Abstract
Aims
This study investigated Cu uptake and accumulation as well as physiological and biochemical changes in grapevines grown in soils containing excess Cu.
Methods
The grapevines were collected during two productive cycles from three vineyards with increasing concentrations of Cu in the soil and at various growth stages, before and after the application of Cu-based fungicides. The Cu concentrations in the grapevine organs and the macronutrients and biochemical parameters in the leaf blades were analyzed.
Results
At close to the flowering stage of the grapevines, the concentration and content of Cu in the leaves were increased. However, the Cu concentrations in the roots, stem, shoots and bunches did not correlate with the metal concentrations in the soil. The application of Cu-based fungicides to the leaves increased the Cu concentrations in the shoots, leaves and rachis; however, the effect of the fungicides on the Cu concentration in the berries was not significant. The biochemical analyses of the leaf blades demonstrated symptoms of oxidative stress that correlated with the Cu concentrations in soil.
Conclusions
The increased availability of Cu in soil had a slight effect on the levels and accumulation of Cu in mature grapevines during the productive season and did not alter the nutritional status of the plant. However, increased Cu concentrations were observed in the leaves. The evidence of oxidative stress in the leaves correlated with the increased levels of Cu in soil.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu)-based fungicides, such as the Bordeaux mixture [CuSO4.5H2O + Ca(OH)2], are applied in vineyards to control fungal diseases such as mildew (Plasmopara vitícola). These fungicides are inexpensive and efficient at controlling diseases, and they demonstrate low toxicity in plants. Therefore, these fungicides are often used in vineyards, including organic vineyards (Council Regulation 2092/91/EEC 1991). However, the successive application of fungicides increases the total and available Cu in the soil (Brun et al. 2001; Pietrzak and McPhail 2004; Fernández-Calviño et al. 2009), reaching high and even excessive concentrations for the plants. The excessive accumulation of Cu in the soil in vineyards is a major environmental problem because it occurs over large areas in many countries (Komárek et al. 2010). Excessive Cu can affect the biological activity of soils (Dumestre et al. 1999), in that it is toxic to plants (Brun et al. 2003; Chaignon and Hinsinger 2003), it is leached into the soil profile, and it pollutes groundwater (Robinson et al. 2006). In enriched sediments, the Cu can be transferred to surface water through runoff (Fernández-Calviño et al. 2008).
Copper is a micronutrient in plants and functions in conjunction with a large number of enzymes related to respiration and photosynthesis (Marschner 1995; Yruela et al. 2000). The main visual symptoms of excess Cu are the impaired growth of the roots and shoots, nutrient deficiency, chlorosis, and, in more severe cases, tissue necrosis and plant death (Marschner 1995; Kopsell and Kopsell 2007). These symptoms are caused by the direct and indirect action of the Cu in plants. The reduction in the growth of the roots results in less exploration of the soil by the roots. This aggravates damage to the cell membranes in the roots (De Vos et al. 1989) and results in a significant decrease in the uptake of nutrients and water (Kopsell and Kopsell 2007). The effect of Cu toxicity in the roots is reflected in indirect symptoms, such as the reduction of branch growth and the chlorosis caused by the generalized deficiency of nutrients and water (Marschner 1995; Yruela et al. 2000). A direct effect of high Cu concentrations at the cellular level is oxidative stress caused by the increased concentration of reactive oxygen species (ROS), such as superoxide anion (O2.-), singlet oxygen (1O2), hydrogen peroxide (H2O2) and hydroxyl radical (OH-) (Apel and Hirt 2004). ROS can damage all biomolecules; however, lipid peroxidation of the cell membranes is one of the most important effects observed. Damage to the cell membranes results in lower selectivity and eventually causes membrane breakage and leakage of the cell contents (De Vos et al. 1989; Yruela 2005).
Studies have been performed on vineyard soils to assess Cu availability and the effect of excess Cu on plant nutrition. Studies using chemical extractors demonstrated that Cu availability is higher in acid and sandy soils containing low concentrations of organic matter (Brun et al. 2001; Chaignon and Hinsinger 2003; Pietrzak and McPhail 2004; Arias et al. 2005). However, estimations of the total concentration of Cu in the soil (or the amount of Cu extractable using various methods) are ineffective for predicting the Cu absorption and its toxicity to plants (Brun et al. 1998). Therefore, studies have investigated plants to assess the Cu availability in soils (Brun et al. 2003; Chaignon et al. 2009). Although the cultivation of grapevines is often associated with the use of fungicides, few studies have used grapevines for the investigation of Cu availability and toxicity.
The effects of excessive Cu in soils on the nutrition of grapevines are unclear. Young grapevine plants grown in soils newly contaminated with Cu demonstrate reduced growth in the roots and shoots, leaf chlorosis and Cu accumulation in the roots. However, a small amount of Cu is translocated to the branches (Toselli et al. 2009). In contrast, adult grapevines during the productive season do not exhibit visual symptoms of toxicity caused by Cu but can uptake and accumulate Cu in the perennial and annual organs (Lai et al. 2010).
Grapevines remain productive for decades in soils with high Cu concentrations, and the plants are often subjected to spraying with Cu-based fungicides. The uptake of Cu from the soil and from the Cu-containing fungicide sprayed directly on the aerial parts of the plant represent two sources of this metal for the plant. Under conditions of high levels of Cu in the soil and the spraying of Cu-based fungicides, grapevines can exhibit the increased uptake and accumulation of Cu and toxicity symptoms of excessive Cu in the tissues. In addition, Cu accumulation in the roots may affect the uptake of other nutrients, causing nutritional problems for the plants. This study evaluated the concentration and content of Cu in the plant organs and the physiological and biochemical changes in the leaf blades of mature plants during the productive season in grapevines grown in soils with increasing Cu levels.
Material and methods
Research locality
The study was performed in vineyards located in the municipality of Santana do Livramento (Rio Grande do Sul State, Brazil). The climate is subtropical, classified as Cfa (Köppen classification), with an average rainfall of 1,388 mm year−1 (IPA 1989). The three vineyards of the variety Cabernet Sauvignon (Vitis vinifera) grafted on rootstock SO4 (Vitis berlandieri x Vitis riparia) were used in the experiments. Vineyard 1 (VN1) was planted at a density of 2,778 plant ha−1 in 2004 (S 30°46′41″, W 55°22′34″); vineyard 2 (VN2) was planted at a density of 2,525 plant ha−1 in 1998 (S 30°47′44″, W 55°21′56″); and vineyard 3 (VN3) was planted in 1977 at a density of 1,429 plant ha−1 (S 30°46′36″, W 55° 22′03″). The vineyards were developed using the vertical shoot position training system with heavy pruning and fertilization with nitrogen (N), phosphorus (P) and potassium (K), performed equally for each vineyard. The control of fungal foliar diseases was performed preventively, including the use of Cu-based fungicides, only after the formation of the berries. In the vineyards, the 2009/2010 and 2010/2011 cycles were assessed. The phenological stages evaluated in the two cycles and the amounts and timing of the application of the Cu fungicides are shown in Table 1.
