Abstract
To test whether shifts in nutrient availability from calcareous to mineral-poor habitats could be a driving force in the evolution of seven closely related wetland brown mosses, we measured soil and vascular plant nutrients and conducted a laboratory incubation experiment with Swedish and some Dutch samples, in which net N and P-mineralization, respiration and microbial characteristics were measured. In spite of high respiration and microbial N, net N-mineralization appeared to be low for the calcareous Palustriella falcata and Scorpidium spp. Net N-mineralization significantly increased (and respiration and microbial N decreased) for the mineral-poor Sarmentypnum exannulatum, Straminergon stramineum and Warnstorfia fluitans, probably due to a decrease in microbial N-demand. Even though values were mainly negative, net P-mineralization showed a similar increase from calcareous to mineral-poor fens, probably due to lower precipitation of calcium phosphate. The calcareous habitat of the early wetland mosses may thus have been nutrient-poor instead of nutrient-rich. Adaptation to mineral-poor habitats, probably driven by expansion of mineral-poor wetlands when the boreal zone became colder and wetter, may have been associated with higher availability of ammonium and phosphate. However, this may have stimulated Sphagnum more than brown mosses, which may have been restricted to particular niches with perhaps some nitrification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In peatlands, mineral-richness is one of the most distinguishing ecological factors (Sjörs 1950; Bridgham et al. 1998; Bayley et al. 2005). Species composition of bryophyte layer and (lignified) vascular plants is basically determined by mineral status and pH. Also, major peatland types, such as bogs and rich, intermediate and poor fens are distinguished by mineral status. Mineral status may also affect nutrient availability. Mineral-rich wetlands not only have higher pH and base cation levels than mineral-poor wetlands, but are also generally considered to be more nutrient-rich, because litter decay and nutrient turnover are faster. While there are indeed reports to support this (Bayley et al. 2005), there are however also many studies which actually showed higher net N-mineralization in mineral-poor instead of mineral-rich wetlands (Verhoeven et al. 1988; 1990; Scheffer et al. 2001; Paulissen et al. 2004). Also, Bridgham et al. (1998) found a rapid turnover of the nutrient pools in ombrotrophic sites, despite low total soil N and P. However, many studies contrasted mineral-rich fens with Sphagnum-peatlands, which not only differ in mineral status, but at least in the bryophyte layer also in litter quality, which may be an important factor to decomposition and mineralization (Aerts et al. 1999). In Sphagnum-dominated sites, with very recalcitrant litter (Clymo and Hayward 1982; Cornelissen et al. 2007), litter quality could have been so low that C was a limiting factor to decomposition instead of N, and N could be net released instead of immobilized.
More subtle changes in nutrient availability could perhaps be studied over habitat gradients of brown mosses, another important group of wetland bryophytes, with genera such as Palustriella, Scorpidium and Warnstorfia. This group contains closely related species of families such as the Amblystegiaceae and Calliergonaceae (Hedenäs 1993, 2003, 2006; Hedenäs and Vanderpoorten 2007), and spans a habitat gradient from calcareous fens to mineral-poor, acidic bog pools. Mineral-rich wetlands have probably been the ancestral habitat, with genera such as Palustriella and Scorpidium (Hedenäs and Kooijman 1996, 2004), but more derived species have adapted to mineral-poor conditions with time. The species are generally good indicators of water chemistry, because they have no roots, only thin leaves without a cuticula, and live in direct contact with the surrounding water. Brown mosses thus offer the opportunity to test whether net mineralization of N and P would decrease or increase from mineral-rich to mineral-poor wetlands, without major changes in litter quality due to a shift to Sphagnum. Such a study would also help to evaluate whether habitat adaptation from mineral-rich to mineral-poor fens could have been affected by changes in nutrient availability.
The objective of this paper was to assess differences in nutrient availability in habitats of related wetland bryophytes over a gradient from calcareous to mineral-poor fens, and to evaluate whether changes in nutrient availability could have been a factor in habitat adaptation and bryophyte evolution. We chose seven relatively common species from the rich-fen genera Palustriella and Scorpidium, and poor-fen Sarmentypnum, Straminergon and Warnstorfia. We collected most samples in Central Sweden, which is relatively undisturbed, but to put the results in a wider perspective, we also included some samples from more polluted habitats in the Netherlands. We measured soil and vascular plant nutrients, and studied net mineralization of N and P and their underlying microbial characteristics in a laboratory incubation experiment.
Methods
Field survey
We sampled 18 wetland sites in central Sweden, in the provinces Västmanland, Närke and Västergötland. We focussed on seven relatively common moss species of different habitats, representative of different groups within the Amblystegiaceae and Calliergonaceae (Hedenäs and Vanderpoorten 2007; Hedenäs et al. 2005): Palustriella falcata (Brid.) Hedenäs, Scorpidium cossoni (Schimp.) Hedenäs, S. scorpioides (Hedw.) Limpr. and S. revolvens (Sw. ex Anonymo) Rubers, Sarmentypnum exannulatum (Schimp.) Hedenäs, Straminergon stramineum (Brid.) Hedenäs and Warnstorfia fluitans (Hedw.) Loeske (Fig. 1). The ancestral Amblystegiaceae and Calliergonaceae species P. falcata and Scorpidium spp., respectively, are characteristic for more calcareous wetlands, while the more derived Calliergonaceae species, S. exannulatum, S. stramineum and W. fluitans occur under mineral-poor conditions (Hedenäs and Vanderpoorten 2007; Hedenäs et al. 2005). To put differences between brown moss species in a wider perspective, we also collected some samples in the Netherlands (NL), for the rich-fen S. scorpioides, which is still relatively common in Northwest-Overijssel, and the poor-fen W. fluitans, which is often found in shallow, acidified lakes in the central part of the country.
