Abstract
Aims and methods
To evaluate the seasonal and spatial variations of methane (CH4) emissions and understand the controlling factors, we measured CH4 fluxes and their environmental variables for the first time by a static chamber technique in high Suaeda salsa marsh (HSM), middle S. salsa marsh (MSM), low S. salsa marsh (LSM) and bare flat (BF) in the northern Yellow River estuary throughout a year.
Results
CH4 emissions from coastal marsh varied throughout different times of the day and significant differences were observed in some sampling periods (p < 0.05). Over all sampling periods, CH4 fluxes averaged between −0.392 mgCH4 m−2 h−1 and 0.495 mgCH4 m−2 h−1, and emissions occurred during spring (0.008 mgCH4 m−2 h−1) and autumn (0.068 mgCH4 m−2 h−1) while sinks were observed during summer (−0.110 mgCH4 m−2 h−1) and winter (−0.009 mgCH4 m−2 h−1). CH4 fluxes from the four marshes were not significantly different (p > 0.05), and emissions occurred in LSM (0.026 mgCH4 m−2 h−1) and BF (0.055 mgCH4 m−2 h−1) while sinks were observed in HSM (−0.035 mgCH4 m−2 h−1) and MSM (−0.022 mgCH4 m−2 h−1). The annual average CH4 flux from the intertidal zone was 0.002 mgCH4 m−2 h−1, indicating that coastal marsh acted as a weak CH4 source. Temporal variations of CH4 emission were related to the interactions of abiotic factors (temperatures, soil moisture and salinity) and the variations of limited C and mineral N in sediments, while spatial variations were mainly affected by the vegetation composition at spatial scale.
Conclusions
This study observed a large spatial variation of CH4 fluxes across the coastal marsh of the Yellow River estuary (CV = 7856.25 %), suggesting that the need to increase the spatial replicates at fine scales before the regional CH4 budget was evaluated precisely. With increasing exogenous nitrogen loading to the Yellow River estuary, the magnitude of CH4 emission might be enhanced, which should also be paid more attentions as the annual CH4 inventory was assessed accurately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methane (CH4) is an important greenhouse gas (GHG) that has more global warming potential than carbon dioxide (CO2) and has been recognized to contribute global warming by 25 % (Mosier 1998). The globally averaged atmospheric CH4 concentration increased from 715 ppb in 1750 to 1,774 ppb in 2005 (IPCC 2007), and increased by 8.3 ± 0.6 ppb during 2007 and 4.4 ± 0.6 ppb in 2008 (Dlugokencky et al. 2009). In 2010, the globally averaged CH4 concentration reached 1,808 ppb, which exceeded the highest annual mean abundance so far (World Meteorological Organization 2011). Emission of CH4 from various natural ecosystems has significant influences on the global climate change since it accounts for 30 ~ 40 % of the total CH4 emissions (150 ~ 237 Tg CH4 year−1) (Allen et al. 2007). Tropical soil and wetlands play an important role in the global carbon biogeochemical cycles and are considered significantly natural sources of CH4, contributing approximately 24 % towards this inventory (Whalen 2005).
CH4 fluxes from wetlands are the result of CH4 production, consumption and transportation from the anaerobic zone to the atmosphere (Bubier and Moore 1994). Considerable efforts have been widely made to investigate the CH4 fluxes and key controlling factors (such as water table, temperature, vegetation, substrate availability for methanogens and redox condition) in different natural wetland ecosystems (Allen et al. 2007; Ganguly et al. 2008; Turetsky et al. 2008; Pennock et al. 2010; Danevčič et al. 2010; Sun et al. 2011). The complex processes of CH4 fluxes and the variability of their controlling factors cause dramatic spatial and temporal variations of CH4 fluxes. The accurate global CH4 budget from different wetlands is very important for forecasting the future climate. However, one of the primary problems in attempting to develop accurate CH4 budgets for global wetlands is the large spatial and temporal variability in CH4 emission rates that are reported from all over the world. A clear understanding these spatial and temporal variations and their primary controlling factors in site measurements will be favorable for obtaining the optimal possible up-scaled emission estimation (Dinsmore et al. 2009).
Coastal marsh is characterized by high temporal and spatial variation related to topographic features, environmental factors and astronomic tidal fluctuation, and is very sensitive to global climate changes and human activities. Above all, the intertidal zone between terrestrial and coastal ecosystems may represent a high dynamic interface of intense material processing and transport, with potentially high GHGs emission (Hirota et al. 2007). In the past two decades, considerable efforts have been made to quantify the CH4 fluxes in different coastal ecosystems, especially in estuarine salt marshes (Shingo et al. 2000; Wang et al. 2009), mangrove swamps (Allen et al. 2007; Ganguly et al. 2008), coastal lagoons (Gregorich et al. 2006; Hirota et al. 2007) and coastal marshes (Sun et al. 2002; Amouroux et al. 2002). In China, the studies on CH4 emission from coastal marshes started quite late (in the 2000s), and the related research mainly focused on the coastal tundra marshes in Antarctica (Sun et al. 2002; Zhu et al. 2008), the mangrove swamps in southeast coastal area (Lu et al. 2000; Ye and Lu 2001), and the salt marshes in the Yangtze River estuary (Yang et al. 2007; Cheng et al. 2007; Wang et al. 2009; Cheng et al. 2010) and the Min River estuary (Tong et al. 2009; Zeng et al. 2010). However, few studies have reported on coastal marshes in northern estuaries, such as Liao River estuary and Yellow River estuary.