Design of grapevines sampling
In each vineyard, two sets of plants were delimited. For the evaluations performed during the 2009/2010 cycle, three blocks consisting of three plots composed of six plants were delimited: one plot for each stage assessed. For both years, the two most representative grapevines in each plot were used for the investigation. This design was necessary to exclude grapevines exhibiting different sizes and vegetative vigor and to decrease variability of the samples. In the 2010/2011 year, the samplings were always performed on the same plants, and three branches were removed at each stage assessed (Fig. 1).
Figures illustrating the organs and parts of grapevines evaluated at various phenological stages during the 2009/2010 cycle (1st year) and the 2010/2011 cycle (2nd year). In the figure representing the 2010/2011 cycle, the letters “L, C and R” indicate the left, central and right positions, respectively, of the plants from which the branches were collected at each collection. For these branches, three groups of leaves were sampled. The samples were defined in accordance with their position: basal (numbers 1, 2 and 3); middle (numbers 8, 9 and 10); and apical (numbers 15, 16 and 17)
Soil sampling and analysis
The soil sampling was performed in July 2009 and was restricted to the area used in the study (approximately 1,000 m2 per vineyard). From the three rows of grapevines reserved for the experiment, three soil samples were collected from the 0.00–0.20 m soil layer. Each sample was composed of nine subsamples, three at each of the following positions: the row, the projection of the canopy of the plants and the middle of the between-row area. Soil samples were also collected in an area of natural field (NF) near the vineyards, which was used as background in the study. The NF was representative of the original vegetation of the soils of this region and was composed primarily of grasses of the genus Paspalum and legumes of the genus Desmodium and Vicia. This vegetation is typical of Pampa Biome and is used as natural pasture; however, this area also exhibits good potential for vineyard cultivation. The samples collected in the vineyards and the NF were air-dried and sieved through a 2-mm mesh. The total concentration of Cu (CuT) was extracted in accordance with the United States Environmental Protection Agency (USEPA) method 3050B (USEPA 1996) by oxidation and acidic attack using HNO3, H2O2 and HCl, concentrated under heating. The available concentrations of Cu (CuA), iron (FeA), manganese (MnA) and zinc (ZnA) were extracted using EDTA (Chaignon et al. 2009). The exchangeable concentrations of calcium (CaE) and magnesium (MgE) were extracted using KCl (1 mol L−1). The determination of the concentrations of Cu, Fe, Mn, Zn, Ca and Mg in the extracts was performed in an atomic absorption spectrophotometer (AAS). The concentrations of available phosphorus (PA) and exchangeable potassium (KE) were extracted using the Mehlich 1 solution (HCl 0.05 mol L−1 + H2SO4 0.0125 mol L−1), the P was measured using a spectrophotometer, and the K was measured using a flame photometer. The pH(H2O) was determined in a soil:water suspension (1:1, m/v). The effective cation exchange capacity (CECeff) was calculated using the sum of CaE, MgE and KE (Tedesco et al. 1995; CQFS-RS/SC 2004). The concentration of organic matter (OM) in the soil was determined in accordance with the Walkley-Black method, and the particle size was analyzed using the pipette method (Embrapa 1997). The soils were classified as Ultisol (SSS 2010).
Sampling of grapevines and chemical and biochemical analyses
The evaluations were performed on the 2009/2010 and 2010/2011 production cycles. In the first cycle, the Cu concentration and Cu content in the various grapevine parts were analyzed. In the second cycle, the Cu concentration and the physiological responses in the annual aerial organs of the grapevine were investigated in detail. In the 2009/2010 cycle, the grapevines were cut, and each organ was analyzed separately, as shown in Fig. 1. At each stage (Table 1), the amount of fresh matter for each organ was determined, and a subsample was removed to determine the dry matter (DM) and the concentration of Cu. The root system was sampled by opening the trenches to a depth of 0.50 m, a length of 1.50–1.75 m, and a width of 0.75–1.00 m (according to the planting spacing) to include an area equivalent to one quarter of the area of one plant. Using a 4-mm mesh sieve, the grapevine roots were separated from the soil. Following the division of the roots into subsamples composed of fresh mass derived from the fine (≤1 mm), medium (1 < and ≤ 5 mm) and coarse (<5 mm) portions of the roots, the subsamples were immersed for 8–10 s in a solution of HCl (0.5 mol L−1) for soil disaggregation, washed in running water, immersed in EDTA (0.02 mol L−1) for 1 min and washed with distilled water three times. Next, the root samples were dried at 60 º C for 72 h, weighed to determine the dry matter (DW) and milled for analysis. The stem subsamples were composed of five segments measuring 10–12 cm in length, the epidermis was removed manually using a utility knife, and the samples were washed three times in distilled water. The subsamples from the leaves and shoots were also washed three times with distilled water.
In the 2010/2011 cycle, only the annual branches were evaluated. At four phenological stages (Table 1), three branches were removed from each plant and were divided into the parts shown in Fig. 1. The leaves corresponding to the basal, middle and apical positions (Fig. 1) were collected only when fully expanded. The samples were washed three times with distilled water. The grapevines samples that were collected in the two harvests were dried at 60 °C to a constant weight and ground for analysis.
Samples from the leaf blades were removed for biochemical analyses at the phenological stages EL31 and EL37 of the 2010/2011 cycle (Table 1). After three washes with distilled water, the blades were dried using absorbent paper and were fractionated into half-longitudinal sections, one of which was used for the biochemical analyses and was immediately frozen in liquid N2 and stored in a freezer. The other half of each blade was oven dried at 60 °C and used to determine the total concentration of nutrients.
Chemical analyses of plant tissues
To determine the total concentration of Cu in all the samples and the concentrations of P, K, Ca and Mg in the leaf blade samples, biochemical analyses were performed after dry digestion of the tissues in a muffle furnace. The samples were calcined at 500–550 °C for 3 h, and the ashes were diluted in HNO3 (1 mol L−1). The total Cu, Ca and Mg concentrations were determined using an AAS, the K concentration was determined using a flame photometer, and the P concentration was determined using a spectrophotometer (Embrapa 1997). The concentrations of N were determined using the semi-micro Kjeldahl method (Tedesco et al. 1995).
Biochemical analyses of leaf blades
The biochemical analyses were performed after maceration of the leaf blades in a mortar using liquid N2. The concentration of hydrogen peroxide (H2O2) was determined by spectrophotometry at 390 nm and was calculated based on the standard calibration curve, in accordance with Loreto and Velikova (2001). The level of lipid peroxidation was estimated using the TBARS assay, which measures the concentration of malondialdehyde (MDA) using a spectrophotometer at 532 nm (El-Moshaty et al. 1993). The effect of turbidity in the samples was reduced by subtracting the absorbances at 532 nm from those obtained at 600 nm. The activity of superoxide dismutase (SOD) was determined by measuring the formation of adrenochrome from epinephrine in alkaline medium using a spectrophotometer at 480 nm for 4 min (Misra and Fridovich 1972). A single unit of SOD activity was defined as the amount of enzyme required to inhibit 50 % of the epinephrine oxidation. The catalase activity (CAT) was determined using a spectrophotometer at 240 nm by monitoring the disappearance of the H2O2 added to the reaction mixture (Aebi 1984). The activity of ascorbate peroxidase (APX) was determined by the ascorbate-dependent oxidation of H2O2, which was measured by the decrease in absorbance at 290 nm (Zhu et al. 2004).
