Abstract
Prolonged storage of rice seeds can lead to a decrease in seed vigor and seedling quality. The Lipoxygenase (LOX) gene family is widely distributed in plants, and LOX activity is closely related to seed viability and stress tolerance. In this study, the lipoxygenase OsLOX10 gene from the 9-lipoxygenase metabolic pathway was cloned from rice, and its roles in determining seed longevity and tolerance to saline-alkaline stress caused by Na2CO3 in rice seedlings were mainly investigated. CRISPR/Cas9 knockout of OsLOX10 increased seed longevity compared with the wild-type and OsLOX10 overexpression lines in response to artificial aging. The expression levels of other 9-lipoxygenase metabolic pathway related genes, such as LOX1, LOX2 and LOX3, were increased in the LOX10 overexpression lines. Quantitative real-time PCR and histochemical staining analysis showed that the expression of LOX10 was highest in seed hulls, anthers and the early germinating seeds. KI-I2 staining of starch showed that LOX10 could catalyze the degradation of linoleic acid. Furthermore, we found that the transgenic lines overexpressing LOX10 showed better tolerance to saline-alkaline stress than the wild-type and knockout mutant lines. Overall, our study demonstrated that the knockout LOX10 mutant increased seed longevity, whereas overexpression of LOX10 enhanced tolerance to saline-alkaline stress in rice seedlings.
Key message
The Lipoxygenase OsLOX10 gene was cloned from rice (Oryza sativa L.). Mutation in LOX10 caused increasing of seed longevity, whereas overexpression of LOX10 enhanced tolerance to saline-alkaline stress in rice seedlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant processes are often affected by environmental stresses, including drought, high and low temperature, saline-alkaline stress, wounding, etc., which have a negative effect on the growth and development of the plant as a result of inhibition of metabolism and changing gene expression patterns. The lipoxygenase pathway (LOX-pathway) metabolites polyunsaturated fatty acids (PUFAs), participate in plant adaptation to stress by sensing and responding to signals, enhancing the expression of defense genes (Feussner and Wasternack 2002; Porta and Rocha-Sosa 2002). The signal molecules generated in plants by the LOXs-pathway are involved in a variety of functions, including growth and development, seed germination, response to biotic and abiotic stresses, wounding, fruit ripening, senescence, cell death, and the biosynthesis of the stress-response phytohormones jasmonic acid and abscisic acid (Viswanath et al. 2020). Previous studies on LOXs have focused mainly on their functions in development and in defense responses to biotic stress (Marla and Singh 2012), where studies showed increases in LOX gene expression and in the corresponding enzyme activity in response to pests and fungal phytopathogens such as blast in rice (Peng et al. 1994). Oxygen free radicals and hydroxide formed by the LOX pathway act as signal transduction substances to participate in plant responses to biotic stresses, but comparatively few studies have been carried out to establish the role of LOXs in plant response to abiotic stress. Studies have shown that the LOX pathway of membrane lipid conversion is an independent signaling pathway (Santino et al. 2013). This signal is enhanced by an autocatalytic cycle involving calcium ions and the calcium-binding protein calmodulin. Hydroperoxides produced by LOX activity on linoleic acid and linoleic acid in plasma membrane transport calcium ions from the outside of cells to the inside of cells (Feussner and Wasternack 2002; Glyan’ko, 2011). The subsequent increase in cytoplasmic calcium concentration leads to the activation of phospholipase A and the release of PUFAs from phospholipids (Babenko et al. 2017; Liavonchanka and Feussner 2006). LOX plays an important role as a key cascade enzyme in this process. Moreover, the intermediate and end products of the LOX pathway can activate protein kinases, sending signals and achieve their transduction. As a consequence, LOX activity is regarded as a molecular marker for plant response to abiotic stresses (Babenko et al. 2017; Pokotylo et al. 2015).
Rice (Oryza sativa L.) is one of the most important crops worldwide, and its yield has been increasing in recent years. Rice seeds are faced with a series of problems in the process of seed production and storage, such as decay, decline in vigor and germination rate, as well as disimprovements in consumer traits such as viscosity, hardness, taste and flavor; these changes are closely related to the oxidative decomposition of membrane lipids in the seeds (Yasumatsu and Moritaka 1964; Yasumatsu et al. 1966). How to maintain seed longevity and prevent quality decline during long-term storage is a universal problem. Many factors affecting seed longevity and quality during storage, including environmental conditions, seed physiological status and sub-species (japonica vs. indica) differences (Vertucci and Roos 1990; Zhang et al. 2007). Lipid degradation is considered to be one of the reasons for the decline in seed longevity and quality during storage (Takano 1993). Lipids can be metabolized by different enzyme systems (LOXs, hydrolases or lyases) into small molecules of alcohols, aldehydes and other volatiles during seed storage, resulting in lower nutritional value (Aibara et al. 1986).
In plants, linolenic acid (LNA) and linoleic acid (LA) are the most common substrates for LOXs; LOXs generate 9- or 13-hydrogen peroxides by adding oxygen at the 9- (9-LOX) or 13- (13-LOX) positions of the substrate carbon chain, respectively (Ivanov et al. 2010; Liavonchanka and Feussner 2006). Inhibition of LOXs activity affects seed storage stability by delaying the metabolism of PUFAs in seeds, maintaining the structual integrity of cell membranes and reducing the formation of cytotoxic derivatives. To date, several LOX family genes have been isolated from rice (Umate 2011). Among them, LOX2 and LOX3 were isolated from rice embryos and their expression is closely related to seed germination and the duration of vigor longevity in response to storage (Huang et al. 2014; Xu et al. 2015), but little is known about the effects of other LOXs on seed longevity during storage.