The Yellow River is well known as a sediment-laden river. Every year, approximately 1.05 × 107 tons of sediment is carried to the delta (Cui et al. 2009) and deposited in the slow flowing landform, resulting in vast floodplain and natural marsh landscape (Xu et al. 2002). Sediment deposition is an important process for the formation and development of coastal marshes in the Yellow River Delta. The deposition rate of sediment in the Yellow River not only affects the formation rate of coastal marshes, but also, to some extent, influences the water or salinity gradient and the succession of plants from the land to the sea. With an area of 964.8 km2, coastal marsh is the main type of marsh in the Yellow River Delta and accounts for 63.06 % of total area (Cui et al. 2009). Suaeda salsa, an annual C3 plant, is one of the most prevalent halophytes in coastal marshes of the Yellow River estuary. As a pioneer plant, it has strong adaptations to environmental stresses, such as high salinity, tidal inundation and sediment burial (Han et al. 2005). In the intertidal zone, three phenotypes are generally formed due to the differences of water and salinity conditions in high, middle and low tidal flats (marshes). In recent years, the nitrogen (N) and organic matter (OM) loadings of the Yellow River estuary have significantly increased due to the effects of human activities, and approximately 4, 650 tons of nutrient and 4.33× 105 tons of CODcr are discharged into Bohai Sea every year (State Oceanic Administration of China 2010). Increases in N and OM loadings to estuarine and coastal marshes can stimulate microbial processes and associated GHGs emission (Seitzinger and Kroeze 1998; Purvaja and Ramesh 2001). However, CH4 fluxes from different coastal S. salsa marshes in the Yellow River estuary remains poorly understood till now.
In this paper, we investigated CH4 fluxes from the coastal marshes in the Yellow River estuary during the spring, summer, autumn and winter of 2010/2011. The objectives were: i) to determine the spatial and temporal variations of CH4 fluxes from different coastal S. salsa marshes and bare flat, ii) to identify the key factors controlling the variations and assess the potential effects of exogenous N loading on CH4 emission.
Materials and methods
Site description
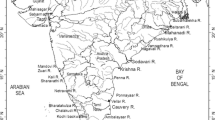
The study was carried out in the northern intertidal zone of the Yellow River estuary, which is located in the Nature Reserve of Yellow River Delta (37°35′N ~ 38°12′N, 118°33′E ~ 119°20′E) in Dongying City, Shandong Province, China. The nature reserve is of typical continental monsoon climate with distinctive seasons. The annual average temperature is 12.1 °C and the frost-free period is 196 day. The average temperature in spring, summer, autumn and winter are 10.7 °C, 27.3 °C, 13.1 °C and −5.2 °C, respectively. The annual evaporation is 1,962 mm, the annual precipitation is 551.6 mm, and about 70 % of precipitation occurring between June and August. The soils are dominated by intrazonal tide soil and salt soil (Tian et al. 2005). The main plant communities include S. salsa-Phragmites australis, S. salsa-Tamarix chinensis and S. salsa.
In the intertidal zone, natural geomorphology and depositing zones are distinct and high, middle and low tidal flats (marshes) develop from the land to the sea. The high tidal flat (marsh) is predominated by S. salsa (> 90 %) and P. australis (< 10 %), while middle tidal flat (marsh) is predominated by S. salsa (> 95 %) and T. chinensis (< 5 %). Low tidal flat (marsh) includes two distinct ecosystem-types. One is pure S. salsa community (100 %), with sparse distribution in tidal flat, and the other is bare flat (Fig. 1). The coverage and maximum aboveground biomass of S. salsa-P. australis, S. salsa-T. chinensis and S. salsa communities are 95 %, 80 %, 60 % and 902.08 ± 195.81, 564.89 ± 99.66, 252.97 ± 29.24 g m−2, respectively (Mou 2010). In this study, four sampling sites were laid in high S. salsa marsh (HSM), middle S. salsa marsh (MSM), low S. salsa marsh (LSM) and bare flat (BF) on the northern coastal marshes of the Yellow River estuary (Fig. 1). The tide in the intertidal zone of the Yellow River estuary is irregular semidiurnal tide (twice a day) and the mean tidal range is 0.73 ~ 1.77 m (Li et al. 1991). The salinity of the floodwater in the intertidal zone is greatly affected by the runoff of Yellow River and the values throughout a year average between 2.2 % and 3.1 % (Tian et al. 2005).
Experimental design
Fluxes of CH4 were measured by using opaque, static, manual stainless steel chambers and gas chromatography techniques. The chamber is an open-bottom a square box (50 cm × 50 cm × 50 cm) and equipped with an electric fan installed on the top wall of each chamber to make turbulence when chamber was closed. Outside of the chamber was covered with 2 cm thickness white foam to reduce the impact of direct radiative heating during sampling. In addition, a thermometer sensor was installed to measure temperature, a three-way stopcocks was fixed to collect gas sample, and a balance pipe was used to equalize the air pressure between the inside and the outside of the chamber. In August 2010, the stainless steel base (50 cm × 50 cm × 20 cm) with a water groove on top was installed at the four sampling sites. During observations, the chamber was placed over the base filled with water in the groove to ensure air-tightness and the plant was covered within the chamber.
Sampling campaigns were undertaken in autumn (September, October and November in 2010), winter (December in 2010), spring (April and May in 2011) and summer (June and July in 2011). Each measurement campaign consisted of 12 chambers set up at four sites (3 chambers per position): HSM and MSM were located near the shore and which were submerged only at high or middle tide, and LSM and BF were adjacent to the sea and which were frequently submerged at low tide. Since the tide in the Yellow River estuary is irregular semidiurnal tide, the sampling campaigns in the LSM and BF were sometimes affected by tidal inundation. In this study, the sampling campaigns in the LSM and BF in May and the BF in June were not carried out due to the influence of tidal inundation. On each sampling date, measurements were conducted at 7:00, 9:30, 12:00, 14:30 and 17:00 h. Measurements representing different times of day were from sediments exposed during low tide, over a number of days. Four air samples inside the chamber were collected every 20 min over a 60 min period by using 100 ml syringe equipped with three-way stopcocks. Samples were injected into pre-evacuated packs, transported back to the laboratory and analyzed within 36 h using gas chromatography (Agilent 7890A) equipped with an FID. The CH4 was separated from the other gases with a 2 m stainless-steel column, with an inner diameter of 2 mm 13XMS column (60/80 mesh). The oven, injector and detector temperatures were 80 °C, 200 °C and 200 °C, respectively. The flow rate of the carrier gas (N2) was 30 ml min−1. Flame gases (H2 and O2) were set at 20 and 30 ml min−1. Gas concentrations were quantified by comparing peak areas of samples against standards run every 8 samples, ensuring each sample run maintained RSD below 6 %. CH4 fluxes were calculated from the linear changes in the chamber concentration over time with an average chamber temperature (Song et al. 2006). Only samples with a regression determination coefficient R 2 greater than 0.95 were used for analysis (2.5 % measurements were rejected).