Statistical analyses
The data are presented as the average plus standard deviations. The ANOVA significance test was used to identify significant differences between the variables. When significant differences were detected, the Tukey test was used at P <0.05 for comparison of the averages. A Pearson’s linear correlation was established between DM, CuT and CuA in the soil and the concentration and content of Cu in the plant tissues.
Results
Concentration of Cu and characteristics of the soils
The soils of the vineyards exhibited similar characteristics and increasing Cu concentrations (Table 2). The soils demonstrated a pH(H2O) of between 5.5 and 6.0, a sandy texture and a small amount of OM. In the VN2 and VN3 soils, a CuT concentration of 20.5 and 62.4 mg kg−1, respectively, was observed, which was equivalent to 6.4 and 19.5 times the concentration found in the natural field soil (background), respectively. Approximately 80 % of the CuT in the VN2 and VN3 soils was CuA and was potentially available to the plants. The PA concentration was interpreted as “High” (> 21.0 mg kg−1) for VN1 and VN2 and “Very High” (> 42.0 mg kg−1) for VN3 (Table 2) (CQFS-RS/SC 2004). The soils from the vineyards exhibited similar CECeff and concentrations of KE, CaE, MgE, FeA, ZnA and MnA, which were all at sufficient or high concentrations for plants (CQFS-RS/SC 2004). A small increase in the concentration of ZnA was observed in the VN3 soil (Table 2).
Concentration, content and partitioning of Cu in grapevine (2009–2010 cycle)
The increased concentrations of CuT and CuA in the soils from the vineyards did not increase the concentration of Cu in the roots, stems, shoots and bunches but were correlated with increased Cu concentrations in the leaves. The Cu concentrations in the roots, collected only at stages EL09 and EL19, were equal for the different vineyards and stages evaluated (Fig. 2). The Cu concentrations in the stems in VN3 were lower than in VN1 and VN2. However, a small change was observed in the three stages evaluated. An average of 5.6, 5.1 and 3.0 mg kg−1 was observed in the stems from VN1, VN2 and VN3, respectively (Fig. 2). The application of Cu-based fungicide in VN2 and VN3 between stages EL19 and EL31, did not affect the Cu concentration in the stem. The Cu concentration in the shoots decreased in the first two stages assessed (Fig. 2), and the values were, on average, 11.7 mg kg−1 in the VN1, 7.3 mg kg−1 in VN2 and 8.0 mg kg−1 in VN3. In contrast, at stage EL31 in VN2 and VN3, an increase in the Cu concentration was observed, most likely because of residual fungicide from the application. In the bunches, the Cu values at stage EL19 were similar for the grapevines from the three vineyards (approximately 7.0 mg kg−1 DW). However, after the application of the Cu-based fungicides, the Cu concentration increased several-fold and reached values above 50 mg kg−1 in VN2 and 90 mg kg−1 in VN3 (Fig. 2). The Cu concentration in the leaves from the three vineyards at stage EL09 was not altered by the Cu in the soil. However, at stage EL19, higher concentrations of Cu were observed in the leaves from VN3, which coincided with the higher concentrations of CuT and CuA in the soil. At this stage, the concentration values for Cu in the leaves were, on average, 7.1, 8.0 and 10.5 mg kg−1 in VN1, VN2 and VN3, respectively. The higher Cu concentration in the leaves observed in VN3, although numerically small, represented an increase of 47 % and 31 % compared to VN1 and VN2, respectively. After the application of the Cu-based fungicide, Cu concentrations above 140 mg kg−1 were observed in the leaves from VN2 and VN3. However, in VN1, without the fungicide application, the Cu concentration was 19.3 mg kg−1.
Dry matter production, copper concentration and content in perennial and annual organs of grapevines grown on soils containing increasing levels of Cu during the 2009/2010 cycle. The same letters on the columns, lowercase for the phenological stages of a single vineyard and uppercase for vineyards and a single phenological stage, indicate no differences according to the Tukey test at P < 0.05. ns not significant.  Application of Cu-based fungicide in the period between the phenological stage indicated and the previous stage (Table 1)
Application of Cu-based fungicide in the period between the phenological stage indicated and the previous stage (Table 1)
In general, the Cu content in the roots from VN3 was higher than in VN1 and VN2, which exhibited similar values (Fig. 2). The Cu content in the shoots at stages EL09 and EL19 was similar for the three vineyards. At stage EL19, the Cu content of the bunches was slightly higher in VN2 compared with VN1 and VN3. Moreover, the Cu content in the leaves was consistently higher in VN3 for the three stages assessed. However, notably, at stage EL31, the Cu content in leaves, shoots and bunches from the VN2 and VN3 plants was considerably higher, likely because of the application of the Cu-based fungicides (Fig. 2).
At stage EL19, when no fungicide was applied, the Cu contents in the bunches from VN1, VN2 and VN3 were 14.8, 14.2 and 28.9 mg plant−1, respectively. The roots and stems exhibited the most Cu accumulation. Together, these organs exhibited a cumulative total per plant of 91.0 % in VN1, 98.7 % in VN2 and 99.2 % in VN3. Individually, the roots and stems represented 57.8 and 33.8 % in VN1, 86.2 and 12.5 % in VN2, and 75.1 and 24.1 % in VN3, respectively. The copper content in the leaves and shoots from VN1 equaled 3.8 % of the total accumulation for the plant and less than 1 % for VN2 and VN3. The shoots represented 0.6 % of the cumulative total in VN1, 0.1 % in VN2 and only 0.03 % in VN3.
The correlation analysis was performed at the EL09 and EL19 stages, when no application of Cu-based fungicides was performed. The CuT and CuA contents in the soil either were weakly correlated with the concentrations of Cu in the roots, stems, shoots and bunches or did not explain these concentrations (Table 3). The concentrations of Cu in the soils was weakly affected by Cu concentration in the leaves at stage EL09 but correlated positively with the concentrations observed at stage EL19. The amount of Cu accumulated in the roots, stems, bunches and leaves correlated positively with the amount of DM, with the exception of the shoots at the EL09 stage (Table 3, Fig. 2). The cumulative amount also correlated with CuT and CuA in the soil; however, the DM was responsible for most of the variance, whereas the total Cu concentration values were altered slightly by the concentrations in the soil.
Cu concentration in annual branches (2010/2011 cycles)
In the second evaluation cycle, the data obtained at phenological stage EL17 corroborated the data obtained at stage EL19 of the 2009/2010 cycle (Fig. 3). The data demonstrated that the Cu concentrations in the leaf blades and shoots were higher in VN3, which demonstrated the highest Cu accumulation in the soil. At stage EL17, the Cu concentrations were 9.4 and 7.9 mg kg−1 in VN1, 9.7 and 9.4 mg kg−1 in VN2, and 12.6 and 26.7 mg kg−1 in VN3 for the shoots and basal leaf blades (BB), respectively (Fig. 3). These concentrations represented an average increase of 31 % for the shoots and a 2-to 3-fold increase for the BB in VN3 compared to VN1 and VN2. In these organs, a positive correlation was observed between the concentrations of CuT and CuA in the soil and the Cu concentration in the tissues (Table 3).