Seed aging is a complex process, which involves physiological and biochemical reactions, signal transduction, metabolic activation, redox homeostasis, etc. (Parkhey et al. 2012). The metabolites produced by lipid peroxidation, such as malondialdehyde (MDA) and acetaldehyde, can react with some macromolecules to cause cell damage, which not only affects the longevity and quality of seeds, but is also closely involved in determining the damage caused to plants by stresses. In the current study, rice OsLOX10 was cloned from three-day-old seedlings of rice cultivar Kitaake with the intention of investigating its function in seed longevity and tolerance of rice seedlings to saline-alkaline stress. Knockout of LOX10 increased seed longevity after artificial aging for 18 days. Lines overexpressing LOX10 showed accelerated seed germination under normal condition and lower seed viability after artificial aging for 18 days; in addition, lines overexpressing LOX10 showed higher tolerance to saline-alkaline stress than the wild-type (WT) and the knockout mutant lines. The practical application of LOX10 might contribute to achieving genetic improvement of rice seed longevity and increased tolerance to saline-alkaline stress.
Materials and methods
Plant materials
The japonica cultivars variety Kitaake was used as the WT material for Agrobacterium tumefaciens-mediated transformation to construct the OsLOX-OE lines and for the generation of the lox10 knockout mutants achieved using the CRISPR/Cas9 system, from which two independent homozygous mutants lox10-1 and lox10-2 were identified using specific primers (Table S1). The overexpression vector of LOX10 was constructed based on the pCAMBIA-1302 plasmid driven by a CaMV 35 S promoter. The transgenic plants of T1 generation were screened with primers of the hygromycin phosphotransferase gene and homozygous T2 generation transgenic seeds were obtained (Table S1). Plants of the lines were grown in an experimental field and harvested uniformly at the maturity stage, with all seeds subsequently undergoing the same storage conditions.
Saline-alkaline stress treatment
To conduct the saline-alkaline stress treatment, rice seedlings were grown for three weeks at a 28℃ temperature, under a 16-h light/8-h dark photoperiod, with approximately 70% relative humidity and 500 µmol photons m− 2s− 1 light intensity. Saline-alkaline stress treatment was conducted as described in a previous reports (Liu et al. 2021). After saline-alkaline stress treatment for 48 h, the MDA concentration was determined by the MDA Assay Kit (Solarbio, Beijing, China), following the manufacturer’s instructions. Three biological replicates were performed for each treatment sample. A section of the flag leaf section from each mature plants was floated in a Petri dish on a solution containing 25 mM Na2CO3 ( pH = 10.0 ) and incubated for three days, after which the leaf was scored for necrosis or spots, and photographic images were taken. Chlorophyll a, chlorophyll b and total chlorophyll content of entire leaves from in WT, lox10 mutants and LOX10 overexpression lines were determined according to Zhang et al. (2015).
Seed germination and artificial aging
Fifty seeds per replicate of each of the lox10 mutants, the LOX10 overexpressed lines and WT ‘Kitaake’ were placed in 9-cm diameter Petri dishes containing 10 ml of distilled water in an incubator at 28 ± 1 °C, 100% relative humidity and 16 h of light per day for seven days. Seed vigor traits were measured according to previous reports, namely germination potential, germination rate and germination index (Fu et al. 2019; He et al. 2019). Three biological replicates were performed for each sample. The artificial aging treatment procedure was based on the method of Zeng et al. (Zeng et al., 2007) with some modifications. Fifty seeds per replicate of the lox10 mutants, the LOX10 overexpression lines and WT were treated at 42 °C and 88% relative humidity for 18 days in a closed desiccator (Binder, Tuttlingen, Germany) with a thermostatic moisture regulator. After artificial aging, the germination potential at 7 days and the germination rate at 14 days were measured according to the above method.
RNA isolation and gene expression analysis
Total RNA was isolated from different tissues (roots, stems, leaves, embryos) of rice seedlings, germinated seeds (after 0, 1, 3, 5 and 7 days of incubation) and transgenic embryos using the TRIzol reagent (Simms et al. 1993). The first-strand cDNA was synthesized from 1 µg of total RNA using ReverTra Ace™ qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). Real-time quantitative PCR was performed using the LightCycler 480 II ( Roche, Jena, Germany) and the FastStart Universal SYBR Green Master (Rox) (Roche, Shanghai, China), with the rice OsActin gene as the internal control. The PCR conditions were as follows: 95℃ for 10 min, followed by 40 cycles at 95℃ for 15 s and 60℃ for 60s. Primers used for qPCR are listed in Supplemental Table S1. Data were analyzed using LightCycler 480 II software.
Cloning of OsLOX10
Total RNA was isolated from three-day-old seedlings of ‘Kitaake’ to carry out OsLOX10 cloning. Based on the OsLOX10 mRNA sequence XM_015759992 in the NCBI database (https://www.ncbi.nlm.nih.gov/), we designed primer pairs LOX10-CDS-F and LOX10-CDS-R (Supplemental Table S1), which amplified the LOX10 coding sequence (CDS) (2607 bp) . Meanwhile, we designed primers LOX10-Pro-F and LOX10-Pro-R (Supplemental Table S1) to amplify the 2000 bp sequence upstream of LOX10-CDS as the promoter of LOX10 gene from the ‘Kitaake’ genome.
GUS staining
The 2-kb promoter region of LOX10 was amplified with the primer sequences listed in Supplemental Table S1. Cloning of the sequence into BamHI and NcoI sites of the pCAMBIA1305 plasmid was conducted using the BamHI and NcoI restriction enzymes. The vector was introduced into ‘Kitaake’ by A.tumefaciens-mediated transformation. GUS staining of different tissues of transgenic plants was conducted overnight using a commercial GUS Staining Solution Kit (Beijing, Solarbio, China) at 37℃ according to the manufacturer’s instructions. Photographic images of the tissues were obtained after decolorization of the tissues with 75% (v/v) alcohol.
