Abstract
Key message
BEIIb plays a specific role in determining the structure of amylopectin in rice endosperm, whereas BEIIa plays the similar role in the culm where BEIIb is absent.
Abstract
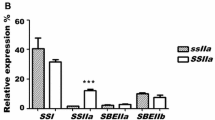
Cereals have three types of starch branching enzymes (BEs), BEI, BEIIa, and BEIIb. It is widely known that BEIIb is specifically expressed in the endosperm and plays a distinct role in the structure of amylopectin because in its absence the amylopectin type changes to the amylose-extender-type (ae-type) or B-type from the wild-type or A-type and this causes the starch crystalline allomorph to the B-type from the wild-type A-type. This study aimed to clarify the role of BEIIa in the culm where BEIIb is not expressed, by using a be2a mutant in comparison with results with be2b and be1 mutants. The results showed that the amylopectin structure exhibited the B-type in the be2a culm compared with the A-type in the wild-type culm. The starch granules from the be2a culm also showed the B-type like allomorph when examined by X-ray diffraction analysis and optical sum frequency generation spectroscopy. Both amylopectin chain-length profile and starch crystalline properties were found to be the A-type at the very early stage of endosperm development at 4–6 days after pollination (DAP) even in the be2b mutant. All these results support a view that in the culm as well as in the endosperm at 4–6 DAP, BEIIa can play the role of BEIIb which has been well documented in maturing endosperm. The possible mechanism as to how BEIIa can play its role is discussed.










Similar content being viewed by others
References
Bogracheva TY, Morris VJ, Ring SG, Hedley CL (1998) The granular structure of C-type pea starch and its role in gelatinization. Biopolymers 45:323–332
Buléon A, Colonna P, Plachot V, Ball S (1998) Starch granules: Structure and biosynthesis. Int J Biol Macromol 23:85–112
Butardo VM, Fitzgerald MA, Bird AR, Gidley MJ, Flanagan BM, Larroque O et al (2011) Impact of down-regulation of starch branching enzyme IIb in rice by artificial microRNA- and hairpin RNA-mediated RNA silencing. J Exp Bot 62:4927–4941
Chonan Y, Matsuba G, Nishida K, Hu W (2021) Crystal methodology of polyurea on rapid quenching. Polymer 213:201
Crofts N, Abe N, Oitome NF, Matsushima R, Hayashi M, Tetlow IJ et al (2015) Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J Exp Bot 66:4469–4482
Flanagan BM, Gidley MJ, Warren FJ (2013) Rapid quantification of starch molecular order through multivariate modelling of 13C CP/MAS NMR spectra. Chem Commun 51(80):14856–14858
French D (1972) Fine structure of starch and its relationship to the organization of starch granules. J Japan Soc Starch Sci 19:8–25
French D (1984) Organization of starch granules. In: Whistler RL, BeMiller JN, Paschall E (eds) Starch: chemistry and technology, 2nd edn. Academic Press, San Diego, pp 183–247
Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140:1070–1084
Gérard C, Planchot V, Colonna P, Bertoft E (2000) Relationship between branching density and crystalline structure of A- and B-type maize mutant starches. Carbohydr Res 326:130–144
Gidley MJ, Bociek SM (1985) Molecular organization in starches: A 13C CP/MAS NMR study. J Am Chem Soc 107:7040–7044
Gidley MJ, Bulpin PV (1987) Crystallization of malto-oligosaccharides at models of the crystalline forms of starch. Carbohydr Res 161:291–300
Guan H, Preiss J (1993) Differentiation of the properties of the branching isozymes from maize (Zea mays). Plant Physiol 102:1269–1273
He W, Wei C (2020) A critical review on structural properties and formation mechanism of heterogeneous starch granules in cereal endosperm lacking starch branching enzyme. Food Hydrocolloids 100:105434
He W, Lin L, Wang J, Zhang L, Liu Q, Wei C (2018) Inhibition of starch branching enzymes in waxy rice increases the proportion of long-chains of amylopectin resulting in the comb-like profiles of starch granules. Plant Sci 277:177–187
