Abstract
Herbal medicinal products are widely considered beneficial and gaining importance in preventing and treating several diseases. Urtica dioica L. (UD) is a medicinal plant that has been used as an herbal remedy and dietary supplement for centuries based on traditional experience or random trials without the know-how of phytoconstituents. UD is one of those herbs with a long record of anti-inflammatory activity and several mechanisms of action have been discussed. Plant part, extraction solvent, and phytoconstituents have a determinant effect on both efficacy and therapeutic objective. Current literature mainly elaborates on the antioxidant effect of Urtica species, with the anti-inflammatory role of UD still being a matter of discussion, as in vitro and in vivo studies have only been characterized to such an extent. In order to elaborate on this topic, the present review aims to characterize the anti-inflammatory action of several UD extracts according to in vitro and in vivo results, as well as the possible molecules and respective mechanism responsible for its anti-inflammatory effect on several pathologies. Despite the knowledge gathered so far surrounding the anti-inflammatory activity of UD, further studies are required to characterize the mechanism of action and discriminate between the molecules underlying the beneficial effects of nettle on inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory diseases are globally prevalent and more than 50% of all deaths are attributed to inflammation-related pathologies (Furman et al. 2019). Inflammation is an evolutionarily conserved host defense mechanism that protects humans from pathogens, internal or external (e.g., bacteria, viruses and toxins) and maintains homeostasis under noxious conditions (Renz et al. 2017; Netea et al. 2017; Bennett et al. 2018; Fleming et al. 2018; Roth et al. 2018). Normally, inflammatory responses are quick in action and self-limited, classified as “acute inflammation”, and initiate during infection via interaction between pattern-recognition-receptors (PPRs) expressed on innate immune cells and pathogen-associated molecular patterns (PAMPs). On the other hand, the acute inflammatory response can also be activated by damage-associated molecular patterns (DAMPs) released in response to physical, chemical, or metabolic noxious stimuli during cellular stress or damage (Netea et al. 2017). Platelets and granulocyte cells, namely, basophils, mast cells, neutrophils, and eosinophils, are activated, producing a large variety of soluble mediators that upregulate the inflammatory response, but neutrophils are recognized as the primary cell modulators of the acute response due to their enriched variety of enzymes, peptides, and proteins (Germolec et al. 2018). However, standard biomarkers for indicating health-damaging chronic inflammation are yet to be identified, but the correlation between the inflammatory process and the increase in mortality and comorbidity appearance has long been investigated. Therefore, canonical biomarkers of the acute inflammatory response are used to measure morbidity and mortality (Germolec et al. 2018; Furman et al. 2019).
Inflammatory pathologies are increasingly becoming harder to treat and require longer duration therapies, in most cases, having a high burden not only on medical services but also on patient quality of life (Ponder and Long 2013; Yatoo et al. 2018). Synthetic drugs are one of the most prescribed medicines for anti-inflammatory purposes, but despite their effectiveness and rapid effect, these medicines have limited application due to their serious side effects, cost, and availability across countries (Brown et al. 2006; Rainsford 2007; Chen et al. 2008; Bacchi et al. 2012; Bindu et al. 2020). Nevertheless, herbal medicines have long been used due to their potential to provide efficacious treatment for inflammatory diseases with traditional medicine and natural herbal products being increasingly used as a natural way of counteracting the limitations of anti-inflammatory drugs.
Urtica dioica L. (UD) is an annual perennial flowering plant belonging to the Urticaceae family, growing in temperate regions in several parts of the globe (e.g., Asia, Europe, North Africa, and North America) with wet, rich soils. It is distinguished by its many stinging hairs on its leaves and stems that, when touched, cause a reaction called “urticaria”, owing to the presence of formic acid and histamine (Roschek et al. 2009; Genc et al. 2011; Hajhashemi and Klooshani 2013; Irgin et al. 2016; Carvalho et al. 2017; Ghasemi et al. 2019; Hodroj et al. 2020; Kasouni et al. 2021; Taheri et al. 2022; Bhusal et al. 2022; Devkota et al. 2022; Namazi et al. 2022). All plant components are used for extract preparation and have several pharmacological properties owing to their etiology (flavonoids, tannins, volatile compounds, fatty acids, steroids, terpenes). It also contains a balanced protein and high mineral and vitamin content, which endows this plant with great dietary capacity (Bhusal et al. 2022).
Current literature regarding the anti-inflammatory characteristics of UD is in expansion but a review is yet to be made as there is an enormous medical prospect for this herb. Over the past few decades, scientific data based on observational studies, clinical trials, reports, and several other investigations have been conducted regarding its anti-inflammatory action on several pathologies. Furthermore, there is an emerging need for clinical trials to evaluate this species’ extracts and possible molecules responsible for its action. Therefore, this review aims to evaluate existing literature regarding the anti-inflammatory action of UD, as well as the inflammatory pathways involved, together with possible phytochemicals responsible for its downregulatory mechanism. Concluding evidence from this review highlights the anti-inflammatory role of UD with both in vitro and in vivo studies further establishing this species as a therapeutic approach to inflammatory-derived diseases.
Methodology
Source and search strategy
An electronic literature search was developed using the following online databases: PubMed, Elsevier, and ResearchGate. The search employed relevant keywords, namely “anti-inflammatory”, “inflammation”, “stinging nettle”, “nettle” and “Urtica dioica.” The resulting literature published in the English language was explored, and pertinent information was included.
Inclusion and exclusion criteria
The primary search results underwent a screening process, wherein irrelevant papers were excluded based on their title, abstract, and overall content. Studies that did not assess inflammatory markers were omitted or earmarked for subsequent analysis. Additionally, articles that explored specific phytochemicals derived from Urtica dioica without explicitly addressing their involvement in inflammation were also excluded because the present review aims to elaborate on the anti-inflammatory potential of UD and its phytochemistry in response to inflammation. Furthermore, investigations focusing on natural, synthetic, or semi-synthetic components isolated from non-herbal sources were also rejected.
Data extraction
A reference manager was employed to comprehensively gather and systematize bibliographic sources. Publications were examined for duplicates and subjected to an in/out process, following the inclusion/exclusion criteria. Each study deemed relevant underwent thorough reading, and pertinent information was highlighted in order to retrieve data for this review. The final selected articles, which will be expounded upon in this work, encompass essential details such as dosage, extract type, methodology, study design, and outcomes. These data have been categorized into Table 1 (bioactive compounds present in aerial parts and roots), Tables 2 (in vitro studies), and 3 (in vivo studies).
Botanics
Urtica dioica L. is a perennial flowering plant belonging to the Urticaceae family. It is a stinging plant that thrives worldwide, primarily in nitrogen-rich humid hills and plains of the Mediterranean soils, except for tropical regions and in the Antarctic continent, favoring temperate and shaded areas. The plant’s stem is characterized by its green color, erect posture, hollow to solid structure, and fibrous composition, adorned with numerous fine stinging hairs and trichomes. The leaves are dark green and paler beneath, possessing stipules and serrated margins, with an oblong or ovate shape featuring a cordate base. From May to September, small reddish to greenish-white flowers emerge, predominantly in a dioecious manner, producing black to dark-brown seeds in early August (Taheri et al. 2022; Bhusal et al. 2022).