Protein subcellular localization
The CDS of LOX10 was amplified with the primer sequences listed in Supplemental Table S1. The pRTVcGFP plasmid was digested with BamHI and HindIII restriction enzymes to construct the LOX10::GFP vector. Rice protoplasts were prepared and transfected as described in a previous report (Zhang et al. 2011). Scanning observation of fluorescent proteins were carried out under a TCS SP8 STED 3X laser confocal microscope ( Leica, Germany).
In-vitro expression and enzyme activity assays
The CDS of LOX10 was inserted into the pET28a plasmid and double digested with NdeI and HindIII to construct the pET28a(+)::LOX10 vector. Then, the pET28a::LOX10 plasmid and the pET28a control plasmid were transformed separately into cells of the Escherichia coli BL21(DE3) prokaryotic expression strain and expression of the fusion protein product was induced by exposure to 0.1 mM isopropylthio-β-D-galactoside (IPTG) for 5 h at 23℃. The E.coli cells were collected, exposed to ultrasonic treatment in phosphate-buffered saline (PBS) on ice and then centrifuged. LOX10 activity was assayed in the supernatant and in transgenic seed embryos using the KI-I2 starch staining method (Xu et al. 2015). The stock LOX10 supernatant, which contained 0.5 µg protein/µl, was diluted to achieve different working concentrations (1×, 2×, 5×dilutions) for the enzyme activity assay and the pET28a(+) supernatant and PBS buffer were used as controls. The color intensity of the different reaction solutions reflected the activity level of LOX10.
Measurement of free fatty acid concentration
The transgenic and WT seeds were artificially aged for 18 days, as described earlier, and approximately 0.5 mg of embryos were then dissected from the seeds, snap frozen in liquid nitrogen and sent to Biotree (Shanghai, China) for determination of free fatty acid concentrations. The embryos from transgenic and WT seeds stored under natural conditions were used as controls. Three biological replicates were performed for each treatment.
SEM analysis
Transgenic and WT seeds before and after artificially aging for 18 days were viewed under a scanning electron microscope (SEM) (Xu et al. 2015). Rice seed endosperm was cut transversely and placed in a S-3500 N SEM (Hitachi, Tokyo, Japan ) and images were recorded according to the manufacturer’s protocol.
Agronomic characteristics of transgenic rice lines and data analysis
The grain length, grain width and 1000-grain weight of transgenic and WT plants were determined. Three biological replicates were performed for each line. Student’s t-test was used to determine whether any pairwise differences between samples were statistically significant, with significance being assessed at the 0.05 and 0.01 levels.
Results
Disruption of LOX10 expression increase seed longevity
Rice LOX10, which is located on chromosome 11 and encodes a protein of 868 amino acids (Fig. S1a,b), was cloned from a japonica variety, Kitaake. LOX10 belongs to the 9-LOX metabolic pathway according to KEGG (https://www.genome.jp/kegg/) database analysis. The phylogenetic tree indicated that a closer genetic relationship of OsLOX10 exists among the monocotyledonous plants rice, wheat, and maize than among the dicotyledonous plants Arabidopsis, soybean, and tomato (Fig. S1c). To extend our understanding of LOX10 functions, we generated knockout mutants (lox10-1 and lox10-2) and overexpression lines (LOX10-OE1 and LOX10-OE2) (Fig. 1a-c). The lox10-1 mutant plants contained a -5 bp deletion and a + 1 bp insertion, whereas the lox10-2 mutant plants contained a -14 bp deletion and a -3 bp deletion (Fig. 1b). In the two knockout mutants, the amino acid sequence of LOX10 suffered premature termination as a result of frame shift mutation of the nucleotide sequence (Fig. S2). These results indicate that the lox10-1 and lox10-2 mutant lines lacked LOX10 activity. In the LOX10-OE1 and LOX10-OE2 overexpression lines, the expression of LOX10 was increased 122- and 574-fold, respectively, compared with the WT (Fig. 1c). The self progeny of these homozygous mutants were used in subsequent experiments.
The mutation ofLOX10affects rice seed vigor. (a) Gene structures of WT and lox10 mutants,. The red box represents the CRISPR/Cas9 target of the LOX10 gene. (b) The lox10-1 mutant plants contained a -5 bp deletion and a + 1 bp insertion, the lox10-2 mutant plants contained a -14 bp and a -3 bp deletion. (c) Relative expression analysis of the LOX10 gene in WT, lox10 knockout mutants and LOX10 overexpression lines. (d) Seed germination of WT, lox10 mutants and LOX10 overexpression lines after 3 and 7 days under normal conditions. (e-g) Germination potential, germination rate and germination index of WT, lox10 mutants and LOX10 overexpression lines after 7 days under normal conditions. Values are means ± SD of three biological replicates; *, ** and *** indicate the significant difference compared to WT at 5%, 1% and 0.1% levels, respectively, according to Student’s t-test
Seed vigor is an important factor affecting seed germination and longevity. Our data showed that there were no significant differences for agronomic traits, including plant architecture, grain length, grain width and 1000-grain weight among the WT, the mutant and transgenic lines(Fig. S3a-f). The seeds of the two lox10 mutants, the two overexpression lines and the WT were compared for the evaluation of seed vigor. Phenotype evaluation indicated that the disruption of LOX10 in the two knockout mutants resulted in decreased seed vigor reflected by slow germination speed and slow seedling growth under normal conditions (Fig.S3g-i). The germination potential, germination rate and seedling vigor index of the two lox10 mutants were significantly lower than the corresponding values of the two LOX10 overexpression lines and the WT plants (Fig. 1d-g), whereas the germination potential of the two LOX10 overexpression lines was significantly greater than that of the WT under normal conditions (Fig. 1e).
