Abstract
Purpose
Hyponatremia after pituitary surgery is a frequent finding with potential severe complications and the most common cause for readmission. Several studies have found parameters associated with postoperative hyponatremia, but no reliable specific predictor was described yet. This pilot study evaluates the feasibility of machine learning (ML) algorithms to predict postoperative hyponatremia after resection of pituitary lesions.
Methods
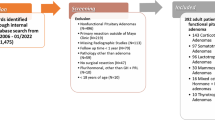
Retrospective screening of a prospective registry of patients who underwent transsphenoidal surgery for pituitary lesions. Hyponatremia within 30 days after surgery was the primary outcome. Several pre- and intraoperative clinical, procedural and laboratory features were selected to train different ML algorithms. Trained models were compared using common performance metrics. Final model was internally validated on the testing dataset.
Results
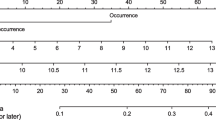
From 207 patients included in the study, 44 (22%) showed a hyponatremia within 30 days postoperatively. Hyponatremic measurements peaked directly postoperatively (day 0–1) and around day 7. Bootstrapped performance metrics of different trained ML-models showed largest area under the receiver operating characteristic curve (AUROC) for the boosted generalized linear model (67.1%), followed by the Naïve Bayes classifier (64.6%). The discriminative capability of the final model was assessed by predicting on unseen dataset. Large AUROC (84.3%; 67.0–96.4), sensitivity (81.8%) and specificity (77.5%) with an overall accuracy of 78.4% (66.7–88.2) was reached.
Conclusion
Our trained ML-model was able to learn the complex risk factor interactions and showed a high discriminative capability on unseen patient data. In conclusion, ML-methods can predict postoperative hyponatremia and thus potentially reduce morbidity and improve patient safety.


Similar content being viewed by others
Availability of data and material
Anonymized raw data files and custom-made code are available from the corresponding author upon reasonable request.
References
Little AS, Kelly DF, White WL, Gardner PA, Fernandez-Miranda JC, Chicoine MR, Barkhoudarian G, Chandler JP, Prevedello DM, Liebelt BD, Sfondouris J, Mayberg MR, Group TS (2019) Results of a prospective multicenter controlled study comparing surgical outcomes of microscopic versus fully endoscopic transsphenoidal surgery for nonfunctioning pituitary adenomas: the Transsphenoidal Extent of Resection (TRANSSPHER) Study. J Neurosurg. https://doi.org/10.3171/2018.11.jns181238
Agam M, Wedemeyer MA, Carmichael JD, Weiss MH, Zada G (2017) 152 Complications associated with transsphenoidal pituitary surgery: experience of 1171 consecutive cases treated at a single tertiary care pituitary center. Neurosurgery 64:237–237. https://doi.org/10.1093/neuros/nyx417.152
Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G (2018) Complications associated with microscopic and endoscopic transsphenoidal pituitary surgery: experience of 1153 consecutive cases treated at a single tertiary care pituitary center. J Neurosurg. https://doi.org/10.3171/2017.12.jns172318
Patel KS, Shu Chen J, Yuan F, Attiah M, Wilson B, Wang MB, Bergsneider M, Kim W (2019) Prediction of post-operative delayed hyponatremia after endoscopic transsphenoidal surgery. Clin Neurol Neurosurg 182:87–91. https://doi.org/10.1016/j.clineuro.2019.05.007
Hussain NS, Piper M, Ludlam WG, Ludlam WH, Fuller CJ, Mayberg MR (2013) Delayed postoperative hyponatremia after transsphenoidal surgery: prevalence and associated factors. J Neurosurg 119(6):1453–1460. https://doi.org/10.3171/2013.8.JNS13411
Zada G, Liu CY, Fishback D, Singer PA, Weiss MH (2007) Recognition and management of delayed hyponatremia following transsphenoidal pituitary surgery. J Neurosurg 106(1):66–71. https://doi.org/10.3171/jns.2007.106.1.66
Tomita Y, Kurozumi K, Inagaki K, Kameda M, Ishida J, Yasuhara T, Ichikawa T, Sonoda T, Otsuka F, Date I (2019) Delayed postoperative hyponatremia after endoscopic transsphenoidal surgery for pituitary adenoma. Acta Neurochir (Wien) 161(4):707–715. https://doi.org/10.1007/s00701-019-03818-3
Krogh J, Kistorp CN, Jafar-Mohammadi B, Pal A, Cudlip S, Grossman A (2018) Transsphenoidal surgery for pituitary tumours: frequency and predictors of delayed hyponatraemia and their relationship to early readmission. Eur J Endocrinol 178(3):247–253. https://doi.org/10.1530/EJE-17-0879
Zoli M, Mazzatenta D, Faustini-Fustini M (2016) Transient delayed hyponatremia after transsphenoidal surgery: attempting to enlighten the epidemiology and management of a still-obscure complication. World Neurosurg 90:654–656. https://doi.org/10.1016/j.wneu.2016.02.015