Nettle holds a profound historical background, tracing its utilization in human populations back to at least 2000 years ago. However, its pharmacological properties and chemical structure were not fully appreciated until a century ago (Ait et al. 2015). Traditionally, this botanical species has served as a source for nourishment and traditional medicinal remedies, with all parts of the plant being employed in a variety of preparations, including teas, salads, ointments, soups, infusions, decoctions, and liquid extracts (Upton 2013). The widespread use of nettle in folk medicine transcends geographical boundaries. Infusions made from nettle leaves have been used to address ailments such as diarrhea, vaginal discharge, and both internal and external bleeding (Tucakov 1997), while the consumption of nettle leaves in soups and dishes is believed to strengthen the body (Gulsel 2003; Council of Europe 2008). Fresh nettle juice is highly regarded as a remedy for various forms of bleeding, whether originating from the nose, lungs, or other internal organs, whereas inhalation of dried nettle leaves offers relief for asthma and similar bronchial conditions, and the entire plant is utilized in addressing sciatica, early stages of wasting, respiratory difficulties, cardiac ailments, coughs, palsy, suppression of menstrual flow, rheumatism, and muscular fatigue (Kirtikar and Basu 2008).
Bioactive compounds
Medicinal plants owe their therapeutic effects to their enriched multi-diverse chemical composition, which can be influenced by factors such as the plant part used, harvesting time, and geographic location. These plant metabolites find applications in both food and pharmaceutical industries, with functional foods and supplements capturing a significant market share, as European consumers alone spend over 2 billion dollars annually on herbal medicinal products (Fisher and Ward 1994; Veiga et al. 2020).
UD has been used as a natural remedy for ages in several treatments (e.g., diabetes, colitis, rhinitis, rheumatism, asthma, cardiovascular diseases, neurodegenerative diseases, and cancer) due to its diverse nutraceutical composition (Ait et al. 2015; Jan et al. 2017). Various species of Urtica are reported to be rich sources of mineral and nutritional content with diverse amino acid, fiber, carbohydrate, lipid, and vitamin composition (Kregiel et al. 2018; Taheri et al. 2022; Bhusal et al. 2022; Devkota et al. 2022). UD has an enormous chemical diversity with immense therapeutic potential. Various phytochemicals are reported from different parts of the plant, including flavonoids, phenolic acids, carotenoids, fatty acids, steroids, and several others (Shibuya et al. 1986; Van Damme et al. 1988; Wagner et al. 1989; Schöttner et al. 1997a; Ait et al. 2015; Jan et al. 2017). However, due to ethnobotanical considerations, its nutraceutical composition may vary across seasons, resulting in fluctuations in phytochemical values (Paulauskienė et al. 2021).
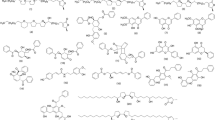
Considering the huge medicinal potential of UD, research has been conducted to catalog and investigate the pharmacological potential of its phytochemical composition. Different species of Urtica have been studied, and their chemically rich and diverse composition can be grouped according to their relative abundance in specific plant parts, with roots and leaves being those with an increased focus. UD has already been substantially characterized and numerous phytochemicals, including flavonoids, phenolic acids (hydroxybenzoic acid and cinnamic acid derivatives), amino acids, carotenoids, organic acids, and fatty acids are described from the different plant parts, although most of the studies are focused on leaves aqueous and hydroalcoholic extracts (Devkota et al. 2022) (Table 1).
Anti-inflammatory potential
The anti-inflammatory activity of UD has long been studied and its effectiveness has been confirmed in various settings, including in vitro, in vivo, and through limited clinical trials. Traditional medicine has also proved to act directly or indirectly in managing diseases with inflammatory background (Klingelhoefer et al. 1999; Riehemann et al. 1999; Broer and Behnke 2002; Konrad et al. 2005; Toldy et al. 2005, 2009; Criado et al. 2006; Roschek et al. 2009; Denzler et al. 2010; Namazi et al. 2011, 2022; Genc et al. 2011; Vafaee et al. 2012; Hajhashemi and Klooshani 2013; Oguz et al. 2013; Johnson et al. 2013; Dar et al. 2013; Yilmaz et al. 2014; Daneshmand et al. 2016; Kaya et al. 2016; Patel et al. 2016, 2018; Zemmouri et al. 2017; Bisht et al. 2017; Pigat et al. 2019; Karami et al. 2020; Saponaro et al. 2020; Kasouni et al. 2021; Shahzad et al. 2021; Mannila et al. 2022).
Inflammation is a complex series of physiological events (Di Lorenzo et al. 2013), with different inflammatory pathways leading to changes in the intended phytochemical extract content (disease type and extent, plant parts, and extraction solvent are crucial variables). Phytoconstituents with proven anti-inflammatory action such as alkaloids, phenolic compounds, and steroids act through different mechanisms to target inflammation by inhibiting one or more enzymes, proteins, factors, or hormones, and may affect inflammatory pathways or favor anti-inflammatory mechanisms (Yatoo et al. 2018). However, the molecular mechanisms by which this species decreases the inflammatory status are still not fully understood and several inflammatory models are being employed for investigating its anti-inflammatory potential (Yatoo et al. 2018).
Most research methods that study the inflammatory mechanism involve induction of inflammation followed by UD administration and monitoring of inflammatory markers using biochemical, chemical, and cell-based assays. Proinflammatory markers are the most used means of investigating inflammatory progression in both in vivo and in vitro models, in addition to providing targets for potential anti-inflammatory phytoconstituents. Technique and parameter optimization are ways of enhancing performance, and changes in inflammatory models allow for comparison of results (Ansar and Ghosh 2016; Germolec et al. 2018).
Inflammation leads to cellular membrane damage, and phospholipids play a key role in this process. Monitoring their metabolism and enzyme activity are essential parameters studied as a way of understanding the inflammatory mechanism. Proinflammatory cytokines, such as interleukins and tumor necrosis factor alpha (TNF-α), are upregulated in inflammation, and most studies monitor these molecules as their decrease is proven to be a signal of inflammatory cease, further supported by histological findings (Walport et al. 1992; Kyriakis 1999; Riehemann et al. 1999; D’Haens 2003; McCoy et al. 2008; Harms et al. 2011; Josiah et al. 2022). Nevertheless, phospholipid degradation also occurs, through phospholipase A2, producing arachidonic acid, but either cyclooxygenases (COXs) or lipoxygenases (LOXs) need to act to promote metabolization. Several UD phytoconstituents act on this pathway, and their anti-inflammatory action on COX and LOX has already been confirmed. As so, these molecules have been extensively quantified to show the progression of inflammatory activity (Clark et al. 1991; Roschek et al. 2009; Dennis et al. 2011; Arnold et al. 2015). Several of the phytoconstituents of UD have demonstrated to possess anti-inflammatory potential, such as kaempferol (Zhang et al. 2022), quercetin (Zhang et al. 2022), rutin (Zhang et al. 2022), baicalein (Liao et al. 2021), isorhamnetin (Gong et al. 2020), luteolin (Aziz et al. 2018), scopoletin (Sakthivel et al. 2022), β-sitosterol (Zhang et al. 2023), stigmasterol (Jie et al. 2022) and campesterol (Nazir et al. 2024).
Additionally, UD has proven antioxidant activity in a variety of diseases by either reducing oxidant levels, or increasing levels of antioxidants, thereby preventing the initiation and progression of inflammation, and further supporting its anti-inflammatory effect (Bisht et al. 2017; Carvalho et al. 2017; Kim et al. 2018; Shabir et al. 2022; Chira et al. 2022; Jaiswal and Lee 2022; Uğur and Güzel 2023).
As inflammation is a complex series of physiological interactions, analysis of inflammatory markers alone may not fully confirm the inflammatory status. As so, additional markers that act indirectly on inflammation, visual observations, and histopathological results are also used for supporting diagnosis.
In vitro data
Multiple sclerosis
Multiple sclerosis is a chronic, inflammatory, and progressive neurodegenerative disease of the central nervous system (CNS) with a likely autoimmune etiology that causes demyelination in white and gray matter, loss of oligodendrocytes and axons, and activation of microglia (Turturici et al. 2014). The disease is believed to be caused by: (1) an inappropriate immune T cell-mediated response (T helper 1 (Th1) and T helper 17 (Th17) cells) against myelin or other antigens (McFarland and Martin 2007); or (2) an inflammatory response caused by the host’s aberrant immune response (Stys et al. 2012). In a recent study (Namazi et al. 2022), a immunoassay analysis of brain tissue from multiple sclerosis (MS) models revealed that UD hydroalcoholic extract administration for 21 days led to a dose-dependent decrease in heat-shock protein (HSP) 70, although no effects were observed in HSP60. Accordingly, histopathological results showed that nettle extracts caused a dose-dependent reduction in myelin degradation and accelerated remyelination.
