Abstract
Background and aims
Rhizodeposition of plants is the most uncertain component of the carbon (C) cycle. By existing approaches the amount of rhizodeposition can only roughly be estimated since its persistence in soil is very short compared to other organic C pools. We suggest an approach to quantify rhizodeposition at the field scale by assuming a constant ratio between rhizodeposited-C to root-C.
Methods
Maize plants were pulse-labeled with 14CO2 under controlled conditions and the soil 14CO2 efflux was separated into root and rhizomicrobial respiration. The latter and the 14C activity remaining in the soil corresponded to total rhizodeposition. By relating rhizodeposited-14C to root-14C a rhizodeposition-to-root ratio of 0.56 was calculated. This ratio was applied to the root biomass C measured in the field to estimate rhizodeposition under field conditions.
Results
Maize allocated 298 kg C ha−1 as root-C and 166 kg C ha−1 as rhizodeposited-C belowground, 50 % of which were recovered in the upper 10 cm. The fate of rhizodeposits was estimated based on the 14C data, which showed that 62 % of total rhizodeposition was mineralized within 16 days, 7 % and 0.3 % was incorporated into microbial biomass and DOC, respectively, and 31 % was recovered in the soil.
Conclusions
We conclude that the present approach allows for an improved estimation of total rhizodeposition, since it accounts not only for the fraction of rhizodeposits remaining in soil, but also for that decomposed by microorganisms and released from the soil as CO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants modify chemical, physical and biological properties of the soil environment surrounding the roots. Organic compounds released from living roots (rhizodeposits), originating from roots exudates of intact cells, from lysates of sloughed-off cells and dead tissues, and from mucilage (Dennis et al. 2010) represent an important carbon (C) flux into the soil. Especially root exudates are a primary source of energy for microorganisms strongly affecting soil organic matter (SOM) dynamics (Kuzyakov et al. 2007). This ecological importance calls for a better estimation of rhizodeposition, which still remains the most uncertain part of the soil C cycle (Nguyen 2003). There are several reasons why it is difficult to reliably quantify rhizodeposition. Organic substances released by living roots occur in a much lower content than other organics in soil and are restricted to the narrow zone around the roots (Kuzyakov and Domanski 2000). Fast decomposition of root-released organics due to their high availability for microorganisms further makes rhizodeposition difficult to assess for analytics (Jones et al. 2005).
To distinguish rhizodeposited-C from native soil organic carbon (SOC), 14C and/or 13C labeling of plants has commonly been applied leading to distinct isotopic differences of root- and SOC-derived C (Werth and Kuzyakov 2008). The portion of root-released C remaining in soil (net rhizodeposition) can thus be quantified. However, these approaches largely underestimate rhizodeposition since they did not account for the amount of rhizodeposits rapidly decomposed by microorganisms (Amos and Walters 2006; Werth and Kuzyakov 2008). The portion of rhizodeposits mineralized to CO2 (rhizomicrobial respiration; RMR) contributes, together with root respiration (RR), to root-derived CO2, a main source of the soil CO2 efflux (Cheng et al. 2003; Kuzyakov 2006). For further partitioning of root-derived CO2 into RMR and RR the isotopic labeling approaches reach their limit since both sources of root-derived CO2 are labeled by the tracer. It is, however, necessary to consider them separately because C input to soil and SOM turnover is only affected by rhizomicrobial C, while root respiration biases the picture of SOM turnover.
C accumulation and consumption in soil are closely coupled with microbial activity and in turn are influenced by rhizodeposition (Kuzyakov et al. 1999). The easily available part of rhizodeposition fuels microbial activity in the rhizosphere and thus represents a direct link between roots and soil microorganisms. Despite the importance of separating rhizomicrobial from root respiration suitable approaches are rare. After 14C pulse labeling of plants, root-derived 14CO2 can be partitioned into CO2 coming from the decomposition of rhizodeposits and CO2 from root respiration by means of a simulation model (Kuzyakov and Domanski 2002; Kuzyakov et al. 1999). The model is based on the assumption that both respiration processes reach their maximum at different times after pulse labeling. While root respiration occurs immediately, rhizomicrobial respiration appears at a later stage after labeling because a chain of successive processes is passed before (exudation, microbial uptake and respiration) (Kuzyakov et al. 2001). Special experiments are necessary to determine the 14CO2 efflux dynamics, which are applicable under controlled conditions, but hardly possible under field conditions. Thus, estimation of rhizodeposition under field conditions remains a challenge for quantification of C budget and fluxes.
Under field conditions, root biomass (RB) was measured to estimate the portion of photosynthetically fixed C allocated to belowground pools. Those measurements alone may greatly underestimate the C input by roots into the soil since rhizodeposition is ignored (Amos and Walters 2006; Johnson et al. 2006). The portion of net photosynthetic C translocated belowground and released by living roots can even be higher as the C retained in the roots (Johnson et al. 2006). However, root biomass contributes more C to SOC than rhizodeposition, because the latter is easily decomposable by microorganisms (Johnson et al. 2006). On average 17 % of net assimilated C is released by roots via rhizodeposition, with 12 % of which being mineralized to CO2 (RMR) and only 5 % remaining in the soil (Nguyen 2003). Attempts to include rhizodeposition in estimates of C inputs into the soil by roots often only very roughly assumed that the quantity of rhizodeposited C equals that of root biomass at harvest (Bolinder et al. 1999; Amos and Walters 2006). However, reliable data on rhizodeposition under field conditions are absent.