In both Sweden and NL, samples were collected in August. In principle, five different localities were selected for each of the seven species. We could however find only four sites for P. falcata. For three of the seven species in Sweden (S. cossoni, S. stramineum and W. fluitans), we had selected one relatively dry site with water tables of 40 cm instead of a few cm below the moss surface, and moisture content around 60% instead of 90%. After analysis, these samples appeared to have much higher mineralization values than all others, probably due to better aeration (Grootjans 1985), and were discarded for net mineralization and variables related to this. In each site, species composition of the vegetation in the general area was recorded. Plots of 30 × 30 cm with dominance of the target brown moss species were randomly selected, height of the water level was recorded to the nearest half centimeter and cover values of bryophyte species were estimated as (1) single shoots, (2) 1–25% cover, (3) 25–50%, (4) 50–75% or (5) 75–100% cover. Aboveground biomass of the vascular (seed) plants was cut at the ground level. Soil samples were collected just below the living moss layer, in metal rings of 5 cm depth and known volume. Water samples were not collected, but for all brown moss species, soil pH was more or less similar to pH values previously measured in water (Hedenäs and Kooijman 1996, 2004). Biomass samples were air dried, and soil samples kept cool until further analysis.
Laboratory analysis
Fresh weight and gravimetric moisture content of soil samples were determined, and dry weight and bulk density calculated. pH-KCL values were determined in water, using a 1:10 weight:volume ratio. Aboveground biomass samples were dried (48 h at 70°C) and dry weight determined. After drying and grinding of biomass and soil subsamples, C and N contents were determined with a CNS analyzer (Westerman 1990). In addition, soil and biomass samples were digested in the microwave with HNO3, and P, K, Ca, Mg, S, Al and Fe content measured ICP (Westerman 1990). Vascular plant N:P, K:P and N:K ratios were used as indicators for which of the three nutrients may limit plant growth (Koerselman and Meuleman 1996; Olde Venterink et al. 2003). N:P ratios around 15 indicate balanced conditions, values around 10 suggest N-limitation, and values around 20 indicate that P is the limiting factor. K:P ratios indicated P-limitation above 3.4, and K-limitation below 3.4. N:K ratios indicated N-limitation below 2.1, and K-limitation above 2.1. To assess whether decrease in pH from mineral-rich to mineral-poor fens could have been affected by oxidation of S and Fe, rather than decrease of base cations, S/(Ca+Mg) and Fe/(Ca+Mg) ratios were calculated (Lucassen et al. 2002). However, Fe/(Ca+Mg) or S/(Ca+Mg) ratios were generally below 1 even in mineral-poor wetlands, which suggests that potential acidification by oxidation of Fe or S was not important.
Incubation experiment
Net N and P-mineralization were studied in a laboratory incubation experiment. To simulate the permanently wet and oxygen-poor natural environment as much as possible, fresh soil samples were put into polyethylene 100 ml bottles, with small perforations to allow some gas exchange (Verhoeven et al. 1988). Soil moisture content was high, with 75% of the fresh weight for P. falcata and a gradual increase to 95% for W. fluitans. The bottles were stored in slightly open plastic bags with moist paper, at 20°C in the dark for two months. Ammonium, nitrate and phosphate concentrations of fresh and incubated samples were determined via extraction with 50 ml 0.5 M K2SO4 solution, using the equivalent of 1.5 g dry material, and measured on a continuous-flow analyzer (Westerman 1990). Net N and P-mineralization were calculated from differences in total inorganic N and P between incubated and fresh samples.
Microbial N and P were measured with chloroform fumigation and extraction (Brooks et al. 1985). Fumigated samples were flushed for 24 h with chloroform and extracted with 0.5 M K2SO4 immediately afterwards, to prevent microbial regrowth. In addition to ammonium, nitrate and phosphate, DON was measured in fumigated and non-fumigated samples, using a continuous-flow analyzer. Microbial N and P concentrations were calculated as differences between fumigated and non-fumigated samples. Microbial N and P did generally not differ between fresh and incubated samples, and average values were used for statistical analysis. Two samples (S. revolvens and S. exannulatum) had undetectable values for microbial P, and could thus not be used for microbial N:P ratios and P-mineralization per unit microbial P.
Respiration was measured at the start and end of the incubation experiments. Fresh or incubated samples were placed in open glass jars during one night, with the equivalent of 5 g dry weight. During measurements, the jars were closed and air samples extracted by needle. CO2-concentrations were measured three times by injecting air samples into a Carlo Erba Varian gas chromotograph (Tietema 1992). CO2-production rates were calculated from the increase in CO2-concentration during the day, volume of the head space and sample dry weight.