Copper concentrations in annual organs of grapevines grown on soils containing increasing levels of Cu during the 2010/2011 cycle. The same letters on the columns, lowercase letters for phenological stages of a single vineyard and uppercase letters for vineyards and a single phenological stage, indicate no difference according to the Tukey test at P < 0.05. ns not significant, NP not present.  Application of Cu-based fungicide in the period between the phenological stage indicated and the previous stage (Table 1)
Application of Cu-based fungicide in the period between the phenological stage indicated and the previous stage (Table 1)
The residual Cu-based fungicides affected the copper concentrations in the tissues at stages EL31, EL33 and EL37. The Cu-based fungicide applications were performed between stages EL17 and EL37, and an increase in the Cu concentrations in mainly the leaf blades, petioles and rachis was observed (Fig. 3). In addition, the fungicides slightly affected the Cu concentrations in the shoots and berries. The Cu concentration in the leaf blades was high, often above 100 mg kg−1, and in some cases, it was more than 400 mg kg−1, as observed in the blades at stage EL33 in VN1. The copper concentration in the petioles was not proportional to the concentrations in the leaf blades. In the petioles from the basal, middle and apical leaves at stage EL33, the maximum concentration of Cu was 80 mg kg−1 in VN1; however, lower values (between 20 and 40 mg kg−1) were often observed in the three vineyards (Fig. 3). At stage EL37, the Cu concentration in the petioles collected from three positions on the branches were, on average, 33.6 mg kg−1 in VN1; however, the concentrations were 12.1 mg kg−1 in VN2 and 16.3 mg kg−1 in VN3. At this stage, the leaf blades from the three vineyards still demonstrated high Cu concentrations, ranging from 50 to greater than 300 mg kg−1. The Cu concentration in the rachis was similar to that in the petioles; however, the concentrations were always lower than in the leaf blades. The Cu concentrations in the shoots of the grapevines from the three vineyards were not affected by the application of the Cu-based fungicides. However, in the final evaluation, the concentration of Cu in the shoots was 18.6 mg kg−1 in VN1, 10.4 mg kg−1 in VN2, and 12.3 mg kg−1 in VN3 (Fig. 3). These data, particularly the data observed in VN2 and VN3, were very close to those observed at stage EL17, before the application of the Cu-based fungicides. In contrast, the Cu concentration in the berries was affected slightly by the concentrations in the soil and the application of the Cu-based fungicides. In general, the Cu concentration in the berries from the three vineyards ranged between 8 and 20 mg kg−1. At stage EL31 (when the berries were not quite ripe), the Cu concentrations in the berries were 12.4 mg kg−1 in the VN1 grapevines, 3.14 mg kg−1 in the VN2 grapevines and 17.3 mg kg−1 in the VN3 grapevines (Fig. 3).
Concentration of macronutrients in leaf blades
The concentration of macronutrients in the leaf blades was very similar for the three vineyards (Table 4). Based on the reference concentrations for the full leaves (normal concentration = 16.0–24.0 mg kg−1 for N; 1.2–4.0 mg kg−1 for P; and 8.0–16.0 mg kg−1 for K), the concentrations of N, P, K were interpreted as normal, and in some cases very high, at both stages that were evaluated (CQFS-RS/SC 2004). The Ca concentrations did not differ between the vineyards. In contrast, the Mg concentration at stage EL37 was slightly higher in VN1 but did not differ from that in VN3. The calcium concentrations between 16.0 and 24.0 mg kg−1 and the Mg concentrations between 2.0 and 6.0 mg kg−1 (CQFS, RS/SC 2004) were considered normal for full leaves. Between stages EL31 and EL37, the concentration of N, P and K decreased, whereas the concentrations of Ca and Mg increased (Table 4).
Biochemical analyses of leaf blades
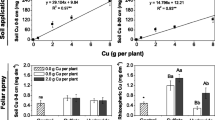
For the 2010/2011 cycle, the biochemical analyses for oxidative stress were performed at stages EL31 in the basal (BB) and middle (MB) leaf blades and at stage EL37 in the BB, MB, and apical blades (AB). An increased concentration of H2O2 was observed in the three vineyards at stage EL31 in the BB and at stage EL37 in particular in the MB; however, statistically, the values were identical to the other assessments (Fig. 4). At stage EL31, the lipid peroxidation (MDA) in the BB was lower in VN2 than in VN1 and VN3. At stage EL37, the lipid peroxidation did not change in the three vineyards (Fig. 4). The SOD activity demonstrated significant variation for the vineyards and stages assessed (Fig. 4). The data revealed a higher activity for this enzyme in the leaf blades from VN2 and VN3 in the MB at stage EL31 and in the BB and AB at stage EL37. In contrast, the SOD activity was lower in VN3 in the BB at stage EL31 and was equal to the activity in VN1 in the MB at stage EL37. The CAT activity at stage EL37 was higher in VN3 in the MB and in VN2 and VN3 in the AB compared with VN1. In the other assessments, the CAT activity demonstrated a slight variation in the different vineyards, with the exception of stage EL31 in the BB, in which the enzyme activity was lower in VN3 compared with VN1 (Fig. 4). The activity of APX was consistently lower in the leaf blades from VN2 and VN3, with the exception of the BB at stage EL31, in which the VN2 demonstrated the lowest activity (Fig. 4). The copper concentration in the leaf blades, largely derived from the residual fungicide, and the macronutrient concentration did not correlate with the concentration of H2O2 and MDA or the activity of SOD, CAT and APX (data not shown).
Concentration of H2O2, level of lipid peroxidation (MDA), and activities of the enzymes SOD, CAT and APX in leaf blades collected at three positions (basal, middle and apical) in the branches and at two vegetative stages (EL31 and EL37) during the 2010/2011 cycle, in three vineyards with increasing levels of Cu in the soil. The same letters on the columns, lowercase for vineyard and a single phenological stage and uppercase for phenological stages and a single vineyard, indicate no difference according to the Tukey test at P < 0.05. NP not present, ns not significant
Discussion
Characteristics of the vineyard soils
The copper accumulation found in the soil in VN2 and VN3 was attributed to the foliar application of Cu-based fungicides over the years. The total copper in the topsoil (20.5 mg kg−1 in VN2 and 62.4 mg kg−1 in VN3, Table 2) was considered to be at a median or low level compared to the levels reported in the literature or by government regulatory agencies. Studies performed in many wine regions of the world have reported maximum concentrations of Cu in vineyard soils ranging from 200 to greater than 600 mg kg−1 (Brun et al. 1998; Arias et al. 2004; Casali et al. 2008). However, according to government regulatory agencies in Brazil, a concentration of 60 mg Cu kg−1 soil indicates the need for preventative measures, although the maximum concentration suggested is 200 mg kg−1 (CETESB 2005; CONAMA 2009). In Australia and New Zealand, a Cu concentration of 60 mg kg−1 in soils necessitates studies of the effect on the environment (ANZECC/NHMRC 1992), whereas the European community advocates that Cu concentrations should be maintained between 40 and 140 mg kg−1 in soils with a pH below 7.0 (Council Directive 86/278/EEC 1986). However, the low concentration of clay and organic matter found in the soils used in this study caused a low sorption capacity (Bradl 2004) and, therefore, a high Cu availability (Table 2). This phenomenon has been reported in other studies of vineyards containing acid and sandy soils and occurs because an acidic pH is one of the most important factors contributing to increased Cu toxicity in soils (Brun et al. 1998; Pietrzak and McPhail 2004).
