Abstract
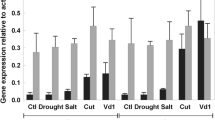
Key message In Verticillium wilt, gene silencing indicates that tomato Ve2-gene expression can have a dramatic effect on many defense/stress protein levels while Ve1-gene induction modulates these effects in a negative fashion.
Abstract
In tomato, Verticillium resistance is dependent on the Ve R-gene locus, which encodes two leucine-rich repeat receptor-like proteins, Ve1 and Ve2. During fungal wilt, Ve1 protein is sharply induced while Ve2 appears expressed constitutively throughout disease development; the disease resistance function usually is attributed to the Ve1 receptor alone. To study Ve2 function, levels of Ve2 mRNA were suppressed using RNAi in both susceptible and resistant Craigella tomato near-isolines and protein changes were evaluated at both the mRNA and protein levels. The results indicate that Ve2-gene expression can have dramatic effects on many defense/stress protein levels while the presence of intact Ve1 protein minimizes these effects in a negative fashion. The data suggest an antagonistic relationship between the Ve proteins in which Ve1 modulates the induction of defense/stress proteins by Ve2.






Similar content being viewed by others
References
Bar M, Sharfman M, Avni A (2011) LeEix1 functions as a decoy receptor to attenuate LeEix2 signaling. Plant Signal Behav 6:455–457
Beckman CH (1987) The nature of wilt diseases of plants. APS Press, St. Paul
Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang X, Sabatino A, Plourde GL (1999) Biochemical characterization of the suberization-associated anionic peroxidase gene of potato. Plant Physiol 121:135–146
Bishop CD, Cooper RM (1983a) An ultrastructural study of vascular colonization in three vascular wilt diseases. I Colonization of susceptible cultivars. Physiol Plant Pathol 23:323–343
Bishop CD, Cooper RM (1983b) An ultrastructure study of root invasion in three vascular wilt diseases. Physiol Plant Pathol 22:15–27
Busch LV, Smith E (1981) Susceptibility of Ontario-grown alflafa cultivars and certain Medicago species to Verticillium albo-atrum. Can J Plant Path 3:169–172
Castroverde CDM, Nazar XX, Robb RN (2017) J: Biotic factors that induce the tomato Ve1 R-gene. Plant Sci 265:61–69
de Jonge R, van Esse HP, Maruthachalam K, Bolton MD, Santhanam P, Saber MK, Zhang Z, Usami T, Lievens B, Subbarao KV, Thomma BPHJ (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc Natl Acad Sci USA 109: 5110–5115
Deng Y, Zhai K, Xie Z, Yang D, Zhu X, Liu J, Wang X, Qin P, Yang Y, Zhang G, Li Q, Zhang J, Wu S, Milazzo J, Mao B, Wang E, Xie H, Tharreau D, He Z (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 335:963–965
Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kühn H, Behrendt A, Dahl SL, Damerell V, Diebel S, Kalman S, Klein S, Knudsen AC, Mäder C, Merrill S, Staudt A, Thiel V, Welti L, Davey NE, Diella F, Gibson T (2016) ELM 2016–data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res 44:D294–D300
Dobinson KF, Tenuta GK, Lazarovits G (1996) Occurrence of race 2 of Verticillium dahliae in processing fields in southwestern Ontario. Can J Plant Pathol 18:55–58
Eckardt NA (2006) Programmed cell death in plants: a role for mitochondrial-associated hexokinases. Plant Cell 18:2097–2099
Fradin EF, Zhang Z, Juarez-Ayala JC, Castroverde CDM, Nazar RN, Robb J, Liu C-M, Thomma BPHJ (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150:320–332
Fradin EF, Zhang Z, Rovenich H, Song Y, Liebrand TW, Masini L, van den Berg GC, Joosten MH, Thomma BPHJ (2014) Functional analysis of the tomato immune receptor Ve1 through domain swaps with its non-functional homologue Ve2. PLoS ONE 9:e88208
Gold J, Robb J (1995) The role of the coating response in Craigella tomatoes infected with Verticillium dahliae, races 1 and 2. Physiol Mol Plant Pathol 47:141–157
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Helliwell C, Waterhouse P (2003) Constructs and methods for high-throughput gene silencing in plants. Methods 30:289–295
Hoagland DR, Arnon DI (1950) The water-culture method of growing plants without soil. California Agricultural Experiment Station, Circular-347
Hu X, Nazar RN, Robb J (1993) Quantification of Verticillium biomass in wilt disease development. Physiol Mol Plant Pathol 42:23–36
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81:802–806
Iida Y, Van‘t Hof P, Beenen H, Kubota M, Stergiopoulos I, Mehrabi R, Notsu A, Fujiwara K, Bahkali A, Abd-Elsalam K, Collemare J, de Wit PJGM (2015) Novel mutations detected in avirulence genes overcoming tomato Cf resistance genes in isolates of a Japanese population of Cladosporium fulvum. PLoS ONE 10:e0123271