HSPs are a molecular chaperone family located both intracellularly and extracellularly in CNS cell types, including neurons, glia, and endothelial cells (Foster and Brown 1997). In response to stressors, such as inflammation, HSPs are thought to exert neuroprotective roles by preventing protein aggregation, misfolding, and inducing antiapoptotic mechanisms (Mosser and Morimoto 2004; Beere 2004; Benn and Woolf 2004; Lanneau et al. 2007). Extracellular HSPs contribute to the induction of the innate immune response through interactions with dendritic cells (DCs) or macrophages, as well as to the enhancement of adaptive immunity and interaction with both tool-like receptors (TLRs) and scavenger receptors (Calderwood et al. 2007). HSP60 can be recognized through TLR2 and TLR4 (Wallin et al. 2002; Roelofs et al. 2006; Warger et al. 2006), and its self-activation due to stress (Quintana and Cohen 2005; Henderson and Pockley 2010) stimulates the release of several proinflammatory cytokines, which are thought to be responsible for neurodegeneration and autoimmunity in MS, as evidenced through analysis of biomarkers in MS patients (Bsibsi et al. 2002; Sloane et al. 2010; Labib et al. 2022). Activation of TLR signaling (mainly via TLR4) in DCs and macrophages through high concentrations of HS60 promotes inflammation via diverse mechanisms, such as maturation of DCs, increased antigen presentation, and secretion of proinflammatory cytokines (Quintana and Cohen 2011). Additionally, autoantibodies to self-HSP60 have been identified in MS (Selmaj et al. 1991, 1992; Quintana et al. 2008), and their immune response-regulating activity has been confirmed in MS animal models (Gao et al. 1995; Birnbaum et al. 1998). HSP60’s inflammatory activity is dose-dependent and may be carried out through signals via monocytes, B cells, and effector T cells. On the other hand, the HSP70 family can be divided into HSP70 intracellular proteins, exerting cytoprotective functions as chaperones, and extracellular HSP70, acting as immunomodulatory factors, triggering immunological responses. HSP70s exacerbate the immune response by acting as adjuvants for myelin peptides and as proinflammatory cytokines (Fleshner and Johnson 2005). Extracellular HSP70s trigger an innate immune response through interaction with TLR-2, TLR-4, and CD14 costimulatory signaling (Asea et al. 2002), and, in addition to DC activation and maturation, they also lead to the activation of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB), inducing proinflammatory cytokine production (Basu et al. 2000; Moroi et al. 2000; Asea et al. 2000) with this overexpression occurring in CNS lesions of MS patients and animal models of MS (Aquino et al. 1993, 1997; Gao et al. 1995; Chabas et al. 2001). Accordingly, one study (Cwiklinska et al. 2020) revealed that inhibition of HSP70 not only reduced Th17 gene expression and alleviated autoimmune demyelination, but also hindered specific microsomal (mi) ribonucleic acid (RNA) (miRNA) functions that cause the expression of Th17 genes. Thus, aberrant HSP70 expression may be involved in MS by exacerbating or contributing to the inflammatory environment. Accordingly, nettle extracts on MS mice models led to a decrease in HSP70 levels, which were significantly elevated before treatment (Namazi et al. 2022).
Considering the results and the aforementioned findings, nettle might downregulate the inflammatory response through HSP expression. However, as there is limited literature regarding UD and its anti-inflammatory role in HSPs, along with an extended debate on the exact mechanism behind HSP’s immunoregulatory function, further studies are required to assess the possible anti-inflammatory mechanism of UD on MS.
Asthma
The downregulatory effect of UD on asthma through its anti-inflammatory mechanism presents a possible therapeutic target for this chronic inflammatory disease. Asthma is characterized by epithelial damage, overproduction of growth factors and proinflammatory cytokines, mucous gland hyperplasia with mucus hypersecretion, airway smooth muscle remodeling with hypertrophy, bronchial hyper-responsiveness and activation of inflammatory cells (e.g., mast cells, T cells, eosinophils, and neutrophils) (Bradding 2008).
A 2021 study (Shahzad et al. 2021) evaluating the antihistaminic potential of several plant extracts, including UD, revealed that treating mice with aqueous nettle extracts increased leukocyte count and decreased eosinophil count and mast cell degranulation. Eosinophil infiltration in the lungs contributes to the release of several cytokines and chemokines. The eosinophil count reduction has shown effective outcomes in asthmatic patients treated with anti-cytokine antibodies, particularly IL-5 (Leckie et al. 2000; Kips et al. 2003; O’Byrne 2006; Flood-Page et al. 2007), which supports the premise that eosinophil count correlates with asthma severity (Bousquet et al. 2000). However, the exact extent to which UD affects eosinophil recruitment remains controversial, as other studies investigating biochemical parameters following UD treatment did not show evident or significant variations in eosinophil concentration (Criado et al. 2006). Conversely, administration of nettle seeds extract in asthmatic mice models has been proven to significantly decrease eosinophil count (Irani et al. 2020). According to these findings, further investigation ought to be carried out to establish the downregulatory action of UD on eosinophil count. Additionally, UD treatment in asthmatic mice decreased mast cell degranulation. Mast cells greatly vary in their cytokine and proteolytic content, with degranulation releasing preformed and newly generated cytokines (Theoharides et al. 2012) that regulate both immunoglobulin (Ig) E synthesis and eosinophilic inflammation development (Bradding and Holgate 1999; Bradding et al. 2006). However, the exact mechanisms leading to chronic mast cell activation in asthma remain unknown, and additional research is required to elucidate the reasons behind the anti-inflammatory effect of UD extracts observed through reduced mast cell degranulation. Finally, blood analysis from asthmatic rodent models also indicated an increase in leukocyte count. Upon inflammation, blood–vascular endothelial cells (ECs) upregulate adhesion molecules and chemokines to support dynamic EC-leukocyte interactions and allow leukocytes to cross the EC barrier and reach the site of inflammation (Hopkin et al. 2019). Dysregulation of these processes occurs in chronic inflammatory diseases, contributing to disease progression. UD extracts have been shown to increase white blood cell recruitment in other species (Saeidi et al. 2017); however, despite extensive literature research, results regarding the correlation between UD and leukocyte count via inflammatory markers remain elusive.
Dermal wounds
A 2021 study (Kasouni et al. 2021) was conducted to assess the wound-healing potential of UD and its correlation with its anti-inflammatory activity. The results revealed that treated rats had more mature inflammatory granulomatous tissue and fewer chronic inflammatory cells, as compared to untreated mice within the same period. Both doses of UD extract administered daily for 5 and 10 days decreased the overall inflammatory status, although a lower dose of UD provided better macro and microscopical results, with treated animals exhibiting reduced edema and capillary blood vessels, along with more mature ECs. Furthermore, complete re-epithelialization was observed with concomitant growth of fibrous connective tissue. Moreover, rat models treated with the extract healed 3–4 days faster as compared to those left untreated and 2 days faster compared to a commercially available formulation, with the new tissue, in those treated with the extract, exhibiting better histopathological characteristics. Additionally, the overall healing and restructuration of the dermal and epidermal tissue was faster and provided better results in UD-treated groups. Moreover, spectrophotometric analysis indicated that the UD extract had a dose-dependent stabilizing effect on erythrocyte membranes in human blood. Stabilization of red blood cells is considered a valid model for indicating anti-inflammatory activity, as it prevents lysosome lysis, and erythrocytes share morphological similarities with these structures (Anosike et al. 2012). Additionally, spectrophotometric analysis of a serum sample from a bovine specimen revealed that mixing the sample with the aqueous nettle extract ceased albumin denaturation in a dose-dependent way. A substance able to inhibit protein denaturation may have anti-inflammatory activity: protein denaturation is correlated with the occurrence of the inflammatory response as inflammation occurs in the vascularized tissue followed by a conspicuous accumulation of leukocytes since the denaturization of albumin constituents of cells or intercellular substance increases permeability of blood vessels(Osman et al. 2016).