In this paper we provide a method for an improved quantification of total rhizodeposition, including C losses by rhizomicrobial respiration, under field conditions. After 14CO2 pulse labeling of maize plants under controlled conditions, we measured the root-derived 14CO2 and determined the contributions of root and rhizomicrobial respiration based on model calculations. The rhizodeposition-to-root ratio determined under controlled conditions was applied to the maize root biomass measured in the field in order to estimate the rhizodeposition at a field scale.
Materials and methods
Determination of rhizodeposition-to-root ratio (R) under controlled conditions
Soil and growing conditions
Intact soil cores were collected with a soil corer (inner diameter 12 cm, height 30 cm) from the upper 30 cm on the experimental site and placed in cylindrical Plexiglas pots (inner diameter 13 cm, height 30 cm, covered with dark foil). Maize seeds (Zea mays L. cv. Ronaldinio) were germinated on wet filter paper and transferred to the 16 pots 3 days after germination. The pots were closed with a plastic lid with holes for the shoots. The soil water content was measured gravimetrically and adjusted daily to 70 % of the water holding capacity (WHC). The plants were grown at 26 to 28 °C day temperature and at 22 to 23 °C night temperature with a day-length of 14 h and a light intensity of about 400 μmol m−2 s−1.
14C pulse labeling
The plants were labeled at the tillering stage, 28 days after germination. The day before labeling, the holes in the plastic lids were sealed around the shoots with silicon paste (NG 3170, Thauer & Co., Germany) and the seals were tested for air leaks. The labeling procedure is described by Kuzyakov et al. (1999). Briefly, eight pots were placed in a Plexiglas chamber (48.1 × 48.1 × 158 cm). The chamber was connected with a flask containing 5 ml of Na 142 CO3 (ARC Inc., USA) solution with a 14C activity of 1.2 MBq per pot. 14CO2 was released into the chamber by addition of 10 ml of 5 M H2SO4 to the labeling solution. The plants were labeled during 4 h in the 14CO2 atmosphere. Thereafter, the chamber air was pumped through 15 ml of 1 M NaOH solution to remove unassimilated 14CO2 for 2 h. Finally, the chamber was opened and trapping of CO2 evolved from the soil started. CO2 produced in four sealed pots was trapped by circulating the air through 15 ml of 1 M NaOH solution. The NaOH solution was changed every two hours after labeling for the first day, then twice daily, then once every 2 days until 16 days after labeling.
Sampling
Plants and soil were sampled 2, 5, 10, and 16 days after labeling with four replicates for each sampling day. At harvest, shoots were cut at the base and roots were separated from the soil of each layer by handpicking. The soil adhering to the roots was shaken gently and termed ‘rhizosphere soil’. The roots were washed with 50 ml deionized water to remove the soil still attached to the roots. The soil was sieved (<2 mm). Shoots, roots, bulk and rhizosphere soil were dried at 60 °C, weighed and pulverized in a ball mill.
Sample analysis
The 14C activity of unassimilated 14CO2 after labeling, trapped in NaOH, and the remaining 14C activity in the tracer solution was measured in 2 ml aliquots added to 4 ml Rothiscint scintillation cocktail (Roth, Germany) with a Liquid Scintillation Counter (LS 6500 Multi-Purpose Scintillation Counter, 217 Beckman, USA) after the decay of chemiluminescence. The 14C activity of soil CO2 trapped in the NaOH solution was measured in the same way. The 14C counting efficiency was about 92 % and the 14C activity measurement error did not exceed 2 %. Total C of soil CO2 was analyzed by an N/C analyzer (Multi N/C 2100, AnalytikJena, Germany)
50 mg of plant samples (shoots, roots) or 500 mg of soil samples (bulk and rhizosphere soil) were combusted in an oxidizer unit (Feststoffmodul 1300, AnalytikJena, Germany) and released CO2 was trapped in 10 ml of 1 M NaOH. The radioactivity was measured by means of a Scintillation Counter (LS 6500 Multi-Purpose Scintillation Counter, 217 Beckman, USA) as described above. Total C concentrations for those samples were measured by a N/C analyzer (Multi N/C 2100, AnalytikJena, Germany)
The 14C activity of the soil microbial biomass C (MBC) was determined for the four replicates sampled on day 16 after labeling by the chloroform fumigation extraction method described by Vance et al. (1987). Briefly, 5 g fresh soil were shaken with 20 ml of 0.05 M K2SO4 for 1 h at 200 rev min−1, centrifuged at 3000 rev min−1 for 10 min, and filtrated. Another 5 g fresh soil were fumigated with chloroform for 24 h and extracted in the same way. The extracts were analyzed for total organic carbon by means of an N/C analyzer (Multi N/C 2100, AnalytikJena, Germany). The 14C activities of the extracts of unfumigated and fumigated soils were measured using a LS 6500 Multi-Purpose Scintillation Counter, 217 Beckman, USA. Measurements were conducted on 1 mL aliquots added to 6 mL scintillation cocktail Rothiscint (Roth, Germany).
