Abstract
Purpose
Given the paucity of reliable predictors of tumor recurrence, progression, or response to somatostatin receptor ligand (SRL) therapy in acromegaly, we attempted to determine whether preoperative MR image texture was predictive of these clinical outcomes. We also determined whether image texture could differentiate somatotroph adenomas from non-functioning pituitary adenomas (NFPAs).
Methods
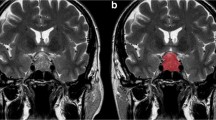
We performed a retrospective study of patients with acromegaly due to a macroadenoma who underwent transsphenoidal surgery at our institution between 2007 and 2015. Clinical data were extracted from electronic medical records. MRI texture analysis was performed on preoperative non-enhanced T1-weighted images using ImageJ (NIH). Logistic and Cox models were used to determine if image texture parameters predicted outcomes.
Results
Eighty-nine patients had texture parameters measured, which were compared to that of NFPAs, while 64 of these patients had follow-up and were included in the remainder of analyses. Minimum pixel intensity, skewness, and kurtosis were significantly different in somatotroph adenomas versus NFPAs (area under the receiver operating characteristic curve, 0.7771, for kurtosis). Furthermore, those with a maximum pixel intensity above the median had an increased odds of IGF-I normalization on SRL therapy (OR 5.96, 95% CI 1.33–26.66), which persisted after adjusting for several potential predictors of response. Image texture did not predict tumor recurrence or progression.
Conclusion
Our data suggest that MRI texture analysis can distinguish NFPAs from somatotroph macroadenomas with good diagnostic accuracy and can predict normalization of IGF-I with SRL therapy.




Similar content being viewed by others
References
Katznelson L, Laws JER, Melmed S, Molitch ME, Murad MH, Utz A, Wass JAH (2014) Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Agustsson TT, Baldvinsdottir T, Jonasson JG, Olafsdottir E, Steinthorsdottir V, Sigurdsson G, Thorsson AV, Carroll PV, Korbonits M, Benediktsson R (2015) The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol 173(5):655–664. https://doi.org/10.1530/eje-15-0189
Bex M, Abs R, T’Sjoen G, Mockel J, Velkeniers B, Muermans K, Maiter D (2007) AcroBel - the Belgian registry on acromegaly: a survey of the ‘real-life’ outcome in 418 acromegalic subjects. Eur J Endocrinol 157(4):399–409. https://doi.org/10.1530/eje-07-0358
Burton T, Le Nestour E, Neary M, Ludlam WH (2016) Incidence and prevalence of acromegaly in a large US health plan database. Pituitary 19(3):262–267. https://doi.org/10.1007/s11102-015-0701-2
Dal J, Feldt-Rasmussen U, Andersen M, Kristensen LO, Laurberg P, Pedersen L, Dekkers OM, Sorensen HT, Jorgensen JO (2016) Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur J Endocrinol 175(3):181–190. https://doi.org/10.1530/eje-16-0117
Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A (2006) High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 91(12):4769–4775. https://doi.org/10.1210/jc.2006-1668
Fernandez A, Karavitaki N, Wass JA (2010) Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol 72(3):377–382. https://doi.org/10.1111/j.1365-2265.2009.03667.x
Gruppetta M, Mercieca C, Vassallo J (2013) Prevalence and incidence of pituitary adenomas: a population based study in Malta. Pituitary 16(4):545–553. https://doi.org/10.1007/s11102-012-0454-0
Hoskuldsdottir GT, Fjalldal SB, Sigurjonsdottir HA (2015) The incidence and prevalence of acromegaly, a nationwide study from 1955 through 2013. Pituitary 18(6):803–807. https://doi.org/10.1007/s11102-015-0655-4