Quercetin is a molecule present within UD with promising dermatological healing characteristics due to its angiogenic and anti-inflammatory activity (Gopalakrishnan et al. 2016). Mi et al. (2022) evaluated the healing effect of quercetin on cutaneous wound models in vivo and in vitro, along with its involvement in the Wingless-related integration site/ beta-catenin (Wnt/β-catenin) pathway and telomerase reverse transcriptase (TERT) mechanisms. It was found that quercetin significantly contributed to wound recovery as it could promote the proliferation and migration ability of skin cells included in the epidermis, dermis, and subcutaneous tissue together with an anti-inflammatory effect. These results were supported by reduced levels of cytokines TNF-α, interleukins (IL) 1 betta (IL-1β) and IL-6, and an increase in expression of fibroblast growth factor (FGF) and smooth muscle actin alpha (α-SMA), together with a reduction in antioxidant factors. In regards to Wnt/β-catenin, in treated groups, its expression was significantly increased. The Wnt signaling pathway is involved in the development of inflammation by interacting with other inflammatory pathways, such as NF-κB and mitogen-activated protein kinases (MAPKs), and cytokines participate in its regulation, such as TNF-α activating β-catenin via the protein kinase B (ATK) pathway and IL-1β activating Wnt/β-catenin via the NF-κB pathway (Moparthi and Koch 2019). Moreover, the synergic role of quercetin in promoting angiogenesis and participating in wound repair has also been described (Zhang et al. 2018). Additionally, quercetin treatment in wounded rats increased the expression of the TERT gene, with studies having reported that TERT inhibition is always associated with the inhibition of Wnt/β-catenin signaling (Yang et al. 2017). Not only, but TERT is also involved in free radical scavenging exerting an antioxidant effect, thus possibly downregulating the inflammatory cascade (Attia et al. 2010).
Considering the results from cutaneous wound models regarding the anti-inflammatory action and ability to heal injured dermal tissue, UD may prove to act on wound recovery favoring an anti-inflammatory effect via quercetin (Gopalakrishnan et al. 2016; Mi et al. 2022). The increased levels of growth factors through Wnt/β-catenin signaling and the elevated expression of TERT protein may reveal the possible anti-inflammatory mechanism of quercetin and its ability to heal injured dermal tissue.
Benign prostatic hyperplasia
Benign prostatic hyperplasia (BPH) cell line BPH-1 and prostate cancer cell line PC3, used as a method for studying the anti-inflammatory impact of UD extracts on the hyperproliferation of prostatic epithelial and stromal cells, showed that reactive oxygen species (ROSs) reduction is implicated in the decrease of NF-κB inflammatory pathway and causes decreased secretion of inflammatory cytokines (Saponaro et al. 2020). A combined mixture containing hydroethanolic UD roots extract revealed through microscopical analysis a decrease in the translocation of NF-κB in the cell nuclei in both in vitro models over a 24-h period. Nevertheless, quantitative polymerase chain reaction qPCR followed by immunoassay revealed that a decrease in IL-6 and IL-8 messenger RNA (mRNA) expression was only observed in BPH-1 cell lines, with the latter having the most notorious influence. The difference in results, by making use of in vitro BPH models, together with an androgen-independent prostate cell model (PC3), highlights the tissue specificity of the commercially approved combined mixture and possibly, for the interest of the present review, the specificity of UD (Saponaro et al. 2020).
Transcription factors of the NF-κB family are essential for the expression of proinflammatory gene products, including cytokines IL-6 and IL-8. In BPH, IL-6 activates B lymphocytes and regulates epithelial cell growth, which is positively associated with a dose-dependent risk of BPH. Conversely, IL-8 is actively secreted by epithelial and stromal cells in response to proinflammatory cytokines, attracting lymphomononuclear cells expressing chemokine receptors into the prostate and inducing stromal and epithelial overgrowth (De Nunzio et al. 2016). This process stimulates the proliferation of senescent epithelial cells (Castro et al. 2004; Fibbi et al. 2010) and the stromal acquisition of a myofibroblast reactive phenotype (Schauer et al. 2008; Fibbi et al. 2010), indirectly promoting fibroblast growth factor secretion (Giri and Ittmann 2001; Fibbi et al. 2010).
Riehemann (Riehemann et al. 1999) provided evidence that UD inhibits the proteolytic degradation of the inhibitor of nuclear factor-κB (IκB) subunit, possibly by direct prevention of degradation or inhibition of kinases or further upstream signaling molecules. A 2019 study (Cicero et al. 2019) reviewing the nutraceutical potential of several plant species for treating BPH and prostate cancer revealed that continuous treatment with UD extracts had a positive impact on symptomatology, International Prostate Symptom Score (IPSS), and flowmetric indices. It was stated that polyphenols, which are abundant in nettle, can act on BPH through a reduction in interleukin levels (including IL-6 and IL-8), providing anti-proliferative and anti-inflammatory activity. Accordingly, in testing this sentence, quercetin was used for testing its sole anti-inflammatory potential. Following treatment, animals had decreased prostate volume, as well as reduced IPSS (Ghorbanibirgani 2012). A series of other molecules also present in UD extracts were tested, but due to the source of material/ plant species, it cannot be concluded whether it would have the same impact as quercetin. Assessing these results, it can be concluded that BPH and chronic prostatic inflammation occur as a consequence of immunological action and UD is expected to exert its anti-neoplasic action through the downregulation of proinflammatory markers via its flavonoid fraction, namely quercetin, which has proven efficacy through validated studies on the effect of BPH in clinical models for this disease.
Acne
Acne proliferation decreases in keratinocyte cell lines upon the application of a combined mixture containing a hydroalcoholic UD extract mixture. One study (Kılıç et al. 2019) reported that, when combined with other plant species, the extract decreased the propagation of skin inflammatory species. Clinical cultivation from both strains of Propionibacterium acnes showed that the antimicrobial efficacy transversely translated in a decrease in keratinocyte cell line inflammatory gene expression, with increasing extract concentration yielding better results on cell proliferation. Additionally, it downregulated IL-1α and steroid-5-alpha-reductase (SRD5A) gene expression, but an increase in TNF-α in a dose-dependent manner was also noted. Hence, the combinatory effect of UD and other herbs in the same extract does not establish whether the anti-inflammatory effect of the anti-acne formulation is exclusively attributed to the phytochemical composition of UD.
Acne, a prevalent skin disease, involves chronic inflammation of pilosebaceous follicles, wherein bacterial proliferation in the skin leads to an increased generation of inflammatory mediators that diffuse via the follicle wall (Toyoda and Morohashi 2001; Kim et al. 2002; Vora et al. 2018). A correlation can be made between inflammation and bacterial growth, as inflammation develops by schematically-attracted neutrophils as bacteria metabolize sebaceous triglycerides into fatty acids (Soleymani et al. 2020). P. acnes activates TLR-2 on neutrophils and monocytes, triggering the release of proinflammatory cytokines as a host defense mechanism (Jeremy et al. 2003; Tanghetti 2013) through the upregulation of inflammatory mediators in the vessels surrounding the pilosebaceous tissue (Jeremy et al. 2003).