Barber SM, Liebelt BD, Baskin DS (2014) Incidence, etiology and outcomes of hyponatremia after transsphenoidal surgery: experience with 344 consecutive patients at a single tertiary center. J Clin Med 3(4):1199–1219. https://doi.org/10.3390/jcm3041199
Kinoshita Y, Tominaga A, Arita K, Sugiyama K, Hanaya R, Hama S, Sakoguchi T, Usui S, Kurisu K (2011) Post-operative hyponatremia in patients with pituitary adenoma: post-operative management with a uniform treatment protocol. Endocr J 58(5):373–379. https://doi.org/10.1507/endocrj.k10e-352
Kristof RA, Rother M, Neuloh G, Klingmuller D (2009) Incidence, clinical manifestations, and course of water and electrolyte metabolism disturbances following transsphenoidal pituitary adenoma surgery: a prospective observational study. J Neurosurg 111(3):555–562. https://doi.org/10.3171/2008.9.JNS08191
Hensen J, Henig A, Fahlbusch R, Meyer M, Boehnert M, Buchfelder M (1999) Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin Endocrinol (Oxf) 50(4):431–439. https://doi.org/10.1046/j.1365-2265.1999.00666.x
Boscoe A, Paramore C, Verbalis JG (2006) Cost of illness of hyponatremia in the United States. Cost Eff Resour Alloc 4:10. https://doi.org/10.1186/1478-7547-4-10
Hollon TC, Parikh A, Pandian B, Tarpeh J, Orringer DA, Barkan AL, McKean EL, Sullivan SE (2018) A machine learning approach to predict early outcomes after pituitary adenoma surgery. Neurosurg Focus 45(5):E8. https://doi.org/10.3171/2018.8.FOCUS18268
Cote DJ, Alzarea A, Acosta MA, Hulou MM, Huang KT, Almutairi H, Alharbi A, Zaidi HA, Algrani M, Alatawi A, Mekary RA, Smith TR (2016) Predictors and rates of delayed symptomatic hyponatremia after transsphenoidal surgery: a systematic review [corrected]. World Neurosurg 88:1–6. https://doi.org/10.1016/j.wneu.2016.01.022
Deaver KE, Catel CP, Lillehei KO, Wierman ME, Kerr JM (2018) Strategies to reduce readmissions for hyponatremia after transsphenoidal surgery for pituitary adenomas. Endocrine 62(2):333–339. https://doi.org/10.1007/s12020-018-1656-7
Jahangiri A, Wagner J, Tran MT, Miller LM, Tom MW, Kunwar S, Blevins L Jr, Aghi MK (2013) Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations. J Neurosurg 119(6):1478–1483. https://doi.org/10.3171/2013.7.JNS13273
van Niftrik CHB, van der Wouden F, Staartjes VE, Fierstra J, Stienen MN, Akeret K, Sebok M, Fedele T, Sarnthein J, Bozinov O, Krayenbuhl N, Regli L, Serra C (2019) Machine learning algorithm identifies patients at high risk for early complications after intracranial tumor surgery: registry-based cohort study. Neurosurgery 85(4):E756–E764. https://doi.org/10.1093/neuros/nyz145
Senders JT, Staples PC, Karhade AV, Zaki MM, Gormley WB, Broekman MLD, Smith TR, Arnaout O (2018) Machine learning and neurosurgical outcome prediction: a systematic review. World Neurosurg 109:476–486. https://doi.org/10.1016/j.wneu.2017.09.149
Serra C, Burkhardt JK, Esposito G, Bozinov O, Pangalu A, Valavanis A, Holzmann D, Schmid C, Regli L (2016) Pituitary surgery and volumetric assessment of extent of resection: a paradigm shift in the use of intraoperative magnetic resonance imaging. Neurosurg Focus 40(3):E17. https://doi.org/10.3171/2015.12.FOCUS15564
Maldaner N, Serra C, Tschopp O, Schmid C, Bozinov O, Regli L (2018) Modern management of pituitary adenomas: current state of diagnosis, treatment and follow-up. Praxis (Bern 1994) 107(15):825–835. https://doi.org/10.1024/1661-8157/a003035
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP (2002) SMOTE: synthetic minority over-sampling technique. J Artif Int Res 16(1):321–357
Menardi G, Torelli N (2014) Training and assessing classification rules with imbalanced data. Data Min Knowl Disc 28(1):92–122. https://doi.org/10.1007/s10618-012-0295-5
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617. https://doi.org/10.1227/00006123-199310000-00008
Hardy J, Vezina JL (1976) Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 15:261–273
Serra C, Staartjes VE, Maldaner N, Muscas G, Akeret K, Holzmann D, Soyka MB, Schmid C, Regli L (2018) Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir (Wien) 160(11):2255–2262. https://doi.org/10.1007/s00701-018-3690-x
Samuel AM, Grant RA, Bohl DD, Basques BA, Webb ML, Lukasiewicz AM, Diaz-Collado PJ, Grauer JN (2015) Delayed surgery after acute traumatic central cord syndrome is associated with reduced mortality. Spine (Phila Pa 1976) 40(5):349–356. https://doi.org/10.1097/brs.0000000000000756
Zada G, Woodmansee WW, Iuliano S, Laws ER (2010) Perioperative management of patients undergoing transsphenoidal pituitary surgery. Asian J Neurosurg 5(1):1–6