The CuA extraction method using EDTA is frequently used to evaluate the Cu availability to plants. In the vineyard soils used in this study, the EDTA extraction method demonstrated a high availability of accumulated CuT. In VN3, the concentrations of CuA were up to 120 times higher than the concentration required by the plants, which is 0.5 mg kg−1 (CQFS-RS/SC 2004), and these high levels can be toxic to grapevines. In other grapevine soils, it has been reported that in soils of an acidic nature and in limestone soils, the EDTA extracts between 10 % and 50 % of the total Cu and frequently demonstrates concentrations between 20 and 60 mg kg−1 (Brun et al. 1998; Chaignon and Hinsinger 2003). This behavior is consistent with the CuA concentrations observed in this study.
Moreover, because of the naturally low P concentrations in the soils used in this study, the addition of P-containing fertilizers resulted in high P availability (Table 2). Phosphorus is a major limiting factor for the growth of plants in soils contaminated with Cu (Nikolic et al. 2011) and has been used to mitigate toxicity (Cao et al. 2003). However, in recent years, organic fungicides containing Zn salts, such as Mancozeb, have replaced Cu-based fungicides. The frequent use of these fungicides may have increased the concentrations of Zn in the soils (Fernández-Calviño et al. 2012), which explains the Zn concentration observed in VN3 (Table 2).
Cu concentration and content in grapevines
The Cu accumulation in the roots of plants grown in the tested soils was consistent with reports in the literature, and Cu accumulation was observed in young grapevines cultivated in soils contaminated with Cu (Toselli et al. 2009). In this study, the increasing Cu concentrations in the soil did not affect the concentration in roots of the grapevines during the productive season (Fig. 2, Table 2). This behavior may have occurred because at the evaluation stage, the annual young roots, which were smaller in diameter, were present in smaller quantities, whereas the thicker and lignified roots prevailed. Copper may have accumulated in the fine roots that are produced annually and are more active at absorbing nutrients. However, these roots have a short lifespan and are renewed every year (Anderson et al. 2003), which results in the elimination of the Cu they have accumulated. Moreover, although the thicker and suberized roots contribute very little to the absorption of nutrients, they are involved mainly in the translocation of water and nutrients, and they store carbohydrates and nutrients (Eissenstat 2007). However, compared with the DM found in the stems, shoots and leaves, the amount of DM in the roots was relatively high (Fig. 2). Therefore, even with Cu concentrations of ~15 mg kg−1, the roots were the grapevine organ that contained the highest amount of Cu.
The main role of the stems is to support the branches and to serve as an organ that transports water and nutrients between the roots and the aerial parts of the plant. Our data demonstrated that the Cu concentration in the stems of the grapevines was not affected by the increased availability of the metal in the soil. In addition, the lowest Cu concentrations were observed in the stems of the VN3 grapevines, which exhibited the highest Cu concentration in the soil. These data can be explained by the dilution of the Cu accumulated in the DM of the stems, which was higher in the VN2 and VN3 plants (Fig. 2, Table 3). In contrast, even compared with the other organs, the stems with the lowest concentrations of Cu exhibited the second highest value for the cumulative amount because of the higher DM (Fig. 2). Our data are inconsistent with a report by Lai et al. (2010), which demonstrated high Cu concentrations (ranging from 200 to 600 mg kg−1 DM) in the perennial organs of grapevines grown in soil containing total Cu concentrations that were similar to the levels observed in this study (an average of ~20 to ~60 mg kg−1 in the 0.00–0.20 m layer).
The Cu concentrations in the shoots in both cycles studied were also affected little by the concentrations of CuT or CuA in the soil. A positive correlation between the Cu concentrations in the shoots and the concentrations in the soil was observed only at stage EL17 in the 2010/2011 cycle (Fig. 3, Table 3). However, the results obtained in the 2009/2010 cycle suggested that at the early stages after the start of sprouting, the concentrations in the shoots were related to the Cu concentration in the stems of the grapevines at stages EL09 and EL19 (Fig. 2). A likely explanation for this observation is that in the early stages of growth, the shoots are tender and maintain a relationship with the stem and the stem Cu concentrations. In the advanced stages, there was a significant increase in DM (Fig. 2) and lignification of tissues and an increase in transpiration because of the increased number of leaves. At this time, the Cu concentration was less affected by the initial Cu level (Figs. 2 and 3).
Similar to the stems, the shoots allow the passage of water and nutrients between the stem and the leaves and bunches. The Cu concentration in the shoots tends to decrease during the vegetative cycle of grapevines because they grow quickly and change from a tender structure that is more metabolically active at the early sprouting stage into a semi-hardwood structure at the end of the cycle. The application of Cu-based fungicides increased the Cu concentration in the shoots of the grapevines and may have been the main contributing factor to the increase observed at stage EL31 in the 2009/2010 cycle and the variations observed in the EL31 stage in the 2010/2011 cycle. However, the increase in Cu concentrations in the shoots due to the applications of Cu-based fungicides was lower than that observed in the leaves (Figs. 2 and 3). This phenomenon can be attributed to the lower surface area of the shoots compared with the leaves and the dilution of the nutrients in the DM. The data obtained in this study were inconsistent with the data obtained by Lai et al. (2010), which demonstrated that the Cu concentrations in the shoots of grapevines grown in conventional and organic systems ranged between ~100 and 200 mg kg−1 DM. The observed Cu concentration may result because in the organic system, Cu is the main fungicide used and can be adsorbed in plant tissues even after washing with water. Studies performed in grapevines and pear trees grown in pots demonstrated that the Cu concentrations in the soil do not correlate with the Cu concentrations in the shoots (Toselli et al. 2008, 2009).
The bunches of grapes are clearly visible from stage EL12 (between five and six leaves are unfolded); however, the highest increase in dry matter and nutrient demand begins only with the emergence of the berries (EL29, small berries) (Eichhorn and Lorenz 1977). This study demonstrated that the Cu concentration in the bunches did not correlate with the Cu concentration in the soil and that the use of Cu-based fungicides increased the Cu concentrations in the bunches, particularly in the rachis (Figs. 2 and 3, Table 3). However, even after the growth of the berries and the consequent accumulation of nutrients from the soil and from various parts of the plant, the Cu concentration remained close to 20 mg kg−1 DM (which is approximately 4–5 mg kg−1 fresh weight (FW)) for the three vineyards. Li (1994) reported that in vineyards using Cu-based fungicides, extremely high Cu concentrations were observed in the leaves and petioles of the grapevines (all >118 mg kg−1), whereas the concentrations in the pulp of the berries (skin and seeds removed) ranged from 0.33 to 1.7 mg kg−1 FW. García-Esparza et al. (2006) demonstrated that the amount of Cu applied via fungicides exhibited a low correlation (R 2 = 0.26) with the metal residues in the grapes. The same study reported Cu concentrations in grapes ranging from 2 to 40 mg kg−1 FW, and after 50 days of fungicide application, the concentrations decreased to values below 10 mg kg−1 FW. These results can be attributed to the smaller surface area of the berries compared to the leaves and their natural waxy coating that prevents the accumulation of the Cu from the fungicides.
