Abstract
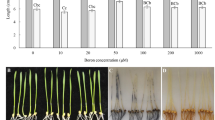
Boron (B) deficiency is a worldwide problem, and Brassica napus is one of the most sensitive crops to B deficiency. To better understand the B starvation response of Brassica napus, we conducted a comparative proteomic analysis of seedling stage Brassica napus root between B-sufficient and B-limited conditions: 45 differentially expressed proteins were successfully identified by 2-DE coupled with MALDI-TOF/TOF-MS and LTQ-ESI-MS/MS analysis. Among these proteins, 10 were down-regulated and 35 were up-regulated under B-limited condition. Combining GO and KEGG analyses with data from previous reports, proteins were categorized into several functional groups, including antioxidant and detoxification, defense-related proteins, signaling and regulation, carbohydrate and energy metabolism, amino acid and fatty acid metabolism, protein translation and degradation, cell wall structure, and transporter. The genes of selected proteins were analyzed by quantitative RT-PCR. Our results provide novel information for better understanding the physiological and biochemical responses to B deficiency in plants.




Similar content being viewed by others
Abbreviations
- AdoHcy:
-
S-adenosyl-l-homocysteine
- AdoMet:
-
S-adenosylmethionine
- APX:
-
Ascorbate peroxidase
- cAcn:
-
Aconitate hydratase
- FBPase:
-
Fructose-1:6-bisphosphatase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GSH:
-
Glutathione
- GST:
-
Glutathione S-transferase
- G6PDH:
-
Glucose 6-phosphate dehydrogenase
- LTQ:
-
Linear ion trap quadrupole
- PGL:
-
6-Phosphogluconolactonase
- PLD:
-
Phospholipase D
- SOD:
-
Superoxide dismutase
- PPP:
-
Pentose phosphate pathway
References
Alves M, Francisco R, Martins I et al (2006) Analysis of Lupinus albus leaf apoplastic proteins in response to boron deficiency. Plant Soil 279:1–11
Armengaud P, Breitling R, Amtmann A (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136:2556–2576
Baxter CJ, Redestig H, Schauer N et al (2007) The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiol 143:312–325
Blevins DG, Lukaszewski KM (1998) Boron in plant structure and function. Annu Rev Plant Physiol Plant Mol Biol 49:481–500
Bongani KN, Stephen C, William JS et al (2005) Identification of Arabidopsis salt and osmotic stress responsive proteins using two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics 5:4185–4196
Brown PH, Bellaloui N, Wimmer MA et al (2002) Boron in plant biology. Plant Biol 4:205–223
Cakmak I, Römheld V (1997) Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 193:71–83
Camacho-Cristóbal JJ, González-Fontes A (2007) Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 226:443–451
Camacho-Cristóbal JJ, Lunar L, Lafont F et al (2004) Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J Plant Physiol 161:879–881
Camacho-Cristóbal JJ, Herrera-Rodríguez MB, Beato VM et al (2008) The expression of several cell wall-related genes in Arabidopsis roots is down-regulated under boron deficiency. Environ Exp Bot 63:351–358
Carrari F, Nunes-Nesi A, Gibon Y et al (2003) Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol 133:1322–1335
Dell B, Huang L (1997) Physiological response of plants to low boron. Plant Soil 193:103–120
Delmas F, Seveno M, Northey JGB et al (2008) The synthesis of the rhamnogalacturonan II component 3-deoxy-d-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. J Exp Bot 59:2639–2647
Dixon D, Lapthorn A, Edwards R (2002) Plant glutathione transferases. Genome Biol 3:3001–3010
Dordas C, Brown PH (2005) Boron deficiency affects cell viability, phenolic leakage and oxidative burst in rose cell cultures. Plant Soil 268:293–301
Du J, Huang Y-P, Xi J et al (2008) Functional gene-mining for salt-tolerance genes with the power of Arabidopsis. Plant J 56:653–664
Feng H, Chen Q, Feng J et al (2007) Functional characterization of the Arabidopsis eukaryotic translation initiation factor 5A-2 that plays a crucial role in plant growth and development by regulating cell division, cell growth, and cell death. Plant Physiol 144:1531–1545
Frans JMM, Victor F, Pawel H et al (2003) Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 35:675–692
Fujita M, Fujita Y, Noutoshi Y et al (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Gorecka KM, Thouverey C, Buchet R et al (2007) Potential role of annexin annAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant Cell Physiol 48:792–803
Han S, Chen L-S, Jiang H-X et al (2008) Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J Plant Physiol 165:1331–1341
Hou FY, Huang J, Yu SL et al (2007) The 6-phosphogluconate dehydrogenase genes are responsive to abiotic stresses in rice. J Integr Plant Biol 49:655–663
Ishii T, Matsunaga T, Hayashi N (2001) Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol 126:1698–1705
Jan T, Erik A, Susanna E et al (1997) Regulation of the wound-induced myrosinase-associated protein transcript in Brassica napus plants. Eur J Biochem 247:963–971
Jean-Emmanuel S, Lauriane K, Céline D et al (2006) The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 6:2180–2198
Jones AM (2002) G-protein-coupled signaling in Arabidopsis. Curr Opin Plant Biol 5:402–407
Jun XY, Robin W, Tom B et al (2000) A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization- mass spectrometry. Electrophoresis 21:3666–3672
Kiddle GA, Bennett BN, Hick AJ et al (1999) C-S lyase activities in leaves of crucifers and non-crucifers, and the characterization of three classes of C-S lyase activities from oilseed rape (Brassica napus L.). Plant Cell Environ 22:433–445
Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110:1017–1020