Statistical analysis
Because data of S. scorpioides and W. fluitans were used in the tests between the seven brownoss habitats in Sweden, but also for a comparison between Sweden and NL, we applied Bonferoni corrections, and considered effects, differences and correlations to be significant if p < 0.025, rather than 0.05. Differences between the seven brown moss species in Sweden with respect to soil, microbial and vascular plant characteristics were tested with one-way ANOVA and least square means tests (Cody and Smith 1987). The relationship between net N and P-mineralization and pH was further tested with linear regression analysis. Relationships between vascular plant nutrient contents and environmental factors were analyzed with linear regression as well. The relationship between aboveground vascular plant biomass and pH was tested with second order regression analysis. Differences between S. scorpioides and W. fluitans in Sweden and NL were tested with two-way ANOVA with species and country as independent factors, and least square means tests.
Results
Habitat differences between brown moss species
The seven moss species clearly differed in pH (Table 1). Values decreased from above 7 for some of the more ancestral P. falcata and Scorpidium spp. of mineral-rich fens, to below 5 for the more derived species S. exannulatum, S. stramineum and W. fluitans of mineral-poor fens. Soil Ca-content decreased accordingly, from 4.4 kg m−2 in the upper 10 cm of calcareous fens with calcite precipitation, to 8 g m−2 in the most acid wetlands.
Bulk density gradually decreased from 0.28 to 0.04 g cm−3 from mineral-rich to mineral-poor fens, and C:N ratio increased from 17 to 48. High bulk density in calcareous fens partly reflected calcite precipitation, but the general decrease towards mineral-poor fens suggested a decrease in decomposition as well. Soil N showed a gradual decrease from 171–248 g m−2 in calcareous to 57 g m−2 in the most acid fens. Soil P decreased from 14–16 g m−2 in calcareous to 2.8 g m−2 in the most acid fens, and soil K from 55–79 to 2 g m−2.
Microbial characteristics and net mineralization of N and P
Like pH and soil nutrients, microbial characteristics differed between the brown moss habitats (Table 2). In accord with expectations, microbial N decreased from mineral-rich to mineral-poor fens. However, microbial P did not change over this gradient. As a result, microbial N:P ratio was high in mineral-rich fens, with values amounting to 41, but significantly decreased to 3 under more acid conditions. In accord with expectations, respiration was significantly higher in the more calcareous fens, and decreased towards mineral-poor wetlands.
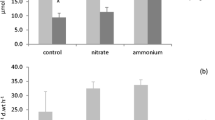
However, patterns in net mineralization of N were different than expected. In fact, despite high microbial and soil N, net mineralization of N was close to zero in calcareous fens, but rapidly increased below pH 6 (Fig. 2). Brown moss habitats also clearly differed in effiency of mineralization per unit microbe (Fig. 3). N-mineralization per unit microbe was close to zero for the mineral-rich P. falcata and Scorpidium spp., but increased to 50 mg g−1 day−1 for the mineral-poor fen species S. exannulatum, S. stramineum and W. fluitans. The increase in net N-mineralization in mineral-poor fens was mainly due to ammonium, but the amount of nitrate released over the incubation period did not differ between species habitats (Table 2). As expected, percentage of nitrification decreased from 46% in calcareous to 4% in the most acid fens, but this was counteracted by the increase in net N-mineralization over this gradient.
Net mineralization per unit microbe for seven related brown moss species with different wetland habitat. a Net N-mineralization per unit microbial N. b net P-mineralization per unit microbial P. Pf = Palustriella falcata, Sc = Scorpidium cossoni, Ss = S. scorpioides, Sr = S. revolvens, Se = Sarmentypnum exannulatum, Sts = Straminergon stramineum and Wf = Warnstorfia fluitans. Mean values (n = 3–5) and standard deviations. Different letters indicate significant differences between species (p < 0.025)
Like N, net mineralization of P also showed an increase from calcareous to acid fens, instead of the decrease expected from lower respiration and soil P (Fig. 2). In calcareous fens, net P-mineralization was strongly negative, but values increased to approximately zero below pH 6. Efficiency of P-mineralization per unit microbe showed a similar pattern, with negative values for the rich-fen species, and almost positive values for poor-fen bryophytes (Fig. 3).
Vascular plants as indicator of nutrient richness
The above patterns in soil nutrients and mineralization were partly reflected in vascular plants. In accord with the gradient in pH, plant Ca-contents clearly decreased from mineral-rich to poor fens (Table 3). Plant Ca-contents were also positively correlated with pH and Ca in the soil (R 2 = 0.54 and 0.44 respectively). Also, as expected, plant Al-contents significantly increased from high to low pH, although patterns were slightly obscured. Plant K-contents significantly decreased from mineral-rich to poor wetlands as well, and were positively correlated with soil K (R 2 = 0.41), even if only slightly with pH (R 2 = 0.15).
Relationships with pH and soil nutrients were more complex for aboveground vascular plant biomass and plant N and P. Plant N and P-contents were significantly higher in the most calcareous fens than in other fen types (Table 3), but did not further correlate with pH or soil N and P. Aboveground biomass even showed an optimum curve over the habitat gradient, with 42 g m−2 in the most calcareous fens, a peak of 207 g m−2 at intermediate pH, and a decrease to 43 g m−2 in the most acid fens (Fig. 4). The total amount of N and P in aboveground vascular plants showed more or less similar distributions, with low values in the most extreme fen types, and a peak around pH 5.8. The increase in biomass and plant nutrients from calcareous to intermediate fens thus corresponded with the increase in net mineralization of N and P, or efficiency of mineralization per unit microbe. However, the subsequent decrease from intermediate to poor fens could not be explained by net mineralization, which only further increased.