Quercetin has been shown to exhibit several biological proprieties, including the downregulation of the inflammatory process. Lim H. (Lim et al. 2021) provided evidence that quercetin suppresses the production of proinflammatory cytokines in P. acnes-stimulated cell lines, as Enzyme-Linked Immunosorbent Assay (ELISA) measurements, RNA expression through real-time qPCR (rt-qPCR), and Western Blot analysis, showed that it acted on dermal inflammations via a significant down-regulation of IL-1β, IL-8, and IL-6, including a suppressed TLR-2 expression. Therefore, quercetin downregulates the inflammatory response, as activation of TLRs is suggested to participate in the activation of NF-κB signaling pathways, stimulating the release of proinflammatory cytokines and chemokines in the same cell lines (Lee et al. 2014). These results thus indicate that quercetin may favor a downregulation in inflammation in response to bacterial dermal conditions, namely acne proliferation caused by P. acnes (Lim et al. 2021).
Osteoarthritis
A research study (Shakibaei et al. 2012) aimed to evaluate the anti-inflammatory capacity of three botanical extracts, including UD, as well as to characterize their effects and mechanism of action in primary canine articular chondrocytes, in order to replicate the inflammatory condition seen in osteoarthritis (OA). Nettle application, as determined through an MTT assay, showed a positive impact on cell proliferation, viability, and function, inhibiting IL-1β-induced cytotoxicity, translated through an increase in mitochondrial activity. Furthermore, the extract blocked IL-1β-induced structural changes, as evidenced by decreased degeneration of cell organelles and apoptosis. Moreover, through western Blot analysis, treatment with nettle, both before and after IL-1β treatment, inhibited the mediated suppression of extracellular matrix (ECM) and signaling proteins in chondrocytes, which translated into an upregulation of collagen type II, chondroitin sulfate proteoglycan (CSPG), and β1-integrin, which are contrary defective in OA (Todhunter et al. 1996; Shakibaei et al. 1999). β1-integrin acts as a transmembrane signal transduction receptor, mediating the interaction between cell chondrocytes and cell–matrix components such as collagen type II and CSPG (Shakibaei 1998; Cao et al. 1999). This mediator can be activated in the MAPK pathway, and its inhibition may lead to chondrocyte apoptosis (Shakibaei et al. 1999, 2001), making the upregulatory effect of nettle on β1-integrin crucial for cell viability. In the same experiment, western Blot also indicated the extract’s inhibitory effect on the upregulation of NF-κB-dependent proinflammatory enzymes and matrix-degrading gene products metalloproteinases (MMPs) 9 (MMP-9) and 13 (MMP-13), and COX-2. Cytokine-induced MMP and COX-2 upregulation is mediated by NF-κB activation (John A. Mengshol et al. 2001), and this transcription factor plays an important role in OA, as it mediates the expression of catabolic and inflammation-related genes (McIntyre et al. 2003). Cell–matrix interactions require a constant remodeling of extracellular proteins executed by MMPs, cleaving ECM molecules. Therefore, dysregulation of MMPs, through upregulation of NF-κB, leads to an augmented concentration of these matrix-degrading enzymes, further enhancing the catabolic events responsible for cartilage matrix degradation (Goldring 1999, 2000). This effect can be seen in OA patients, as MMPs are abundantly found in serum samples (Manicourt et al. 1995; Chen et al. 2006). Additionally, COX-2 is an important mediator of pain and inflammation in OA (Chikanza and Fernandes 2000), causing prostaglandin E2 (PGE2) and thromboxane production to induce several other pathological catabolic events in cartilage, such as decreased proliferation of chondrocytes and inhibition of ECM synthesis (Nédélec et al. 2001). UD treatment in canine chondrocytes led to a downregulation of MMPs and COX-2, at least in part, via NF-κB inhibition, as the expression of these enzymes is regulated by NF-κB. Thus, an anti-inflammatory effect can be observed, leading to a more favorable prognosis regarding cell degradation. Furthermore, treatment with UD in conjunction with IL-1β led to an inhibitory effect on the downregulation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling, SRY-Box transcription factor 9 (SOX-9) protein levels, and src homology collagen (Shc) adaptor protein, aligning with the observed effect on chondrocyte differentiation in the same experiment, further indicating the extract’s inhibitory effect on IL-1β (Shakibaei et al. 2012). Moreover, SOX-9 plays a significant role in the expression of ECM genes and chondrocyte differentiation, and cytokines have been shown to reduce SOX-9 levels through an NF-κB dependent, posttranscriptional mechanism (Murakami et al. 2000). Conversely, reduced cell–matrix interactions, such as in the case of OA, lead to the inhibition of Erk1/2 signaling and stimulate the apoptotic pathway in chondrocytes (Shakibaei et al. 2001). Additionally, treating cells with the nettle extract provoked an inhibition of the IL-1β-induced NF-κB-dependent downregulation of collagen type II and SOX-9 expression. Results of this study also suggest that the extract suppressed cytokine-induced activation and upregulation of proinflammatory enzymes such as MMPs and COX-2, the transcription factor NF-κB, and downregulation of cartilage-specific matrix components and important signaling proteins in chondrocytes. Moreover, the chloroformic extract inhibited the degradation and phosphorylation of the IκBα subunit and, thus, its dissociation from the p65-p50 complex, preventing chondrocyte degradation, as seen through high cytoplasmatic IκBα concentrations and decreased levels of phosphorylated p65 in nucleus (Shakibaei et al. 2012. p 65) is located as an inactive complex on NF-κB, together with an inhibitory IκBα subunit. In response to phosphorylation, IκBα dissociates from the complex, and NF-κB translocates to the cell nucleus, binding to target genes and inducing an upstream of inflammatory events, with previous studies having shown that nettle inhibits p65 phosphorylation and nuclear translocation, thus blocking the release of NF-κB (Riehemann et al. 1999). Results also showed that IκBα was completely inhibited in the cytoplasmic extracts of chondrocyte cultures treated with IL-1β alone, indicating cytokine-induced degradation and NF-κB activation (Shakibaei et al. 2012). Treatment with UD resulted in high concentrations of IκBα in the cytoplasm and decreased levels of phosphorylated p65 in nuclear extracts. These findings strongly suggest that nettle inhibits the IL-1β-induced downregulation of cartilage-specific ECM compounds, MAPK-signaling proteins, cartilage-specific transcription factors, and the upregulation of proinflammatory and degrading enzymes, by preventing, at least in part, IκBα phosphorylation and degradation through NF-κB activation. Finally, to validate the viability of these results, and as chondrocytes proliferate in high-density monolayers in vivo, when the extract was administered to high-density culture models it was also capable to produce cartilage-specific ECM and cartilage, which indicates that the UD extract does inhibit IL-1β-induced inflammation and apoptosis, allowing the cells in high-density cultures to redifferentiate back into chondrocytes. These results suggest the presence of active components that can inhibit the IL-1β-induced inflammatory process upstream of the IκBα phosphorylation. UD extract had a positive effect on chondrocyte viability, differentiation, and function as well as having inhibitory effects on IL-1β-induced suppression of proliferation and viability. Thus, UD may well provide an alternative plant-based approach for the downregulation of the inflammatory pathway seen in OA.
