Abstract
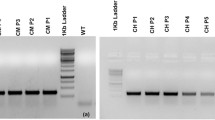
Artificial microRNAs (amiRNA) provide a new feature in the gene silencing era. Concomitantly, reducing the amount of lignin in fiber-yielding plants such as jute holds significant commercial and environmental potential, since this amount is inversely proportional to the quality of the fiber. The present study aimed at reducing the lignin content in jute, by introducing amiRNA based vectors for down-regulation of two monolignoid biosynthetic genes of jute, coumarate 3-hydroxylase (C3H) and ferulate 5-hydroxylase (F5H). The transgenic lines of F5H–amiRNA and C3H–amiRNA showed a reduced level of gene expression, which resulted in about 25 % reduction in acid insoluble lignin content for whole stem and 12–15 % reduction in fiber lignin as compared to the non-transgenic plants. The results indicate successful F5H–amiRNA and C3H–amiRNA transgenesis for lignin reduction in jute. This is likely to have far-reaching commercial implications and economic acceleration for jute producing countries.










Similar content being viewed by others
References
Ai T, Zhang L, Gao Z, Zhu C, Guo X (2011) Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants. Plant Biol 13:304–316
Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, Eshed Y (2006) Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell Online 18:1134–1151
Anderson NA, Chapple C (2014) Perturbing lignin biosynthesis: metabolic changes in response to manipulation of the phenylpropanoid pathway. Rec Adv Polyphen Res 4:39–59
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Baucher M, Halpin Petit-Conil M, Boerjan W (2003) Lignin: genetic engineering and impact on pulping. Crit Rev Biochem Mol Biol 38:305–350
Bhagwat B, Chi M, Su L, Tang H, Tang G, Xiang Y (2013) An in vivo transient expression system can be applied for rapid and effective selection of artificial microRNA constructs for plant stable genetic transformation. J Genet Genom 40:261–270
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Cantó-Pastor A, Mollá-Morales A, Ernst E et al (2015) Efficient transformation and artificial miRNA gene silencing in Lemna minor. Plant Biol 17:59–65
Carbonell A, Takeda A, Fahlgren N et al (2014) New generation of artificial microRNA and synthetic trans-acting small interfering RNA vectors for efficient gene silencing in Arabidopsis. Plant Physiol 165:15–29
Cesarino I, Araújo P, Domingues Júnior AP, Mazzafera P (2012) An overview of lignin metabolism and its effect on biomass recalcitrance. Braz J Bot 35:303–311
Chapple C, Ladisch M, Meilan R (2007) Loosening lignin’s grip on biofuel production. Nat Biotechnol 25:746–748
Chen PY, Wang CK, Soong SC, To KY (2003) Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol Breed 11:287–293
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Das SS, Sanan-Mishra N (2015) A direct method for genetically transforming rice seeds modelled with FHVB2, a suppressor of RNAi. Plant Cell Tissue Organ Cult (PCTOC) 120(1):277–289
Day A, Ruel K, Neutelings G, Crônier D et al (2005) Lignification in the flax stem: evidence for an unusual lignin in bast fibers. Planta 222:234–245
Del Rio JC, Rencoret J, Marques G (2009) Structural characterization of the lignin from jute (Corchorus capsularis) fibers. J Agric Food Chem 57:10271–10281
Eamens AL, Agius C, Smith NA, Waterhouse PM, Wang MB (2010) Efficient silencing of endogenous microRNAs using artificial microRNAs in Arabidopsis thaliana. Mol Plant 4:157–170
Edeerozey AM, Akil HM, Azhar A, Ariffin MZ (2007) Chemical modification of kenaf fibers. Mater Lett 61:2023–2025
Esteves B, Marques AV, Domingos I, Pereira H (2007) Influence of steam heating on the properties of pine (Pinus pinaster) and eucalypt (Eucalyptus globulus) wood. Wood Sci Technol 41:193–207
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Franke R, McMichael CM, Meyer K, Shirley AM, Cusumano JC, Chapple C (2000) Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. Plant J 22:223–234
Giwa A, Akwu P (2007) Enhancement of colourfastness properties of direct dyed jute fabric by ammonium molybdate. Niger J Sci 41:57–64
Halpin C (2004) Re-designing lignin for industry and agriculture. Biotechnol Genet Eng 21:229–248
Hammond SM, Bernstein E, Beach D, Hannon GJ (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293–296