Relationship between aboveground vascular biomass and pH in wetland habitats of seven related brown moss species (R 2 = 0.70). Pf = Palustriella falcata, Sc = Scorpidium cossoni, Ss = S. scorpioides, Sr = S. revolvens, Se = Sarmentypnum exannulatum, Sts = Straminergon stramineum and Wf = Warnstorfia fluitans. Values are mean values for each bryophyte species, each based on n = 5
Plant N:P ratios did not show a consistent change along the habitat gradient either. Values ranged from 20 to 26, independent of pH, which suggested that P was the main limiting factor in all fens. This was supported by K:P ratios of 11–16, which were clearly above the critical limit of 3.4. Plant N:K ratios did not show consistent differences between species either. However, values of 1.2–2.0 further supported that, compared to N and P, K was not a limiting factor in any of the fens, despite low K-contents in some of them. The only ratio clearly changing across the habitat gradient was plant N:Ca, which was around 2 in calcareous fens and increased to 5.5 in the most acid habitat, and significantly correlated with pH (R 2 = 0.34).
Comparison between Sweden and the Netherlands (NL)
To put the above picture in a wider perspective, we compared central Swedish and Dutch habitats of the rich- and poor-fen species S. scorpioides and W. fluitans, which both occur in NL. Differences between species were consistent in both countries for pH, vascular plant Ca-content, microbial N, net N-mineralization and efficiency of N-mineralization (Table 4). The rich-fen S. scorpioides had significantly higher pH, plant Ca-content and microbial N than the poor-fen W. fluitans, but lower net N-mineralization and efficiency of N-mineralization.
However, there were differences between the two countries as well. Plant K-contents and net P-mineralization were higher in NL than in Sweden for both species, and net P-mineralization showed clearly positive values instead of negative or close to zero. Soil P and K also showed a significant country effect, especially for W. fluitans, with higher values in NL. Other variables related to P-dynamics differed between country and species. For S. scorpioides, vascular plant P-content was low in both Sweden and NL, and plant N:P ratios still indicating P-limitation, despite higher microbial P in NL. Warnstorfia fluitans, however, showed clearly higher vascular plant P-content in NL, and lower plant and soil N:P ratios. Plant N:P ratio was even so low that it suggested excess of P compared to N, and N-limitation instead of the P-limitation found in all other situations. Efficiency of P-mineralization per unit microbe was higher in NL than in Sweden for both brown moss species, but in both countries also higher for W. fluitans than for S. scorpioides.
Discussion
Low instead of high nutrient availability in calcareous fens
As expected from the general decrease in pH (Aerts and Chapin 2000; Schimel and Bennett 2004; Bayley et al. 2005), microbial N and respiration decreased from mineral-rich to poor fens. However, despite this decrease in biological activity, net mineralization of N and P increased from high to low pH, not only for central Sweden, but also for Dutch samples. Efficiency of mineralization per unit microbe increased from mineral-rich to poor fens as well. Even though based on laboratory incubation experiments, high net mineralization of N and P in mineral-poor fens is probably no artefact. Although Bayley et al. (2005) reported that decomposition rates were significantly correlated with the mean daily net N mineralization rate, many studies actually measured high net mineralization in acid peatlands, and low values under mineral-rich conditions (Verhoeven et al. 1988, 1990; Scheffer et al. 2001; Paulissen et al. 2004). Also, Bridgham et al. (1998) found a rapid turnover of the nutrient pools in ombrotrophic sites, despite low total soil N and P. High instead of low net mineralization under acid conditions has also been reported for field and laboratory studies in forests (Zöttle 1960; Davy and Taylor 1974; Kooijman et al. 2008) and coastal dune grasslands (Kooijman and Besse 2002).
In terrestrial soils, increase in net N-mineralization from high to low pH seemed associated with a shift in microbial community (Kooijman et al. 2008, 2009). Bacteria usually predominate at high pH, and fungi under more acid conditions (Bååth and Anderson 2003). In wetlands, heterotrophic bacteria and fungi are dominant groups of micro-organisms as well, and even though microfungi may be abundant in fens (Thormann et al. 2004), fungi may be more important in bogs (Tsuneda et al. 2001; Thormann 2006). Bacteria have generally lower C:N ratio and higher N-requirements than fungi (Hassink et al. 1993; Moore et al. 2005), possibly due to a more rapid life style and osmoregulation with amino acids, rather than carbohydrates (Measures 1975; Kuehn et al. 1998). In the present study, we did not measure bacteria and fungi, but lower effiency of N-mineralization per unit microbe in mineral-rich fens at least supported higher microbial N-demand than in poor fens. In mineral-poor fens, microbial N and respiration were low, and biological activity and gross N-mineralization probably reduced. In that case, high net N-mineralization may only be explained by low microbial N-demand, which was supported by the high efficiency of N-mineralization per unit microbe.