Calculation of the 14C budget
A 14C budget was compiled for each sampling day separately. The percentage of 14C recovered in a C pool (\( r{\left( {{}^{{14}}C} \right)_p} \), %) was calculated by relating the 14C activity of the respective C pool (\( a{\left( {{}^{{14}}C} \right)_p} \), kBq) to the total 14C recovery after each harvest (\( a{\left( {{}^{{14}}C} \right)_T} \), kBq), i.e. to the sum of the 14C activity in shoot, root, bulk soil, rhizosphere soil and CO2:
Note, CO2 measurements started directly after labeling, but only for the pots harvested 16 days after labeling. Therefore, from those pots the cumulative 14CO2 efflux after 2, 5 and 10 days of labeling was added to the total 14C recovery on the respective day.
The 14C results obtained from the measurement of the extracts of fumigated and un-fumigated soil were converted to the 14C activity in microbial biomass (\( {}^{{14}}{C_{{mic}}} \)) using the following equation:
where \( {}^{{14}}{C_{{flush}}} \) is the difference between the 14C activity in fumigated and in unfumigated samples (kBq) and 0.45 is the conversion factor (Wu et al. 1990). As a measure for the fraction of dissolved organic carbon (DOC) we used the 14C activity of the unfumigated soils.
The percentage of 14C recovered in MBC and DOC on day 16 after labeling was calculated using Eq. (1).
Model calculations for separating root and rhizomicrobial respiration
In order to estimate the percentage of root respiration and rhizomicrobial respiration on total 14CO2 efflux a model approach was applied. The model design is described in detail by Kuzyakov and Domanski (2002). The 14C activity of total CO2 \( a{\left( {{}^{{14}}C} \right)_{{COz}}} \) (kBq) was converted into percentage of total assimilated CO2 \( r{\left( {{}^{{14}}C} \right)_{{C{O_z}}}} \) before using it in the model. The amount of total assimilated 14C \( a{\left( {{}^{{14}}C} \right)_{{TA}}} \) (kBq) was assumed to be equal to the 14C activity of the tracer introduced into the chamber \( a{\left( {{}^{{14}}C} \right)_C} \) (kBq) at the beginning of labeling minus the 14C activity remaining in the chamber \( a{\left( {{}^{{14}}C} \right)_{{RC}}} \) (kBq) and in the tracer solution \( a{\left( {{}^{{14}}C} \right)_{{RS}}} \) (kBq) after labeling (Kuzyakov and Domanski 2002).
The model parameters (Table 1) were adjusted based on 1) the 14CO2 efflux rate from soil, expressed in % of assimilated per hour, and based on 2) the cumulative 14CO2 efflux, expressed as % of assimilated. Thereby, the cumulative 14CO2 efflux allows to adjust parameters responsible for the amount of respired 14CO2, while the 14CO2 efflux rate was used to adjust parameters responsible for the dynamics of the respiration rates (Kuzyakov and Domanski 2002). The distribution between above- and belowground C pools was considered by the shoot-to-root ratio. The parameters shoot growth rate, short-term shoot respiration and long-term shoot respiration were not considered here since they did not affect the belowground 14C fluxes. RR and RMR were simulated based on the Model-maker (3) software (ModelKinetix, Oxford, UK; www.modelkinetix.com).
Rhizodeposition-to-root ratio
The contribution of rhizomicrobial respiration (\( r{\left( {{}^{{14}}C} \right)_{{RMR}}} \), % of assimilated) to total root-derived 14CO2, simulated by the 14CO2 efflux model, was converted into the 14C activity of rhizomicrobial respiration (\( a{\left( {{}^{{14}}C} \right)_{{RMR}}} \), kBq):
where \( {\left( {{}^{{14}}C} \right)_{{CumC{O_z}}}} \) (kBq) is the fitted 14C activity of the cumulative 14CO2 efflux at day 16 after labeling.
The rhizodeposition-to-root ratio (R) was calculated as follows:
where \( a{\left( {{}^{{14}}C} \right)_{{BS}}} \) and \( a{\left( {{}^{{14}}C} \right)_{{RS}}} \) are the 14C activities in kBq of the bulk and the rhizosphere soil, respectively, and \( a{\left( {{}^{{14}}C} \right)_{{Root}}} \) is the 14C activity in kBq of the root.
Root biomass measurements in the field-experimental design and root sampling
The experimental site was established on an arable field in the north-west of Göttingen, Germany (51°33′36.8″N, 9°53′46.9″E) in 2009. The soil type was classified as a haplic Luvisol. Detailed information about soil properties and the experimental site are given by Kramer et al. (2012). Maize (Zea mays L. cv. Ronaldinio) was planted on a 24 × 240 m plot in April 2009 after removing wheat seedlings sown in October 2008 with a non-selective herbicide (“Round-up”, Monsanto Agrar, Germany). The mean distance between the maize rows was 0.8 m and the mean distance between the plants in row was 0.5 m. Maize plants on the experimental plots were harvested in November 2009.