Kwon O, Song YD, Kim SY, Lee EJ (2013) Nationwide survey of acromegaly in South Korea. Clin Endocrinol 78(4):577–585. https://doi.org/10.1111/cen.12020
Mestron A, Webb SM, Astorga R, Benito P, Catala M, Gaztambide S, Gomez JM, Halperin I, Lucas-Morante T, Moreno B, Obiols G, de Pablos P, Paramo C, Pico A, Torres E, Varela C, Vazquez JA, Zamora J, Albareda M, Gilabert M (2004) Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 151(4):439–446
Raappana A, Koivukangas J, Ebeling T, Pirila T (2010) Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab 95(9):4268–4275. https://doi.org/10.1210/jc.2010-0537
Tjornstrand A, Gunnarsson K, Evert M, Holmberg E, Ragnarsson O, Rosen T, Filipsson Nystrom H (2014) The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur J Endocrinol 171(4):519–526. https://doi.org/10.1530/eje-14-0144
Petrossians P, Daly AF, Natchev E, Maione L, Blijdorp K, Sahnoun-Fathallah M, Auriemma R, Diallo AM, Hulting AL, Ferone D, Hana V Jr, Filipponi S, Sievers C, Nogueira C, Fajardo-Montanana C, Carvalho D, Hana V, Stalla GK, Jaffrain-Rea ML, Delemer B, Colao A, Brue T, Neggers S, Zacharieva S, Chanson P, Beckers A (2017) Acromegaly at diagnosis in 3173 patients from the Liege Acromegaly Survey (LAS) Database. Endocr Relat Cancer 24(10):505–518. https://doi.org/10.1530/erc-17-0253
Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, Marshall JC, Laws ER Jr, Vance ML (2011) Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 96(9):2732–2740. https://doi.org/10.1210/jc.2011-0554
Kreutzer J, Vance ML, Lopes MB, Laws ER Jr (2001) Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab 86(9):4072–4077. https://doi.org/10.1210/jcem.86.9.7819
Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr (2013) Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab 98(8):3190–3198. https://doi.org/10.1210/jc.2013-1036
Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB (1998) Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab 83(10):3411–3418. https://doi.org/10.1210/jcem.83.10.5111
Fernandez Mateos C, Garcia-Uria M, Morante TL, Garcia-Uria J (2017) Acromegaly: surgical results in 548 patients. Pituitary 20(5):522–528. https://doi.org/10.1007/s11102-017-0813-y
Freda PU, Wardlaw SL, Post KD (1998) Long-term endocrinological follow-up evaluation in 115 patients who underwent transsphenoidal surgery for acromegaly. J Neurosurg 89(3):353–358. https://doi.org/10.3171/jns.1998.89.3.0353
Nomikos P, Buchfelder M, Fahlbusch R (2005) The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol 152(3):379–387. https://doi.org/10.1530/eje.1.01863
Swearingen B, Barker FG 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT (1998) Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab 83(10):3419–3426. https://doi.org/10.1210/jcem.83.10.5222
Bates PR, Carson MN, Trainer PJ, Wass JA (2008) Wide variation in surgical outcomes for acromegaly in the UK. Clin Endocrinol 68(1):136–142. https://doi.org/10.1111/j.1365-2265.2007.03012.x
Schofl C, Franz H, Grussendorf M, Honegger J, Jaursch-Hancke C, Mayr B, Schopohl J (2013) Long-term outcome in patients with acromegaly: analysis of 1344 patients from the German Acromegaly Register. Eur J Endocrinol 168(1):39–47. https://doi.org/10.1530/eje-12-0602
Sun H, Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M (2014) Factors associated with biochemical remission after microscopic transsphenoidal surgery for acromegaly. J Neurol Surg Part B Skull Base 75(1):47–52. https://doi.org/10.1055/s-0033-1354578