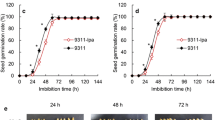
Seed longevity was measured as the germination potential, germination rate and germination index after artificial aging (high temperature and high humidity treatment to accelerate seed aging) or natural aging treatments. After storage for 6 months under natural conditions, the germination potential of two lox10 mutants was significantly higher than the WT, while the two LOX10 overexpression lines were significantly lower than the WT (Fig. 2a,b). The germination rate and germination index of the two lox10 mutants and WT were all remain above 90%, while the two LOX10 overexpression lines were significantly reduced (Fig. 2a,c,d). Overall, the transgenic lines and WT exhibited similar trends with little difference under both natural and artificial aging conditions (Fig. 2). The germination potential and germination rate of the two lox10 mutants were significantly higher than those of the two LOX10 overexpression lines and WT plants after artificial aging for 18 days (Fig. 2e-g). All these results suggest that LOX10 plays an important role in regulating seed vigor and longevity.
Comparison of rice seed vigor among WT, lox10knockout mutants andLOX10overexpression lines under naturally aged and artificially aged conditions. (a) Seed germination of WT, lox10 mutants and LOX10 overexpression lines after 7 days following natural aging for 6 months; (b-d) Germination potential, germination rate and germination index of WT, lox10 mutants and LOX10 overexpression lines after 7 days following natural aging for 6 months; (e) Seed germination of WT, lox10 mutants and LOX10 overexpression lines after 7 and 14 days following artificial aging for 18 days; (f-g) Germination potential and germination rate of WT, lox10 mutants and LOX10 overexpression lines after 14 days following artificial aging for 18 days. Values are means ± SD of three biological replicates; *, ** and *** indicate the significant difference compared to WT at 5%, 1% and 0.1% levels, respectively, according to Student’s t-test
Expression pattern of LOX10 and subcellular localization
To further understand the physiological function of LOX10 in rice, the expression patterns of LOX10 during seed germination and seedling development were analyzed using quantitative real-time PCR (qPCR). LOX10 transcripts were detected during the early stage of seed germination (Fig. 3a). Histochemical staining for GUS activity was performed in LOX10 promoter::GUS transgenic lines, and observed that GUS activity was relatively high in hull tissue, anther tissue and during the early seed germination stage (Fig. 3b-i). The elevated expression of LOX10 in germinated seeds suggests that this gene might play an important role in modulating seed vigor in rice. We then further constructed a recombinant LOX10 tagged with GFP at the C-terminus and expressed it transiently in rice protoplasts to determine the subcellular localization of LOX10. We observed the green fluorescence of GFP-tagged LOX10 to be distributed throughout the cytoplasm (Fig. 3j), indicating that LOX10 protein is present in the cytoplasm.
Expression patterns ofLOX10and subcellular localization in rice. (a) Expression patterns of LOX10 in different tissues and germination stages of in rice using the quantitative real-time PCR (qPCR) approach; (b-i)Histochemical staining for GUS activity of LOX10 in seedling, root, stem, leaf, young panicle, mature panicles, anther and early seed germination stage; (j) Subcellular localization in rice protoplasts of LOX10 tagged with green fluorescent protein (GFP) at the C-terminus. Bar = 5 μm
LOX10 activity assay on α-linoleic acid (LA)
We introduced complete LOX10 cDNA into pET28a(+) for in-vitro expression analysis. Using SDS-PAGE and western blotting, a specific band corresponding to 100 kDa was observed, consisting of LOX10 ( about 98 kDa ) and a His tag (Fig. 4a). Most of LOX10 recombinant proteins were present in the supernatant of the cell lysate under moderate-temperature induction conditions (23℃) and the recombinant protein exhibited high LOX10 activity (Fig. 4a). The supernatant was shown to contain the soluble LOX10-His fusion protein ( diluted to achieve different concentrations ) and the His tag protein were obtained in the supernatant for enzyme activity analysis. The KI-I2 staining showed that LOX10 could metabolize the substrate LA to produce starch, which stained dark purple, with the color becoming lighter (reflecting lower enzyme activity) as the protein concentration decreased (Fig. 4b). We separately extracted the embryo proteins from the lines lox10-1, lox10-2, LOX10-OE1, LOX10-OE2 and WT for KI-I2 staining, and found that the two LOX10-OE overexpression lines showed deeper purple (higher activity), compared with the two lox10 mutants and the WT (Fig. 4c). We speculated that the cause of the light purple staining by the lox10 mutants and WT may be functional redundancy among LOX family members. These results showed that LOX10 encoded an authentic LOX protein, which exhibited enzymatic activity.