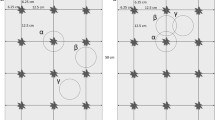
Root biomass was sampled in July 2009, at the silking stage of the maize plants, 12 weeks after planting. To investigate the spatial distribution of maize roots we sampled direct at the position of the maize plant, 12.5 cm and 25 cm away from the plant in row, 20 cm and 40 cm away in the inter-row and 23.5 cm and 47 cm away from the plant at the diagonal between row and inter-row. Soil samples were taken with an auger (Riverside auger, Eijkelkamp, The Netherlands) at each position up to 50 cm depth in 10 cm layers. This sampling procedure allowed to cover spatial variability of maize roots under the plant, within and between the maize rows.
Each fresh sample was weighed and a subsample of the soil (without roots) was dried for 3 days at 60 °C. The water content of the subsample was used to determine the dry weight of the total sample. All roots were carefully washed free from soil using the method described by Smucker et al. (1982). The remaining non-root material was separated from the sample by handpicking. The samples were dried at 60 °C for 3 days and weighed. The C content of the roots was determined on five replicates using a multi N/C 2100S analyzer (Analytik, Jena, Germany). Root biomass was expressed as mg C per g dry soil. Note, in the present study only the portion of the root system below the soil surface was considered as root biomass and thus, the aboveground crown was not included.
Upscaling: Root biomass C and total C from rhizodeposition in the field
The amount of maize root C (\( n{\left( {{C_{{Root}}}} \right)_F} \), kg C ha−1) was calculated for each 10 cm layer until 50 cm.
where z (cm) is the thickness of the respective soil layer (10 cm), ρ (g cm−3) is the bulk density of the layer and \( n\left( {{C_{{Root}}}} \right) \) is the C (\( {\text{mg}}\,{\text{C}}\,{\text{g}}_{\text{soil}}^{{ - 1}} \)) content of the roots. Bulk density values were taken from Kramer et al. (2012) and are 1.4 ± 0.0 g cm−3 for the Ap1 horizon (0–25 cm), 1.6 ± 0.0 g cm−3 for the Ap2 horizon (25–37 cm) and 1.7 ± 0.0 g cm−3 for the Btw1 horizon (37–65 cm).
The amount (\( n{\left( {{C_{{RD}}}} \right)_F} \)) of root released C in the field during one growing season was estimated by multiplying the amount of maize root \( C\left( {n{{\left( {{C_{{Root}}}} \right)}_F}} \right) \) with the rhizodeposition-to-root ratio (R) and was expressed as kg C ha−1:
Statistics
The values presented in the figures are given as means ± standard errors of means (±SEM). A one-way analysis of variance (ANOVA) was conducted to test for significant differences in root biomass C between the sampling depths. The significance of differences between the depths at individual sampling positions was obtained by the post hoc Tukey HSD test for unequal N, while the significance of differences for the mean root biomass C between depths was calculated by the post hoc Tukey HSD test. Significant differences in the 14C recovery between the sampling dates were also obtained by a one-way ANOVA in combination with a post hoc Tukey HSD test for unequal N. All statistical analysis were performed with the statistical package statistica for Windows (version 7.0; StatSoft Inc., OK, USA).
Results
14C pulse labeling under controlled conditions
Budget of assimilated 14C
The precondition for the determination of the rhizodeposition-to-root ratio at the end of the chase period was that the 14C allocation between above- and belowground C pools was mostly completed. To demonstrate this, pots were sampled 2, 5, 10 and 16 days after the labeling and the 14C budgets of the individual sampling dates were checked for statistical differences. We could not find significant differences between 14C budgets of the investigated sampling dates with the exception of the difference in the 14C recovery of the bulk soil between day 2 and 5 (Table 2). Thus, the main part of the tracer was allocated to various pools already in the first two days after the labeling.
At all sampling dates about half of the tracer was incorporated into the shoot biomass and 21–28 % was recovered in the roots (Table 2). While about 7 % of 14C retained in the bulk soil, the 14C recovered in the rhizosphere soil was only about 0.1 % because of its small volume. The missing portion of 14C in the complete balance is connected with the CO2 efflux from soil, which was included in the calculations of the 14C recovery.
Separating root and rhizomicrobial respiration
An earlier developed model of belowground C fluxes was applied to determine the contributions of RR and RMR to total root-derived CO2.
Cumulative 14CO2 efflux and the 14CO2 efflux rate measured under controlled conditions were used to fit most of the model parameters (Table 1). The root growth rate (h−1) was measured as root biomass increase between the sampling dates. For parameters which can be varied over a wide range we used the values from the previous model parameterization (Kuzyakov and Domanski 2002) (Table 1). A good correlation between the measured and the fitted data were obtained, for the cumulative 14CO2 efflux (Fig. 1a) as well as for the 14CO2 efflux dynamics (Fig. 1b). The model is based on the finding that the 14C activity of the CO2 efflux after pulse labeling shows two peaks (Warembourg and Billes 1979; Nguyen et al. 1999; Kuzyakov et al. 1999, 2001; Kuzyakov and Domanski 2002). Warembourg and Billes (1979) assumed that the second peak of 14C activity can be attributed to the decomposition of rhizodeposits by microorganisms, and is delayed compared to root respiration because of the time necessary for roots to synthesize and release substances which are decomposed later on. We found the highest 14C activity of the CO2 efflux already 6 h after the start of the labeling (Fig. 1). Thereafter, the 14C activity strongly decreased within the first 20 h. The data did not show a distinctive second peak. However, the CO2 efflux rate remained on a constant level between 20 h and 24 h after the labeling before it gradually declined. The measured kinetics is similar to that reported by Nguyen et al. (1999) and Todorovic et al. (2001). The assumption of different process rates of root and rhizomicrobial respiration allow to separate both based on the simulation model. During the 16 days after labeling about 16.2 % of total assimilated 14C was detected in root-derived CO2 (Fig. 1a). Rhizomicrobial respiration accounted for 9.2 % of assimilated 14CO2, which equals 56.8 % of total root-derived 14CO2.