Environmental measurements
Air temperature and soil temperatures (0, 5, 10 and 15 cm) were measured in each position during gas sampling. Soil volumetric moisture and electrical conductivity (EC) in 0–5, 5–10 and 10–15 cm depths were determined in situ by high-precision moisture measuring instrument (AZS-2) and soil & solution EC meter (Field Scout), respectively. Soil moisture and EC were not determined in December 2010 since the topsoil (0–10 cm) was frozen. On each sampling date, two soil samples (0–10, 10-20 cm) per position were taken for analyzing total carbon (TC) and total nitrogen (TN) contents by element analyzer (Elementar Vario Micro, German) and ammonium (\( \mathrm{NH}_4^{+}-\mathrm{N} \)) and nitrate (\( \mathrm{NO}_3^{-}-\mathrm{N} \)) contents by sequence flow analyzer (San++ SKALAR, Netherlands). The total sulfur (TS) and sulfate (\( \mathrm{SO}_4^{2- } \)) data in the coastal marshes of the Yellow River estuary were cited from Sun et al. (2009) and Fan et al. (2010).
Statistical analysis
The results were presented as means of the replications, with standard error (S.E). Statistical significance of differences at p < 0.05 between samples were analyzed using analysis of variance (ANOVA). Multiple comparison of samples was undertaken by Tukey’s test with a significance level of p = 0.05. Correlation analyses and stepwise linear regression analyses were used to examine the relationship between fluxes and the measured environmental variables. In all tests, differences were considered significantly only if p < 0.05.
Results
Spatial variation of CH4 fluxes
Variation of CH4 fluxes in spring
CH4 fluxes in spring averaged between −0.781 mgCH4 m−2 h−1 and 0.822 mgCH4 m−2 h−1, but the values among the four marshes showed no significant difference (p > 0.05) (Fig. 2). Similar variations of CH4 flux in HSM and MSM were observed both in April and May. In April, with the exception of 12:00 sampling, the other sampling periods showed CH4 consumptions. In May, both HSM and MSM showed consumptions before 9:30 sampling and emissions afterward. CH4 fluxes from LSM ranged from −0.067 mgCH4 m−2 h−1 to 0.190 mgCH4 m−2 h−1 and a significant peak occurred in 14:30. The BF was found to release CH4 with the exception of 7:00 sampling, with the maximum occurred in 17:00. The mean CH4 fluxes from HSM, MSM, LSM and BF in spring were −0.056, 0.049, 0.034 and 0.027 mgCH4 m−2 h−1, respectively, indicating that coastal marshes represented weak emission except HSM.
Variation of CH4 fluxes in summer
CH4 fluxes in summer ranged from −1.128 mgCH4 m−2 h−1 to 0.380 mgCH4 m−2 h−1, but the values among the four marshes were not significantly different (p > 0.05) (Fig. 2). Similar variations of CH4 flux in June and July were observed in HSM except for 17:00 sampling. CH4 fluxes from MSM in June and July were −1.128 ~ 0.261 and −0.244 ~ 0.261 mgCH4 m−2 h−1, respectively, and they had no significant difference (p > 0.05). CH4 fluxes from LSM in June and July were in opposite except for 14:30 sampling and the ranges were −0.762–0.328 and −0.239–0.218 mgCH4 m−2 h−1, respectively. With the exception of July 14:30 sampling, the BF was found to consume CH4 in other periods. The mean CH4 fluxes from HSM, MSM, LSM and BF in summer were −0.083, −0.175, −0.083 and −0.083 mgCH4 m−2 h−1, respectively, indicating that coastal marshes acted as a CH4 sink.
Variation of CH4 fluxes in autumn
Although CH4 fluxes in autumn covered a wide range, from −0.742 mgCH4 m−2 h−1 to 1.767 mgCH4 m−2 h−1, the values among the four marshes were not significantly different (p > 0.05) (Fig. 2). Over all sampling periods, the variations of positive and negative CH4 fluxes were observed irregularly in each coastal marsh and the maximum and minimum occurred in BF. CH4 fluxes from each coastal marsh showed no significant difference among September, October and November (p > 0.05). The ranges of CH4 flux in HSM, MSM, LSM and BF in autumn were −0.176 ~ 0.335, −0.162 ~ 0.321, −0.125 ~ 0.660 and −0.742 ~ 1.767 mgCH4 m−2 h−1, and the means were 0.001, 0.030, 0.121 and 0.119 mgCH4 m−2 h−1, respectively, indicating that coastal marshes represented weak CH4 emission.
Variation of CH4 fluxes in winter
CH4 fluxes in winter covered a range of −0.092 mgCH4 m−2 h−1 to 0.117 mgCH4 m−2 h−1, but the values among the four marshes showed no significant difference (p > 0.05) (Fig. 2). With the exception of LSM that showed CH4 consumption during all times of day sampled, the other marshes were found to release CH4 in some sampling periods. Although the variations of CH4 flux in HSM and BF were opposite, both positive and negative values alternated regularly. With the exception of 7:00 sampling, the MSM was found to consume CH4 in other sampling periods. The mean CH4 fluxes from HSM, MSM, LSM and BF in winter were −0.004, −0.016, −0.046 and 0.031 mgCH4 m−2 h−1, respectively, indicating that coastal marshes acted as weak CH4 sink except BF.
Temporal variation of CH4 fluxes
CH4 fluxes from different coastal marshes varied throughout different times of the day and significant differences were observed in some sampling periods (p < 0.05) (Fig. 2). Although the temporal variations of CH4 fluxes were not significant in each coastal marsh (p > 0.05), significant differences still could be observed among different months (p < 0.05) (Fig. 3). Over all seasons, CH4 fluxes averaged between −0.392 mgCH4 m−2 h−1 and 0.495 mgCH4 m−2 h−1, with the maximum and minimum occurred in September (in BF) and June (in MSM), respectively. CH4 emissions occurred during spring (0.008 mgCH4 m−2 h−1) and autumn (0.068 mgCH4 m−2 h−1) while CH4 sink were observed during summer (−0.110 mgCH4 m−2 h−1) and winter (−0.009 mgCH4 m−2 h−1) (Fig. 3). Over all sampling periods, CH4 fluxes from the four marshes were not significantly different (p > 0.05). CH4 emissions occurred in LSM (0.026 mgCH4 m−2 h−1) and BF (0.055 mgCH4 m−2 h−1) while CH4 sink were observed in HSM (−0.035 mgCH4 m−2 h−1) and MSM (−0.022 mgCH4 m−2 h−1). The annual average CH4 flux from the intertidal zone was 0.002 mgCH4 m−2 h−1, indicating that coastal marsh acted as a weak source.
