Abstract
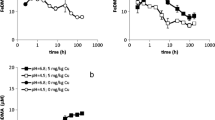
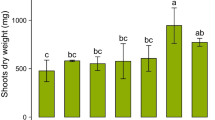
The impact of a large rhizosphere alkalisation on copper (Cu) bioavailability to durum wheat (Triticum turgidum durum L.) initially exposed to a broad range of bulk soil pH (4.8–7.5) was studied. Plants were exposed to a Cu-contaminated soil treated with eight levels of lime (Ca(OH)2) and supplied with NO3 − or NH4 +-NO3 −. Nitrate-fed plants strongly increased their rhizosphere pH to about 6.9–7.6, whatever the initial pH. NH4 +-NO3 −-fed plants slightly acidified their rhizosphere down to 3.9. Free Cu2+ concentration in the rhizosphere was 3 orders of magnitude larger for NH4 +-NO3 − than NO3 −fed plants. Consequently, Cu bioavailability was 2.4- to 4.2-fold larger for NH4 +−NO3 −-fed plants which demonstrates the importance of rhizosphere alkalisation to restrict metal bioavailability in acidic soils. Copper bioavailability of NO3 −-fed plants initially exposed to a broad range of bulk soil pH was insensitive to bulk soil pH, as rhizosphere pH was ultimately neutral in any case.






Similar content being viewed by others
References
AFNOR (1999) Recueil de normes françaises. Qualité des sols, Paris
Bagayoko M, Alvey S, Neumann G, Buerkert A (2000) Root-induced increases in soil pH and nutrient availability to field-grown cereals and legumes on acid sandy soils of Sudano-Sahelien West Africa. Plant Soil 225:117–127 doi:10.1023/A:1026570406777
Bravin MN (2008) Processus rhizosphériques déterminant la biodisponibilité du cuivre pour le blé dur cultivé en sols à antécédent viticole. PhD Thesis, Montpellier SupAgro. Electronic version available through the corresponding author
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584 doi:10.1078/0176-1617-0774
Carrillo-Gonzalez R, Simunek J, Sauvé S, Adriano D (2006) Mechanisms and pathways of trace element mobility in soils. Adv Agron 91:111–178 doi:10.1016/S0065-2113(06)91003-7
Cattani I, Fragoulis G, Boccelli R, Capri E (2006) Copper bioavailability in the rhizosphere of maize (Zea mays L.) grown in two Italian soils. Chemosphere 64:1972–1979 doi:10.1016/j.chemosphere.2006.01.007
Chaignon V, Hinsinger P (2003) A biotest for evaluating copper bioavailability to plants in a contaminated soil. J Environ Qual 32:824–833
Chaignon V, Bedin F, Hinsinger P (2002) Copper bioavailability and rhizosphere pH changes as affected by nitrogen supply for tomato and oil seed rape cropped on an acidic and a calcareous soil. Plant Soil 243:219–228 doi:10.1023/A:1019942924985
Chaignon V, Quesnoit M, Hinsinger P (2008) Rhizosphere pH, bioavailability and extractability of Cu in an acidic Cu-contaminated soil as affected by liming. Environ Pollut (submitted)
Cheng Y, Howieson JG, O’Hara GW, Watkin ELJ, Souche G, Jaillard B, Hinsinger P (2004) Proton release by roots of Medicago murex and Medicago sativa growing in acidic conditions, and implications for rhizosphere pH changes and nodulation at low pH. Soil Biol Biochem 36:1357–1365 doi:10.1016/j.soilbio.2004.04.017
Cornu JY, Denaix L (2006) Prediction of zinc and cadmium phytoavailability within a contaminated agricultural site using DGT. Environ Chem 3:61–64 doi:10.1071/EN05050
Cornu JY, Staunton S, Hinsinger P (2007) Copper concentration in plants and in the rhizosphere as influenced by the iron status of tomato (Lycopersicum esculentum L.). Plant Soil 292:63–77 doi:10.1007/s11104-007-9202-z
Costigan PA, Rose JA, McBurney T (1982) A microcomputer based method for the rapid and detailed measurement of seedling root systems. Plant Soil 69:305–309 doi:10.1007/BF02374528
Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminium resistance in the Arabidopsis mutant alr-104 is caused by an aluminium-induced increase in rhizosphere pH. Plant Physiol 117:19–27 doi:10.1104/pp.117.1.19
Degryse F, Smolders E, Parker DR (2006) Metal complexes increase uptake of Zn and Cu by plants: implications for uptake and deficiency studies in chelator-buffered solutions. Plant Soil 289:171–185 doi:10.1007/s11104-006-9121-4
Degryse F, Verma VK, Smolders E (2008) Mobilization of Cu and Zn by root exudates of dicotyledonous plants in resin-buffered solutions and in soil. Plant Soil 306:69–84 doi:10.1007/s11104-007-9449-4
Haynes RJ (1990) Active ion uptake and maintenance of cation-anion balance: a critical examination of their role in regulating rhizosphere pH. Plant Soil 126:247–264 doi:10.1007/BF00012828
Hinsinger P, Courchesne F (2008) Biogeochemistry of metals and metalloids at the soil-root interface. In: Violante A, Huang PM, Gadd GM (eds) Biophysic-chemical processes of heavy metals and metalloids in soil environments. Wiley & Sons, Hoboken, USA, pp 267–311
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59 doi:10.1023/A:1022371130939
Huang JW, Chen J (2003) Role of pH in phytoremediation of contaminated soils. In: Rengel Z (ed) Handbook of soil acidity. Marcel Dekker, New York, pp 449–472
ISO (1999) Soil quality. Guidance on the ecotoxicological characterisation of soils and soil materials. ISO/DIS 15799 Geneva, Switzerland
Jarvis SC, Robson AD (1983) The effects of nitrogen nutrition of plants on the development of acidity in western Australian soils. II. Effects of differences in cation/anion balance between plant species grown under non-leaching conditions. Aust J Agric Res 34:355–365 doi:10.1071/AR9830355
Khalid M, Soleman N, Jones DL (2007) Grassland plants affect dissolved organic carbon and nitrogen dynamics in soil. Soil Biol Biochem 39:378–381 doi:10.1016/j.soilbio.2006.07.007
Kinraide TB, Ryan PR, Kochian LV (1992) Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol 99:1461–1468
Lebourg A, Sterckmann T, Ciesielski H, Proix N (1998) Trace metal speciation in three unbuffered salt solutions used to assess their bioavailability. J Environ Qual 27:584–590
Lexmond TM (1980) The effect of soil pH on copper toxicity to forage maize grown under field conditions. Neth J Agric Sci 28:164–183
Lexmond TM, van der Vorm PDJ (1981) The effect of pH on copper toxicity to hydroponically grown maize. Neth J Agric Sci 29:217–238
Lofts S, Spurgeon DJ, Svendsen C, Tipping E (2004) Deriving soil critical limits for Cu, Zn, Cd and Pb: a method based on free ion concentrations. Environ Sci Technol 38:3623–3631 doi:10.1021/es030155h
Loosemore N, Straczek A, Hinsinger P, Jaillard B (2004) Zinc mobilisation from a contaminated soil by three genotypes of tobacco as affected by soil and rhizosphere pH. Plant Soil 260:19–32 doi:10.1023/B:PLSO.0000030173.71500.e1
Ma YB, Lombi E, Oliver IW, Nolan AL, McLaughlin MJ (2006) Long-term aging of copper added to soils. Environ Sci Technol 40:6310–6317 doi:10.1021/es060306r
McBride MB, Blasiak JJ (1979) Zinc and copper solubility as a function of pH in an acid soil. Soil Sci Soc Am J 43:866–870
Michaud AM, Bravin MN, Galleguillos M, Hinsinger P (2007) Copper uptake and phytotoxicity as assessed in situ for durum wheat (Triticum turgidum durum L.) cultivated in Cu-contaminated, former vineyard soils. Plant Soil 298:99–111 doi:10.1007/s11104-007-9343-0
Miller AJ, Cramer MD (2004) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36 doi:10.1007/s11104-004-0965-1
Mullins GL, Sommers LE (1986) Cadmium and zinc influx characteristics by intact corn (Zea mays L.) seedlings. Plant Soil 96:153–164 doi:10.1007/BF02374760