Henmi K, Sato H, Matsuba G, Tsuji H, Nishida K, Kanaya T, Toyohara K, Oda A, Endou K (2016) Isothermal crystallization process of poly(l-lactic acid)/ply(d-lactic acid) blends after rapid cooling from the melt. ACS Omega 1:476–482
Hieu HC, Li H, Miyauchi Y, Mizutani G, Fujita N, Nakamura Y (2015) Wetting effect on optical sum frequency generation (SFG) spectra of D-glucose, D-fructose, and sucrose. Spectrochim Acta A138:834–839
Hizukuri S (1986) Polymodal distribution of the chain lengths of amylopectin, and its significance. Carbohydr Res 147:342–347
Horie T, Shiraiwa T, Homma K, Katsura K, Maeda S, Yoshida H (2005) Can yields of lowland rice resume the increases that they showed in the 1980s? Plant Prod Sci 8:259–274
Imberty A, Buléon A, Tran V, Tran V, Pérez S (1991) Recent advances in knowledge of starch structure. Starch/stärke 43:375–384
Jane JL, Wong KS, McPherson AE (1997) Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr Res 300:219–227
Kainuma K, French D (1972) Naegeli amylodextrin and its relationship to starch granule structure. II. Role of water in crystallization of B-starch. Biopolym 11:2241–2250
Kikumoto S, French D (1983) Naegeli amylodextrin: Large scale preparation of fractions by step-wise precipitation using organic solvents. J Jpn Soc Starch Sci 30:69–75
Kong L, Lee C, Kim SH, Ziegler GR (2014) Characterization of starch polymorphic structures using vibrational sum frequency generation spectroscopy. J Phys Chem B 118:1775–1783
Matsuki J, Wada M, Sasaki T, Yoza K, Tokuyasu K (2019) Purification of branched dexrin from Nägeli amylodextrin by ethanol precipitation and characterization of its aggregation property by methanol-water. J Appl Glycosci 66:97–102
Matsushima R, Maekawa M, Sakamoto W (2015) Geometrical formation of compound starch grains in rice implements Voronoi diagram. Plant Cell Physiol 56:2150–2157
Matsushima R, Maekawa M, Kusano M, Tomita K, Kondo H, Nishimura H, Crofts N, Fujita N, Sakamoto W (2016) Amyloplast membrane protein SUBSTANDARD STARCH GRAIN6 controls starch grain size in rice endosperm. Plant Physiol 170:1445–1459
Miyauchi Y, Sano H, Mizutani G (2006) Selective observation of starch in a water plant using optical sum-frequency microscopy. J Opt Soc Am A 23:1687–1690
Morita R, Crofts N, Shibatani N, Miura S, Hosaka Y, Oitome NF, Ikeda K, Fujita N, Fukayama H (2019) CO2-responsive CCT protein stimulates the ectopic expression of particular starch biosynthesis-related enzymes, which markedly change the structure of starch in the leaf sheaths of rice. Plant Cell Physiol 60:961–972
Nagasaki A, Matsuba G, Ikemoto Y, Moriwaki T, Ohta N, Osaka K (2021) Analysis of the sol and gel structures of potato starch over a wide spatial scale. Food Sci Nutr 9:4916–4926
Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: Rice endosperm as a model tissue. Plant Cell Physiol 43:718–725
Nakamura Y (2015) Biosynthesis of reserve starch. In: Nakamura Y (ed) Starch: metabolism and structure. Springer, Tokyo, pp 161–209
Nakamura Y (2018) Rice starch biotechnology: Rice endosperm as a model of cereal endosperms. Starch 70:1600375
Nakamura Y, Kainuma K (2021) On the cluster structure of amylopectin. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01183-3
Nakamura Y, Yuki K, Park SY, Ohya T (1989) Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol 30:833–839
Nakamura Y, Sakurai A, Inaba Y, Kimura K, Iwasawa N, Nagamine T (2002) The fine structure of amylopectin in endosperm from Asian cultivated rice can be largely classified into two classes. Starch 54:117–131
Nakamura Y, Utsumi Y, Sawada T, Aihara S, Utsumi C, Yoshida M, Kitamura S (2010) Characterization of the reactions of starch branching enzyme from rice endosperm. Plant Cell Physiol 51:776–794
Nakamura Y, Aihara S, Crofts N, Sawada T, Fujita N (2014) In vitro studies of enzymatic properties of starch synthases and interactions between starch synthase I and starch branching enzymes in rice. Plant Sci 224:1–8
Nakamura Y, Ono M, Hatta T, Kainuma K, Yashiro K, Matsuba G, Matsubara A, Miyazato A, Mizutani G (2020) Effects of BEIIb-deficiency on the cluster structure of amylopectin and the internal structure of starch granules in endosperm and culm of japonica-type rice. Front Plant Sci 11:571346