Temporal variations of CH4 fluxes (mgCH4 m−2 h−1) from high S. salsa marsh (HSM), middle S. salsa marsh (MSM), low S. salsa marsh (LSM) and bare flat (BF). Bars with different letters (a, b for HSM; x, y for MSM; m, n for LSM; o, p for BF) are significantly different at the level of p < 0.05; bars with same letters are not significantly different at the level of p < 0.05
Environmental variables in coastal marsh
Similar variations of air temperature and ground temperature (0, 5, 10 and 15 cm) in the four marshes were observed over all sampling periods (Fig. 4a). Air temperature did not show significant difference among the four marshes (p > 0.05). Ground temperatures generally decreased with increasing soil depth, but no significant differences were found within the four marshes (p > 0.05). Significant correlations between CH4 fluxes and temperatures were observed in different marshes during spring or summer (p < 0.05 or p < 0.01). By comparison, most correlations between CH4 fluxes and temperatures were not significant during autumn or winter (p > 0.05) (Table 1). Dissimilar variations of soil moisture and EC (0–5 and 5–10 cm) in the four marshes were observed over all sampling periods (Fig. 4b,c). With increasing depth, soil moisture increased (Fig. 4b), while EC generally decreased (Fig. 4c). Soil moisture did not show significant differences among the four marshes (p > 0.05), while significant differences of EC were observed (p < 0.05). Although both positive and negative influences of soil moisture and EC on CH4 emissions were observed within the four marshes, only the correlations between soil moisture (5–10, 10–15 cm) and CH4 fluxes in HSM (p < 0.01) and between EC (0–5 cm) and CH4 fluxes in LSM (p < 0.05) were significant (Table 2).
Seasonal dynamics of sediment substrate in the four marshes were observed over all sampling period (Fig. 5). TC, TN and NH4 +-N in the surface (0–10 cm) and subsurface sediment (10–20 cm) of BF were generally higher than those in other marshes (Fig. 5a, b, c). Both TC in surface and subsurface sediment had significant differences among the four marshes (p < 0.05), while only TN in subsurface sediment showed significant difference (p < 0.01). Both NH4 +-N and NO3 −-N in sediment (0–10, 10–20 cm) were not significantly different within the four marshes (p > 0.05). Lacks of correlations between CH4 fluxes and substrate variables were observed (p > 0.05) except the correlations occurred in subsurface sediment of HSM (p < 0.01) (Table 3). Moreover, the correlations between environmental variables determined during all times of day and CH4 diurnal emissions were not significant (p > 0.05).
Discussion
Temporal variations of CH4 fluxes
The magnitudes of CH4 fluxes from coastal marshes in the Yellow River estuary were in the range of −0.392 mgCH4 m−2 h−1 to 0.495 mgCH4 m−2 h−1, which were generally lower than those from salt marshes in the Yangtze River estuary and the Min River estuary, and mangrove swamps in the Brisbane River and Puerto Rico, but approximated emissions recorded at the Macrotidal Salt Marsh Bay, the sand shore of Lake Nakaumi, the intertidal zone of Maritime Antarctica, and the mangrove swamps in the Moreton Bay and the Dong zhai Harbor (Table 4). Differently, CH4 emissions from the salt marshes (Carex rugulosa, P. australis, Solidago altissima) of Lake Nakaumi under light condition recorded by Hirota et al. (2007) were 184 ~ 495 times greater than the maximum CH4 emission reported by our study, which was probably owing to large aerobic respiration by plant and microbes with continuous anaerobic condition. Existing abundant both live and dead aboveground biomass in salt marsh were important supporting evidences (Hirota et al. 2007).
Seasonal variations in CH4 emissions from coastal marshes were observed in this study (Fig. 3) and also reported by others (Inubushi et al. 2003; Allen et al. 2007; Zhu et al. 2008). Whalen (2005) observed that seasonal patterns of trace gas emission were influenced by latitude, with arctic, austral and some temperate regions characterized by pronounced CH4 emission (Gregorich et al. 2006; Allen et al. 2007), which are governed by seasonal variability in temperatures affecting water availability, production of substrate precursors and microbial activity. However, CH4 emissions from the coastal marshes in the Yellow River estuary seemed not to be affected by seasonal variability in temperatures though the estuary located in temperate region (37°35′N ~ 38°12′N). CH4 emissions generally occurred during spring and autumn while CH4 sink were observed during summer and winter. We considered that the seasonal variations in CH4 emissions observed in this paper were probably related to the complex interactions of temperatures and other biotic/abiotic factors, such as water and salinity status (Whalen 2005; Tong et al. 2009), plants (Tong et al. 2009) and sediment substrate (Allen et al. 2007). Although significant positive/negative correlations between CH4 fluxes and temperatures were observed in some periods (Table 1), the effects of seasonal variability in temperatures on CH4 emissions, to a great extent, might be covered by above biotic/abiotic parameters.
We found that CH4 emissions occurred during spring and autumn while CH4 sink were observed during summer and winter. Because the environmental variables determined in coastal marshes were all excluded in the stepwise liner regression, we considered that CH4 fluxes in different seasons were controlled by the interactions of multiple factors. Among them, the variations of limited C and mineral N in the sediments might have significant influences on CH4 emissions. As was shown in Fig. 5, TC, TN and NH4 +-N in different coastal marsh sediments (0–10, 10–20 cm) were higher in spring and autumn (especially in autumn), while \( \mathrm{NO}_3^{-}-\mathrm{N} \) were generally higher in summer and winter, indicating that the increase of C and N (especially \( \mathrm{NH}_4^{+}-\mathrm{N} \)) during spring and autumn might promote CH4 production and inhibit CH4 uptake, while the increase of \( \mathrm{NO}_3^{-}-\mathrm{N} \) during summer and winter was unfavorable for CH4 production. Similar results were drawn by Mancinelli (1995) and Bodelier and Laanbroek (2004). For one thing, the increase of C and N (NH4 +-N) improved the limited status of C and N in sediment and provided enough C and N sources for the growth of methanogens, which was favorable for the production of CH4. For another, the \( \mathrm{NH}_4^{+}-\mathrm{N} \) addition could inhibit the uptake of CH4, which was related to two underlying mechanisms: i) NH4 + is a competitive inhibitor of CH4 oxidation due to lack of specificity of methane monooxygenase (MMO) in methanotroph (Saari et al. 2004); ii) hydroxylamine and nitrite produced by methanotrophic ammonia oxidation are toxic to methanotrophic bacteria (Jiang et al. 2010). The inhibition of \( \mathrm{NO}_3^{-}-\mathrm{N} \) on CH4 production has been reported by many studies (Banik et al. 1996; Kluber and Conrad 1998; Chidthaisong and Conrad 2000), which could be partly applied to explain the formation of CH4 sink during summer and winter.