van Bunderen CC, van Varsseveld NC, Baayen JC, van Furth WR, Aliaga ES, Hazewinkel MJ, Majoie CB, Freling NJ, Lips P, Fliers E, Bisschop PH, Drent ML (2013) Predictors of endoscopic transsphenoidal surgery outcome in acromegaly: patient and tumor characteristics evaluated by magnetic resonance imaging. Pituitary 16(2):158–167. https://doi.org/10.1007/s11102-012-0395-7
Almeida JP, Ruiz-Trevino AS, Liang B, Omay SB, Shetty SR, Chen YN, Anand VK, Grover K, Christos P, Schwartz TH (2018) Reoperation for growth hormone-secreting pituitary adenomas: report on an endonasal endoscopic series with a systematic review and meta-analysis of the literature. J Neurosurg 129(2):404–416. https://doi.org/10.3171/2017.2.jns162673
Anthony JR, Alwahab UA, Kanakiya NK, Pontell DM, Veledar E, Oyesiku NM, Ioachimescu AG (2015) Significant elevation of growth hormone level impacts surgical outcomes in acromegaly. Endocr Pract 21(9):1001–1009. https://doi.org/10.4158/ep14587.or
Briceno V, Zaidi HA, Doucette JA, Onomichi KB, Alreshidi A, Mekary RA, Smith TR (2017) Efficacy of transsphenoidal surgery in achieving biochemical cure of growth hormone-secreting pituitary adenomas among patients with cavernous sinus invasion: a systematic review and meta-analysis. Neurol Res 39(5):387–398. https://doi.org/10.1080/01616412.2017.1296653
Hofstetter CP, Mannaa RH, Mubita L, Anand VK, Kennedy JW, Dehdashti AR, Schwartz TH (2010) Endoscopic endonasal transsphenoidal surgery for growth hormone-secreting pituitary adenomas. Neurosurg Focus 29(4):E6. https://doi.org/10.3171/2010.7.focus10173
Braileanu M, Hu R, Hoch MJ, Mullins ME, Ioachimescu AG, Oyesiku NM, Pappy A 2nd, Saindane AM (2019) Pre-operative MRI predictors of hormonal remission status post pituitary adenoma resection. Clin Imaging 55:29–34. https://doi.org/10.1016/j.clinimag.2019.01.020
Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M (2013) Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary 16(4):490–498. https://doi.org/10.1007/s11102-012-0445-1
Heck A, Ringstad G, Fougner SL, Casar-Borota O, Nome T, Ramm-Pettersen J, Bollerslev J (2012) Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin Endocrinol 77(1):72–78. https://doi.org/10.1111/j.1365-2265.2011.04286.x
Puig-Domingo M, Resmini E, Gomez-Anson B, Nicolau J, Mora M, Palomera E, Marti C, Halperin I, Webb SM (2010) Magnetic resonance imaging as a predictor of response to somatostatin analogs in acromegaly after surgical failure. J Clin Endocrinol Metab 95(11):4973–4978. https://doi.org/10.1210/jc.2010-0573
Bonneville F, Riviere LD, Petersenn S, Bevan J, Houchard A, Sert C, Caron PJ (2018) MRI T2 signal intensity and tumor response in patients with GH-secreting pituitary macroadenoma: PRIMARYS post hoc analysis. Eur J Endocrinol. https://doi.org/10.1530/eje-18-0254
Iacovazzo D, Carlsen E, Lugli F, Chiloiro S, Piacentini S, Bianchi A, Giampietro A, Mormando M, Clear AJ, Doglietto F, Anile C, Maira G, Lauriola L, Rindi G, Roncaroli F, Pontecorvi A, Korbonits M, De Marinis L (2016) Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: an immunohistochemical study. Eur J Endocrinol 174(2):241–250. https://doi.org/10.1530/eje-15-0832
Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S (2005) The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab 90(11):6290–6295. https://doi.org/10.1210/jc.2005-0998
Heck A, Emblem KE, Casar-Borota O, Bollerslev J, Ringstad G (2016) Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine 52(2):333–343. https://doi.org/10.1007/s12020-015-0766-8