The increase in net P-mineralization from mineral-rich to poor fens seems in accord with the generally lower P-availability in calcareous fens (e.g., Boyer and Wheeler 1989; Rozbrojova and Hajek 2008). Similar to N, higher P-availability may also be explained by high microbial demand. Richardson and Marshall (1986) showed that most of the P added to a fen ecosystem was rapidly removed from the water column by microorganisms. However, in contrast to N, chemical processes are important as well. In calcareous fens, P may be fixed in calcium phosphate (Lindsay and Moreno 1966; Boyer and Wheeler 1989; Reddy and DeLaune 2008), which reduces P-availability to the vegetation. In mineral-poor fens, calcium phosphates are highly soluble, but P-fixation in iron phosphates may play a role (Lamers et al. 1998, Reddy and DeLaune 2008). However, iron contents and Fe/(Ca+Mg) ratios were low, and Fe:P weight ratios generally below 15, which suggests that P-fixation in iron phosphate is limited (Jensen et al. 1992). However, even if P-availability increased from mineral-rich to poor fens, net P-mineralization was still very low, and plant N:P ratios still above 20, which supports that P is generally the most limiting factor in peatlands (Gore 1961; Olde Venterink et al. 2003).
Relationships with plant and soil nutrients
The increase in net mineralization of N and P from mineral-rich to poor fens may seem contradictory with the decrease in soil N and P. However, a large part of soil N and P may actually be unavailable to the vegetation, because it is incorporated in stable soil organic matter, or chemically precipitated. During decomposition, most microbial N may be recycled, but small amounts of N are probably incorporated in low-degradable substances, which become part of the stable soil organic matter within a few months (Sjöberg and Persson 1998). In mineral-rich fens, with higher biological activity and microbial N than in poor fens, N-storage may be relatively high. This is supported by Phoenix et al. (2003), who reported higher storage of experimentally supplied N in calcareous than in acid grassland soils. High soil P in calcareous fens may also mainly reflect higher storage, due to higher biological activity and precipitation of calcium phosphate (Lindsay and Moreno 1966; Reddy and DeLaune 2008). In mineral-poor fens, N-storage instable soil organic matter may be reduced by low decomposition and microbial N-uptake. Also, the only partially decomposed dead bryophytes may be a more available substrate than the well decomposed organic matter of calcareous fens. In mineral-poor fens, low soil P may also reflect low chemical P-fixation. Thus, in accord with Bridgham et al. (1998), high amounts of nutrients in the soil and low availability may not actually contradict each other, but reflect different sides of the same coin.
In contrast to soil nutrients, plant nutrients showed at least some correspondence with net mineralization of N and P. Even though vascular plant N and P contents were higher in the most calcareous fens, in accord with generally higher nutrient contents for calcicole species (Ellenberg 1974; Aerts and Chapin 2000), aboveground biomass and total plant N and P were actually lower than in most other fens. Similar to coastal dune grasslands (Kooijman and Besse 2002), aboveground biomass in fens showed an optimum at intermediate pH, associated with an increase in net mineralization of N and P. In intermediate fens, biomass and plant nutrients may increase by dissolution of calcium phosphate, and higher efficiency of mineralization per unit microbe. In mineral-poor fens, however, biomass and plant nutrients decreased again. In dune grasslands, this decrease seemed mainly due to P-fixation in iron phosphate (Kooijman and Besse 2002), but in the mineral-poor fens of this study, with low iron contents, iron phosphates were probably unimportant. Biomass decreased from intermediate to poor fens, despite a further increase in net mineralization of N and P. Aboveground biomass may have been reduced by Al-toxicity (Zvereva et al. 2007), or to some extent by Fe-toxicity (Rozbrojova and Hajek 2008), even though iron contents were relatively low. Also, high uptake of N and P may not be useful when other nutrients, such as base cations, are limiting. Dwarf shrubs like Vaccinium spp., common species in mineral-poor wetlands, did at least not take up additional supplies of N (Nordin et al. 2005). Like in Rozbrojova and Hajek (2008), plant Ca clearly decreased from mineral-rich to mineral-poor fens, and Ca is often a limiting factor in peatlands (Gore 1961). Calcium plays a role in the structural rigidity of cell walls, which need to be strong to support the plant shape (Hepler 2005). In mineral-poor fens, plant support may be improved by structural carbohydrates, which may lead to higher C, but lower nutrient contents. Carbon contents indeed significantly increased from 45% in calcareous to 49% in the most acid fens.
Nutrient availability as an evolutionary driving force?
The shift from mineral-rich to mineral-poor species in the boreal zone may have been driven by the expansion of mineral-poor wetlands when the climate became colder and wetter in Late Tertiary and Pleistocene times (Elliott-Fisk 1988; Potts and Behrensmeyer 1992). The creation of young calcareous soils at the expense of mature acid soils through periglacial processes of denudation, solifluction and sedimentation of löss may have temporarily favoured evolution of calciphilous species (Ewald 2003), but acid soils and mineral-poor wetlands have probably prevailed in the boreal zone for long time periods. During that shift in habitat conditions, nutrient availability seemed to have increased, which may have been a beneficial factor to bryophyte evolution.
