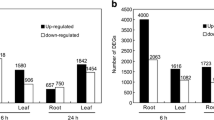
Abstract
Background and aims
Two major adaptive strategies used by Zygophyllum xanthoxylum, a C3 succulent xerophyte, against arid environments are absorbing a great quantity of Na+ from low-salinity soil which is efficiently transported to the leaves, and maintaining the stability of K+ concentration in those leaves. The plasma membrane Na+/H+ antiporter SOS1 has been suggested to be involved in Na+ transport and correlated with K+ nutrition in glycophytes. In this study, we investigated the function of the plasma membrane Na+/H+ antiporter ZxSOS1 in long-distance transport and spatial distribution of Na+ and K+ in the xerophyte Z. xanthoxylum.
Methods
The responses of ZxSOS1 to NaCl, KCl treatments and osmotic stress were investigated by semi-quantitative RT-PCR, then the role of ZxSOS1 in regulating plant growth and Na+, K+ transport and spatial distribution in Z. xanthoxylum was studied by using post-transcriptional gene silencing.
Results
We found that ZxSOS1 was preferentially expressed in roots and was induced and regulated by salt treatments and osmotic stress. Using post-transcriptional gene silencing, we found that ZxSOS1-silenced plants exhibited reduced growth rate compared to wild-type (WT) plants under both normal and saline conditions. ZxSOS1-silenced plants accumulated more Na+ in their roots but less Na+ in leaves and stems than WT under 50 mM NaCl. Furthermore, ZxSOS1-silenced plants had a lower net K+ uptake rate than WT plants under both normal and saline conditions, and more interestingly, accumulated less K+ in leaves under normal conditions than WT plants. ZxSOS1-silenced plants also showed a decreased concentration and spatial distribution of K+ in leaves and roots than WT under 50 mM NaCl. In addition, ZxSOS1-silenced plants possessed an increased selective transport (ST) capacity for K+ over Na+ from root to stem while a decreased ST value from stem to leaf compared with WT plants when both were grown in 50 mM NaCl.
Conclusions
These results demonstrate that ZxSOS1 is not only essential in long-distance transport and spatial distribution of Na+ and even K+, but also vital for regulating K+ and Na+ transport system and maintaining Na+ and K+ homeostasis in Z. xanthoxylum, thereby regulating its normal growth.







Similar content being viewed by others
References
Adolf VI, Jacobsen SE, Shabala S (2013) Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ Exp Bot 92:43–54
Amtmann A (2009) Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants. Mol Plant 2:3–12
Anderson WP, Willcocks DA, Wright BJ (1977) Electrophysiological measurements on root of Atriplex hastata. J Exp Bot 28:894–901
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581:2247–2254
Ashraf M (2010) Inducing drought tolerance in plants: Recent advances. Biotechnol Adv 28:169–183
Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24:1127–1142
Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23:3482–3497
Ben Hassine A, Bouzid S, Lutts S (2010) Does habitat of Atriplex halimus L. affect plant strategy for osmotic adjustment? Acta Physiol Plant 32:325–331
Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, Gosti F, Simonneau T, Essah PA, Tester M, Véry AA, Sentenac H, Casse F (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22:2004–2014
Britto DT, Kronzucker HJ (2008) Cellular mechanisms of potassium transport in plants. Physiol Plant 133:637–650
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water saving agriculture. J Exp Bot 55:2365–2384
Chen J, Xiao Q, Wu F, Dong X, He J, Pei Z, Zheng H (2010) Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol 30:1570–1585
Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi H (2008) Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J 53:554–565
Cohen E, Okon Y, Kigel J, Nur I, Henis Y (1980) Increase in dry weight and total nitrogen content in Zea mays and Setaria italica associated with nitrogen-fixing Azospirillum spp. Plant Physiol 66:746–749