This study also showed that the coastal marsh in the Yellow River estuary represented weak CH4 emission (0.002 mgCH4 m−2 h−1) throughout the year and there were three probable causes. Firstly, although the high soil moisture in sediment (Fig. 4b) was favorable for CH4 production, the interaction of moisture and salinity might reduce CH4 emissions. Magenheimer et al. (1996) showed that the CH4 fluxes from a macrotidal salt marsh (Bay of Fundy) were inversely correlated (r 2 = 0.23, p = 0.001) with salinity of the upper porewater at the sampling site. Chidthaisong and Conrad (2000) indicated that high salinity could inhibit the activities of methanogens or did harm to methanogens which reduced CH4 emission. In this paper, the salinity (represented by EC) of the coastal marshes in the Yellow River estuary were high (0–5 cm,14.44 ± 3.96 mS cm−1; 5–10 cm, 12.10 ± 2.96 mS cm−1; 10–15 cm, 11.50 ± 2.63 mS cm−1) (Fig. 4c), which might reduce CH4 production. Secondly, the \( \mathrm{SO}_4^{2- } \) concentrations in sediment substrate resulting from high \( \mathrm{SO}_4^{2- } \) content in seawater (Ivanou 1992) were also considered dominant factor controlling CH4 emissions from coastal marsh (Kreuzwieser et al. 2003). Because sulfate reducing bacteria (SRB) compete for H2/CO2 and ethylic acid (CH3COOH) with methanogen and the former had more strong affinity to the reaction substrates, the coexistence of SRB and \( \mathrm{SO}_4^{2- } \) would inhibit the production and emission of CH4 (van der Gon et al. 2001). In this study, the TS and \( \mathrm{SO}_4^{2- } \) contents in the coastal marshes of the Yellow River estuary were 3.0 ~ 6.2 % (Sun et al. 2009) and 0.62 ~ 1.50 % (Fan et al. 2010), respectively, indicating that the high S content and the anoxic condition in sediment could enhance the dissimilatory reduction of \( \mathrm{SO}_4^{2- } \), which inhibited the CH4 emission from S. salsa marsh. Thirdly, the microbially mediated AOM (anaerobic oxidation of methane) process occurring mainly in anoxic sediments also has crucial effects on CH4 emission (Raghoebarsing et al. 2006). AOM coupled to \( \mathrm{SO}_4^{2- } \) reduction is performed by a consortium of anaerobic methanotrophic archaea (ANME) and SRB (Strous and Jetten 2004; Mileto et al. 2008; Knittel and Boetius 2009). During AOM, CH4 is oxidized with \( \mathrm{SO}_4^{2- } \) as the terminal electron acceptor (CH4 + SO4 2− → HCO3 − + HS− + H2O) (Knittel and Boetius 2009). As mentioned above, the high S content in sediment might enhance the AOM process under anoxic condition, which further inhibited the CH4 emission from S. salsa marsh.
We also demonstrated that CH4 emissions from coastal marshes varied throughout different times of the day and significant differences were observed in some sampling periods (p < 0.05) (Fig. 2). Diurnal variations in trace gas flux have been reported in other coastal marsh studies. Hirota et al. (2007) indicated that the diurnal variation of CH4 fluxes from salt marsh in dark contrition was influenced by soil temperature (r = 0.47, p < 0.05). Lu et al. (1999) showed that CH4 fluxes from Bruguiera sexangula mangrove swamp sediment had large diurnal fluctuation, which was caused by the changes of tidal inundation rather than the changes of air or sediment temperatures. Since environmental factors are involved directly with different microbe and plant activities and are changeable in relative short term (diurnal) scale, the diurnal variations of CH4 emission from different coastal marshes are affected by different principal factors. But this study showed that any environmental variables determined during all times of day had no significant correlations with CH4 diurnal emissions (p > 0.05). Similar result was drawn by Hirota et al. (2007) who found that any environmental factors seemed to not affect diurnal variation of CH4 flux under light condition. Because our study did not measure CH4 fluxes and environmental variables over the complete tidal inundation cycles, the variations of some major factors during all times of night might be missed, which probably covered the main factors. Besides, the variations of environmental variables in the coastal marshes of the Yellow River estuary might be more complicated than those in other coastal marshes, which caused the CH4 diurnal emissions to be affected by two or more factors. However, these explanations require to be verified in the following study.
Spatial variations of CH4 fluxes
Over all sampling periods, we found that the physical (temperature, soil moisture and EC) and chemical (TC, TN, NH4-N and NO3-N) parameters of sediment differed in their magnitude among the four marshes. Significant differences in TC, TN and EC in sediment were observed (p < 0.05). Such differences among the four marshes would be due to the site-specific conditions such as topography, slope, hydrology and species composition which determine the magnitudes and variations of CH4 at spatial scale (Allen et al. 2007; Hirota et al. 2007). From the stepwise linear regression analysis, soil moisture (X1) and EC (X2) were the dominant factors that controlled the CH4 emissions (Y) in HSM (Y = −3.091 + 0.080X1, R 2 = 0.773, p = 0.004) and LSM (Y = −0.566 + 0.060X2, R 2 = 0.682, p = 0.043), respectively, while in MSM and BF, the environmental variables determined during sampling periods were all excluded, indicating that CH4 fluxes were controlled by multiple factors.
