Abstract
Aims
The study aims (1) to evaluate the effect of Mesorhizobium tianshanense on plant proline and polyamine levels of Lotus tenuis and its modulatory effect during plant response to short-term salt stress and (2) to compare these effects with those caused by mycorrhizal symbiosis.
Methods
Experiments consisted of a randomized factorial design of two factors: salinity (two levels, 0 and 150 mM NaCl) and symbiosis (three levels, uninoculated, Glomus intraradices, and M. tianshanense).
Results
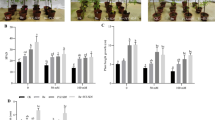
Salinization led to increased proline levels regardless of plant organ and symbiotic status, excepting mycorrhizal L. tenuis roots. Salinity diminished the total polyamine level of control and rhizobial plants but not in mycorrhizal ones. Variations in the pattern response of the three individual polyamines (putrescine, spermidine, and spermine) differed in accordance with the symbiotic status of the plant, highlighting a divergence on proline and polyamine metabolisms between rhizobial and mycorrhizal symbiosis.
Conclusions
Spermidine and spermine contributed the most with the salt-induced root polyamine increment observed upon salinization in roots of nodulated plants, suggesting that these polyamines might mediate an adaptive role of the plant–M. tianshanense symbiosis in L. tenuis plants growing in a saline environment.





Similar content being viewed by others
Abbreviations
- DAO:
-
Diamine oxidase
- PA:
-
Polyamines
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
References
Allan GJ, Francisco-Ortega J, Santos-Guerra A, Boerner E, Zimmer EA (2004) Molecular phylogenetic evidence for the geographic origin and classification of Canary Island Lotus (Fabaceae: Loteae). Mol Phylogenet Evol 32:123–138
Aziz A, Martin-Tanguy J, Larher F (1998) Stress-induced changes in polyamine and tyramine levels can regulate proline accumulation in tomato leaf discs treated with sodium chloride. Physiol Plantarum 104:195–202
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Campestre MP, Bordenave CD, Origone AC, Menéndez AB, Ruiz OA, Rodríguez AA, Maiale SJ (2011) Polyamine catabolism is involved in response to salt stress in soybean hypocotyls. J Plant Physiol 168:1234–1240
Chattopadhyay MK, Tabor CW, Tabor H (2002) Absolute requirement of spermidine for growth and cell cycle progression of fission yeast (Schizosaccharomyces pombe). P Natl Acad Sci 99:10330–10334
Cohen SS (1998) A guide to the polyamines. Oxford University Press, New York
Colmer TD, Epstein E, Dvorak J (1995) Differential solute regulation in leaf blades of various ages in salt-sensitive wheat and a salt-tolerant wheat × Lophopyrum elongatum (host) A. Löve amphiploid. Plant Physiol 108:1715–1724
da Rocha IMA, Vitorello VA, Silva JS, Ferreira-Silva SL, Viégas RA, Silva EN, Silveira JAG (2012) Exogenous ornithine is an effective precursor and the δ-ornithine amino transferase pathway contributes to proline accumulation under high N recycling in salt-stressed cashew leaves. J Plant Physiol 169:41–49
Diouf D, Duponnois R, Ba AT, Neyra M, Lesueur D (2005) Symbiosis of Acacia auriculiformis and Acacia mangium with mycorrhizal fungi and Bradyrhizobium spp. improves salt tolerance in greenhouse conditions. Aust J Plant Physiol 32:1143–1152
Erdei L, Szegletes Z, Barabas K, Pestenacz A (1996) Responses in polyamine titer under osmotic and salt stress on sorghum and maize seedlings. J Plant Physiol 147:599–603
Estrella MJ, Muñoz S, Soto MJ, Ruiz O, Sanjuan J (2009) Genetic diversity and host range of rhizobia nodulating Lotus tenuis in typical soils of the Salado River Basin (Argentina). Appl Environ Microbiol 75:1088–1098
Federico R, Angelini R (1991) Polyamine catabolism in plants. In: Slocum RD, Flores HE (eds.) Biochemistry and physiology of polyamines in plants. Boca Raton, FL,CRC Press, pp 41-56
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Angel Torres M, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Fujihara S (2009) Biogenic amines in rhizobia and legume root nodules. Microbes Environ 24:1–13
Gaspar T, Kevers C, Hausman J-F, Faivre-Rampant O, Boyer N, Dommes J, Penel C, Greppin H (2000) Integrating phytohormone metabolism and action with primary biochemical pathways. I. Interrelationships between auxins, cytokinins, ethylene and polyamines in growth and development processes. In: Greppin H, Penel C, Broughton WJ, Strasser R (eds) Integrated plant systems. University of Geneva, Switzerland, pp 163–191