Induction of LOX10 proteinin vitroand determination of enzyme activity using the KI-I2starch staining method. (a) Induction of production of LOX10 protein in vitro; m:protein marker; a: pET28a(+) precipitate; b: pET28a(+) supernatant; c: pET28a(+)::LOX10 precipitate; d: pET28a(+)::LOX10 supernatant; red arrow represents LOX10 protein. (b) Enzymatic activity assay of LOX10 on α-linoleic acid (LA) in vitro; control:pET28a(+) supernatant; PBS: PBS buffer; 1×dilution: the 0.5 µg protein/µl supernatant containing LOX10; 2×dilution: the 0.25 µg protein/µl supernatant containing LOX10; 5×dilution: the 0.1 µg protein/µl supernatant containing LOX10. (c) Enzyme activity assay of LOX10 on LA in WT, lox10 mutants and LOX10 overexpression rice seed embryos
Disruption of LOX10 affects grain starch granule structure and free fatty acid content
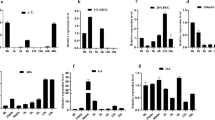
Lipid peroxidation and degradation mediated by enzymes of the LOX pathway are thought to be important causes of seed aging. Therefore, we detected the expression levels of LOX pathway-related genes and the downstream metabolism-related genes related to seed vigor in the rice endosperm, and found that the expression levels of the LOX pathway-related genes LOX1, LOX2 and LOX3 and of the downstream metabolism-related genes OsAOS2, OsHPL1 and OsHPL3 were significantly higher in the two overexpression lines than in the WT (Fig. 5a-f). In plants, LOX usually catalyzes the oxidation of polyunsaturated fatty acids ( PUFAs ) and free fatty acids to produce unsaturated fatty acid hydroperoxides (Feussner and Wasternack 2002; Stelmach et al. 2001). Thus, we speculate that the free fatty acids concentration in the rice embryo may be associated with seed longevity during storage. To test this hypothesis, we used metabolomics to detect the concentration of free fatty acids in the embryos of WT, mutant lox10-1 and overexpression line LOX10-OE1 before and after aging. Interestingly, the concentrations of many different types of free fatty acids, including LNA and LA, were significantly lower in lox10-1 than in LOX10-OE1 and WT before as well as after aging (Fig. 5 g-j and S4). Furthermore, the concentrations of cis-11-Octadecenoic acid and stearic acid were much higher in LOX10-OE1 seeds after artificial aging for 18 days (Fig.S4). These results suggest that when the expression level of LOX10 is inhibited, the hydroperoxide produced by LOX10 on linoleic acid and linoleic acid is also decreased, which affects the transport of calcium ions into the cells, and finally affects the activation of phospholipase A and the release of PUFAs from phospholipids. Overall, LOX10 plays a critical role by influencing the LOX pathway and activating downstream metabolism-related genes, so that the disruption of LOX10 increases seed longevity through reducing free fatty acid content in seed embryos before and after aging.
Observation of starch granule structure in endosperm and determination of free fatty acid concentration in embryo. (b) (a-c) Expression analysis of 9-LOX metabolic pathway-related genes LOX1, LOX2 and LOX3 in WT, lox10 mutants and LOX10 overexpression rice seed embryos. (d-f) Expression analysis of 9-LOX downstream metabolism-related genes AOS2, HPL1 and HPL3 in WT, lox10 mutants and LOX10 overexpression rice seed embryos. (g, h) Linolenic acid and linoleic acid concentrations in rice embryos of WT, lox10-1 mutant and LOX10-OE1 overexpression lines with or without artificial aging for 18 days. Values are means ± SD of three biological replicates; * and *** indicate the significant difference compared to WT at 5% and 0.1% levels, respectively, according to Student’s t-test. (i, j) Heatmap of metabolite clustering in rice embryos of WT, lox10-1 mutant and LOX10-OE1 overexpression lines with or without artificial aging for 18 days. (k) The observation of starch granule structure in rice endosperm of WT, lox10-1 mutants and LOX10-OE1 overexpression lines with or without artificial aging for 18 days. Bar = 10 μm
Previous studies had reported that down-regulation of LOX3 expression affected grain starch granule structure (Xu et al. 2015). To investigate whether LOX10 also affected starch granule morphology, we performed scanning electron microscopic (SEM) analysis on transverse sections of WT, lox10-1, lox10-2, LOX10-OE1 and LOX10-OE2 seed endosperms before and after artificial aging. Before artificial aging, the structure of the seed endosperms of WT, lox10-1, lox10-2, LOX10-OE1 and LOX10-OE2 was tightly arranged and the shape and size of cells were uniform and regular. After artificial aging for 18 days, the endosperm structure of the two LOX10 overexpression lines and WT appeared to be loosely packed, fragile and irregular. In contrast, the endosperm structure of two lox10 mutants after aging maintained a relatively complete and regular starch granule structure (Fig. 5k and S5). Therefore, the disruption of LOX10 was closely associated with decreased stability of the morphological changes of rice endosperm starch granules in the presence or absence of artificial aging.
Overexpression of LOX10 increases tolerance of rice seedlings to saline-alkaline stress
Saline-alkaline soil is characterized by a combination of high sodium ion (Na+) concentration and high pH at the same time, which has more complex, adverse effects on plant growth and development than pH-neutral saline soils (Tang et al. 2014). It was found that LOX activity was higher and lipid peroxide oxidation was activated under salt stress conditions (Ben-Hayyim et al. 2001). As part of an investigation of the role of LOX10 in saline-alkaline stress tolerance, the two LOX10-OE overexpression lines showed increased seedling survival rates under saline-alkaline conditions (25 mM Na2CO3, pH = 10.0), comparing with the two lox10 mutants and WT lines (Fig. 6a, c). At the 25 mM Na2CO3 (pH = 10.0) concentration, the flag leaves of the mature plants of the LOX10 overexpression lines remained green and with less necrosis or fewer spots than in WT or lox10 mutant plants (Fig. 6b). To examine the concentration of malondialdehyde (MDA), a marker of oxidative stress-related lipid peroxidation, we performed MDA quantification after three-week-old rice seedlings had been treated with sodium carbonate for 48 h. The results showed lower concentrations of MDA in the two LOX10-OE overexpression lines than in the two lox10 mutants and the WT lines (Fig. 6d). Meanwhile, we have measured and compared Chlorophyll a, chlorophyll b and total chlorophyll contents in WT, lox10 knockout mutants and LOX10 overexpression lines. And found that chlorophyll b and total chlorophyll contents of LOX10 overexpression lines were significantly higher than WT and lox10 knockout mutants (Fig. 6e-g). Taken together, these results indicate that the overexpression of LOX10 plays an important role in determining rice tolerance to saline-alkaline stress.