Rhizodeposition-to-root ratio
The 14C activities of the bulk soil, of the rhizosphere soil and of microbially respired 14CO2 were considered as total 14C rhizodeposition and related to the 14C activity of the roots. The respective rhizodeposition-to-root ratio was on average 0.56 ± 0.2 (Fig. 2). To investigate the fate of the rhizodeposits, total rhizodeposition (16 days after labeling) was partitioned into four C fluxes. The largest portion of rhizodeposits was respired by microorganisms and released as CO2. This portion accounted for 61.8 % of total rhizodeposition. About 30.6 % of the 14C released by roots retained in the soil longer than 16 days, with further 7.3 % being incorporated into the microbial biomass and only 0.3 % recovered in DOC.
Root biomass in the field
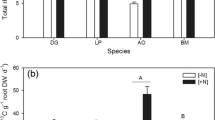
Root biomass sampled directly over the plant showed a decline with depth (Fig. 3). About 50 % of the roots were distributed in the upper 10 cm of the Ap horizon. The decline was still present 12.5 cm away from the plant in row and 23.5 cm away on the diagonal between row and inter-row. The RB did not differ significantly at one depth between the seven sampling positions (aboveground crown not included; Fig. 3). The weighted average biomass C also declined with depth, from 104 kg C h−1 at the 0–10 cm to 15 kg C h−1 at the 40–50 cm depth (Fig. 4).
Vertical and horizontal root biomass C distribution (±SEM) of maize sampled in July 2009. Different letters indicate significant differences (P ≤ 0.05) of root biomass C between the depths (vertical). The samples of a single depth did not differ significantly between different positions (horizontal). The sampling design is shown bottom left
Rhizodepositon at field scale. Weighted maize root biomass C (±SEM, kg C ha−1) measured in July 2009 and C released by roots via rhizodeposition (±SEM, kg C ha−1) during the growing season 2009. The extrapolated values, shown in the frame, correspond to a soil depth of 50 cm, a plant population of 25,000 and a growing period from April to July 2009. Different letters indicate significant differences between the depths
Upscaling: Root biomass C and total C from rhizodeposition in the field
Considering the spatial variability of roots between and within the rows, the measured maize root C in the upper 50 cm was 298 ± 64 kg C ha−1 (Fig. 4). By applying the rhizodeposition-to-root ratio of 0.56 ± 0.2 analyzed by 14C labeling under controlled conditions (Fig. 3) to the root C measured in field we estimated that 166 ± 53 kg C ha−1 was released from living roots as rhizodeposits between April and July 2009 in the upper 50 cm of the soil. Half of these rhizodeposits was released into the upper 10 cm soil. The portion declined with depth (Fig. 4).
Discussion
Root biomass determination in the field
A reliable quantification of the maize root biomass in the field, as a precondition to estimate rhizodeposition, strongly depends on 1) the sampling design and time of sampling and on 2) losses during the root washing procedure. The sampling design must cover the spatial variability of the RB to accurately extrapolate to the basis of RB per hectare. It was shown that the maize RB was highest close to the base of the plants, than decreased with distance from the plant and increased again at the mid-row position where adjacent plants contribute to the RB (Gajri et al. 1994). RB sampling at different positions in row, in inter-row and on the diagonal between row and inter-row allows to cover the spatial variability. However, in the present experiment with already developed maize plants, the RB did not differ significantly between the sampling locations (Fig. 3). Furthermore, the rooting depth must be considered. The portion of roots grown deeper than the sampling depth of 50 cm was not considered and therefore, the total RB may be slightly underestimated. However, the majority of roots were allocated directly below the soil surface. The upper 30 cm contain 70–90 % of the RB of maize (reviewed by Amos and Walters 2006). Therefore, samples were taken up to 50 cm depth included the main part of the RB. Root biomass was sampled on day 84 after planting, at the silking stage of the plant growth. This stage was chosen since it has been reported that the maize root biomass is maximal just after anthesis (Amos and Walters 2006; Anderson 1988).
The second source of uncertainty of RB determination may have been the washing procedure. Despite a considerable loss of root hairs and fine roots during the washing procedure (see discussion below), 93 to 96 % of total maize RB has been recovered when using a sieve with a mesh size of 0.5 mm (Livesley et al. 1999), as done in the present experiment.
The root-to-shoot ratio (R/S) measured in the current study was compared with literature data. For calculating R/S, the aboveground biomass (without the crown) of nine representative maize plants was measured. The dry weight per plant was on average 173 g plant−1. The average distance between maize rows (inter-row) in the field was 0.8 m and the average distance between plants in row was 0.5 m, resulting in a theoretical number of 25,000 plants ha−1. Therefore, the shoot biomass accounts for 4325 kg dry weight ha−1. We calculated a spatially weighted mean RB of 960 kg dry weight ha−1 with reference to the upper 50 cm of soil. The resulting R/S of 0.22 is in agreement with the R/S ratios reviewed by Amos and Walters (2006). With plant age a decrease in the root-to-shoot ratio has been reported, from 0.68 at emergence to 0.16 at physiological maturity (Amos and Walters 2006).