Ghosh N, Adak MK, Ghosh PD, Gupta S, Sen Gupta DN, Mandal C (2011) Differential responses of two rice varieties to salt stress. Plant Biotechnol Rep 5:89–103
Goicoechea N, Szalai NG, Antolín MC, Sánchez-Díaz M, Paldi E (1998) Influence of arbuscular mycorrhizae and Rhizobium on free polyamines and proline levels in water-stressed alfalfa. J Plant Physiol 153:706–711
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Phys 51:463–499
Hennion F, Frenot Y, Martin-Tanguy J (2006) High flexibility in growth and polyamine composition of the crucifer Pringlea antiscorbutica in relation to environmental conditions. Physiol Plant 127:212–224
Hoagland DR, Arnon DL (1950) The water culture method for growing plants without soil. Calif Agricult Exp Stat Circ 374:1–39
Jiménez-Bremont JF, Ruiz OA, Rodríguez-Kessler M (2007) Modulation of spermidine and spermine levels in maize seedlings subjected to long-term salt stress. Plant Physiol Bioch 45:812–821
Jiménez-Zurdo JI, García-Rodríguez FM, Toro N (1997) The Rhizobium meliloti putA gene: its role in the establishment of the symbiotic interaction with alfalfa. Mol Microbiol 23:85–93
Jindal V, Atwala A, Sekhon BS, Singh R (1993) Effect of vesicular-arbuscular mycorrhizae on metabolism of moong plants under NaCl salinity. Plant Physiol Bioch 31:475–481
King ND, Hojnacki D, O'Brian MR (2000) The Bradyrhizobium japonicum proline biosynthesis gene proC is essential for symbiosis. Appl Environ Microbiol 66:5469–5471
Kirkbride JH Jr (2006) The scientific name of narrow-leaf trefoil. Crop Sci 46:2169–2170
Kohl DH, Schubert KR, Carter MB, Hagedorn CH, Shearer G (1988) Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. P Natl Acad Sci 85:2036–2040
Kytöviita M-M, Sarjala T (1997) Effects of defoliation and symbiosis on polyamine levels in pine and birch. Mycorrhiza 7:107–111
Legocka J, Kluk A (2005) Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus seedlings. J Plant Physiol 162:662–668
Liptay A, Davidson D (1971) Coleoptile growth: variation in elongation patterns of individual coleoptiles. Ann Bot 35:91–1002
Maiale S, Sanchez D, Guirado A, Vidal A, Ruiz O (2004) Spermine accumulation under salt stress. J Plant Physiol 161:35–42
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Marcé M, Brown DS, Capell T, Figueras X, Tiburcio AF (1995) Rapid high-performance liquid chromatographic method for the quantitation of polyamines as their dansyl derivatives: application to plant and animal tissues. J Chromatogr B Biomed Appl 666:329–335
Márquez AJ, Betti M, García-Calderón M, Pal'ove-Balang P, Díaz P, Monza J (2005) Nitrate assimilation in Lotus japonicus. J Exp Bot 56:1741–1749
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Pérez-Amador MA, Carbonell J, Granell A (1995) Expression of arginine decarboxylase is induced during early fruit development and in young tissues of Pisum sativum (L.). Plant Mol Biol 28:997–1009
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. T Brit Mycol Soc 55:158–161
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4:210–220
Radyukina NL, Mapelli S, Ivanov YV, Kartashov AV, Brambilla I, Kuznetsov VV (2009) Homeostasis of polyamines and antioxidant systems in roots and leaves of Plantago major under salt stress. Russ J Plant Physl 56:323–331
Rodríguez AA, Maiale SJ, Menéndez AB, Ruiz OA (2009) Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J Exp Bot 60:4249–4262
Sannazzaro A, Ruiz O, Albertó E, Menéndez A (2004) Presence of different arbuscular mycorrhizal infection patterns in roots of Lotus glaber plants growing in the Salado River basin. Mycorrhiza 14:139–142
Sannazzaro AI, Echeverria M, Albertó EO, Ruiz OA, Menéndez AB (2007) Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol Bioch 45:39–46
Sannazzaro A, Bergottini V, Paz R, Castagno L, Menéndez A, Ruiz O, Pieckenstain F, Estrella M (2011) Comparative symbiotic performance of native rhizobia of the Flooding Pampa and strains currently used for inoculating Lotus tenuis; in this region. Anton Leeuw Int J G 99:371–379
Santa-Cruz A, Acosta M, Rus A, Bolarin MC (1999) Short-term salt tolerance mechanisms in differentially salt tolerant tomato species. Plant Physiol Bioch 37:65–71