Cosentino C, Fischer-Schliebs E, Bertl A, Thiel G, Homann U (2010) Na+/H+ antiporters are differentially regulated in response to NaCl stress in leaves and roots of Mesembryanthemum crystallinum. New Phytol 186:669–680
Craig PD, Møller IS (2010) Na+ transport in glycophytic plants: what we know and would like to know. Plant Cell Environ 33:612–626
Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ 30:497–507
Ding L, Zhu JK (1997) Reduced Na+ uptake in the NaCl-hypersensitive sos1 mutant of Arabidopsis thaliana. Plant Physiol 113:795–799
Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133:307–318
Estañ MT, Martinez-Rodriguez MM, Perez-Alfocea F, Flowers TJ, Bolarin MC (2005) Grafting raises the salt-tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J Exp Bot 56:703–712
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol Plant Mol Biol 28:89–121
Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud JB, Sentenac H (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94:647–655
Guerrier G (1996) Fluxes of Na+, K+ and Cl-, and osmotic adjustment in Lycopersicon pimpinellifolium and L. esculentum during short- and long-term exposures to NaCl. Physiol Plant 97:583–591
Guo KM, Babourina O, Rengel Z (2009) Na+ ⁄H+ antiporter activity of the SOS1 gene: lifetime imaging analysis and electrophysiological studies on Arabidopsis seedlings. Physiol Plant 137:155–165
Guo Q, Wang P, Ma Q, Zhang JL, Bao AK, Wang SM (2012) Selective transport capacity for K+ over Na+ is linked to the expression levels of PtSOS1 in halophyte Puccinellia tenuiflora. Funct Plant Biol 39:1047–1057
Hajibagheri MA, Yeo AR, Flowers TJ (1985) Salt tolerance in Suaeda maritima (L.) Dum. Fine structure and ion concentrations in the apical region of roots. New Phytol 99:331–343
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33:552–565
Janz D, Polle A (2012) Harnessing salt for woody biomass production. Tree Physiol 32:1–3
Kronzucker HJ, Britto DT (2011) Sodium transport in plants: a critical review. New Phytol 189:54–81
Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB (2000) A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12:837–851
Lebaudy A, Véry AA, Sentenac H (2007) K+ channel activity in plants: genes, regulations and functions. FEBS Lett 581:2357–2366
Li W, Chen L, Cai DT, Yang GP (1997) A new approach for gene transfer into citrus. Acta Bot Sin 39:782–784
Liu JQ, Pu JC, Liu XM (1987) Comparative studies on water relations and xeromorphic structures of some plant species in the middle part of the desert zone in China. Acta Bot Sin 29:662–673
Liu JQ, Li ZJ, Pu JC, Liu XM (1988) Comparative studies on relationships between proline accumulation and photosynthesis, respiration and chlorophyll content of some plant species in the middle part of the desert zone in China. Acta Bot Sin 30:85–95
Ma Q, Lou JQ, Wang SM (2010) Effects of Na+ on photosynthetic characteristics of Zygophyllum xanthonylon seedlings under osmotic stress. Acta Prata Sin 19:198–203
Ma Q, Yue LJ, Zhang JL, Wu GQ, Bao AK, Wang SM (2012) Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol 32:4–13
Martínez JP, Kinet JM, Bajji M, Lutts S (2005) NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J Exp Bot 419:2421–2431
Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ (2007) Conservation of the salt overly sensitive pathway in rice. Plant Physiol 143:1001–1012
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, Quintero FJ, Jiang X, D’Urzo MP, Lee SY, Zhao Y, Bahk JD, Bressan RA, Yun DJ, Pardo JM, Bohnert HJ (2009) Loss of halophytism by interference with SOS1 expression. Plant Physiol 151:210–222
Oh DH, Lee SY, Bressan RA, Yun DJ, Bohnert HJ (2010) Intracellular consequences of SOS1 deficiency during salt stress. J Exp Bot 61:1205–1213
Olías R, Eljakaoui Z, Li J, De Morales PA, Marín-Manzano MC, Pardo JM, Belver A (2009a) The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ 32:904–916