Johnson et al. (2013) conducted a different study to evaluate the anti-inflammatory potential of various UD parts using different extract solvents on pair with a commercially available anti-inflammatory agent, in an effort to obtain an anti-inflammatory extract that could be used as a therapeutic agent to provide treatment for inflammatory disorders, particularly rheumatoid or osteoarthritis (Johnson et al. 2013), as a previous clinical trial had demonstrated that the topical application of a proprietary ethanol-based UD extract for treating OA was well tolerated and potentially effective in reducing pain and improving function(Rayburn et al. 2009). Using an NF-κB luciferase assay to assess the anti-inflammatory potential of nettle extracts on lipopolysaccharide (LPS)-stimulated macrophage cell lines, it was revealed that all extracts (methanol, hexane, and chloromethane), except for the more polar water one, exhibited inhibitory activity on NF-κB, with all of the lipophilic extracts displaying minimal cytotoxicity. Among them, the lipophilic dichloromethane extract exhibited the highest inhibitory activity on LPS-induced cells, similar or even higher to that of the commercially available ethanolic extract, along with the least observable cytotoxicity. However, hexane, dichloromethane, and methanol, as opposed to water extract, were all promising in terms of anti-inflammatory activity having little to no effect on cell viability, with one of them, dichloromethane, reaching an inhibitory activity close and even better to that reported as effective. In treating arthritic diseases, some clinical trials on UD have been made, but its efficacy remains unclear (Chrubasik et al. 2007; Rayburn et al. 2009; Jacquet et al. 2009), along with an unknown phytochemical content responsible for its anti-inflammatory action and the improvement in the state of arthritic diseases (Schulze-Tanzil et al. 2002). Nevertheless, different extract preparations have exhibited clinical success, with water and ethanol being the most used solvents for evaluating the anti-inflammatory effect, as increasing polarity shows no efficacy (Chrubasik et al. 2007; Randall et al. 2008; Cameron et al. 2009). Moreover, previous studies (Shakibaei et al. 2012) had already demonstrated the possible anti-inflammatory effect of UD on osteoarthritic cells from canine specimens at the same dose, using a different extract that revealed an inhibitory effect on NF-κB translocation, together with decreased cell degeneration and apoptosis. Concludingly, as this study states, further preclinical work needs to be undertaken regarding the structure of phytochemical components of nettle responsible for its anti-inflammatory action in OA, as well as to determine the appropriate dosage and extract solvent.
Arthritis
Chronic administration of UD hydroalcoholic leaf extract on in vitro human joint osteoarthritic chondrocytes yielded positive results (Schulze-Tanzil et al. 2002). Arthritis is an inflammatory disease characterized by the gradual enzymatic degradation of joint cartilage in which chondrocytes, synoviocytes, and macrophages produce proinflammatory cytokines such as IL-1β and TNF-α, which promote cartilage cells to produce destructive enzymes, including MMPs, who break down cartilage-specific ECM (Gowen et al. 1984; Walport et al. 1992; Martel-Pelletier 1998). MMPs ought to be increased in osteoarthritic cartilage and rheumatoid arthritis (Freemont et al. 1997; Horton et al. 1998; Saito et al. 1998), and are initially expressed in their inactive form. Upon proteolytic activation, they are capable of degrading ECM components responsible for structural integrity (Martel-Pelletier et al. 1994; Cawston 1996; Yong et al. 1998; Murphy and Gavrilovic 1999; Schulze-Tanzil et al. 2001). Accordingly, MMP expression can serve as a marker for proinflammatory cytokine release following OA, as these proinflammatory molecules are key for mediating cartilage degradation (Gowen et al. 1984; Walport et al. 1992; Feldmann et al. 1996; Martel-Pelletier 1998). In IL-1β-treated chondrocytes, immunofluorescence analysis revealed a marked inhibition in cell surface expression of MMP-1, 3, and 9 following UD administration. Furthermore, the immunoblot method for detecting MMP expression by IL-1β-stimulated human chondrocytes cultured on collagen type-II demonstrated a decrease in MMP expression after 24 h when coincubated with the nettle extract and compared to control groups, which was in accordance with densitometric results. Hence, UD significantly decreased MMP expression in cytokine-stimulated chondrocytes. The interaction between chondrocytes and their matrix is essential for the proliferation, differentiation, and survival of cells (Kosher et al. 1973; Kosher and Church 1975; Hewitt et al. 1982; Shakibaei et al. 1999, 2001), and a well-regulated turnover of ECM, mediated through MMPs, is required (Schulze-Tanzil et al. 2001). However, an imbalance between these molecules and their natural inhibitors leads to pathological conditions (Dean et al. 1989; Martel-Pelletier et al. 1994), namely, OA, where high levels of MMPs lead to structural degradation (Kolkenbrock et al. 1993; Manicourt et al. 1995; Freemont et al. 1997; Keyszer et al. 1999), as a differential regulation of mRNA levels of MMPs and aggrecanases in response to IL-1β is seen in this disease (Bluteau et al. 2001). The results obtained from immunofluorescence experiments and quantitative densitometry through western Blot implicate cytokine IL-1β regulation in MMP expression in human cartilage tissue when UD extract treatment is applied, which is consistent with previous studies(Horton et al. 1998; Robbins et al. 2000).
Collectively, these results further validate the anti-inflammatory role of UD extracts in accordance with disease mechanisms and inflammatory pathways.
Allergic rhinitis
Nettle leaves ethanolic extracts acted as a possible antihistaminic and anti-inflammatory agent following administration on HEK cells as fluorescence emission ratio measurements showed that when the extracts were administered, both H1 receptor antagonism and negative agonism, and inhibitory activity on COX-1, COX-2, and Hematopoietic Prostaglandin D2 synthase (HPGDS) were observed (Roschek et al. 2009). Additionally, when the extract was further diluted, absorbance levels reflecting chromophore p-nitroaniline (pNA) production revealed significant inhibition of mast cell tryptase, preventing mast cell degranulation and consequent release of proinflammatory cytokines and chemokines. Using mass spectrometry for characterizing the chemical profile of nettle leaves it was revealed that several compounds possessed theoretical IC50 values lower than those used for control purposes, possibly contributing to the observed in vitro antihistaminic and anti-inflammatory activity. As so, several bioactive compounds in nettle extracts contribute to in vitro anti-inflammatory properties as they are able to inhibit COX production: 4-shogaol, deoxyharringtonine, carnosol, resorcinol (Mutoh et al. 2000), vitamin B3 (Jonas et al. 1996), gingerols, particularly 4-shogaol and 8-dehydrogingerdione (Grzanna et al. 2005), shikimic acid (Pajonk et al. 2006), DL-methyl-m-tyrosine (Palmer and Balacescu 1977), and coumarin derivatives (Liu et al. 1998; Matsuda et al. 2002). Among the bioactives identified, DL-methyl-m-tyrosine, isopropyl-β-D-thioglactopyranoside, phosphotadilcholyne, 4-shogaol, piperine, 8-dehydrogineridone, deoxyharrangitone and carnosol all had theoretical values that contributed to anti-H1 receptor activity. Phenols and alkaloids, including shogaol, dehydrogingerdione, piperine, deoxyharringtonine, and carnosol, and the amino acid tyrosine had theoretical IC50 values lower than those used for comparison of HPGDS inhibitory activity, indicating that these compounds are the major contributors to the nettle in vitro effect on HPGDS activity. This enzyme is located in mast cells and converts COX-1 and COX-2 prostaglandin products into prostaglandin D2, a specific proinflammatory allergic mediator released from mast cells and basophils (FitzGerald 2003; Nantel et al. 2004). As so, the effect seen on HPGDS might provide the anti-inflammatory effect of nettle on mast cells (Roschek et al. 2009). Additionally, the aforementioned synergistic interactions of functional bioactives which impact on the inflammatory cascade must be taken into account, as all IC50 values for indicating inhibitory activity were attributed to individual bioactivities. Nonetheless, these results provide that UD acts through several classes of compounds to deliver an antihistaminic and anti-inflammatory activity through endpoints associated with allergic rhinitis.