On the other hand, brown mosses tend to be more common when nutrient availability is low. Scorpidium spp. generally occur at low P-levels, and disappear when P-availability becomes too high (Kooijman and Hedenäs 1991; Kooijman and Westhoff 1995). In the present study, even the Dutch S. scorpioides fens were (still) P-limited. High nutrient availability may lead to increased growth of vascular plants, and reduced light conditions in the bryophyte layer. Brown mosses may thus be relatively common in calcareous fens, their ancestral habitat, because productivity is reduced by low availability of N and P. In intermediate fens, both bryophytes and vascular plants may profit from increased availability of N and P, but increased vascular plant growth leads to reduced light for bryophytes. In the relatively mild climate of NL, intermediate fen bryophytes are very rare (van Tooren and Sparrius 2007), partly because habitats with such poor buffer capacity have mostly acidified due to atmospheric deposition (Leuven et al. 1986), but possibly also because vascular plant productivity is higher than in the colder climate of the boreal zone.
In the more acid fens, vascular plant productivity may have been limited by low Ca or high Al, and brown mosses actually profited from higher availability of N and P. However, they may have mainly been stimulated by P, which was the main limiting factor, and slightly increased in poor fens. If brown mosses prefer nitrate to ammonium, then N-availability may not actually have been higher at all. Rich-fen species such as S. scorpioides may generally be nitrate users, and sensitive to ammonium toxicity (Paulissen et al. 2004). In mineral-poor fens, brown moss species have likely adapted to ammonium toxicity to some extent, but it is not known whether they have also shifted in preference from nitrate to ammonium. Distribution patterns of Warnstorfia fluitans in the Netherlands at least suggest that the species is doing well in areas with high atmospheric N-deposition (van Tooren and Sparrius 2007), where nitrification is relatively high even in the most acid soils. Release of nitrate in Dutch W. fluitans samples amounted to 12 (±15) mg g−1 day−1.
However, even if they are able to use ammonium, brown mosses of mineral-poor habitats may have a disadvantage compared to Sphagnum spp., which are probably much more efficient ammonium users. Sphagnum spp. preferentially use ammonium to nitrate (Paulissen et al. 2004), and generally have a much higher cation exchange complex than other bryophytes, which can absorb ammonium rapidly (Clymo and Hayward 1982). Given the general success of Sphagnum spp. in nutrient uptake and acidification of the surroundings, it may actually be surprising that mineral-poor brown mosses have evolved at all. Sphagnum spp. were probably already there when the boreal zone became colder and wetter in Late Tertiary and Pleistocene times (Elliott-Fisk 1988; Potts and Behrensmeyer 1992). The Sphagnaceae presumably diverged from all other mosses during the Devonian (Newton et al. 2007), and the rate of diversification in Sphagnum most likely decreased later in its evolutionary history (Shaw et al. 2004). Brown moss evolution is probably much more recent, as diversification of most pleurocarpous moss families took place only during the Cretaceous (Newton et al. 2007). Scorpidium scorpioides existed in northernmost North America three Myr BP, having evolved from ancestral northern populations of S. cossoni almost one Myr earlier (Hedenäs 2009). Possibly, poor-fen brown mosses have evolved or invaded in particular niches, marginal to Sphagnum. Sarmentypnum exannulatum may have exploited a niche in mineral-poor springs, where some base cations are continuously supplied, and some nitrification has occurred through time. Straminergon stramineum may have its stronghold in intermediate and poor fens, where it occurs in pure stands, even if single and often poorly developed shoots among Sphagnum are found as well. Warnstorfia fluitans may have been able to occupy bog pools, which are slightly richer in minerals, and perhaps in nitrification, than the surrounding Sphagnum bogs (Crushell et al. 2006). Naturally, it is impossible to reconstruct evolutionary development and habitat adaptation directly. It is also not clear whether brown mosses have learned to use ammonium, or whether they still depend on nitrate. Nevertheless, we think it likely that habitat adaptation to mineral-poor conditions has included responses to shifts in nutrient availability.
References
Aerts MAPA, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of process and patterns. Adv Ecol Res 30:1–67. doi:10.1016/S0065-2504(08) 60016-1
Aerts R, Verhoeven JTA, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80:2170–2181
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963. doi:10.1016/S0038-0717(03) 00154-8