This study observed a large spatial variation of CH4 fluxes in the coastal marshes of the Yellow River estuary. The coefficient of variations (CVs) of CH4 fluxes in the four marshes were 133.70 %, 712.06 %, 562.02 % and 425.49 %, respectively, while the value across the coastal marsh was 7856.25 %, indicating that, to evaluate the regional budget of CH4 emissions precisely, measurements should be designed at fine scales and the number of spatial replicates should be increased. Although CH4 fluxes from the four marshes had no significant differences (p > 0.05), CH4 emission patterns were different during all times of day and the seasons measured (Fig. 2). Previous studies have indicated that temperatures had great influence on CH4 emissions at spatial scale (Sun et al. 2002; Gregorich et al. 2006). Although air temperature and ground temperatures did not show significant difference among the four marshes during all the seasons measured (p > 0.05), strong positive/negative correlations still could be found in some sampling periods (p < 0.05) (Table 1). This indicated that thermal condition was an important factor affecting CH4 emission across the coastal marsh, but its function might be covered by the interactions of other biotic or abiotic factors, such as moisture, salinity, sediment substrate and plant. For spatial variations of CH4 fluxes in coastal marsh, we considered that the differences of vegetation composition were the main driving forces. van den Pol-van Dasselaar et al. (1999) suggested that vegetation was the most important factor in predicting the spatial variability of CH4 fluxes because it was a comprehensive reflection of environmental conditions such as climate, soil moisture and nutrient status. The importance of vegetation for carbon fluxes in wetlands also has been described in many studies (Joabsson et al. 1999; Hirota et al. 2006). Especially, it has been well known that wetland plants have complex gas transport system via their body and emit CH4 from soil to the atmosphere (Schimel 1995; Hirota et al. 2004). As mentioned before, because the vegetation composition and biomass in the four marshes were different, the plant distributed continuously across the coastal marsh would be one of the key factors for the CH4 emissions at spatial scale.
Site-level control of CH4 emission was also attributed to the effects of soil moisture, salinity and nutrient status. Although soil moisture did not show significant differences among the four marshes (p > 0.05), strong positive correlations were observed between soil moisture (5–10, 10–15 cm) and CH4 fluxes in HSM (p < 0.01) (Table 2). This is common because higher moisture creates a reduction condition that is beneficial for the production and emission of CH4. In contrast, EC showed significant differences within the four marshes (p < 0.05) and significant positive correlation was observed between EC and CH4 emission in LSM (p < 0.05) (Table 2). Generally, both positive and negative impacts of soil moisture and EC on CH4 emissions were found in coastal marshes (Table 2), which induced the formation of different CH4 emission patterns at spatial scale. As discussed before, the negative impact of soil moisture or the positive effect of EC on CH4 emission might be related to the interaction of soil moisture and salinity in sediment. Similar result was drawn by Hirota et al. (2007) who found that, in coastal ecosystems subjected to the fluctuation of water level (or soil moisture) by astronomic tide, there will be both positive and negative impact on CH4 emissions. Site-level nutrient status also influenced the spatial variations of CH4 fluxes (Ding et al. 2004). In this study, both positive and negative correlations between CH4 emission and nutrient status were observed, and significant correlation between CH4 emission and NO3-N occurred in HSM (p < 0.01) (Table 3). Although lacks of correlations between CH4 fluxes and substrate variables were observed (p > 0.05), local nutrient differences due to topography, aspect, slope, hydrology and vegetation, to some extent, influenced the spatial differences of CH4 emissions at spatial level.
Our study showed that the coastal marsh acted as a weak CH4 source in the present N loading of the Yellow River estuary. Numerous studies have demonstrated that N was an important regulatory factor for the production and consumption of CH4 (Bodelier and Laanbroek 2004; Lebauer and Treseder 2008), and the response of CH4 flux to N enrichment, both the magnitude and the direction, might vary due to the N addition level and N forms (Liu and Greaver 2009). Jiang et al. (2012) studied the responses of N enrichment (\( \mathrm{NH}_4^{+}-\mathrm{N} \) and \( \mathrm{NO}_3^{-}-\mathrm{N} \)) on CH4 production and consumption of the different coastal marsh sediments in the Yellow River estuary, and found that \( \mathrm{NH}_4^{+}-\mathrm{N} \) addition caused a general stimulation on CH4 emission while \( \mathrm{NO}_3^{-}-\mathrm{N} \) addition generally inhibited CH4 production. Moreover, with increasing \( \mathrm{NH}_4^{+}-\mathrm{N} \) addition, the suppression of CH4 uptake enhanced (Jiang 2012). Similar results were drawn by Le Mer and Roger (2001), and Bodelier and Laanbroek (2004). The increase in CH4 emssion under N addition probably was caused by the activities of both methanogenic archaea and methanotropic bacteria (Liu and Greaver 2009). At present, the exogenous N loading (\( \mathrm{NH}_4^{+}-\mathrm{N} \) is dominated) of the Yellow River estuary is increasing due to human activities (State Oceanic Administration of China 2010). Since N is a very limited nutrient in the coastal marshes of the Yellow River estuary (Mou 2010), increases in exogenous N loading to estuarine and coastal marshes will stimulate microbial processes and CH4 emission. In addition, increases in exogenous \( \mathrm{NH}_4^{+}-\mathrm{N} \) loading will also enhance the suppression of CH4 uptake (Jiang 2012) and the multiple mechanisms have been declared as mentioned before. Based on the above analysis, we can conclude that the CH4 emission in the future will be enhanced with increasing N loading to the Yellow River estuary (especially \( \mathrm{NH}_4^{+}-\mathrm{N} \) is the major pollutant), and the magnitude of CH4 emission should be paid more attentions as the annual CH4 inventory was assessed accurately.
Conclusions
This paper studied the seasonal and spatial variations of CH4 emissions and associated environmental factors in the coastal marshes (HSM, MSM, LSM and BF) of the Yellow River estuary. Results have demonstrated that: i) CH4 fluxes averaged between −0.392 mgCH4 · m−2 · h−1 and 0.495 mgCH4 · m−2 · h−1, and emissions occurred during spring and autumn while sinks were observed during summer and winter. CH4 emissions occurred in LSM and BF while sinks were observed in HSM and MSM. The annual average CH4 flux from the intertidal zone was 0.002 mgCH4 · m−2 · h−1 and coastal marsh acted as a weak CH4 source; ii) Temporal variations of CH4 emission were related to the interactions of abiotic factors (temperatures, soil moisture and salinity) and the variations of limited C and mineral N in sediments, while spatial variations were mainly affected by the vegetation composition and biomass at spatial scale; iii) Both the large spatial variation of CH4 fluxes across the coastal marsh (CV = 7856.25 %) and the potential effect of exogenous N loading to the Yellow River estuary on CH4 emission should be considered before the annual CH4 inventory was evaluated accurately.