Factors affecting root biomass and/or rhizodeposition
The type of study, i.e. field or controlled conditions, may affect root biomass and rhizodeposition through differences in growth conditions. To keep the soil conditions as comparable as possible, intact soil cores from the field site were used for the controlled conditions experiment. To exclude plant genetic influences on the root system and on rhizodeposition the same maize variety as in the field was used.
The root biomass and the quantity of C released by living roots depend on the plant phenology and on environmental factors (Grayston et al. 1996; Hütsch et al. 2002; Nguyen 2003). Plant phenology may influence root biomass as well as rhizodeposition, mainly through root growth dynamics and differences in the quantity of rhizodeposits (Vancura 1964; Klein et al. 1988; Van der Krift et al. 2001; Jones et al. 2004). At young age, plants translocate more carbon to the roots, whereas older plants preferably retain newly assimilated C in the shoots (Keith et al. 1986; Gregory and Atwell 1991; Palta and Gregory 1997; Gransee and Wittenmayer 2000) thus, leading to decreased C inputs into the soil due to a decreased assimilates allocation to the roots (reviewed by Nguyen 2003). Aging of plants decreases the exudation intensity, however, if this decrease is slower than the root growth total rhizodeposition will increase (Kuzyakov 2002). On the other hand an enhanced die of root material with plant age increased C inputs into the soil. It was shown that rhizodeposition is positively correlated to root biomass (Van der Krift et al. 2001). To adequately estimate the rhizodeposition under field conditions, we sampled root biomass at the maximum development stage of the root system. However, under controlled conditions the size of the pots may restrict the rooting volume and the amount of nutrients available for plants. To overcome these restrictions an earlier stage of plant development were studied under controlled conditions as compared to the field. Here we assumed that changes in the root biomass between field and controlled conditions are accompanied with the same relative changes in rhizodeposition. This assumption allows to conclude that despite the differences between the root biomass (and rhizodeposition) in field and under controlled conditions, the rhizodeposition-to-root ratio remains nearly constant and its changes are much lower than variations in the both C pools.
Furthermore, not only plant phenology but also environmental factors may alter the root growth pattern and the amount of rhizodeposition (Grayston et al. 1996; Hütsch et al. 2002; Nguyen 2003). The release of C by living roots is driven by the allocation of recently assimilated C belowground and thus, depends to a large degree on the intensity of photosynthesis (Kuzyakov and Cheng 2001) and growth rates of individual organs. It was suggested that plants grown under natural sunlight released a higher amount of C compared to plants grown under artificial light, the latter showing highly variable values (Amos and Walters 2006). On the other hand, consistently lower light conditions may not only reduce the rhizodeposition of maize plants (Kuzyakov and Cheng 2004), but may also lead to a lower root biomass (Hébert et al. 2001).
The main assumption involved in the current study was, that the ratio of rhizodeposition-to-root is much more stable than changes in the C amount of roots and rhizodeposits between field and controlled conditions.
14C-Partitioning
Sixteen days after the labeling about 51 % of the 14C activity was recovered in the maize shoot, 28 % in the roots, 5 % in the soil and 16 % in the CO2 efflux. The 14C recovery in the CO2 efflux was within the range of 21 % reported by Werth and Kuzyakov (2008) and of 14 % measured by Todorovic et al. (2001). Based on a 14CO2 efflux model we found that root respiration accounts for 7 % of total assimilated 14C and about 9.2 % of assimilated 14C was released as CO2 from rhizomicrobial respiration. These values are close to the results obtained for Lolium perenne, ranging 1.4–7.6 % and 0.9–8 % of assimilated 14C for RR and RMR, respectively (Kuzyakov et al. 1999, 2001; Kuzyakov and Domanski 2002). In a further study, conducted with wheat, Cheng et al. (1993) used the isotope dilution method to separate root and rhizomicrobial respiration and found that 59 % of root-derived C is coming from rhizomicrobial respiration. This is in accordance to our value of 57 % rhizomicrobial respiration on total root-derived CO2.
Rhizodeposition at field scale
The main obstacle to quantify total rhizodeposition is the separation of root-derived CO2 into root and rhizomicrobial respiration. Limitations and advantages of methods used to separate the sources of root-derived CO2 were reviewed earlier (Hanson et al. 2000; Kuzyakov and Larionova 2005; Sapronov and Kuzyakov 2007). Due to methodological difficulties and various assumptions involved in the separation methods, most studies, aiming to quantify the amount of C released from living roots, are focusing on the net rhizodeposition, i.e. on the amount of rhizodeposits that remained in the soil at harvest. In order to compare our data from the 14C labeling experiment with data from the literature we calculated a net rhizodeposition-to-root ratio based on the results of eight studies conducted with maize. CO2 from rhizomicrobial respiration was not included (Table 3). Thus, net rhizodeposition is equal to the portion of 14C measured in the soil at harvest.