Bayley SE, Thormann MN, Szumigalski AR (2005) Nitrogen mineralization and decomposition in western boreal bog and fen peat. Ecoscience 12:455–465. doi:10.2980/i1195-6860-12-4-455.1
Boyer MLH, Wheeler BD (1989) Vegetation patterns in spring-fed calcareous fens: calcite precipitation and constraints on fertility. J Ecol 77:597–609. doi:10.2307/2260772
Bridgham SD, Updegraff K, Pastor J (1998) Carbon, Nitrogen, and Phosphorus mineralization in northern wetlands. Ecology 79:1545–1561
Brooks PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. doi:10.1016/0038-0717(85) 90144-0
Clymo RS, Hayward PM (1982) The ecology of Sphagnum. In: Smith AJE (ed) Byrophyte ecology. Chapman & Hall, London, UK, pp 229–289
Cody RP, Smith JK (1987) Applied statistics and the SAS programming Language. Elsevier Science Publishing Co, New York
Cornelissen JHC, Lang SI, Soudzilovskaia NA, During HJ (2007) Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Ann Bot (Lond) 99:987–1001. doi:10.1093/aob/mcm030
Crushell PH, Schouten MGC, Smolders AJP, Roelofs JGM, Giller PS (2006) Restoration of minerotrophic vegetation within an Irish raised bog soak. Proc R Ir Acad, Syst biol Environ 106B:371–385
Davy AJ, Taylor K (1974) Seasonal patterns of nitrogen availability in contrasting soils in the Chiltern Hills. J Ecol 62:793–807. doi:10.2307/2258955
Ellenberg H (1974) Zeigerwerte der Gefässplanzen Mitteleuropas. Scripta Geobotanica 9. p. 97
Elliott-Fisk DL (1988) Chapter 2. The boreal forest. In: Barbour MG, Billings WD (eds) North American terrestrial vegetation. Cambridge University Press, Cambridge, pp 33–62
Ewald J (2003) The calcareous riddle: why are there so many calciphilous species in the Central European Flora? Folia Geobot 38:357–366. doi:10.1007/BF02803244
Gore AJP (1961) Factors limiting plant growth on high-level blanket peat: I. calcium and phosphate. J Ecol 49:399–402. doi:10.2307/2257272
Grootjans AP (1985) Changes of groundwater regime in wet meadows. PhD-thesis University of Groningen
Hassink J, Bouwman LA, Zwart KB, Bloem J, Brussaard L (1993) Relationships between soil texture, soil structure, physical protection of organic matter, soil biota and C and N mineralization in grasslands soils. Geoderma 57:105–128. doi:10.1016/0016-7061(93) 90150-J
Hedenäs L (1993) A generic revision of the Warnstorfia-Calliergon group. J Bryol 17:447–479
Hedenäs L (2003) The European species of the Calliergon-Scorpidium-Drepanocladus complex, including some related or similar species. Meylania 28:1–116
Hedenäs L (2006) Additional insights into the phylogeny of Calliergon, Loeskypnum, Straminergon, and Warnstorfia (Bryophyta: Calliergonaceae). J Hattori Bot Lab 100:125–134
Hedenäs L (2009) Relationships among Arctic and non-Arctic haplotypes of the moss species Scorpidium cossonii and S. scorpioides (Calliergonaceae). Plant Syst Evol (in press).
Hedenäs L, Kooijman AM (1996) Phylogeny and habitat adaptations within a monophyletic group of wetland moss genera (Amblystegiaceae). Plant Syst Evol 199:33–52. doi:10.1007/BF00985916
Hedenäs L, Kooijman AM (2004) Habitat differentiation within Palustriella. Lindbergia 29:40–50
Hedenäs L, Vanderpoorten A (2007) The Amblystegiaceae and Calliergonaceae. In: Newton AE, Tangney R (eds) Pleurocarpous mosses: systematics and evolution. The Systematics Association Special Volume Series 71. Taylor & Francis/CRC Press, Boca Raton, pp 163–176
Hedenäs L, Olivan G, Eldenäs P (2005) Phylogeny of the Calliergonaceae (Bryophyta) based on molecular and morphological data. Plant Syst Evol 252:49–61. doi:10.1007/s00606-004-0281-5
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155. doi:10.1105/tpc.105.032508
Jensen HS, Kristensen P, Jeppesen E, Skytthe A (1992) Iron:phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia 235–236:731–743. doi:10.1007/BF00026261
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450. doi:10.2307/2404783
Kooijman AM, Besse M (2002) On the higher availability of N and P in lime-poor than in lime-rich coastal dunes in the Netherlands. J Ecol 90:394–403. doi:10.1046/j.1365-2745.2001.00661.x
Kooijman AM, Hedenäs L (1991) Differentiation in habitat requirements within the genus Scorpidium, especially between S. revolvens and S. cossoni. J Bryol 16:619–627
Kooijman AM, Westhoff V (1995) Variation in habitat factors and species composition of Scorpidium scorpioides communities in NW-Europe. Vegetatio 117:133–150. doi:10.1007/BF00045505
Kooijman AM, Kooijman-Schouten MM, Martinez-Hernandez GB (2008) Alternative strategies to sustain N-fertility in acid and calcaric beech forests: low microbial N-demand versus high biological activity. Basic Appl Ecol 9:410–442. doi:10.1016/j.baae.2007.05.004
Kooijman AM, van Mourik JM, Schilder MLM (2009) The relationship between N-mineralization or microbial biomass N with micromorphological properties in beech forest soils with different texture and pH. Biol Fertil Soils (in press).