Studies performed under field conditions generally report high Cu concentrations in the leaves of grapevines. Pinamonti et al. (1999) reported Cu concentrations close to 270 mg kg−1 in the leaves of grapevines cultivated in soils contaminated by the addition of organic compounds. Higher concentrations of Cu were found by Lai et al. (2010) in grapevines cultivated in organic or conventional systems and observed concentrations of 400–1600 mg kg−1 in the leaves of the grapevines. Similarly, Li (1994) reported Cu concentrations between 118 and 9845 mg kg−1 in the petioles and leaves of grapevines grown in soils with an accumulation of Cu. In contrast, Toselli et al. (2009) reported that in grapevines grown in pots containing soil with up to 1,000 mg kg−1 Cu, reduced plant growth and chlorosis of the leaves was observed with Cu concentrations between 10 and 20 mg kg−1. The data obtained in this study demonstrated that the concentrations of Cu in the leaves effectively originated from the Cu in the soil at a concentration ranging from 7 to 10 mg kg−1; however, levels up to 25 mg kg−1 were found in the leaf blades in response to increased concentrations of Cu in the soil (Figs. 2 and 3). In spite of this correlation with the Cu concentrations in the soil, the Cu concentration and content in the leaves was weak and represented a small fraction of the Cu content of the plants. However, after the application of Cu-based fungicides, the concentrations in the leaves increased several-fold, reaching concentrations higher than the concentrations found in the soil. This observation can be explained by the fact that the Cu applied via the fungicide was absorbed by the apoplast of the leaves and was not removed by washing the plant with water, which has also been reported by Li (1994). Because of their smaller surface area, the petioles of the leaves in the grapevines were less contaminated by spraying with the Cu-based fungicides and demonstrated Cu concentrations that were several times lower than observed in the leaf blades. The petiole nutrient concentrations are related to the concentration in the leaves, and this concentration is used to diagnose the nutritional status of grapevines (CQFS-RS/SC 2004). The difference between the Cu concentration in the petioles and blades (Fig. 3) supported the hypothesis that the Cu applied via fungicide was not taken up by the plant or redistributed in the tissues.
Nutritional status of the grapevines
The toxic effects of Cu on the root system of plants affect the absorption of water and nutrients. Reduced root growth affects mainly the uptake of the nutrients supplied by diffusion, such as K and P (Marschner 1995), because of the reduced volume of soil explored by the smaller roots. In studies of soils contaminated with Cu, a P deficiency is the most limiting factor that hinders the growth of annual plants such as wheat (Nikolic et al. 2011). Moreover, excess Cu also impairs nitrogen assimilation, especially in the root system, which was observed in a study of young grapevines performed by Llorens et al. (2000). However, our data demonstrated that the grapevine exhibited similar concentrations of N, P, K, Ca and Mg in the leaf blades, indicating that the excess of Cu in the soil did not affect the uptake of these nutrients. During the productive season, grapevines exhibit a broad and deep root system and may take up nutrients from soil layers with lower Cu levels. In addition, constant fertilization performed to maintain soil fertility increases the availability of nutrients in the soil, therefore mitigating the toxic effects of Cu through better plant nutrition (Marschner 1995). The toxic effects of Cu in the roots also reduce the absorption of micronutrients, mainly Fe, Zn and Mn (Marschner 1995; Toselli et al. 2008, 2009). The concentration of these nutrients was not investigated in this study; however, visual symptoms of a deficiency in these nutrients were not observed.
Biochemical parameters in leaf blades
The excessive formation of ROS results from an imbalance in electron flow in oxidation-reduction reactions in the presence of excessive concentrations of Cu, Fe, Zn and other metals and factors (Apel and Hirt 2004). These oxidation-reduction reactions occur mostly in the peroxisomes and mitochondria and particularly in the chloroplasts in photosynthetic organs (Dat et al. 2000). Under conditions of oxidative stress, increased concentrations of H2O2 and other ROS are observed, leading to lipid peroxidation, which results from the attack of the hydrogens in fatty acid chains and the consequent formation of lipid radicals and aldehydes (Apel and Hirt 2004). Lipid peroxidation, estimated by the MDA concentration, is an important parameter for assessing cell damage because of the toxic effects of heavy metals (Islam et al. 2008; Yang et al. 2011). In this study, the leaf blades exposed to direct insolation exhibited higher lipid peroxidation. Changes were also observed in the concentration of H2O2 and in the activity of CAT and APX. This condition was observed mainly in the BB and MB at stage EL31 and in the MB and AB at stage EL37 (Fig. 4). This phenomenon was related to the growth of the shoots and the emergence of new leaves, with consequent shading of the basal leaves. The changes in the cellular metabolism of the leaf blades directly exposed to solar radiation correlated with the excessive Cu in soils (data not shown) and were not related to the Cu concentrations in the leaves, which were mostly caused by the application of Cu-based fungicides (Figs. 2 and 3). After the fungicide applications, the highest concentrations of Cu in the leaves of the grapevines were observed in VN1, which had the lowest concentration of the metal in the soil and MDA in the leaf blades. These results suggested that the Cu applied via fungicide did not affect the cellular metabolism. Therefore, the Cu can remain in the apoplast of the leaves or in other organs. However, increased concentrations of Cu in the leaves because of an excess of the metal in the soil (observed at stage EL19 in the 2009/2010 cycle; and at stage EL17 in the 2010/2011 cycle) could be the main factor contributing to the changes in metabolism and the increased MDA.
In our study, the concentration of MDA in the MB and AB was consistently higher in VN3 (Fig. 4), which demonstrated the highest concentrations of Cu in the soil (Fig. 4, Table 2). However, the H2O2 concentrations were higher in VN3 only in the BB at stage EL31 and especially in the MB at stage EL37. SOD, CAT and APX are important key enzymes in the removal of ROS (Apel and Hirt 2004), and the activities of these enzymes are generally increased under conditions of high levels of metals (Zhang et al. 2010). However, in this study, the SOD activity was variable and was explained partially by the concentrations of H2O2 or Cu in the soil and leaves. In addition, the concentration of H2O2 between stages EL31 and EL37 increased more than three-fold, likely because of the older leaves, without a corresponding increase in SOD activity (Fig. 4). Yang et al. (2011) also observed no changes in SOD activity in grapevines grown with an excess of Zn in soil. Excess Cu in cells can differently affect the activity of the SOD isoforms in the cytoplasm, chloroplasts, mitochondria and peroxisomes (Møller et al. 2007), which were not evaluated separately in this study. The formation of H2O2 from O2.- can also be catalyzed by transition metals, such as Fe3+ and Cu2+, via the Fenton reaction (Møller et al. 2007), without requiring enzymes.