Kobayashi M, Mutoh T, Matoh T (2004) Boron nutrition of cultured tobacco BY-2 cells IV. Genes induced under low boron supply. J Exp Bot 55:1441–1443
Kruger NJ, von Schaewen A (2003) The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol 6:236–246
Less H, Galili G (2008) Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol 147:316–330
Li J, Wu XD, Hao ST et al (2008) Proteomic response to iron deficiency in tomato root. Proteomics 8:2299–2311
Li M, Hong Y, Wang X (2009) Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochimica et Biophysica Acta (BBA) Mol Cell Biol L 1791:927–935
Loenen WAM (2006) S-adenosylmethionine: jack of all trades and master of everything? Biochem Soc Trans 34:330–333
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, San Diego, CA, pp 379–396
Matsuura K, Miyagawa I, Kobayashi M et al (2003) Arabidopsis 3-deoxy-D-manno-oct-2-ulosonate-8-phosphate synthase: cDNA cloning and expression analyses. J Exp Bot 54:1785–1787
Misson J, Raghothama KG, Jain A et al (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci 102:11934–11939
Moeder W, del Pozo O, Navarre D et al (2007) Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol Biol 63:273–287
Moffatt BA, Stevens YY, Allen MS et al (2002) Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol 128:812–821
Morant AV, Jørgensen K, Jørgensen C et al (2008) Beta-glucosidases as detonators of plant chemical defense. Phytochem 69:1795–1813
Nagib A, Dong-Gi L, Iftekhar A et al (2008) Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during as stress. Proteomics 8:3561–3576
Nutricati E, Miceli A, Blando F et al (2006) Characterization of two Arabidopsis thaliana glutathione S -transferases. Plant Cell Rep 25:997–1005
O’Neill MA, Warrenfeltz D, Kates K et al (1996) Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem 271:22923–22930
O’Neill MA, Eberhard S, Albersheim P et al (2001) Requirement of borate cross-linking of cell wall Rhamnogalacturonan II for Arabidopsis Growth. Science 294:846–849
Pradet-Balade B, Boulm F, Beug H et al (2001) Translation control: bridging the gap between genomics and proteomics? Trends Biochem Sci 26:225–229
Qiaosong Y, Yuqi W, Jianjun Z et al (2007) Identification of aluminum-responsive proteins in rice roots by a proteomic approach: cysteine synthase as a key player in Al response. Proteomics 7:737–749
Rüdiger H, Gabius H-J (2001) Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconjugate J 18:589–613
Shorrocks VM (1997) The occurrence and correction of boron deficiency. Plant Soil 193:121–148
Shu S, Mahadeo DC, Liu X et al (2006) S-adenosylhomocysteine hydrolase is localized at the front of chemotaxing cells, suggesting a role for transmethylation during migration. Proc Natl Acad Sci 103:19788–19793
Sondergaard TE, Schulz A, Palmgren MG (2004) Energization of transport processes in plants. Roles of the plasma membrane H+ -ATPase. Plant Physiol 136:2475–2482
Sunkar R, Kapoor A, Zhu J-K (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18:2051–2065
Sweetlove LJ, Heazlewood JL, Herald V et al (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32:891–904
Taipalensuu J, Falk A, Rask L (1996) A wound- and methyl jasmonate-inducible transcript coding for a myrosinase-associated protein with similarities to an early nodulin. Plant Physiol 110:483–491
Takano J, Noguchi K, Yasumori M et al (2002) Arabidopsis boron transporter for xylem loading. Nature 420:337–340
Takano J, Miwa K, Yuan L et al (2005) Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci 102:12276–12281
Takano J, Wada M, Ludewig U et al (2006) The Arabidopsis major intrinsic protein nip5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18:1498–1509
Tanaka M, Fujiwara T (2008) Physiological roles and transport mechanisms of boron: perspectives from plants. Eur J Physiol 456:671–677
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biol 16:123–132
Warington K (1923) The effect of boric acid and borax on the broad bean and certain other plants. Ann Bot 37:629–672
Warren A, Olivier L, Johann J et al (2009) Comparative proteomics of leaf, stem, and root tissues of synthetic Brassica napus. Proteomics 9:793–799
Wei W, Rita V, Monica S et al (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27:2782–2786
Wimmer MA, Lochnit G, Bassil E et al (2009) Membrane-associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant Cell Physiol 50:1292–1304
Xu FS, Yang YH, Wang YH (2002) Boron uptake and retranslocation in cultivars of Brassica napus differing in boron efficiency. In: Goldbach HeinerE et al (eds) Boron in plant and animal nutrition. Kluwer Academic/Plenum Publisher, New York, pp 127–135
Xu Z, Escamilla-Treviño L, Zeng L et al (2004) Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol Biol 55:343–367
Yuko S-S, Nozomi T, Takeshi O et al (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:653–668
Zhe W, Wei H, Qishan L et al (2009) Understanding rice plant resistance to the Brown Planthopper (Nilaparvata lugens): a proteomic approach. Proteomics 9:2798–2808
Acknowledgments
This work was supported by grants from the National 863 High Technology Program (2007AA10Z117), the National Natural Science Foundation of China (30771283), and the Specialized Research Fund for the Doctoral Program of Higher Education (20090504009), China. We thank the two anonymous reviewers for the critical comments and valuable suggestions for revising the paper.
Conflicts of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, Z., Shi, L. et al. Proteomic alterations of Brassica napus root in response to boron deficiency. Plant Mol Biol 74, 265–278 (2010). https://doi.org/10.1007/s11103-010-9671-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9671-y