References
Allen DE, Dalal RC, Rennenberg H et al (2007) Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol Biochem 39:622–631
Alongi DM, Pfitzner J, Trott LA et al (2005) Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang estuary, China. Estuar Coast Shelf S 63:605–618
Amouroux D, Roberts G, Rapsomanikis S et al (2002) Biogenic Gas (CH4, N2O, DMS) emission to the atmosphere from near-shore and shelf waters of the North-western Black Sea. Estuar Coast Shelf Sci 54(3):575–587
Banik B, Sen M, Sen SP (1996) Effects of inorganic fettilizers and micronutrients on methane production from wetland rice (Oryza sativa L). Biol Fertil Soils 21(4):319–322
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277
Bubier JL, Moore TR (1994) An ecological perspective on methane emissions from northern wetlands. Trends Ecol Evol 9:460–464
Cheng XL, Peng R, Chen J et al (2007) CH4 and N2O emissions from Spartina alterniflora and Phragmites australis in experimental mesocosms. Chemosphere 68:420–427
Cheng XL, Luo YQ, Xu Q et al (2010) Seasonal variation in CH4 emission and its 13C-isotopic signature from Spartina alterniflora and Scirpus mariqueter soils in an estuarine wetland. Plant Soil 327:85–94
Chidthaisong A, Conrad R (2000) Turnover of glucose and acetate coupled to reduction of nitrate, ferric iron and sulfate and to methanogenesis in anoxic rice field soil. FEMS Microbiol Ecol 31:73–76
Cui BS, Yang QC, Yang ZF et al (2009) Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecol Eng 35:1090–1103
Danevčič T, Mandic-Mulec I, Stres B (2010) Emissions of CO2, CH4 and N2O from Southern European peatlands. Soil Biol Biochem 42:1437–1446
Ding WX, Cai ZC, Tsuruta H (2004) Diel variation in methane emissions from the stands of Carex lasiocarpa and Deyeuxia angustifolia in a cool temperate freshwater marsh. Atmos Environ 38:181–188
Dinsmore KJ, Skiba UM, Billett MF et al (2009) Spatial and temporal variability in CH4 and N2O fluxes from a Scottish ombrotrophic peatland. Soil Biol Biochem 41:1315–1323
Dlugokencky EJ, Bruhwiler L, White JWC et al (2009) Observational constraints on recent increases in the atmospheric CH4 burden. Geophys Res Lett 36:L18803
Fan XM, Liu GH, Tang ZP et al (2010) Analysis on main contributors influencing soil salinization of Yellow River Delta. J Soil Water Conservat 24(1):139–144
Ganguly D, Dey M, Mandal SK et al (2008) Energy dynamics and its implication to biosphere-atmosphere exchange of CO2, H2O and CH4 in a tropical mangrove forest canopy. Atmos Environ 42:4172–4184
Gregorich EG, Hopkins DW, Elberling B et al (2006) Emission of CO2, CH4 and N2O from lakeshore soils in an Antarctic dry valley. Soil Biol Biochem 38:3120–3129
Han N, Shao Q, Lu CM et al (2005) The leaf tonoplast V-H+-ATPase activity of a C3 halophyte Suaeda salsa is enhanced by salt stress n a Ca-dependent mode. J Plant Physiol 162:267–274
Hirota M, Tang Y, Hu Q et al (2004) Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland. Soil Biol Biochem 36:737–748
Hirota M, Tang Y, Hu Q et al (2006) Carbon dioxide dynamics and controls in a deep water wetland on the Qinghai-Tibetan Plateau. Ecosystems 9:673–688
Hirota M, Senga Y, Seike Y et al (2007) Fluxes of carbon dioxide, methane and nitrous oxide in two contrastive fringing zones of coastal lagoon, Lake Nakaumi, Japan. Chemosphere 68:597–603
Inubushi K, Furukawa Y, Hadi A et al (2003) Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere 52:603–608
IPCC (2007) Climate change: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Ivanou M (1992) Sulphur cycling in the continents. John Wiley and Sons, New York
Jiang HH (2012) Study on the flux characteristics and influence mechanisms of carbon dioxide and methane emission of tidal wetland ecosystem in the Yellow River estuary. Master degree dissertation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai
Jiang CM, Yu GY, Fang HJ et al (2010) Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China. Atmos Environ 44:2920–2926
Jiang HH, Sun ZG, Wang LL et al (2012) A preliminary study on methane production potential of soil in tidal wetlands of the Yellow River estuary and its responses to organic matter and nitrogen import. Wetland Sci 10(4) (Online First) http://www.cnki.net/kcms/detail/22.1349.P.20121101.1059.003.html
Joabsson A, Cheristensen TR, Wallen B (1999) Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14:385–388
Kluber DH, Conrad R (1998) Inhibition effect of nitrate, nitrite, NO and N2O on methanogenesis by by Methanosarcina mazei. FEMS Microbiol Ecol 25(3):331–339
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol 63:311–334
Kreuzwieser J, Buchholz J, Rennenberg H (2003) Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biol 5:423–431
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37:25–50
Lebauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Li YF, Huang YL, Li SK (1991) A primarily analysis on the coastal physiognomy and deposition of the modern Yellow River Delta. Acta Oceanol Sin 13(5):662–671
Liu LL, Greaver TL (2009) A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emmision. Ecol Lett 12:1103–1117
Lu CY, Wong YS, Tam NFY et al (1999) Methane flux and production from soils of a mangrove wetland on Hainan Island, China. Mangroves Salt Marsh 3:41–49
Lu CY, Ye Y, Wong YS et al (2000) Methane fluxes from mangrove communities at Dongzhai harbour, Hainan. Acta Phytophysiol Sin 24:87–90
Magenheimer JF, Moore TR, Chmura GL et al (1996) Methane and carbon dioxide flux from a macrotidal salt marsh, Bay of Fundy, New Brunswick. Estuar Coast 19:139–145
Mancinelli RL (1995) The regulation of methane oxidation in soil. Annu Rev Microbiol 49:581–605