Oste LA, Temminghoff EJM, Van Riemsdijk WH (2002) Solid-solution partitioning of organic matter in soils as influenced by an increase in pH and Ca concentration. Environ Sci Technol 36:208–214 doi:10.1021/es0100571
Parker DR, Pedler JF, Thomason DN, Li H (1998) Alleviation of copper rhizotoxicity by calcium and magnesium at defined free metal-ion activities. Soil Sci Soc Am J 62:965–972
Rachou J, Hendershot W, Sauvé S (2004) Effects of pH on fluxes of cadmium in soils measured by using diffusive gradients in thin films. Commun Soil Sci Plant Anal 35:2655–2673 doi:10.1081/CSS-200030438
Riley D, Barber SA (1971) Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root-induced pH changes at the root-soil interface. Soil Sci Soc Am Proc 35:301–306
Ritsema CJ (1993) Estimation of activity coefficients of individual ions in solutions with ionic strengths up to 0.3 mol dm−3. J Soil Sci 44:307–315
Salam AK, Helmke PA (1998) The pH dependence of free ionic activities and total dissolved concentrations of copper and cadmium in soil solution. Geoderma 83:281–291 doi:10.1016/S0016-7061(98)00004-4
Sauvé S, Dumestre A, McBride M, Hendershot W (1998) Derivation of soil quality criteria using predicted chemical speciation of Pb2+ and Cu2+. Environ Toxicol Chem 17:1481–1489 doi:10.1897/1551-5028(1998)017<1481:DOSQCU>2.3.CO;2
Sauvé S, Hendershot W, Allen HE (2000) Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1131 doi:10.1021/es9907764
Taylor GJ, Foy CD (1985) Mechanisms of aluminium tolerance in Triticum aestivum (wheat). IV. The role of ammonium and nitrate nutrition. Can J Bot 63:2181–2186
Thakali S, Allen HE, Di Toro DM, Ponizovsky AA, Rooney CP, Zhao FJ, McGrath SP (2006) A terrestrial biotic ligand model. 1. Development and application to Cu and Ni toxicities to barley root elongation in soils. Environ Sci Technol 40:7085–7093 doi:10.1021/es061171s
Tyler G, Olsson T (2001) Concentrations of 60 elements in the soil solution as related to the soil acidity. Eur J Soil Sci 52:151–165 doi:10.1046/j.1365-2389.2001.t01-1-00360.x
Youssef RA, Chino M (1989) Root-induced changes in the rhizosphere of plants. I. pH changes in relation to bulk soil. Soil Sci Plant Nutr 35:461–468
Zhang H, Lombi E, Smolders E, McGrath S (2004) Kinetics of Zn release in soils and prediction of Zn concentration in plants using diffusive gradients in thin. Environ Sci Technol 38:3608–3613 doi:10.1021/es0352597
Zhao FJ, Rooney CP, Zhang H, McGrath SP (2006) Comparison of soil solution speciation and diffusive gradients in thin-films measurement as an indicator of copper bioavailability to plants. Environ Toxicol Chem 25:733–742 doi:10.1897/04-603R.1
Zhao LYL, Schulin R, Nowack B (2007) The effects of plants on the mobilization of Cu and Zn in soil columns. Environ Sci Technol 41:2770–2775 doi:10.1021/es062032d
Acknowledgements
Edith Le Cadre-Barthélémy is acknowledged for her advices for estimating soil pH buffering capacity. Nicole Balsera, Joëlle Toucet-Louri and Bruno Buatois are also thanked for technical support. Pierre Berthomieu is acknowledged for providing free access to F-AAS. Financial support was provided by the PNETOX programme of the French Ministry of Ecology and Sustainable Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Reid.
Rights and permissions
About this article
Cite this article
Bravin, M.N., Martí, A.L., Clairotte, M. et al. Rhizosphere alkalisation — a major driver of copper bioavailability over a broad pH range in an acidic, copper-contaminated soil. Plant Soil 318, 257–268 (2009). https://doi.org/10.1007/s11104-008-9835-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9835-6