Haney CH, Long SR (2010) Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc Natl Acad Sci 107:478–483
Hauser F, Chen W, Deinlein U, Chang K et al (2013) A genomic-scale artificial microRNA library as a tool to investigate the functionally redundant gene space in Arabidopsis. Plant Cell Online 25:2848–2863
Huntley SK, Ellis D, Gilbert M, Chapple C, Mansfield SD (2003) Significant increases in pulping efficiency in C4H-F5H-transformed poplars: improved chemical savings and reduced environmental toxins. J Agric Food Chem 51:6178–6183
Islam A, Sarkanen KV (1993) The isolation and characterization of the lignins of jute (Corchorus capsularis). Holzforsch Int J Biol Chem Phys Technol Wood 47:123–132
Jelly NS, Schellenbaum P, Walter B, Maillot P (2012) Transient expression of artificial microRNAs targeting grapevine fanleaf virus and evidence for RNA silencing in grapevine somatic embryos. Transgenic Res 21:1319–1327
Johansen LK, Carrington JC (2001) Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol 126:930–938
Karjee S, Islam MN, Mukherjee SK (2008) Screening and identification of virus-encoded RNA silencing suppressors. Methods Mol Biol 442:187–203
Khraiwesh B, Ossowski S, Weigel D, Reski R, Frank W (2008) Specific gene silencing by artificial microRNAs in Physcomitrella patens: an alternative to targeted gene knockouts. Plant Physiol 148:684–693
Kline LM, Hayes DG, Womac AR, Labbe N (2010) Simplified determination of lignin content in hard and soft woods via UV-spectrophotometric analysis of biomass dissolved in ionic liquids. Bio Resour 5:1366–1383
Kung YJ, Lin SS, Huang YL, Chen TC, Harish SS, Chua NH, Yeh SD (2012) Multiple artificial microRNAs targeting conserved motifs of the replicase gene confer robust transgenic resistance to negative-sense single-stranded RNA plant virus. Mol Plant Pathol 13:303–317
Kuroda KI, Izumi A, Mazumder BB, Ohtani Y, Sameshima K (2002) Characterization of kenaf (Hibiscus cannabinus) lignin by pyrolysis-gas chromatography-mass spectrometry in the presence of tetramethylammonium hydroxide. J Anal Appl Pyrolysis 64:453–463
Li X, Tabil LG, Panigrahi S (2007) Chemical treatments of natural fiber for use in natural fiber-reinforced composites: a review. J Polym Environ 15:25–33
Li C, Knierim B, Manisseri C, Arora R et al (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101:4900–4906
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Current protocols in food analytical chemistry (CPFA). Wiley, New York, pp F4.3.1–F4.3.8
Liu Q, Chen YQ (2010) A new mechanism in plant engineering: the potential roles of microRNAs in molecular breeding for crop improvement. Biotechnol Adv 28:301–307
Liu C, Zhang L, Sun J, Luo Y et al (2010) A simple artificial microRNA vector based on ath-miR169d precursor from Arabidopsis. Mol Biol Rep 37:903–909
Maity S, Chowdhury S, Datta AK (2012) Jute biology, diversity, cultivation, pest control, fiber production and genetics. In: Lichtfouse E (ed) Organic fertilisation, soil quality and human health, vol 9. Springer, Netherland, pp 227–262
Meng X, Muszynski MG, Danilevskaya ON (2011) The FT-like ZCN8 gene functions as a floral activator and is involved in photoperiod sensitivity in maize. Plant Cell Online 23:942–960
Michniewicz M, Zago MK, Abas L et al (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130:1044–1056
Molnar A, Bassett A, Thuenemann E, Schwach F et al (2009) Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant J 58:165–174
Niu QW, Lin SS, Reyes JL, Chen KC, Wu HW, Yeh SD, Chua NH (2006) Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat Biotechnol 24(11):1420–1428
Ossowski S, Schwab R, Weigel D (2008) Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53:674–690
Parizotto EA, Dunoyer P, Rahm N, Himber C, Voinnet O (2004) In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev 18:2237–2242
Qu J, Ye J, Fang R (2007) Artificial microRNA-mediated virus resistance in plants. J Virol 81:6690–6699
Ralph J, Akiyama T, Kim H et al (2006) Effects of coumarate 3-hydroxylase down-regulation on lignin structure. J Biol Chem 281:8843–8853
Reddy MS, Chen F, Shadle G, Jackson L, Aljoe H, Dixon RA (2005) Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc Natl Acad Sci USA 102:16573–16578
Sajib AA, Islam MS, Reza MS, Bhowmik A, Fatema L, Khan H (2008) Tissue culture independent transformation for Corchorus olitorius. Plant Cell Tissue Organ Cult 95:333–340