Characterization ofLOX10overexpression plants under saline-alkaline stress conditions (a) Images of WT, lox10 mutants and LOX10 overexpression lines before and after recovery from the saline-alkaline treatment (25mM Na2CO3, pH = 10.0); (b) Images of flag leaf sections of WT, lox10 mutants and LOX10 overexpression lines before and after saline-alkaline treatment (25mM Na2CO3, pH = 10.0); (c) Survival rates; (d) Malondialdehyde (MDA) content of WT, lox10 mutants and LOX10 overexpression lines after 48 h of the saline-alkaline treatment (25 mM Na2CO3, pH = 10.0). (e-g) Chlorophyll a, chlorophyll b and total chlorophyll contents in WT, lox10 knockout mutants and LOX10 overexpression lines were determined after 48 h of the saline-alkaline treatment (25 mM Na2CO3, pH = 10.0). Values are means ± SD of three biological replicates; ** and *** indicate the significant difference compared to WT at 1% and 0.1% levels, respectively, according to Student’s t-test
Discussion
Seed storability is an important agronomic and physiological trait in crop production. Seed storability has been shown to be regulated by genetic and environmental factors, but the mechanisms involved remain unclear. Previous studies had shown that temperature, moisture content and oxidation processes are the main factors affecting seed longevity (Ellis and Roberts 1980; Smith and Berjak 2017). In recent years, several studies have detected a number of quantitative trait loci (QTLs) related to seed storage longevity in rice, such as qLG-2, qLG-4 and qLG-9 (Miura et al. 2002). Artificial aging is widely used to study rice seed longevity by simulating natural conditions because of its simple operation, stable environment and ease of control (Priestley and Leopold 1983; Salama and Pearce 1993). In the current study, we cloned LOX10 from rice ‘Kitaake’ and found that this gene played an major role in seed longevity and seedling salt-alkaline stress tolerance. Our study suggests that LOX10 is located in the cytoplasm, and expression of the corresponding gene is induced to express during seed germination (Fig. 3j). Disruption of LOX10 inhibits seed germination under normal conditions, on the contrary, after natural and artificial aging of seeds, the lox10 knockout mutants maintained a higher germination potential than those of the WT and the overexpression lines (Fig. 2), without affecting other agronomic traits (Fig.S3a-f). These results are consistent with those from previously reported roles of LOX2 and LOX3 in the regulation of rice seed longevity (Huang et al. 2014; Xu et al. 2015). In addition, we also showed that LOX10 overexpression lines enhanced the tolerance of rice seedlings to saline-alkaline stress (Fig. 6a-c).
In plants, the expression of the LOX genes is regulated by different factors, including phytohormones (Creelman and Mullet, 1997; Melan et al. 1993), biotic and abiotic stresses (Porta et al. 1999), and the plant species being studied. LOX gene show different, organ-specific expression patterns (Kolomiets et al. 2001). The hydroperoxy fatty acid products of LOX reaction can be further converted into different compounds through different enzyme pathways and thus have diverse functions (Feussner et al. 2001; Porta and Rocha-Sosa 2002). Our data showed that the expression levels of the LOX pathway-related genes LOX1, LOX2 and LOX3 and the downstream metabolism-related genes AOS2, HPL1 and HPL3 were significantly higher in the overexpression lines than in the WT and the lox10 mutants (Fig. 5a-f). It is generally considered that LOXs in plant seeds act as a storage protein and do not have a clear physiological role (Siedow 1991). During germination, LOX proteins and their corresponding mRNAs accumulate and new LOXs are synthesized in seedlings and cotyledons (Melan et al. 1994; Porta et al. 1999). The storage lipids are mobilized from lipid bodies to release free fatty acids for further oxidation metabolism (Feussner et al. 2001). In vitro, most LOXs prefer free fatty acids as substrates (Feussner et al. 2001; Stelmach et al. 2001). By quantification of free fatty acid concentration in embryos before and after aging, we found that the free fatty acid concentrations of lox10 mutant seeds embryos, including LNA and LA, were lower than in WT and the LOX10 overexpression lines, through the detection of free fatty acid content in embryos before and after aging (Fig. 5 g-j). These results suggest that the concentration of free fatty acids in the embryo may be an important factor affecting seed storability. Seed germination is closely related to lipid degradation and hense LOX activity, but the underlying mechanism needs further study.
The LOX pathway of PUFAs plays an important role in regulating plant growth and development, and tolerance to biotic and abiotic stresses. The metabolites produced in the LOX pathway act as stress signaling molecules to perform diverse functions (Babenko et al. 2017; Viswanath et al. 2020), so that LOX activity can be used as a molecular marker for plant response to stress (Pokotylo et al. 2015; Porta and Rocha-Sosa 2002). The effects of cold and salt stress on 9-LOX and 13-LOX activity were different, suggesting that different abiotic stresses may regulate different LOX pathways (Babenko et al. 2017). It was reported that the LOX activity of salt-tolerant plants increased under salt stress conditions (Ben-Hayyim et al. 2001), whereas the expression of OsLOX2 in rice was induced under drought stress (Du et al. 2013).
Our results showed that overexpression of LOX10 increased the tolerance of rice seedlings to saline-alkaline stress, whereas the accumulation of MDA in the overexpression lines was also lower after 48 h exposure to sodium carbonate treatment compared with WT and the knockout mutants (Fig. 6d). At the same time, chlorophyll b and total chlorophyll contents of LOX10 overexpression lines were significantly higher than WT and lox10 knockout mutants (Fig. 6e-g). Thus, we confirmed that the overexpression of LOX10 enhance the resistance of rice seedlings to alkaline stress. We also found that overexpression of LOX10 enhance tolerance of rice seedlings to saline-alkaline, but the mutants showed no significant difference with WT, this may due to the decrease of LOX10 expression in the two mutants was not as significant as the increase in the LOX10 overexpression lines. Previous studies had reported that the concentration of free polyunsaturated fatty acids from LOX substrates increased in response to stress effects (Porta and Rocha-Sosa 2002). This indicates that the free fatty acid content of the LOX substrate has a positive effect on plant tolerance to stress.