Daly AF, Tichomirowa MA, Petrossians P, Heliovaara E, Jaffrain-Rea ML, Barlier A, Naves LA, Ebeling T, Karhu A, Raappana A, Cazabat L, De Menis E, Montanana CF, Raverot G, Weil RJ, Sane T, Maiter D, Neggers S, Yaneva M, Tabarin A, Verrua E, Eloranta E, Murat A, Vierimaa O, Salmela PI, Emy P, Toledo RA, Sabate MI, Villa C, Popelier M, Salvatori R, Jennings J, Longas AF, Labarta Aizpun JI, Georgitsi M, Paschke R, Ronchi C, Valimaki M, Saloranta C, De Herder W, Cozzi R, Guitelman M, Magri F, Lagonigro MS, Halaby G, Corman V, Hagelstein MT, Vanbellinghen JF, Barra GB, Gimenez-Roqueplo AP, Cameron FJ, Borson-Chazot F, Holdaway I, Toledo SP, Stalla GK, Spada A, Zacharieva S, Bertherat J, Brue T, Bours V, Chanson P, Aaltonen LA, Beckers A (2010) Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab 95(11):E373–E383. https://doi.org/10.1210/jc.2009-2556
Ganeshan B, Burnand K, Young R, Chatwin C, Miles K (2011) Dynamic contrast-enhanced texture analysis of the liver: initial assessment in colorectal cancer. Investig Radiol 46(3):160–168. https://doi.org/10.1097/RLI.0b013e3181f8e8a2
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22(4):796–802. https://doi.org/10.1007/s00330-011-2319-8
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K (2012) Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 67(2):157–164. https://doi.org/10.1016/j.crad.2011.08.012
Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR (2009) Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250(2):444–452. https://doi.org/10.1148/radiol.2502071879
Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V (2013) Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266(1):177–184. https://doi.org/10.1148/radiol.12120254
Tourassi GD (1999) Journey toward computer-aided diagnosis: role of image texture analysis. Radiology 213(2):317–320. https://doi.org/10.1148/radiology.213.2.r99nv49317
Galm BP, Martinez-Salazar EL, Swearingen B, Torriani M, Klibanski A, Bredella MA, Tritos NA (2018) MRI texture analysis as a predictor of tumor recurrence or progression in patients with clinically non-functioning pituitary adenomas. Eur J Endocrinol 179(3):191–198. https://doi.org/10.1530/eje-18-0291
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER Jr (1996) Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38(1):99–106. https://doi.org/10.1097/00006123-199601000-00024discussion 106-107
Mazal PR, Czech T, Sedivy R, Aichholzer M, Wanschitz J, Klupp N, Budka H (2001) Prognostic relevance of intracytoplasmic cytokeratin pattern, hormone expression profile, and cell proliferation in pituitary adenomas of akromegalic patients. Clin Neuropathol 20(4):163–171
Obari A, Sano T, Ohyama K, Kudo E, Qian ZR, Yoneda A, Rayhan N, Mustafizur Rahman M, Yamada S (2008) Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol 19(2):82–91. https://doi.org/10.1007/s12022-008-9029-z
Hagiwara A, Inoue Y, Wakasa K, Haba T, Tashiro T, Miyamoto T (2003) Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 228(2):533–538. https://doi.org/10.1148/radiol.2282020695
Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Into Imaging 3(6):573–589. https://doi.org/10.1007/s13244-012-0196-6
Zhang S, Chiang GC, Magge RS, Fine HA, Ramakrishna R, Chang EW, Pulisetty T, Wang Y, Zhu W, Kovanlikaya I (2019) MRI based texture analysis to classify low grade gliomas into astrocytoma and 1p/19q codeleted oligodendroglioma. Magn Reson Imaging 57:254–258. https://doi.org/10.1016/j.mri.2018.11.008
Fujima N, Homma A, Harada T, Shimizu Y, Tha KK, Kano S, Mizumachi T, Li R, Kudo K, Shirato H (2019) The utility of MRI histogram and texture analysis for the prediction of histological diagnosis in head and neck malignancies. Cancer Imaging 19(1):5. https://doi.org/10.1186/s40644-019-0193-9