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Laboratory Press, New York
Sarkanen KV, Ludwig CH (1971) Lignins: occurrence, formation, structure and reactions. Wiley-Interscience, New York
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Dev Cell 8:517–527
Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell Online 18:1121–1133
Schwab R, Ossowski S, Warthmann N, Weigel D (2010) Directed gene silencing with artificial microRNAs. In: Plant microRNAs. Methods Mol Biol, vol 592, pp 71–88
Sengupta G, Palit P (2004) Characterization of a lignified secondary phloem fibre-deficient mutant of jute (Corchorus capsularis). Ann Bot 93:211–220
Shi R, Yang C, Lu S, Sederoff R, Chiang VL (2010) Specific down-regulation of PAL genes by artificial microRNAs in Populus trichocarpa. Planta 232:1281–1288
Tang G, Galili G, Zhuang X (2007) RNAi and microRNA: breakthrough technologies for the improvement of plant nutritional value and metabolic engineering. Metabolomics 3:357–369
Tang Y, Wang F, Zhao J, Xie K, Hong Y, Liu Y (2010) Virus-based microRNA expression for gene functional analysis in plants. Plant Physiol 153:632–641
Tanmoy A, Alum M, Islam M, Farzana T, Khan H (2015) Jute (Corchorus olitorius var. O-72) stem lignin: variation in content with age. Bangladesh J Bot 43:309–314
Templeton D, Ehrman T (1995) Determination of acid-insoluble lignin in biomass. In: Laboratory analytical procedure LAP-003. National Renewable Energy Laboratory (NREL), Golden, CO
Tiwari M, Sharma D et al (2014) Artificial microRNA mediated gene silencing in plants: progress and perspectives. Plant J Plant Mol biol 86(1–2):1–18
Toppino L, Kooiker M, Lindner M, Dreni L, Rotino GL, Kater MM (2011) Reversible male sterility in eggplant (Solanum melongena L.) by artificial microRNA-mediated silencing of general transcription factor genes. Plant Biotechnol J 9:684–692
Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, Boerjan W (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196:978–1000
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136(4):669–687
Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33:949–956
Wang L, Luo YZ, Zhang L, Jiao XM, Wang MB, Fan YL (2008) Rolling circle amplification-mediated hairpin RNA (RMHR) library construction in plants. Nucleic Acids Res 36:e149
Warthmann N, Chen H, Ossowski S, Weigel D, Hervé P (2008) Highly specific gene silencing by artificial miRNAs in rice. PLoS One 3:e1829
Warthmann N, Ossowski S, Schwab R, Weigel D (2013) Artificial microRNAs for specific gene silencing in rice. Methods Mol Biol 956:131–149
Yan H, Deng X, Cao Y, Huang J, Ma L, Zhao B (2011) A novel approach for the construction of plant amiRNA expression vectors. J Biotechnol 151:9–14
Zhu L, O’Dwyer JP, Chang VS, Granda CB, Holtzapple MT (2008) Structural features affecting biomass enzymatic digestibility. Bioresour Technol 99:3817–3828
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgements
The authors would like to thank Prof. Sudhir K. Sopory and V. S. Chauhan of ICGEB, New Delhi for providing Farhana Shafrin the facilities required for her stay and work at the ICGEB. Farhana Shafrin gratefully acknowledges the Bangladesh Academy of Sciences (BAS) for the fellowship she received as a Ph.D. Research Fellow. The authors also thank the BJRI (Bangladesh Jute Research Institute) for providing the jute seeds. We also appreciate Md. Tariqul Islam and Ahlan Sabah Ferdous for their kind support. The research was supported by a financial grant received from the BAS-USDA-PALS Program.
Author contributions
FS, HK and N. Sanan-Mishra designed the experiment. FS performed the experimental research, data analysis and manuscript writing. HK and N. Sanan-Mishra performed the manuscript editing. SSD contributed during Southern, northern and modified 5′ RACE experiment and analysis with FS. All the authors have approved the final manuscript for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shafrin, F., Das, S.S., Sanan-Mishra, N. et al. Artificial miRNA-mediated down-regulation of two monolignoid biosynthetic genes (C3H and F5H) cause reduction in lignin content in jute. Plant Mol Biol 89, 511–527 (2015). https://doi.org/10.1007/s11103-015-0385-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0385-z