The net rhizodeposition-to-root ratio ranged from 0.04 to 0.84 (Table 3), with a mean value of 0.34 and a median of 0.35. In our study the net rhizodeposition-to-root ratio (decomposition to CO2 is not included) was on average 0.29. However, when including the CO2 from RMR the ratio was almost twice as high since about 62 % of released rhizodeposits were decomposed within 16 days (Fig. 2).
The amounts of rhizodeposition and root biomass C are influenced by various biotic and abiotic factors in the plant-soil system (Jones et al. 2004; Amos and Walters 2006). The soil environment can affect rhizodeposition and root biomass through physical aspects (e.g. water availability, temperature, soil texture) and chemical conditions (e.g. pH, availability of nutrient ions), as well as through the activity and diversity of microbial populations (Lynch et al. 2002). Moreover, plant-mediated factors, like the maize variety and the plant phenological stage, are influencing the root biomass and the rhizodeposition. The mentioned factors may alter the rhizodeposition-to-root ratio and thus, may provide the explanation for the variability in the literature data (Table 3). This variability underlines the necessity for future experiments assessing the effects of various factors influencing rhizodeposition and/or root biomass on the robustness of the rhizodeposition-to-root ratio. In our study, however, the same plant species and variety and the same soil as in the field was used, thus, we assumed a constant rhizodeposition-to-root ratio for controlled and field conditions.
By applying the rhizodeposition-to-root ratio of 0.56 to the root biomass determined in the field, we estimated that about 166 kg C ha−1 was released by living roots into the soil in the time from planting to sampling (April to July 2009) for a theoretical plant population of 25,000 plants ha−1. Amos and Walters (2006) concluded in their review, that net belowground C accounts for about 29 ± 13 % of shoot biomass C of maize when assuming similar C contents of roots and shoots. By doing so, we found 34.6 % of the shoot biomass C allocated belowground (roots + rhizodeposition). Root respiration was not included. This value, however, includes RMR and may thus slightly be higher than the reported average.
Conclusion
We showed that the combination of root biomass data from the field with the rhizodeposition-to-root ratio determined under controlled condition allow to quantify rhizodeposition at a field scale. The advantage of the present approach compared to recent estimates is that the portion of rhizodeposits, which are quickly mineralized by microorganisms (rhizomicrobial respiration), is considered. Thus, in contrast to previous studies estimating net rhizodeposition, here the gross rhizodeposition was measured and upscaled to the field. The portion of rhizodeposits decomposed to CO2 within 16 days accounts for about 57 % of total root-derived CO2. Therefore, including RMR led to an improved estimation of the total rhizodeposition under field conditions. Our data showed a total rhizodeposition by maize of 166 ± 53 kg C ha−1. If the rhizodeposition-to-root ratio is known for particular plants, the new approach offers a promising estimation of rhizodeposition at field scale as a huge data base of root biomass distributions already exists in the literature.
References
Amos B, Walters DT (2006) Maize root biomass and net rhizodeposited carbon: an analysis of the literature. Soil Sci Soc Am J 70:1489–1503
Anderson EL (1988) Tillage and N fertilization effects on maize root growth and root:shoot ratio. Plant Soil 108:245–251
Bolinder MA, Angers DA, Giroux M, Laverdiere MR (1999) Estimating C inputs retained as soil organic matter from corn (Zea Mays L.). Plant Soil 215:85–91
Cheng W, Coleman DC, Carroll CR, Hoffman CA (1993) In situ measurement of root respiration and soluble carbon concentrations in the rhizosphere. Soil Biol Biochem 25:1189–1196
Cheng W, Johnson DW, Fu S (2003) Rhizosphere effects on decomposition: controls of plant species, phenology, and fertilization. Soil Sci Soc Am J 67:1418–1427
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72:313–327
Gajri PR, Arora VK, Kumar K (1994) A procedure for determining average root length density in row crops by single-site augering. Plant Soil 160:41–47
Gransee A, Wittenmayer L (2000) Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J Plant Nutr Soil Sc 163:381–385
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Gregory PJ, Atwell BJ (1991) The fate of carbon in pulse-labelled crops of barley and wheat. Plant Soil 136:205–213
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146
Hébert Y, Guingo E, Loudet O (2001) The response of root/shoot partitioning and root morphology to light reduction in maize genotypes. Crop Sci 41:363–371
Helal HM, Sauerbeck D (1986) Effect of plant roots on carbon metabolism of soil microbial biomass. Z Pflanz Bodenkunde 149:181–188
Helal HM, Sauerbeck D (1989) Carbon turnover in the rhizosphere. Z Pflanz Bodenkunde 152:211–216
Hütsch BW, Augustin J, Merbach W (2002) Plant rhizodeposition—an important source for carbon turnover in soils. J Plant Nutr Soil Sc 165:397–407
Johnson JM-F, Allmaras RR, Reicosky DC (2006) Estimating source carbon from crop residues, roots and rhizodeposits using the national grain-yield database. Agron J 98:622–636