Rheumatoid arthritis
In the treatment of rheumatoid arthritis, a standardized extract from UD leaves suppressed the activation of the NF-κB reporter gene in response to several stimuli through inhibition on the degradation of its inhibitory subunit, IκBα (Riehemann et al. 1999). Nettle inhibited activation of NF-κB, as human T lymphocytes showed no desoxyribonucleic acid (DNA) complex formation after being stimulated with TNF, with the water-soluble fraction having a more pronounced effect, as it had been previously demonstrated (Obertreis et al. 1996a, b; Teucher et al. 1996). Additionally, UD extracts partially inhibited DNA probes specific for activator protein 1 (AP-1), which goes to show that DNA inhibition not only occurs on NF-κB, with AP-1 possibly being an additional target of UD extracts. AP-1 has been implicated in the hyperplasia of synovial tissues (Asahara et al. 1997), and its target genes include MMPs, responsible for the degradation of cartilage matrix molecules, with a previous study having demonstrated that inhibition of AP-1 activity prevented collagen-induced arthritis (Shiozawa et al. 1997). Furthermore, nettle inhibition on NF-κB DNA binding was proven, as NF-κB controlled gene expression in another cell line was functionally and almost completely inhibited in a dose-dependent manner, with the water-soluble fraction reaching lower expression levels. This effect was similarly supported in other cell lines in response to stimuli other than TNF, demonstrating that nettle extracts inhibit NF-κB activation in response to several stimuli. However, the inhibition does not directly modify NF-κB DNA binding as even high extract concentrations from the water-soluble fraction did not prevent binding; instead, nettle extracts act through direct prevention of proteolytic degradation of IκBα or the inhibiting of kinases or further upstream signaling molecules because treating cells with phosphorus substrate led to the rapid degradation of IκBα, whereas a protein that reacted non-specifically with the anti-IκBα antibody was not affected. UD dose-dependently inhibited the proteolytic degradation of IκBα as a lower chemiluminescence was observed through western Blot analysis. Activation of NF-κB requires phosphorylation and subsequent degradation of IkBα by the proteasome pathway (Traenckner et al. 1995), with the active form translocating to the nucleus and binding to regulatory sequences of target genes, including those encoding for the upregulation of proinflammatory genes and chemotactic cytokines (Baeuerle and Henkel 1994; Baeuerle and Baichwal 1997). As so, NF-κB activation ceases and UD effectively stops the course of inflammation. NF-κB activity is elevated in rheumatoid arthritis and its downregulation is a suitable target for anti-inflammatory therapeutics (Handel et al. 1995; Fujisawa et al. 1996; Marok et al. 1996). Additionally, TNF expression in macrophages is dependent on NF-κB, and the effect of nettle on the inhibition of the proteolytic degradation of subunit IκBα is evident, which supports previous findings (Foxwell et al. 1998). However, as previously stated, AP-1 inhibition could also be a possible target of UD for an anti-inflammatory approach as TNF activation of AP-1 is important to elements involved in the proinflammatory response including the expression of proinflammatory cell adhesion molecules (Karin et al. 1997; Kyriakis 1999). Concludingly, the anti-inflammatory effect of UD may be ascribed to its inhibitory effect on NF-κB and AP-1 activation.
In vivo data
Central nervous system diseases
A 2018 study (Patel et al. 2018) demonstrated that UD extract administration on streptozotocin (STZ)-induced mice attenuated anxiogenic and depressive-like behavior together with autophagic stimulation, antiapoptotic, and anti-inflammatory effects. Chronic diabetes increased TNF-α expression in several regions of the hippocampus, as previously seen (Jawale et al. 2016), with depressive-like behaviors supporting this fact, as increased immobility following behavioral experiments was seen in untreated rats. The hippocampus is a region known for mediating anxiogenic and depressive behaviors (Jangra et al. 2016), and UD extracts have been found to exert anti-inflammatory and antiapoptotic effects, promoting neuronal survival (Toldy et al. 2005; Patel and Udayabanu 2014), along with a significant reversal of depressive-like behavior in diabetic mice (Patel and Udayabanu 2014). In the same way, following UD treatment, TNF-α levels markedly decreased, showing an anti-inflammatory effect that could reverse depressive behaviors in mice models. Additionally, chronic diabetes also mediated an anxiogenic effect, as mice decreased the number of entries and time spent in the elevated maze task, as it had been previously shown (Tang et al. 2015). However, UD reversed this effect.
Among flavonoids, the major compound identified in UD extracts is quercetin in the form of its glycosides (Carvalho et al. 2017). Quercetin is recorded as a therapeutic agent in CNS disorders via its anti-inflammatory and antioxidant activity in the hippocampus. Furthermore, its neuromodulating activity is described to upregulate neurotrophic factors (Liu et al. 2015) and alleviate depressive-like behavior (Holzmann et al. 2015) and anxiety in rodents (Merzoug et al. 2014). Altogether, the potential therapeutic role of quercetin within the CNS has been discussed in several disease models, and using different cell lines it has been shown to prevent TNF-α from directly activating extracellular signal-related kinase (ERK), transcription factor Jun (c-Jun), NH2-terminal kinase (JNK), and NF-κB. In addition, it may indirectly prevent inflammation via peroxisome proliferator-activated receptor gamma (PPARγ) activity, thereby antagonizing NF-κB or AP-1 transcriptional activation of inflammatory genes, thus blocking the inflammatory cascade (Li et al. 2016). As so, UD may prove to have neuroprotective efficacy through an anti-inflammatory pathway via the therapeutic role of quercetin.
Asthma
One study involving ovalbumin (OVA)-induced inflammation, which served as a disease model for asthma, revealed a positive anti-inflammatory effect on airway inflammation via UD treatment (Zemmouri et al. 2017). Following OVA administration and blood and tissue sample collection, a significant increase was seen in the rate of eosinophils and leukocytes in sensitized rats as compared to those who were left untreated. However, daily administration of UD aqueous extract reduced leukocyte and eosinophil levels, returning their rates to baseline values. Additionally, lymphocyte levels significantly decreased in the UD-treated group compared to those in the control and the sensitized groups. Leukocytes are important mediators in the inflammatory response, and their role in asthma is correlated with chronic inflammation (Hwang et al. 2010), which is in accordance with previous studies, as OVA synthetization in experimental asthmatic models showed an increase in the leukocyte ratio (Li et al. 2009; Wadibhasme et al. 2011). Hence, this study (Zemmouri et al. 2017) shows that UD administration in asthmatic models decreases leukocyte count in the same way, as lymphocyte recruitment was less visible in both cytological and bronchoalveolar lavage fluid (BALF) analyses. During asthmatic inflammation, proinflammatory cells infiltrating the lungs, such as eosinophils and lymphocytes, secrete immune-recruited Th2 cytokines (e.g., IL-1, IL-13, and IL-4) (Yuk et al. 2007). Accordingly, following treatment with UD extract, BALF, and cytological samples showed a marked decrease in the pro-inflammatory cytokine IL-4 (Zemmouri et al. 2017), which acts as a growth factor for Th2 cells and promotes eosinophil migration into the lungs, adhesion to endothelial cells, and mucus production. It also stimulates the profibrotic transformation of transforming growth factor beta (TGF-β), inducing increased accumulation of the ECM and thickening of the septa (Halwani et al. 2011). In a similar pattern (Zemmouri et al. 2017), histological observations in the OVA-sensitized rats showed structural changes characteristic of airway remodeling following chronic inflammation, such as inflammatory cell infiltration, edema, thickened epithelium, and mucus production (Ra et al. 2010). Nevertheless, UD extract administration showed its anti-inflammatory and anti-asthmatic activity, since a decrease in the influx of inflammatory cells to the lungs, as well as in the signs of inflammation and airway obstruction was similarly observed. These results agreed with body weight measurements as rats sensitized to OVA and treated with nettle showed weight gain compared to those sensitized to OVA and left untreated, suggesting that UD has a protective effect, which is in accordance with previous studies (KK et al. 2015). These results, as henceforth supported (Shahzad et al. 2021), provide evidence that further establishes the traditional therapeutic indications of UD extracts for asthmatic airway inflammation.