Kuehn KA, Churchill PF, Suberkropp K (1998) Osmoregulatory responses of fungi inhabiting standing litter of the freshwater emergent macrophyte Juncus effusus. Appl Environ Microbiol 64:607–612
Lamers LPM, Tomassen HBM, Roelofs JGM (1998) Sulfate-induced eutrophication and phytotoxicity in fresh-water wetlands. Environ Sci Technol 32:199–205. doi:10.1021/es970362f
Leuven RSEW, Kersten HLM, Schuurkes JAAR, Roelofs JGM, Arts GHP (1986) Evidence for recent acidification of lentic soft waters in the Netherlands. Water Air Soil Pollut 30:387–392. doi:10.1007/BF00305209
Lindsay WL, Moreno EC (1966) Phosphate phase equilibria in soils. Proc Soil Sci Soc Am 24:177–182
Lucassen ECHET, Smolders AJP, Roelofs JGM (2002) Potential sensitivity of mires to drought, acidification and mobilisation of heavy metals: the sediment S/(Ca+Mg) ratio as diagnostic tool. Environ Pollut 120:635–646
Measures JC (1975) Role of amino acids in osmoregulation of non-halophilic bacteria. Nature 257:398–400. doi:10.1038/257398a0
Moore JC, McCann K, de Ruiter PC (2005) Modelling trophic pathways, nutrient cycling, and dynamic stability in soils. Pedobiologia 49:499–510
Newton AE, Wikström N, Bell N, Forrest LL, Ignatov M (2007) Dating the diversification of the pleurocarpous mossses. Syst Assoc Spec Vol 71:337–366
Nordin A, Strengbom J, Ericson L (2005) Responses to ammonium and nitrate additions by boreal plants and their natural enemies. Environ Pollut 141:167–174. doi:10.1016/j.envpol.2005.08.017
Olde Venterink H, Wassen MJ, Verkroost AWM, de Ruiter PC (2003) Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84:2191–2199. doi:10.1890/01-0639
Paulissen MPCP, van der Ven PJM, Dees AJ, Bobbink R (2004) Differential effects of nitrate and ammonium on three fen bryophyte species in relation to pollutent nitrogen input. New Phytol 164:451–458. doi:10.1111/j.1469-8137.2004.01196.x
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2003) Effects of enhanced nitrogen deposition and phosphorus limitation on nitrogen budgets of semi-natural grasslands. Glob Change Biol 9:1309–1321. doi:10.1046/j.1365-2486.2003.00660.x
Potts R, Behrensmeyer AK (1992) Late Cenozoic terrestrial ecosystems. In: Behrensmeyer AK, Damuth JD, DiMichele WA, Potts R, Sues H-D, Wing SL (eds) Terrestrial ecosystems through time. Evolutionary paleoecology of terrestrial plants and animals. The University of Chicago Press, Chicago, pp 419–541
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC, Taylor & Francis, Boca Raton, p 800
Richardson CJ, Marshall PE (1986) Processes controlling movement, storage, and export of phosphorus in a fen peatland. Ecol Monogr 56:280–302. doi:10.2307/1942548
Rozbrojova Z, Hajek M (2008) Changes in nutrient limitation of spring fen vegetation along environmental gradients in the West Carpathians. J Veg Sci 19:613–620
Scheffer RA, Logtestijn RSP, Verhoeven JTA (2001) Decomposition of Carex and Sphagnum litter in two mesotrophic fens differing in dominant plant species. Oikos 92:44–54. doi:10.1034/j.1600-0706.2001.920106.x
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. doi:10.1890/03-8002
Shaw AJ, Cox CJ, Melosik I (2004) Diversification of peatmosses: a phylogenetic approach. Monogr Syst Bot Mo Bot Garden 98:240–254
Sjöberg RM, Persson T (1998) Turnover of carbon and nitrogen in coniferous forest soils of different N-status and under different 15NH4-N application rate. Environ Pollut 102:385–393. doi:10.1016/S0269-7491(98) 80058-4
Sjörs H (1950) On the relation between vegetation and electrolytes in north Swedish mire water. Oikos 2:241–258. doi:10.2307/3564795
Thormann MN (2006) The role of fungi in Boreal Peatlands. In: Wieder KR and Vitt DH (eds) Boreal Peatland ecosystems. Ecological Studies 188. Springer Verlag, pp 101–123
Thormann MN, Currah RS, Bayley SE (2004) Patterns of distribution of microfungi in decomposing bog and fen plants. Can J Bot 82:710–720. doi:10.1139/b04-025
Tietema A (1992) Nitrogen cycling and soil acidification in forest ecosystems in the Netherlands. PhD thesis University of Amsterdam
Tsuneda A, Thormann MN, Currah RS (2001) Modes of cell-wall degradation of Sphagnum fuscum by Acremonium cf. curvulum and Oidiodendron maius. Can J Bot 79:93–100. doi:10.1139/cjb-79-1-93
van Tooren BF, Sparrius LB (2007) Voorlopige verspreidingsatlas van de Nederlandse mossen. Bryologische en Lichenologische Werkgroep van de KNNV, p 350
Verhoeven JTA, Kooijman AM, van Wirdum G (1988) Mineralization of N and P along a trophic gradient in a freshwater mire. Biogeochemistry 6:31–43. doi:10.1007/BF00002931
Verhoeven JTA, Maltby E, Schmitz MB (1990) Nitrogen and phosphorus mineralization in fens and bogs. J Ecol 78:713–726. doi:10.2307/2260894
Westerman RL (1990) Soil testing and plant analysis, Thirdth edn. Soil Science Society of America, Madison, Wisconsin
Zöttle H (1960) Dynamik der Stickstoffmineralisation im Waldbodenmaterial. Plant Soil 8:207–223. doi:10.1007/BF01677502
Zvereva EL, Toivonen E, Kozlov MV (2007) Changes in species richness of vascular plants under the impact of air pollution: a global perspective. Glob Ecol Biogeogr 17:305–319. doi:10.1111/j.1466-8238.2007.00366.x
Acknowledgements
We thank Leo Hoitinga, Leen de Lange and Piet Warthenberg for help with laboratory analyses, and Mari-Carmen Lopez-Navarro and Maleni Munoz-Celdron for their pilot study in the Weerribben.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kooijman, A., Hedenäs, L. Changes in nutrient availability from calcareous to acid wetland habitats with closely related brown moss species: increase instead of decrease in N and P. Plant Soil 324, 267–278 (2009). https://doi.org/10.1007/s11104-009-9954-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-9954-8