Olías R, Eljakaoui Z, Pardo JM, Belver A (2009b) The Na+/H+ exchanger SOS1 controls extrusion and distribution of Na+ in tomato plants under salinity conditions. Plant Signal Behav 4:973–976
Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57:1181–1199
Pei SF, Fu H, Cen YM, Li JB (2004) Influence of Z. xanthoxylum shrubs on soil fertility in enclosure and grazing conditions. J Desert Res 24:763–767
Qi Z, Spalding EP (2004) Protection of plasma membrane K+ transport by the salt overlay sensitive Na+/H+ antiporter during salinity stress. Plant Physiol 136:2548–2555
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci U S A 99:8436–8441
Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci U S A 99:9061–9066
Rozema J, Flowers T (2008) Crops for a salinized world. Science 322:1478–1480
Sambatti JB, Caylor KK (2007) When is breeding for drought tolerance optimal if drought is random? New Phytol 175:70–80
Schulze LM, Britto DT, Li M, Kronzucker HJ (2012) A pharmacological analysis of high-affinity sodium transport in barley (Hordeum vulgare L.): a 24Na+/42K+ study. J Exp Bot 63:2479–2489
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Shabala S, Mackay A (2011) Ion transport in halophytes. Adv Bot Res 57:151–187
Shabala L, Cuin TA, Newman IA, Shabala S (2005) Salinity induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222:1041–1050
Shabala S, Shabala S, Cuin TA, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner LH (2010) Xylem ionic relations and salinity tolerance in barley. Plant J 61:839–853
Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci U S A 97:6896–6901
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long distance Na+ transport in plants. Plant Cell 14:465–477
Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009a) NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149:1141–1153
Sun J, Dai S, Wang R, Chen S, Li N, Zhou X, Lu C, Shen X, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009b) Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol 29:1175–1186
Sunarpi HT, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, Konomi M, Osumi M, Yamagami M, Schroeder JI, Uozumi N (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44:928–938
Takahashi R, Liu SK, Takano T (2009) Isolation and characterization of plasma membrane Na+/H+ antiporter genes from salt-sensitive and salt-tolerant reed plants. J Plant Physiol 166:301–309
Taleisnik E, Grunberg K (1994) Ion balance in tomato cultivars differing in salt tolerance. I. Sodium and potassium accumulation and fluxes under moderate salinity. Physiol Plant 92:528–534
Tester M, Davenport RJ (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Wang SM, Zheng WJ, Ren JZ, Zhang CL (2002) Selectivity of various types of salt-resistant plants for K+ over Na+. J Arid Environ 52:457–472
Wang SM, Wan CG, Wang YR, Chen H, Zhou ZY, Fu H, Sosebee RE (2004) The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J Arid Environ 56:525–539
Wang SM, Zhang JL, Flowers TJ (2007) Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol 145:559–571
Wang CM, Zhang JL, Liu XS, Li Z, Wu GQ, Cai JY, Flowers TJ, Wang SM (2009) Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant Cell Environ 32:486–496
Weeks JT, Ye J, Rommens CM (2008) Development of an in planta method for transformation of alfalfa (Medicago sativa). Transgenic Res 17:587–597
Wegner LH, De Boer AH (1997) Properties of two outward rectifying channels in root xylem parenchyma cells suggest a role in K+ homeostasis and long-distance signaling. Plant Physiol 115:1707–1719
Wegner LH, Raschke K (1994) Ion channels in the xylem parenchyma of barley roots- a procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiol 105:799–813
Wegner LH, Stefano G, Shabala L, Rossi M, Mancuso S, Shabala S (2011) Sequential depolarization of root cortical and stelar cells induced by an acute salt shock-implications for Na+ and K+ transport into xylem vessels. Plant Cell Environ 34:859–869
Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27:581–590
Wu SJ, Ding L, Zhu JK (1996) SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8:617–627
Wu CX, Zhou ZY, Zhang GH, Bao P, Hou CL (2004) Aboveground nutrient content and its seasonal change in the strong xerophils. Pratacul Sci 21:30–34
Wu GQ, Xi JJ, Wang Q, Bao AK, Ma Q, Zhang JL, Wang SM (2011) The ZxNHX gene encoding tonoplast Na+/H+ antiporter from the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. J Plant Physiol 168:758–767
Xu H, Jiang X, Zhan K, Cheng X, Chen X, Pardo JM, Cui D (2008) Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast. Arch Biochem Biophys 473:8–15
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunity. Trends Plant Sci 10:615–620
Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong ZZ (2009) Overexpression of SOS (salt overly sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol Plant 2:22–31
Yue LJ, Li SX, Ma Q, Zhou XR, Wu GQ, Bao AK, Zhang JL, Wang SM (2012) NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. J Arid Environ 87:153–160
Zhou XR, Zhou ZY, Wu CX (2006) The research of the breeding characters of Zygophyllum xanthoxylum. Pratacul Sci 23:38–41
Zhu JK (2000) Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol 124:941–948
Zhu JK, Liu J, Xiong L (1998) Genetic analysis of salt tolerance in Arabidopsis: evidence for a role of potassium nutrition. Plant Cell 10:1181–1191
Acknowledgments
We are very grateful to Professor Timothy J. Flowers from University of Sussex, UK, for critically reviewing the manuscript and for valuable suggestions. This work was supported by the National Basic Research Program of China (973 Program, grant No. 2014CB138701), the National Natural Science Foundation of China (grant No. 31072073) and the Scholarship Award for Excellent Doctoral Student granted by Ministry of Education (grant No. 224000–860016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Herbert J. Kronzucker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table S1
Primer sequences used in this study. (DOC 29 kb)
Supplemental Fig. S1
The expression level of ZxSOS1 in root tissue of Z. xanthoxylum under different concentrations of NaCl. (a) Semi-quantitative RT-PCR analysis of ZxSOS1 mRNA in root tissue of 3-week-old plants treated with 0, 5, 25, 50, 100 and 150 mM NaCl for 48 h, respectively. ACTIN was used as an internal control. Experiments were repeated at least three times (with similar results). (b) The relative expression level of ZxSOS1 (related to ACTIN) in root tissue of Z. xanthoxylum under different concentrations of NaCl. Values are means ± SD (n = 3) and bars indicate SD. Columns with different letters indicate significant differences at P < 0.05 (Duncan test). (JPEG 19 kb)
Supplemental Fig. S2
The expression level of ZxSOS1 in root, stem, and leaf tissue of Z. xanthoxylum under different concentrations of KCl. (a) Semi-quantitative RT-PCR analysis of ZxSOS1 mRNA in root, stem, and leaf tissue of 3-week-old plants treated with 0, 0.1, 0.5, 1, 5 and 10 mM KCl for 48 h, respectively. Plants were treated with modified half strength Hoagland nutrient solutions deprived of KNO3 for 3 days, where 2 mM KNO3 was substituted by 1 mM NH4NO3. Then different concentrations of K+ treatments were supplemented. ACTIN was used as an internal control. Experiments were repeated at least three times (with similar results). (b) The relative expression level of ZxSOS1 (related to ACTIN) in the root, stem, and leaf of Z. xanthoxylum under different concentrations of KCl. Values are means ± SD (n = 3) and bars indicate SD. (JPEG 23 kb)
Rights and permissions
About this article
Cite this article
Ma, Q., Li, YX., Yuan, HJ. et al. ZxSOS1 is essential for long-distance transport and spatial distribution of Na+ and K+ in the xerophyte Zygophyllum xanthoxylum . Plant Soil 374, 661–676 (2014). https://doi.org/10.1007/s11104-013-1891-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1891-x