The increased CAT activity observed at stage EL37 in the MB and AB also coincided with the full exposure of the leaves to the sun; however, this effect was not observed at stage EL31 when the leaves were younger. CAT has high affinity for H2O2 (mM range) and catalyzes the direct conversion of H2O2 into H2O and O2. Because of these characteristics, an increased CAT activity is observed under conditions of oxidative stress (Dat et al. 2000; Apel and Hirt 2004). Yang et al. 2011 observed that increased Zn in the soil resulted first in increased CAT activity in the leaves of grapevines, followed by a sharp decrease in the CAT activity because of the high degree of toxicity. In the same study, increased concentrations of Zn in the soil caused the progressive reduction in peroxidase activity. This behavior was also observed in this study; the APX activity in the leaf blades was lower in VN2 and VN3, in which the highest concentrations of Cu in the soil were found. The reduced APX activity could have been a direct or indirect effect of the excess Cu on the functioning of this enzyme. APX is part of the glutathione-ascorbate cycle (Dat et al. 2000) and is very sensitive to reduced concentrations of oxidized ascorbate, which is one of its substrates (Shigeoka et al. 2002). Excessive Cu in cells leads to the formation of phytochelatins from glutathione and SH groups, which are present when these molecules are chelated (De Vos et al. 1989). This process reduces the ascorbate-glutathione cycle activity, and the lower concentrations of oxidized ascorbate reduce APX activity.
The increased MDA, H2O2 concentrations and changes in the CAT and APX enzymes observed in this study were indications of the effect of excess Cu in the mature leaves, which exhibited full photosynthetic activity. However, the damage to the leaves tended to be small because decreased vegetative vigor or chlorosis, classic symptoms of toxicity by Cu and other metals, were not observed in the grapevines (Marschner 1995; Toselli et al. 2009; Yang et al. 2011). Additionally, the excellent nutritional status of the plants and the satisfactory production of grapes in these vineyards indicated that the adult grapevines were tolerant to the excess Cu in the soil.
Conclusion
The increased Cu concentrations observed in the leaves and the evidence of oxidative stress correlated with the increased Cu concentrations in the soil. However, the increased Cu availability in the soils only slightly affected the concentrations and accumulation of Cu in the grapevines during the productive season and did not change the nutritional status of the plants. The use of Cu-based fungicides was the main determinant for the increased Cu concentrations in the annual organs of the grapevines, especially in the leaves and rachis; however, the residual Cu in the berries was not related to the amount of Cu applied.
References
Aebi H (1984) Catalase: in vitro. Methods Enzymol 105:121–126
Anderson LJ, Comas LH, Lakso AN, Eissenstat DM (2003) Multiple risk factors in root survivorship: a 4-year study in Concord grape. New Phytol 158:489–501. doi:10.1046/j.1469-8137.2003.00757.x
ANZECC/NHMRC (Australian and New Zealand Environment and Conservation Council, National Health and Medical Research Council) (1992) Australian and New Zealand guidelines for the assessment and management of contaminated sites
Apel K, Hirt H (2004) Reactiven oxygen species: Metabolism, oxidative Stress, and signal transduction. An Rev Plant Biol 55:373–C-372. doi:10.1146/annurev.arplant.55.031903.141701
Arias M, López E, Fernández D, Soto B (2004) Copper distribution and dynamics in acid vineyard soils treated with copper-based fungicides. Soil Sci 169:796–805
Arias M, Pérez-Novo C, Osorio F, López E, Soto B (2005) Adsorption and desorption of copper and zinc in the surf ace layer of acid soils. J Colloid Interf Sci 288:21–29. doi:10.1016/j.jcis.2005.02.053
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. Colloid Interf Sci 27:1–18. doi:10.1146/annurev.arplant.55.031903.141701
Brun LA, Maillet J, Richarte J, Herrmann P, Rémy JC (1998) Relationships between extractable copper, soil properties and copper uptake by wild plants in vineyard soils. Environ Pollut 102:151–161. doi:10.1016/s0269-7491(98)00120-1
Brun LA, Maillet J, Hinsinger P, Pépin M (2001) Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ Pollut 111:293–302. doi:10.1016/S0269-7491(00)00067-1
Brun LA, Corff JLE, Maillet J (2003) Effects of elevated soil copper on henology, growth and reproduction of five ruderal plant species. Environ Pollut 122:361–368. doi:10.1016/S0269-7491(02)00312-3
Cao RX, Lena LQ, Chen M, Singh SP, Harris WG (2003) Phosphateinduced metal immobilization in a contaminated site. Environ Pollut 122:19–28. doi:10.1016/S0269-7491(02)00283-X
Casali CA, Moterle DF, Rheinheimer DS, Brunetto G, Mello ALC, Kaminski J, Melo GWB (2008) Formas e dessorção de cobre em solos cultivados com videira na Serra Gaúcha do Rio Grande do Sul. Rev Bras Ciênc Solo 32:1479–1487. doi:10.1590/S0100-06832008000400012
CETESB (Companhia de Tecnologia de Saneamento Ambiental) (2005) Decisão de Diretoria Nº 195-2005- E, 23/11/2005. Dispõe sobre a aprovação dos valores orientadores para solos e águas subterrâneas no Estado de São Paulo, em substituição aos valores orientadores de 2001, e dá outras providências. (In Portuguese.) Governo do Estado de São Paulo, São Paulo
Chaignon V, Hinsinger P (2003) A biotest for evaluating copper bioavailability to plants in a contaminated soil. J Enviro Qual 32:824–833. doi:10.2134/jeq2003.8240
Chaignon V, Quesnoit M, Hinsinger P (2009) Copper availability and bioavailability are controlled by rhizosphere pH in rape grown in an acidic Cu-contaminated soil. Environ Pollut 157:3363–3369. doi:10.1016/j.envpol.2009.06.032
CONAMA (Conselho Nacional do Meio Ambiente) (2009) Resolução 420, de 28/12/2009 Dispõe sobre critérios e valores orientadores de qualidade do solo quanto à presença de substâncias químicas e estabelece diretrizes para o gerenciamento ambiental de áreas contaminadas por essas substâncias em decorrência de atividades antrópicas. Diário Oficial da Repúbica Federativa do Brasil, 249, Brasília
Council Regulation 2092/91/EEC (1991) of 24 June 1991 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. Off J L, 198, Anexo II
Council Directive 86/278/EEC (1986) Protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off J Eur Union 181
CQFS RS/SC (Comissão de Química e Fertilidade do Solo - RS/SC) (2004) Manual de adubação e calagem para os Estados do Rio Grande do Sul e Santa Catarina. Núcleo Regional Sul - Sociedade Brasileira de Ciência do Solo, Porto Alegre, p 400