Schmidt G, Zotz G (2001) Ecophysiological consequences of differences in plant size: in situ carbon gain and water relations of the epiphytic bromeliad, Vriesea anguinolenta. Plant Cell Environ 24:101–112
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin and abscisic acid. Plant Physiol 125:1591–1602
Shamseldin A, Nyalwidhe J, Werner D (2006) A proteomic approach towards the analysis of salt tolerance in Rhizobium etli and Sinorhizobium meliloti strains. Curr Microbiol 52:333–339
Shaul-Keinan O, Gadkar V, Ginzberg I, Grünzweig JM, Chet I, Elad Y, Wininger S, Belausov E, Eshed Y, Atzmon N, Ben-Tal Y, Kapulnik Y (2002) Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol 154:501–507
Smith TA (1985) Polyamines. Ann Rev Plant Physio 36:117–143
Soliman ASh, Shanan NT, Massoud ON, Swelim DM (2012) Improving salinity tolerance of Acacia saligna (Labill.) plant by arbuscular mycorrhizal fungi and Rhizobium inoculation. Afr J Biotechnol 11:1259–1266
Sotiropoulos TE, Therios IN, Tsirakoglou V, Dimassi KN (2007) Response of the quince genotypes BA 29 and EMA used as pear rootstocks to boron and salinity. Int J Fruit Sci 6:93–101
Stetsenko LA, Rakitin VY, Shevyakova NI, Kuznetsov VV (2009) Organ-specific changes in the content of free and conjugated polyamines in Mesembryanthemum crystallinum plants under salinity. Russ J Plant Physl+ 56:808–813
Stewart GR, Larher F (1980) Accumulation of amino acids and related compounds in relation to environmental stress. In: Miflin BJ (ed) The biochemistry of plants, Vol 5. Academic Press, New York, pp 609–635
Su G, Bai X (2008) Contribution of putrescine degradation to proline accumulation in soybean leaves under salinity. Biol Plantarum 52:796–799
Tal M, Katz A, Heikin H, Dehan K (1979) Salt tolerande in the wild relatives of the cultivated tomato: porline accumulation in Lycopersicon esculentum Mill., L. peruvianum Mill. and Solanum pennelli Cor. treated with NaCI and polyethylene glycol. New Phytol 82:349–355
Theiss C, Bohley P, Voigt J (2002) Regulation by polyamines of ornithine decarboxylase activity and cell division in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol 128:1470–1479
Tonon G, Kevers C, Faivre-Rampant O, Graziani M, Gaspar T (2004) Effect of NaCl and mannitol iso-osmotic stresses on proline and free polyamine levels in embryogenic Fraxinus angustifolia callus. J Plant Physiol 161:701–708
Trotel P, Bouchereau A, Niogret MF, Larher F (1996) The fate of osmo-accumulated proline in leaf discs of rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci 118:31–45
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)—differential response in salt-tolerant and sensitive varieties. Plant Sci 165:1411–1418
Wang K, Liu Y, Dong K, Dong J, Kang J, Yang Q, Zhou H, Sun Y (2011) The effect of NaCl on proline metabolism in Saussurea amara seedlings. Afr J Biotechnol 10:2886–2893
Zhang J, Zhang Y, Du Y, Chen S, Tang H (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10:1904–1914
Zhao F-G, Sun C, Liu Y-L (2001) Ornithine pathway in proline biosynthesis activated by salt stress in barley seedlings. Acta Bot Sin 43:36–40
Zotz G (1997) Photosynthetic capacity increases with plant size. Bot Acta 110:306–308
Zotz G (2000) Size dependence in the reproductive allocation of Dimerandra emarginata, an epiphytic orchid. Ecotropica 6:95–99
Zotz G, Ziegler H (1999) Size-related differences in carbon isotope discrimination in the epiphytic orchid, Dimerandra emarginata. Naturwissenschaften 86:39–40
Zotz G, Thomas V, Hartung W (2001) Ecophysiological consequences of differences in plant size: abscisic acid relationships in the epiphytic orchid Dimerandra emarginata. Oecologia 129:179–185
Acknowledgments
This work was supported by: Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (PICT 20517), UBACYT x143. M.E is CONICET scholarship holder. A.B.M. is a Universidad de Buenos Aires (UBA) and CONICET researcher, and O.A.R. and A.I.S are CONICET researchers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Rights and permissions
About this article
Cite this article
Echeverria, M., Sannazzaro, A.I., Ruiz, O.A. et al. Modulatory effects of Mesorhizobium tianshanense and Glomus intraradices on plant proline and polyamine levels during early plant response of Lotus tenuis to salinity. Plant Soil 364, 69–79 (2013). https://doi.org/10.1007/s11104-012-1312-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1312-6