Kelly DF, Laws ER Jr, Fossett D (1995) Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma. Report of nine cases. J Neurosurg 83(2):363–367. https://doi.org/10.3171/jns.1995.83.2.0363
Burke WT, Cote DJ, Iuliano SI, Zaidi HA, Laws ER (2018) A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary 21(1):25–31. https://doi.org/10.1007/s11102-017-0843-5
Skinner DC (2009) Rethinking the stalk effect: a new hypothesis explaining suprasellar tumor-induced hyperprolactinemia. Med Hypotheses 72(3):309–310. https://doi.org/10.1016/j.mehy.2008.08.030
Kruse A, Astrup J, Gyldensted C, Cold GE (1995) Hyperprolactinaemia in patients with pituitary adenomas. The pituitary stalk compression syndrome. Br J Neurosurg 9(4):453–457. https://doi.org/10.1080/02688699550041089
Olson BR, Gumowski J, Rubino D, Oldfield EH (1997) Pathophysiology of hyponatremia after transsphenoidal pituitary surgery. J Neurosurg 87(4):499–507. https://doi.org/10.3171/jns.1997.87.4.0499
Kovacs KJ, Foldes A, Sawchenko PE (2000) Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci 20(10):3843–3852
Kalogeras KT, Nieman LK, Friedman TC, Doppman JL, Cutler GB Jr, Chrousos GP, Wilder RL, Gold PW, Yanovski JA (1996) Inferior petrosal sinus sampling in healthy subjects reveals a unilateral corticotropin-releasing hormone-induced arginine vasopressin release associated with ipsilateral adrenocorticotropin secretion. J Clin Invest 97(9):2045–2050. https://doi.org/10.1172/JCI118640
Oelkers W (1989) Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med 321(8):492–496. https://doi.org/10.1056/NEJM198908243210802
Green HH, Harrington AR, Valtin H (1970) On the role of antidiuretic hormone in the inhibition of acute water diuresis in adrenal insufficiency and the effects of gluco- and mineralocorticoids in reversing the inhibition. J Clin Invest 49(9):1724–1736. https://doi.org/10.1172/JCI106390
Kleeman CR, Czaczkes JW, Cutler R (1964) Mechanisms of impaired water excretion in adrenal and pituitary insufficiency. IV. Antidiuretic hormone in primary and secondary adrenal insufficiency. J Clin Invest 43:1641–1648. https://doi.org/10.1172/jci105039
Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH (2016) Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 101(11):3888–3921. https://doi.org/10.1210/jc.2016-2118
Amann K, Benz K (2013) Structural renal changes in obesity and diabetes. Semin Nephrol 33(1):23–33. https://doi.org/10.1016/j.semnephrol.2012.12.003
Coiro V, Chiodera P (1987) Effect of obesity and weight loss on the arginine vasopressin response to insulin-induced hypoglycaemia. Clin Endocrinol (Oxf) 27(2):253–258. https://doi.org/10.1111/j.1365-2265.1987.tb01151.x
Kuhn M, Johnson K (2013) Applied predictive modeling, vol 26. Springer, Berlin
Senders JT, Arnaout O, Karhade AV, Dasenbrock HH, Gormley WB, Broekman ML, Smith TR (2018) Natural and artificial intelligence in neurosurgery: a systematic review. Neurosurgery 83(2):181–192. https://doi.org/10.1093/neuros/nyx384
Staartjes VE, Serra C, Muscas G, Maldaner N, Akeret K, van Niftrik CHB, Fierstra J, Holzmann D, Regli L (2018) Utility of deep neural networks in predicting gross-total resection after transsphenoidal surgery for pituitary adenoma: a pilot study. Neurosurg Focus 45(5):E12. https://doi.org/10.3171/2018.8.FOCUS18243
Staartjes VE, Zattra CM, Akeret K, Maldaner N, Muscas G, Bas van Niftrik CH, Fierstra J, Regli L, Serra C (2019) Neural network-based identification of patients at high risk for intraoperative cerebrospinal fluid leaks in endoscopic pituitary surgery. J Neurosurg. https://doi.org/10.3171/2019.4.jns19477
Liu Y, Liu X, Hong X, Liu P, Bao X, Yao Y, Xing B, Li Y, Huang Y, Zhu H, Lu L, Wang R, Feng M (2019) Prediction of recurrence after transsphenoidal surgery for cushing’s disease: the use of machine learning algorithms. Neuroendocrinology 108(3):201–210. https://doi.org/10.1159/000496753
Funding
No funding was received for this research
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No Conflicts of Interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and local ethics review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study and data was treated according to the local ethical regulations and the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Voglis, S., van Niftrik, C.H.B., Staartjes, V.E. et al. Feasibility of machine learning based predictive modelling of postoperative hyponatremia after pituitary surgery. Pituitary 23, 543–551 (2020). https://doi.org/10.1007/s11102-020-01056-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01056-w