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795. doi:10.1007/s000180050041
De Vos CHR, Schat H, Vooijs R, Ernst WHO (1989) Copper induced damage to the permeability barrier in roots of Silene cucubalus. J Plant Physiol 135:164. doi:10.1016/S0176-1617(89)80171-3
Dumestre A, Sauve S, McBride M, Baveye P, Berthelin J (1999) Copper speciation and microbial activity in long-term contaminated soil. Arch Environ Contant Toxicol 36:24–131. doi:10.1007/s002449900451
Eichhorn KW, Lorenz H (1977) Phaenologische Entwicklungstadiender Rebe. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 21:119–120
Eissenstat DM (2007) Dinamica di crescita delle radici nelle coltura da frutto. Italus Hortus 14:1–8
El-Moshaty FIB, Pike SM, Novacky AJ, Sehgal OP (1993) Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ringspot virus or southern bean mosaic virus. Physiol Mol Plant Pathol 43:109–119. doi:10.1006/pmpp.1993.1044
Embrapa (Empresa Brasileira de Pesquisa Agropecuária), (1997) Manual de métodos de análise de solo. CNPS, Rio de Janeiro, p 212
Fernández-Calviño D, Rodríguez-Suárez JA, López-Periago E, Arias-Estévez M, Simal-Gándara J (2008) Copper content of soils and river sediments in a winegrowing area, and its distribution among soil or sediment components. Geoderma 145:91–97. doi:10.1016/j.geoderma.2008.02.011
Fernández-Calviño D, Pérez-Novo C, Nóvoa-Muñoz JC, Arias-Estévez M (2009) Copper fractionation and release from soils devoted to different crops. J Hazard Mater 167:797–802. doi:10.1016/j.jhazmat.2009.01.054
Fernández-Calviño D, Pateiro-Moure M, Nóvoa-Muñoz JC, Garrido-Rodríguez B, Arias-Estévez M (2012) Zinc distribution and acid–base mobilisation in vineyard soil and sediments. Sci Total Environ 414:470–479. doi:10.1016/j.scitotenv.2011.10.033
García-Esparza MA, Capria E, Pirzadeha P, Trevisana M (2006) Copper content of grape and wine from Italian farms. Food Addit Contam. doi:10.1080/02652030500429117
IPA - Instituto de Pesquisas Agronômicas (1989) Atlas Agroclimático do Estado do Rio Grande do Sul. Porto Alegre. http://www.cpact.embrapa.br/agromet/tab/tabela19.html. Accessed 05 June 2012
Islam E, Liu D, Li T, Yang X, Jin X, Mahmood Q, Tian S, Li J (2008) Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 154:914–926. doi:10.1016/j.jhazmat.2007.10.121
Komárek M, Cadková E, Chrastný V, Bordas F, Bollinger JC (2010) Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Enviro Int 36:138–151. doi:10.1016/j.envint.2009.10.005
Kopsell DE, Kopsell DA (2007) Copper. In: Barker AV, Pilbeam DJ (eds) Handbook of plant nutrition. Boca Raton, Taylor and Francis Group, pp 293–328
Lai H-Y, Juang K-W, Chen BC (2010) Copper concentrations in grapevines and vineyard soils in central Taiwan. Soil Sci Plant Nutr 56:601–606. doi:10.1111/j.1747-0765.2010.00494
Li R (1994) Effect of long-term applications of copper on soil and grape copper (Vitis vinifera). Can J Soil Sci 74:345–347. doi:10.4141/cjss94-047
Llorens N, Arola L, Blade C, Mas A (2000) Effects of copper exposure upon nitrogen metabolism in tissue cultured Vitis vinifera. Plant Sci 160:159–163. doi:10.1016/S0168-9452(00)00379-4
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787. doi:10.1104/pp. 010497
Marschner H (1995) Mineral Nutrition of Higher Plants. Academic, San Diego
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Møller LM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Ann Rev Plant Biol 58:459–481. doi:10.1146/annurev.arplant.58.032806.103946
Nikolic N, Kostic L, Djordjevic A, Nikolic M (2011) Phosphorus deficiency is the major limiting factor for wheat on alluvium polluted by the copper mine pyrite tailings: a black box approach. Plant and Soil 339:485–498. doi:10.1007/s11104-010-0605-x
Pietrzak U, McPhail DC (2004) Copper accumulation, distribution and fractionation in vineyard soils of Victoria, Australia. Geoderma 122:151–166. doi:10.1016/j.geoderma.2004.01.005
Pinamonti F, Nicolini G, Dalpiaz A, Stringari G, Zorzi G (1999) Compost use in viticulture: effect on heavy metal levels in Soil and plants. Commun Soil Sci Plant Anal 30:1531–1549
Robinson B, Greven M, Green S, Sivakumaran S, Davidson P, Clothier B (2006) Leaching of copper, chromium and arsenic from treated vineyard posts in Marlborough, New Zealand. Sci Total Environ 364:113–123. doi:10.1016/j.scitotenv.2005.07.012
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319. doi:10.1093/jexbot/53.372.1305
SSS (Soil Survey Staff) (2010) Soil Taxonomy Agricultural Handbook. United States Department of Agriculture, Washington
Tedesco MJ, Gianello C, Bissani C, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais. UFRGS/FA/DS, Porto Alegre, p 174, Boletim técnico 5
Toselli M, Baldi E, Marcolini G, Malaguti D, Marangoni B, Quartieri M (2008) Response of potted pear trees to increasing copper concentration in sandy and clay-loam soils. J Plant Nutr 2089–2104 doi:10.1080/01904160802459609
Toselli M, Baldi E, Marcolini G, Malaguti D, Quartieri M, Sorrenti G, Marangoni B (2009) Response of potted grapevines to increasing soil concntation. Aust J Grape Wine Res 15:85–92. doi:10.1111/j.1755-0238.2008.00040.x
USEPA (United States Environmental Protection Agency) (1996) Method 3050B. Acid digestion of sediments, sludges, and soils. Revision 2, Washington
Yang Y, Sun C, Yao Y, Zhang Y, Achal V (2011) Growth and physiological responses of grape (Vitis vinifera “Combier”) to excess zinc. Acta Physiologiae Plantarum 33:1483–1491. doi:10.1007/s11738-010-0687-3
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156. doi:10.1590/S1677-04202005000100012
Yruela I, Alfonso M, Barón M, Picorel R (2000) Copper effect on the protein composition of photosystem II. Physiol Plant 110:551–557. doi:10.1111/j.1399-3054.2000.1100419.x
Zhang H, Zhang F, Xia Y, Wang G, Shen Z (2010) Excess copper induces production of hydrogen peroxide in the leaf of Elsholtzia haichowensis through apoplastic and symplastic CuZn-superoxide dismutase. J Hazard Mater 178:834–843. doi:10.1016/j.jhazmat.2010.02.014
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533. doi:10.1016/j.plantsci.2004.04.020
Acknowledgments
We are grateful to FAPERGS, the CNPq and CAPES for the scholarships provided and the financial resources made available for this study. We are also grateful to the Almadén Wine Company for making the experimental vineyards available.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yong Chao Liang.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Miotto, A., Ceretta, C.A., Brunetto, G. et al. Copper uptake, accumulation and physiological changes in adult grapevines in response to excess copper in soil. Plant Soil 374, 593–610 (2014). https://doi.org/10.1007/s11104-013-1886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1886-7