Kawchuk L, Hachey J, Lynch DR, Klcsar F, van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, Fischer R, Prufer D (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA 98:6511–6515
Kourelis J, van der Hoorn RAL (2018) Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell. https://doi.org/10.1105/tpc.17.00579
Kruijt M, Kip DJ, Joosten MHAJ, Brandwagt BF, De Wit PJGM (2005) The Cf-4 and Cf-9 resistance genes against Cladosporium fulvum are conserved in wild tomato species mol. Plant Microbe Interact 18:1011–1021
Liebrand TWH, van den Berg GCM, Zhang Z, Smit P, Cordewener JHG, America AHP, Sklenar J, Jones AME, Tameling WIL, Robatzek S, Thomma BPHJ, Joosten MHAJ (2013) Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc of Natl Acad Sci USA 110:10010–10015
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5:81–84
Nazar RN, Xu X, Shittu HO, Kurosky A, Robb EJ (2018a) Tomato Ve resistance locus; defense or growth. Planta 247:1339–1350
Pegg GF, Brady BL (2002) Verticillium wilts. CABI Publishing, Wallingford
Pegg GF, Jonglaekha N (1981) Assessment of colonization in chrysanthemum grown under different photoperiods and infected with Verticillium dahliae. Trans Br Mycol Soc 76:353–360
Pegg GF, Street PFS (1984) Measurement of Verticillium albo-atrum in high and low resistance hop cultivars. Trans Br Mycol Soc 82:99–106
Preston GM (2000) Pseudomonas syringae pv. tomato: the right pathogen, of the right plant, at the right time. Mol Plant Pathol 1:263–275
Robb J, Busch LV (1983) Structure and significance of “blocked” veins in Verticillium-infected chrysanthemum. Physiol Plant Pathol 23:35–53
Robb EJ, Street PFS, Busch LV (1983) Basic fuchsin: a vascular dye in studies of Verticillium-infected chrysanthemum and tomato. Can J Bot 61:3355–3365
Robb EJ, Powell DA, Street PFS (1989) Vascular coating: a barrier to colonization by the pathogen in Verticillium wilt of tomato. Can J Bot 67:600–607
Robb EJ, Lee S-W, Mohan R, Kolattukudy PE (1991) Chemical characterization of stress-induced vascular coating in tomato. Plant Physiol 97:528–536
Robb J, Lee B, Nazar RN (2007) Gene suppression in a tolerant tomato-vascular pathogen interaction. Planta 226:299–309
Robb EJ, Shittu HO, Soman KV, Kurosky A, Nazar RN (2012) Elevated defense protein fails to protect tomato against Verticillium dahliae. Planta 236:623–633
Roberts E, Kutchan T, Kolattukudy PE (1988) Cloning and sequencing of cDNA for a highly anionic peroxidase from potato and the induction of its mRNA in suberizing potato tubers and tomato fruits. Plant Mol Biol 11:15–26
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sharfman M, Bar M, Ehrlich M, Schuster S, Melech-Bonfil S, Ezer R, Sessa S, Avni A (2011) Endosomal signaling of the tomato leucine-rich repeat receptor-like protein LeEix2. Plant J 68:418–423
Shittu HO, Castroverde CDM, Nazar RN, Robb J (2009) Plant-endophyte interplay protects tomato against a virulent Verticillium. Planta 229:415–426
Song Y, Zhang Z, Boshoven JC, Rovenich H, Seidl MF, Jakše J, Maruthachalam K, Liu C-M, Subbarao KV, Javornik B, Thomma BPHJ (2017) Tomato immune receptor Ve1 recognizes surface-exposed co-localized N- and C-termini of Verticillium dahliae effector. Ave1. https://doi.org/10.1101/103473
Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z (2017) agriGO v2.0: a GO analysis toolkit for the gricultural community, 2017 update. Nucleic Acids Res 45:W122–W129
Tsai SJ, Wiltbank MC (1996) Quantification of mRNA using competitive RT-PCR with standard-curve methodology. Biotechniques 21:862–866
Van Ooijin G, Van der Burg HA, Cornelissen BJC, Takken FLW (2007) Structure and function of resistance proteins in Solanaceous plants. Ann Rev Phytopathol 45:43–72
Young DH, Pegg GF (1982) The action of tomato and Verticillium albo-atrum glycosidases on the hyphal wall of Verticillium albo-atrum. Physiol. Plant Pathol 21:411–423
Acknowledgements
We thank Drs. X. Luo and K.V. Soman (UTMB Proteomics Center) for the peptide mass spectrometry and help with the proteomic analyses. This study was supported by NSERC Canada (R.N.N. and J.R.) and NIH, NHLBI (A.K.).
Author information
Authors and Affiliations
Contributions
RNN and JR conceived the experiments and prepared the manuscript, XX conducted the experiments. RNN conducted the data analyses and AK was responsible for the peptide mass spectrometry.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Nazar, R.N., Xu, X., Kurosky, A. et al. Antagonistic function of the Ve R-genes in tomato. Plant Mol Biol 98, 67–79 (2018). https://doi.org/10.1007/s11103-018-0764-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0764-3