilis roots in the shortgrass steppe. Plant Soil 241:233–242
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat DM (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monogr 81:89–102
Gorissen A, Cotrufo M (2000) Decomposition of leaf and root tissue of three perennial grass species grown at two levels of atmospheric CO2 and N supply. Plant Soil 224:75–84
Guo DL, Mitchell RJ, Hendricks JJ (2004) Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 140:450–457
Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z (2008) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Hättenschwiler S, Coq S, Barantal S, Handa IT (2011) Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Hobbie SE (2005) Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8:644–656
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513
John MG, St. Orwin KH, Dickie IA (2011) No ‘home’ versus ‘away’ effects of decomposition found in a grassland-forest reciprocal litter transplant study. Soil Biol Biochem 43:1482–1489
Johnsen K, Maier C, Kress L (2005) Quantifying root lateral distribution and turnover using pine trees with a distinct stable carbon isotope signature. Funct Ecol 19:81–87
Joslin J, Gaudinski JB, Torn MS, Riley W, Hanson PJ (2006) Fine-root turnover patterns and their relationship to root diameter and soil depth in a 14C–labeled hardwood forest. New Phytol 172:523–535
Kemp P, Reynolds J, Virginia R, Whitford W (2003) Decomposition of leaf and root litter of Chihuahuan desert shrubs: effects of three years of summer drought. J Arid Environ 53:21–39
King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty P (2002) Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol 154:389–398
Knorr M, Frey S, Curtis P (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Kramer C, Trumbore S, Fröberg M, Cisneros Dozal LM, Zhang D, Xu X, Santos GM, Hanson PJ (2010) Recent (<4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem 42:1028–1037
Langley JA, Hungate BA (2003) Mycorrhizal controls on belowground litter quality. Ecology 84:2302–2312
Ludovici KH, Zarnoch SJ, Richter DD (2002) Modeling in-situ pine root decomposition using data from a 60-year chronosequence. Can J For Res 32:1675–1684
Majdi H (2004) Root and needle litter decomposition responses to enhanced supplies of N and S in a Norway spruce forest in southwest Sweden. Plant Biosystems 138:225–230
Mambelli S, Bird JA, Gleixner G, Dawson TE, Torn MS (2011) Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Org Geochem 42:1099–1108
Melin Y, Petersson H, Nordfjell T (2009) Decomposition of stump and root systems of Norway spruce in Sweden—A modelling approach. Forest Ecol Manag 257:1445–1451
Milcu A, Manning P (2011) All size classes of soil fauna and litter quality control the acceleration of litter decay in its home environment. Oikos 120:1366–1370
Mo JM, Zhang W, Zhu WX, Fang YT, Li DJ, Zhao P (2007) Response of soil respiration to simulated N deposition in a disturbed and a rehabilitated tropical forest in southern China. Plant Soil 296:125–135
Moretto AS, Distel RA (2003) Decomposition of and nutrient dynamics in leaf litter and roots of Poa ligularis and Stipa gyneriodes. J Arid Environ 55:503–514
Nambiar EKS (1987) Do nutrients retranslocate from fine roots? Can J For Res 17:913–918
Nelson DW, Sommers LE, Sparks D, Page A, Helmke P, Loeppert R, Soltanpour P, Tabatabai M, Johnston C, Sumner M (1996) Total carbon, organic carbon, and organic matter. Methods of soil analysis Part 3‐chemical methods, pp 961–1010
Olajuyigbe S, Tobin B, Hawkins M, Nieuwenhuis M (2012) The measurement of woody root decomposition using two methodologies in a Sitka spruce forest ecosystem. Plant Soil 360:77–91
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Paquette A, Messier C (2010) The role of plantations in managing the world's forests in the Anthropocene. Front Ecol Environ 8:27–34
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Park BB, Yanai RD, Fahey TJ, Bailey SW, Siccama TG, Shanley JB, Cleavitt NL (2008) Fine root dynamics and forest production across a calcium gradient in northern hardwood and conifer ecosystems. Ecosystems 11:325–341
Powers JS, Montgomery RA, Adair EC, Brearley FQ, DeWalt SJ, Castanho CT, Chave J, Deinert E, Ganzhorn JU, Gilbert ME (2009) Decomposition in tropical forests: a pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. J Ecol 97:801–811
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of forest litter. Can J For Res 20:166–171
Santiago LS (2007) Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology 88:1126–1131
Seastedt TR, Parton WJ, Ojima DS (1992) Mass-loss and nitrogen dynamics of decaying litter of grasslands - the apparent low nitrogen immobilization potential of root detritus. Can J Bot 70:384–391
Silver WL (1998) The potential effects of elevated CO2 and climate change on tropical forest soils and biogeochemical cycling. Climatic change 39:337–361
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Sun T, Mao ZJ, Dong LL, Hou LL, Song Y, Wang XW (2013a) Further evidence for slow decomposi5on of very fine roots using two methods: litterbags and intact cores. Plant Soil 366:633–646
Sun T, Mao Z, Han Y (2013b) Slow decomposition of very fine roots and some factors controlling the process: a 4-year experiment in four temperate tree species. Plant Soil. doi:10.1007/s11104-013-1755-4
Tobin B, Nieuwenhuis M (2007) Biomass expansion factors for Sitka spruce (Picea sitchensis (Bong.) Carr.) in Ireland. Eur J For Res 126:189–196
Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM (2008) Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am J Bot 95:1506–1514
Vitousek P, Turner D, Parton W, Sanford R (1994) Litter decomposition on the Mauna Loa environmental matrix, Hawai’i: patterns, mechanisms, and models. Ecology 75:418–429
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Wang H, Liu S, Mo J (2010) Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 335:289–298
Xiong Y, Fan P, Fu S, Zeng H, Guo D (2013) Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 363:19–31
Xu XN, Hirata EJ (2005) Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 273:279–289
Acknowledgments
This research was supported by the National Basic Research Program of China (2013CB956303 and 2010CB950600), projects of the National Natural Science Foundation of China (31222011, 31270363 and 31070428) and projects supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (31021001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Wang, W., Zhang, X., Tao, N. et al. Effects of litter types, microsite and root diameters on litter decomposition in Pinus sylvestris plantations of northern China. Plant Soil 374, 677–688 (2014). https://doi.org/10.1007/s11104-013-1902-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1902-y