Guo CG, Ren S, Chen X, Wang QD, Xiao WB, Zhang JF, Duan SF, Wang ZQ (2019) Pancreatic neuroendocrine tumor: prediction of the tumor grade using magnetic resonance imaging findings and texture analysis with 3-T magnetic resonance. Cancer Manag Res 11:1933–1944. https://doi.org/10.2147/cmar.s195376
Ditmer A, Zhang B, Shujaat T, Pavlina A, Luibrand N, Gaskill-Shipley M, Vagal A (2018) Diagnostic accuracy of MRI texture analysis for grading gliomas. J Neurooncol 140(3):583–589. https://doi.org/10.1007/s11060-018-2984-4
Meyer HJ, Schob S, Hohn AK, Surov A (2017) MRI texture analysis reflects histopathology parameters in thyroid cancer - a first preliminary study. Transl Oncol 10(6):911–916. https://doi.org/10.1016/j.tranon.2017.09.003
Jakola AS, Zhang YH, Skjulsvik AJ, Solheim O, Bo HK, Berntsen EM, Reinertsen I, Gulati S, Forander P, Brismar TB (2018) Quantitative texture analysis in the prediction of IDH status in low-grade gliomas. Clin Neurol Neurosurg 164:114–120. https://doi.org/10.1016/j.clineuro.2017.12.007
Cannella R, Borhani AA, Tublin M, Behari J, Furlan A (2019) Diagnostic value of MR-based texture analysis for the assessment of hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Abdom Radiol 44(5):1816–1824. https://doi.org/10.1007/s00261-019-01931-6
Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA (2013) Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266(1):326–336. https://doi.org/10.1148/radiol.12112428
Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ (2007) Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol 67(1):65–70. https://doi.org/10.1111/j.1365-2265.2007.02836.x
Chanson P, Arnoux A, Mavromati M, Brailly-Tabard S, Massart C, Young J, Piketty ML, Souberbielle JC (2016) Reference values for IGF-I serum concentrations: comparison of six immunoassays. J Clin Endocrinol Metab 101(9):3450–3458. https://doi.org/10.1210/jc.2016-1257
Mavromati M, Kuhn E, Agostini H, Brailly-Tabard S, Massart C, Piketty ML, Arnoux A, Young J, Souberbielle JC, Chanson P (2017) Classification of patients with GH disorders may vary according to the IGF-I assay. J Clin Endocrinol Metab 102(8):2844–2852. https://doi.org/10.1210/jc.2017-00202
Cuevas-Ramos D, Carmichael JD, Cooper O, Bonert VS, Gertych A, Mamelak AN, Melmed S (2015) A structural and functional acromegaly classification. J Clin Endocrinol Metab 100(1):122–131. https://doi.org/10.1210/jc.2014-2468
Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O (2013) Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 168(4):491–499. https://doi.org/10.1530/eje-12-0864
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
NAT and MAB originally conceived of the study. BPG extracted clinical data, performed the statistical analyses, and wrote the first draft of the manuscript. CB performed the image texture analyses. All authors were involved in study design and interpretation of results. All authors revised the manuscript critically for important intellectual content, agreed on the final content of the manuscript, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
AK serves on the scientific advisory board for Crinetics, which works on developing acromegaly-targeted pharmaceuticals, but does not have any conflicts of interest related to the present work. There are no conflicts of interest related to this study reported by any of the other authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galm, B.P., Buckless, C., Swearingen, B. et al. MRI texture analysis in acromegaly and its role in predicting response to somatostatin receptor ligands. Pituitary 23, 212–222 (2020). https://doi.org/10.1007/s11102-019-01023-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-019-01023-0