Mileto M, Loy A, Antheunisse AM et al (2008) Biogeography of sulphate-reducing bacteria in river floodplains. FEMS Microbiol Ecol 64:395–406
Mosier AR (1998) Soil processes and global change. Biol Fertil Soils 27:221–229
Mou XJ (2010) Study on the nitrogen biological cycling characteristics and cycling model of tidal wetland ecosystem in Yellow River estuary. Master degree dissertation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai
Pennock D, Yates T, Bedard-Haughn A et al (2010) Landscape controls on N2O and CH4 emissions from freshwater mineral soil wetlands of the Canadian Prairiee Pothole region. Geoderma 155:308–319
Purvaja R, Ramesh R (2001) Natural and anthropogenic methane emission from coastal wetlands of South India. Environ Manag 27:547–557
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT et al (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921
Saari A, Rinnan R, Martikainen PJ (2004) Methane oxidation in boreal forest soils: kinetics and sensitivity to pH and ammonium. Soil Biol Biochem 36:1037–1046
Schimel J (1995) Plant transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry 28:183–200
Seitzinger SP, Kroeze C (1998) Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochem Cy 12:93–113
Shingo U, Chun-sim UG, Takahito Y (2000) Dynamics of dissolved O2, CO2, CH4 and N2O in a tropical coastal swamp in southern Thailand. Biogeochemistry 49:191–215
Song CC, Wang YS, Wang YY et al (2006) Emission of CO2, CH4 and N2O from freshwater marsh during freeze-thaw period in Northeast of China. Atmos Environ 40:6879–6885
Sotomayor D, Corredor JE, Morell JM (1994) Methane flux from mangrove sediments along the southwestern coast of Puerto Rico. Estuar Coast 17:140–147
State Oceanic Administration of China (2010) Ocean Environmental Quality Communique of Shandong Province in 2009. http://www.soa.gov.cn/oa/hygb/hgb/nine/webinfo/2010/06/1281687829218830.htm
Strous M, Jetten MS (2004) Anaerobic oxidation of methane and ammonium. Annu Rev Microbiol 58:99–117
Sun LG, Zhu RB, Xie ZQ et al (2002) Emissions of nitrous oxide and methane from Antarctic Tundra: role of penguin dropping deposition. Atmos Environ 36:4977–4982
Sun WL, Sun ZG, Mou XJ et al (2009) Distribution characteristics of phosphor and sulfur in different wetland soils of the Yellow River Delta. Bull Soil Water Conserv 30(4):104–108
Sun XJ, Mu CC, Song CC (2011) Seasonal and spatial variations of methane emissions from montane wetland in Northeast China. Atmos Environ 45:1809–1816
Tian JY, Wang XF, Cai XJ (2005) Protection and restoration technique of wetland ecosystem in Yellow River Delta. China Ocean University Press, Qingdao
Tong C, Zeng CS, Wang WQ et al (2009) Main factors influencing CH4 flux from a Phragmites australis wetland in the Min River estuary. Acta Sci Circums 29:207–216
Turetsky MR, Treat CC, Waldrop MP et al (2008) Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. J Geophys Res 113:G00A10. doi:10.1029/2002JG000496
van der Gon HAD, van Bodegom PM, Wassmann R et al (2001) Sulfate-containing amendments to reduce methane emissions from rice fields: mechanisms, effectiveness and costs. Mitigation Adaptation Strat 6:71–89
van den Pol-van Dasselaar A, van Beusichem ML, Oenema O (1999) Determinants of spatial variability of methane emissions from wet grasslands on peat soil. Biogeochem 44:221–237
Wang DQ, Chen ZL, Sun WW et al (2009) Methane and nitrous oxide concentration and emission flux of Yangtze Delta plain river net. Sci China Ser B 52:652–661
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
World Meteorological Organization (2011) WMO Greenhouse Gas Bulletin No.7 (21 November 2011). http://www.wmo.int/pages/prog/arep/gaw/ghg/documents/GHGbulletin_7_en.pdf
Xu XG, Guo HH, Chen XL et al (2002) A multi-scale study on land use and land cover quality change: the case of the Yellow River Delta in China. GeoJournal 56:177–183
Yang HX, Wang DQ, Chen ZL et al (2007) Seasonal variation of CH4 emission from Chongming east intertidal flat of Yangtze River estuary. Sci Geogr Sin 27:408–413
Ye Y, Lu CY (2001) Dynamics of CH4 in soil under Aegiceras corniculatum mangrove at Changning estuary of Hainan Island. J Trop Oceanogr 20:35–42
Ye Y, Lu CY, Lin P et al (2000) Diurnal change of CH4 fluxes from estuarine mangrove wetlands. Acta Oceanol Sin 22:103–109
Zeng CS, Wang WQ, Zhang LH et al (2010) Methane fluxes of Cyperusma laccensis tidal wetland in Minjiang River estuary. Chin J Appl Ecol 21:500–504
Zhu RB, Liu YS, Ma J et al (2008) Nitrous oxide flux to the atmosphere from two coastal tundra wetlands in eastern Antarctica. Atmos Environ 42:2437–2447
Acknowledgements
The authors would like to acknowledge the two anonymous reviewers for their constructive comments on this paper. This study was financially supported by the Strategy Guidance Program of the Chinese Academy of Sciences (No. XD05030404), the “1-3-5” Strategy Plan Program of the Yantai Institute of Coastal Zone Research of the Chinese Academy of Sciences (No. Y254021031), the National Nature Science Foundation of China (No. 41171424), the Ocean Public Welfare Scientific Research Project, State Oceanic Administration, People’s Republic of China (No. 2012418008–3), the Key Program of Natural Science Foundation of Shandong Province (No. ZR2010DZ001) and the Talents Program of the Youth Innovation Promotion Association, Chinese Academy of Sciences (No. Y129091041).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sun, Z., Jiang, H., Wang, L. et al. Seasonal and spatial variations of methane emissions from coastal marshes in the northern Yellow River estuary, China. Plant Soil 369, 317–333 (2013). https://doi.org/10.1007/s11104-012-1564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1564-1