Nakamura Y, Ono M, Suto M, Kawashima H (2020b) Analysis of malto-oligosaccharides and related metabolites in rice endosperm during development. Planta 241:110
Nishi A, Nakamura Y, Tanaka N, Satoh H (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127:459–472
O’Shea MG, Samuel MS, Konik CM, Morell MK (1998) Fluorophore-assisted carbohydrate electrophoresis (FACE) of oligosaccharides: efficiency of labeling and high-resolution separation. Carbohydr Res 307:1–12
Ohdan T, Francisco PB Jr, Sawada T, Hirose T, Terao T, Satoh H et al (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56:3229–3244
Peat S, Whelan WJ, Thomas GJ (1952) Evidence of multiple branching in waxy maize starch. J Chem Soc 4546–4548
Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y et al (2003a) Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol 133:1111–1121
Satoh H, Nishi A, Fujita N, Kubo A, Nakamura Y, Kawasaki T, Okita TW (2003b) Isolation and characterization of starch mutants in rice. J Appl Glycosci 50:223–230
Sawada T, Nakamura Y, Ohdan T, Saitoh A, Francisco PB Jr, Suzuki E et al (2014) Diversity of reaction characteristics of glucan branching enzymes and the fine structure of α-glucan from various sources. Arch Biochem Biophys 562:9–21
Sawada T, Itoh M, Nakamura Y (2018) Contributions of three starch branching enzyme isozymes to the fine structure of amylopectin in rice endosperm. Front Plant Sci 9:1536
Shannon JC, Garwood DL, Boyer CD (2009) Genetics and physiology of starch development. In: BeMiller J, Whistler R (eds) In starch, chemistry and technology, 3rd edn. Academic Press, New York, pp 23–82
Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M, Kawasaki S, Nakamura Y (2004) The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotechnol J 2:507–516
Tetlow IJ, Emes MJ (2017) Starch biosynthesis in the developing endosperms of grasses and cereals. Agronomy 7:1
Thompson DB (2000) On the non-random nature of amylopectin branching. Carbohydr Polym 43:223–239
Toyosawa Y, Kawagoe Y, Matsushima R, Crofts N, Ogawa M, Fukuda M, Kumamaru T, Okazaki Y, Kusano M, Saito K, Toyooka K, Sato M, Ai Y, Jane JL, Nakamura Y, Fujita N (2016) Deficiency of starch synthase IIIa and IVb alters starch granule morphology from polyhedral to spherical in rice endosperm. Plant Physiol 170:1255–1270
Wei C, Xu B, Qin F, Yu H, Chen C, Meng X, Zhu L, Wang Y, Gu M, Liu Q (2010) C-type starch from high-amylose rice resistant starch granules modified by antisense RNA inhibition of starch branching enzyme. Agric Food Chem 58:7383–7388
Yamaguchi M, Kainuma K, French D (1979) Electron microscopic observations of waxy maize starch. J Ultrastr Res 69:249–261
Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol 33:985–991
Yano M, Okuno K, Kawakami J, Satoh H, Omura T (1985) High amylose mutants of rice, Oryza sativa L. Theor Appl Genet 69:253–257
Acknowledgements
We thank Dr. Hikaru Satoh for providing us with rice mutants used in this study. We also thank Dr. Naoko Fujita for help to use facilities in Akita Prefectural University, and Dr. Satoko Miura for instruction of the method for measurement of amylose content of the starch sample. This study was funded by JSPS KAKENHI Grant Number JP19H05721 (GMa).
Author information
Authors and Affiliations
Contributions
YN conceived and designed the study. Those who conducted the research and analyzed the data were YN, AK, and MO (biochemical experiments), KY and GMa (XRD), and YW, AMa, and GMi (SFG). JM prepared standard glucans, BD4A and DB4B, whereas YN prepared glucans from rice. YN, JM, KK, GMa, and GMi wrote, read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakamura, Y., Kubo, A., Ono, M. et al. Changes in fine structure of amylopectin and internal structures of starch granules in developing endosperms and culms caused by starch branching enzyme mutations of japonica rice. Plant Mol Biol 108, 481–496 (2022). https://doi.org/10.1007/s11103-021-01237-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01237-6