In summary, our results provide new insights into the regulatory role of LOX10 on seed longevity during storage and on the stress tolerance of rice seedlings. The knockout lox10 mutants increase seed longevity by reducing the decomposition of free fatty acids during seed storage. However, the overexpression of LOX10 increased the metabolism of free fatty acids, which produced different metabolites and activated various signaling molecules to enhance the tolerance of rice seedlings to saline-alkaline stress, but the functions of LOX10 toward seed storability and stress response of rice seedlings warrant further investigation.
Accession numbers
Sequence data used in this study are from the Rice Genome Annotation Project website (MSU, http://rice.uga.edu/index.shtml). The accession numbers are as follows: LOX10, LOC_Os11g36719; LOX1, LOC_Os03g49380; LOX2, LOC_Os03g5286; LOX3, LOC_Os03g49260; OsAOS2, LOC_Os03g12500; OsHPL1, LOC_Os02g12690; OsHPL3, LOC_Os02g02000.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Aibara S, Ismail IA, Yamashita H, Ohta H, Sekiyama F, Morita Y (1986) Changes in Rice Bran lipids and free amino acids during storage. Agric Biol Chem 50:665–673. https://doi.org/10.1271/bbb1961.50.665
Babenko L, Shcherbatiuk MM, Skaterna T, Kosakivska IV (2017) Lipoxygenases and their metabolites in formation of plant stress tolerance. Ukr Biochem J 89(1):5–21. https://doi.org/10.15407/ubj89.01.005
Ben-Hayyim G, Gueta-Dahan Y, Avsian-Kretchmer O, Weichert H, Feussner I (2001) Preferential induction of a 9-lipoxygenase by salt in salt-tolerant cells of Citrus sinensis L. Osbeck Planta 212(3):367–375. https://doi.org/10.1007/s004250000397
Creelman RA, Mullet JE, ACTION OF JASMONATES IN PLANTS (1997) BIOSYNTHESIS AND. Annu Rev Plant Physiol Plant Mol Biol 48:355–381. https://doi.org/10.1146/annurev.arplant.48.1.355
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4:39. https://doi.org/10.3389/fpls.2013.00397
Ellis RH, Roberts EH (1980) Improved equations for the prediction of seed longevity. Ann Bot 45:13–30. https://doi.org/10.1093/oxfordjournals.aob.a085797
Feussner I, Kühn H, Wasternack C (2001) Lipoxygenase-dependent degradation of storage lipids. Trends Plant Sci 6(6):268–273. https://doi.org/10.1016/s1360-1385(01)01950-1
Feussner I, Wasternack C (2002) The lipoxygenase pathway. Ann Rev Plant Biol 53:275–297. https://doi.org/10.1146/annurev.arplant.53.100301.13s5248
Fu Y, Gu Q, Dong Q, Zhang Z, Lin C, Hu W, Pan R, Guan Y, Hu J (2019) Spermidine enhances Heat Tolerance of Rice Seeds by modulating endogenous starch and polyamine metabolism. Molecules 24(7):1395. https://doi.org/10.3390/molecules24071395
Glyan’ko AK, Kolupaev YuYe, Karpets YuV (2011) Formation of adaptive reactions of plants on the action of abiotic stress factors. Kiev: Osnova, 2010. Appl Biochem Microbiol 47:328–331. https://doi.org/10.1134/S0003683811030069
He Y, Cheng J, He Y, Yang B, Cheng Y, Yang C, Zhang H, Wang Z (2019) Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotechnol J 17:322–337. https://doi.org/10.1111/pbi.12979
Huang J, Cai M, Long Q, Liu L, Lin Q, Jiang L, Chen S, Wan J (2014) OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Res 23:643–655. https://doi.org/10.1007/s11248-014-9803-2
Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H, Walther M (2010) Molecular enzymology of lipoxygenases. Arch Biochem Biophys 503(2):161–174. https://doi.org/10.1016/j.abb.2010.08.016
Kolomiets MV, Hannapel DJ, Chen H, Tymeson M, Gladon RJ (2001) Lipoxygenase is involved in the control of Potato Tuber Development. Plant Cell 13:613–626. https://doi.org/10.1105/tpc.13.3.613
Liavonchanka A, Feussner I (2006) Lipoxygenases: occurrence, functions and catalysis. J Plant Physiol 163(3):348–357. https://doi.org/10.1016/j.jplph.2005.11.006
Liu Y, Chen X, Xue S et al (2021) SET DOMAIN GROUP 721 protein functions in saline–alkaline stress tolerance in the model rice variety kitaake. Plant Biotechnol J 19:2576–2588. https://doi.org/10.1111/pbi.13683
Marla SS, Singh VK (2012) LOX genes in blast fungus (Magnaporthe grisea) resistance in rice. Funct Integr Genomics 12:265–275. https://doi.org/10.1007/s10142-012-0268-1
Melan MA, Dong XN, Endara ME, Davis K, Ausubel FM, Peterman TK (1993) An Arabidopsis thaliana Lipoxygenase Gene can be Induced by Pathogens, Abscisic Acid, and Methyl Jasmonate. Plant Physiol 101(2):441–450. https://doi.org/10.1104/pp.101.2.441
Melan MA, Enriquez A, Peterman TK (1994) The LOX1 gene of Arabidopsis is temporally and spatially regulated in germinating seedlings. Plant Physiol 105(1):385–393. https://doi.org/10.1104/pp.105.1.385
Miura K, Lin SY, Yano M, Nagamine T (2002) Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.). Theor Appl Genet 104(6–7):981–986. https://doi.org/10.1007/s00122-002-0872-x
Parkhey S, Naithani SC, Keshavkant S (2012) ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol Biochem: PPB 57:261–267. https://doi.org/10.1016/j.plaphy.2012.06.008
Peng YL, Shirano Y, Ohta H, Hibino T, Tanaka KS, Shibata D (1994) A novel lipoxygenase from rice. Primary structure and specific expression upon incompatible infection with rice blast fungus. J Biol Chem 269(5):3755–3761