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones DL, Kemmitt SJ, Wright D, Cuttle SP, Bol R, Edwards AC (2005) Rapid intrinsic rates of amino acid biodegradation in soils are unaffected by agricultural management strategy. Soil Biol Biochem 37:1267–1275
Keith H, Oades JM, Martin JK (1986) Input of carbon to soil from wheat plants. Soil Biol Biochem 18:445–449
Kisselle KW, Garrett CJ, Fu S, Hendrix PF, Crossley DA Jr, Coleman DC, Potter RL (2001) Budgets for root-derived C and litter-derived C: comparison between conventional tillage and no tillage soils. Soil Biol Biochem 33:1067–1075
Klein DA, Frederick BA, Biondini M, Trlica MJ (1988) Rhizosphere microorganisms effects on soluble amino acids, sugars and organic acids in the root zone of Agropyron cristatum. A. smithii and Bouteloua gracilis. Plant Soil 110:19–25
Kramer S, Marhan S, Ruess L, Armbruster W, Butenschoen O, Haslwimmer H, Kuzyakov Y, Pausch J, Schoene J, Scheunemann N, Schmalwasser A, Totsche KU, Walker F, Scheu S, Kandeler E (2012) Carbon flow into microbial and fungal biomass as a basis to understand the belowground food web in an agroecosystem. Pedobiologia 55:111–119
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sc 165:382–396
Kuzyakov Y (2006) Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38(3):425–448
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33(14):1915–1925
Kuzyakov Y, Cheng W (2004) Photosynthesis controls of CO2 efflux from maize rhizosphere. Plant Soil 263:85–99
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. J Plant Nutr Soil Sc 163:421–431
Kuzyakov Y, Domanski G (2002) Model for rhizodeposition and CO2 efflux from planted soil and its validation by 14C pulse labelling of ryegrass. Plant Soil 239:87–102
Kuzyakov Y, Larionova AA (2005) Root and rhizomicrobial respiration: a review of approaches to estimate respiration of autotrophic and heterotrophic organisms in soil. J Plant Nutr Soil Sc 168(4):503–520
Kuzyakov Y, Kretzschmar A, Stahr K (1999) Contribution of Lolium perenne rhizodeposition to carbon turnover of pasture soil. Plant Soil 213:127–136
Kuzyakov Y, Ehrensberger H, Stahr K (2001) Carbon partitioning and below-ground translocation by Lolium perenne. Soil Biol Biochem 33:61–74
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305
Livesley SJ, Stancey CL, Gregory PJ, Buresh RJ (1999) Sieve size effects on root length and biomass measurements of maize (Zea mays) and Grevillea robusta. Plant Soil 207:183–193
Lynch JM, Brimecombe M, De Leij FAAM (2002) Rhizosphere. In: eLS. Wiley: Chichester. doi:10.1002/9780470015902.a0000403.pub2
Martens R (1990) Contribution of rhizodeposits to the maintenance and growth of soil microbial biomass. Soil Biol Biochem 22:141–147
Merckx R, van Ginkel JH, Sinnaeve J, Cremers A (1986) Plant-induced changes in the rhizosphere of maize and wheat. Plant Soil 96:85–93
Nguyen C (2003) Rhizodeposition of organic C by plant: mechanisms and controls. Agronomie 23:375–396
Nguyen C, Todorovic C, Robin C, Christophe A, Guckert A (1999) Continuous monitoring of rhizosphere respiration after labelling of plant shoots with 14CO2. Plant Soil 212:191–201
Palta JA, Gregory PJ (1997) Drought affects the fluxes of carbon to roots and soil in 13C pulse-labelled plants of wheat. Soil Biol Biochem 29(9/10):1395–1403
Sapronov DV, Kuzyakov Y (2007) Separation of root and microbial respiration: comparison of three methods. Eurasian Soil Sci 40(7):775–784
Smucker AJM, McBurney SL, Srivastava AK (1982) Quantitative separation of roots from compacted soil profiles by the Hydropneumatic Elutriation System. Agron J 74:500–503
Todorovic C, Nguyen C, Robi C, Guckert A (2001) Root and microbial involvement in the kinetics of 14C-partitioning to rhizosphere respiration after a pulse labelling of maize assimilates. Plant Soil 228:179–189
Tubeileh A, Groleau-Renaud V, Plantureux S, Guckert A (2003) Effect of soil compaction on photosynthesis and carbon partitioning within a maize-soil system. Soil Till Res 71:151–161
Van der Krift T, Kuikman PJ, Möller F, Berendse F (2001) Plant species and nutritional-mediated control over rhizodeposition and root decomposition. Plant Soil 228:191–200
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(9):703–707
Vancura V (1964) Root exudates of plants. I. Analysis of root exudates of barley and wheat in their initial phases of growth. Plant Soil 21(2):231–248
Warembourg FR, Billes G (1979) Estimating carbon transfers in the plant rhizosphere. In: Harley JL, Russel RS (eds) The soil–root interface. Academic, London, pp 183–196
Werth M, Kuzyakov Y (2008) Root-derived carbon in soil respiration and microbial biomass determination by 14C and 13C. Soil Biol Biochem 40:625–637
Wu J, Jörgensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass-C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Acknowledgments
We would like to thank Michaela Dippold for suggestions on the earlier version of the manuscript. This study was supported by the German Research Foundation (DFG) within the Research Unit “Carbon Flow in Belowground Food Webs assessed by Isotope Tracers”.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pausch, J., Tian, J., Riederer, M. et al. Estimation of rhizodeposition at field scale: upscaling of a 14C labeling study. Plant Soil 364, 273–285 (2013). https://doi.org/10.1007/s11104-012-1363-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1363-8