Pokotylo I, Kolesnikov YS, Derevyanchuk M, Kharitonenko AI, Kravets VS (2015) [LIPOXYGENASES AND PLANT CELL METABOLISM REGULATION]. Ukr Biochem J 87(2):41–55
Porta H, Rocha-Sosa M (2002) Plant Lipoxygenases. Physiological and molecular features. Plant Physiol 130:15–21. https://doi.org/10.1104/pp.010787
Porta H, Rueda-Benítez P, Campos F et al (1999) Analysis of lipoxygenase mRNA accumulation in the common bean (Phaseolus vulgaris L.) during development and under stress conditions. Plant Cell Physiol 40(8):850–858. https://doi.org/10.1093/oxfordjournals.pcp.a029614
Priestley DA, Leopold AC (1983) Lipid changes during natural aging of soybean seeds. Physiol Plant 59:467–470. https://doi.org/10.1111/j.1399-3054.1983.tb04231.x
Salama AM, Pearce RS (1993) Ageing of Cucumber and Onion Seeds: phospholipase D, lipoxygenase activity and changes in Phospholipid Content. J Exp Bot 44:1253–1265. https://doi.org/10.1093/jxb/44.8.1253
Santino A, Taurino M, De Domenico S, Bonsegna S, Poltronieri P, Pastor V, Flors V (2013) Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep 32:1085–1098. https://doi.org/10.1007/s00299-013-1441-2
Siedow JN (1991) PLANT LIPOXYGENASE: STRUCTURE AND FUNCTION. Annu Rev Plant Physiol. Plant Mol Biol 42:145–188. https://doi.org/10.1146/annurev.pp.42.060191.001045
Simms D, Cizdziel PE, Chomczyński PA (1993) TRIzol: a new reagent for optimal single-step isolation of RNA
Smith MT, Berjak P (2017) Deteriorative changes Associated with the loss of viability of. Stored Desiccation-Tolerant and Desiccation-Sensitive Seeds
Stelmach BA, Müller A, Hennig P, Gebhardt S, Schubert-Zsilavecz M, Weiler EWJ (2001) A Novel Class of Oxylipins, sn1-O-(12-Oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl Diglyceride, from Arabidopsis thaliana. J Biol Chem 276:12832–12838. https://doi.org/10.1074/jbc.M010743200
Takano K (1993) Advances in cereal chemistry and technology in Japan. Cereal Foods World
Tang L, Cai H, Zhai H, Luo X, Wang Z, Cui L, Bai X (2014) Overexpression of Glycine soja WRKY20 enhances both drought and salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Cell Tiss Organ Cult 118:77–86. https://doi.org/10.1007/s11240-014-0463-y
Umate P (2011) Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signal Behav 6(3):335–338. https://doi.org/10.4161/psb.6.3.13546
Vertucci CW, Roos EE (1990) Theoretical basis of protocols for seed storage. Plant Physiol 94(3):1019–1023. https://doi.org/10.1104/pp.94.3.1019
Viswanath KK, Varakumar P, Pamuru RR, Basha SJ, Mehta S, Rao AD (2020) Plant Lipoxygenases and their role in Plant Physiology. J Plant Biol 63:83–95. https://doi.org/10.1007/s12374-020-09241-x
Xu H, Wei Y, Zhu Y et al (2015) Antisense suppression of LOX3 gene expression in rice endosperm enhances seed longevity. Plant Biotechnol J 13(4):526–539. https://doi.org/10.1111/pbi.12277
Yasumatsu K, Moritaka S (1964) Fatty acid compositions of Rice lipid and their changes during storage. Agric Biol Chem 28:257–264. https://doi.org/10.1080/00021369.1964.10858241
Yasumatsu K, Moritaka S, Wada S (1966) Studies on Cereals Part V:Stale Flavor of Stored Rice. Agric Biol Chem 30:483–486
Zhang Y, Yu Z, Lu Y, Wang Y, She D, Song M, Wu Y (2007) Effect of the absence of lipoxygenase isoenzymes on the storage characteristics of rice grains. J Stored Prod Res 43:87–91. https://doi.org/10.1016/j.jspr.2005.11.004
Zhang Y, Su J, Duan S et al (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30–30. https://doi.org/10.1186/1746-4811-7-30
Zhang Y, Liu Z, Chen Y, He J-X, Bi Y (2015) PHYTOCHROME-INTERACTING FACTOR 5 (PIF5) positively regulates dark-induced senescence and chlorophyll degradation in Arabidopsis. Plant Science 237:57–68 S0168945215001351 https://doi.org/10.1016/j.plantsci.2015.05.010
Acknowledgements
This work was supported by the Key Program of Science and Technology in Fujian Province, China (grant no. 2020NZ08016), the National Major Projects of Cultivated Transgenic New Crop Varieties Foundation of China (grant no. 2016ZX08001-006), the National Rice Industry Technology System of Modern Agriculture for China (grant no. CARS-01-20), the “5511” Collaborative Innovation Project for High-Quality Development and Surpasses of Agriculture between the Government of Fujian and the Chinese Academy of Agricultural Sciences (grant no. XTCXGC2021001) and the Special Foundation of Non-Profit Research Institutes of Fujian Province (grant no. 2020R1023008).
Author information
Authors and Affiliations
Contributions
JZ conceived and designed the project. FW, HX, LZ, YS, YS and XW performed the experiments. QC and HX created the rice genetic materials. JZ and FW wrote the manuscript. JZ revised and edited the manuscript. All authors read and approved the version of the manuscript submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, F., Xu, H., Zhang, L. et al. The lipoxygenase OsLOX10 affects seed longevity and resistance to saline-alkaline stress during rice seedlings. Plant Mol Biol 111, 415–428 (2023). https://doi.org/10.1007/s11103-023-01334-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-023-01334-8