Benign prostatic hyperplasia
Pigat et al. (Pigat et al. 2019) unveiled that a botanical combination comprising a hydroethanolic UD extract diminished various hallmarks of BPH in a validated preclinical model, including tissue growth and stromal inflammation. Cluster of differentiation (CD) 45 is exclusively expressed in hematopoietic cells and stands as one of the most abundant leukocyte cell surface glycoproteins, with its increase implying the rise of one or more inflammatory cells correlating with the severity of fibrosis (De Vito et al. 2012). Using CD45 for monitoring tissue inflammation, it was revealed that UD-treated mice exhibited diminished prostate inflammation in a dose-dependent manner, as evidenced by lower CD45 content when compared to control groups and mice treated with a commercially available medicine. Fibrosis can ensue as sequelae of healing inflammation, characterized by the deposition of extracellular collagen by activated fibroblasts, constituting a wound-healing process characterized by activation and accumulation of myofibroblasts produced in several tissues (Rodriguez-Nieves and Macoska 2013). Additionally, visualization and quantification of fibrosis using tissue staining revealed a dose-related escalation in treated groups, reflected by collagen upregulation that was significant at the highest doses, both at the histological and molecular levels. Inflammatory infiltrates lead to tissue damage and trigger a chronic process of wound healing that may foster prostatic enlargement (Gandaglia et al. 2013), with infiltrated immune cells associated with high levels of cytokines/chemokines and their receptors (Kramer et al. 2002; Robert et al. 2011), including IL-2, IL-6 (Royuela et al. 2004), IL-7 (Mengus et al. 2011), IL-8 (Giri and Ittmann 2001), IL-15 (Mengus et al. 2011), and IL-17 (Steiner et al. 2003). These factors are suspected to directly impact prostatic cells by altering common cellular mechanisms participating in the initiation or promotion of prostate lesions (Kramer and Marberger 2006; Wang et al. 2008; Penna et al. 2009; Robert et al. 2009). Hence, the downregulation of these proinflammatory genes provides a target for therapeutical management. Accordingly, UD reduced the proinflammatory profile of Pb-PRL mice as it downregulated overall chemokine and cytokine proinflammatory gene expression in the three lobes, in many instances with the lower dose being as efficient as the highest dose and with better outcomes in comparison to standard medicine. IL-1β, IL-15, CCL2, CXL1, CXL6, CXL12, and the chemokine receptor CXCR4 were among the prominent targets displaying the highest inhibitory effect, as they were downregulated in ≥ 2 lobes and/or ≥ 2 doses. IL-1β, IL-15, and CCL2 are secreted by stromal cells, and their production is increased when BPH cells are incubated with activated CD4 + cells (Vignozzi et al. 2012), suggesting their potential contribution to a proinflammatory vicious circle. On the other hand, CXCL1, CXCL6 (Begley et al. 2008), and CXCL12 (Begley et al. 2005) are secreted by stromal prostate fibroblasts and were shown to promote a low-level increase in the proliferation of primary prostate epithelial cells. As so, the downregulation of these receptors and ligands is a major beneficial effect seen from UD treatment. Additionally, UD also reduced COX-2 and iNOS levels, with the lowest dosage having the highest efficacy. COX-2 activity is upregulated in proliferative inflammatory prostate lesions(Sciarra et al. 2007) and in inflammatory cells that infiltrate the prostate, and inducible nitric oxide synthase (iNOS) is the principal factor activating reactive nitrogen that leads to cells injury (Baltaci et al. 2001), with its expression heightened in BPH (Gradini et al. 1999). These findings might support a stronger impact of nettle on these interconnected pathways, which may contribute to the anti-inflammatory effect, accompanied by fibrosis, reduced prostate tissue weight, and reduced proliferation, as evidenced through histological and molecular analyses. However, it is of notice that this experiment used combined extracts, and inflammatory markers could not allow for direct results of nettle’s sole anti-inflammatory action on BPH.
Parkinson’s disease
Pathological and systemic symptoms of Parkinson’s disease (PD) involve: severe motor deficits, oxidative stress, mitochondrial dysfunction, excitotoxicity, and neuroinflammation (Warner et al. 2003). A 2017 (Bisht et al. 2017) study revealed that chronic intragastric administration of hydroalcoholic UD extracts in a body-weight-dependent manner in Parkinson’s models dose-dependently reduced neuroinflammatory markers TNF-α and IL-β. Additionally, the anti-inflammatory effect of UD on PD models was tested through behavioral tests, along with body weight measurements. Results provided that post-treatment with UD significantly and dose-dependently reversed deviations in body weight and motor coordination. As the anti-inflammatory effect of UD occurs, neuronal cell death ceases and the ability to promote recovery from neuromuscular damage increases, resulting in an interruption in motor deficit, which was visually manifested by motor activity tests.
PD is a multisystem disorder associated with neuroinflammation via immune responses, which leads to striatal dopamine depletion and mismanagement through microglial activation (Jung and Kim 2018; Tseng et al. 2020). The inflammatory activation of microglial cells increases the levels of pro-inflammatory cytokines TNF, IL-1β, TGF-β, IL-6, reactive oxygen species (ROS), and other proinflammatory molecules, inducing an inflammatory environment and contributing to neuronal death (Nagatsu et al. 2000; Harms et al. 2021). The upregulation of cytokines can be a result of immune dysregulation, leading to a cascade of events that result in neurotoxicity (Tansey et al. 2022), with expression of these proinflammatory proteins being implicated in the degeneration of dopaminergic neurons following microglia activation (Karpenko et al. 2018). However, even though different signaling pathways have been discussed in PD pathogenesis, TNF is one molecule that has received increased attention as it has a role in nigral degeneration, with selective neutralization significantly attenuating dopaminergic neuron death (McCoy et al. 2006, 2008; Harms et al. 2011; Barnum et al. 2014). Furthermore, TNF-α has been implicated in the decrease of dopaminergic neurons through the activation of intracellular molecular markers, including NF-κB signaling pathways, further amplifying the inflammatory cascade (Singh et al. 2019; Tseng et al. 2020). In the event of microglial activation, redox-sensitive NF-κB translocates to the nuclear compartment resulting in the activation of various pro-inflammatory genes, including cytokines IL-1β and TNF-α (Magalingam et al. 2015).
As a way of investigating the correlation between the anti-inflammatory action and the phytochemical composition of UD, results from a 2022 study (Josiah et al. 2022), which addressed the neurochemical modulatory properties of quercetin on PD models, suggested that the anti-inflammatory effect seen after treatment was mediated by the modulation of canonical NF-κB. Additionally, results also showed the anti-apoptotic potential via inhibition of the NF-κB-dependent p53-signaling pathway, which had already been seen (Zhang et al. 2017; Farombi et al. 2019; Pogačnik et al. 2020). These results were in accordance with previous in vitro reports, indicating that quercetin has a role in reducing cell loss in striatal dopamine (Karuppagounder et al. 2013). According to these findings, quercetin may have an anti-inflammatory effect in UD extracts decreasing the extent of PD, providing evidence of UD’s potential as an anti-inflammatory agent in the treatment of PD, supporting its use as a therapeutic intervention to mitigate neuroinflammation and related motor deficit.
Alzheimer’s disease
One of the major neuropathological changes observed in Alzheimer’s disease (AD) is the extensive deposition of amyloid beta (Aβ) plaques in the brain parenchyma and vessel walls, leading to neurodegradation and toxicity in the hippocampus and cerebral cortex (Theuns and Van Broeckhoven 2000). Chronic neurodegenerative disorders are related to synapse malfunction, and AD may be evaluated according to synaptophysin (Syp) mRNA expression levels in the hippocampus, as it usually occurs before Aβ accumulation, playing a role in Aβ synthesis (Callahan et al. 1999; Liu et al. 2008). When STZ-induced mice were treated with ethanolic-mixed UD-containing extracts it was seen an upregulation in SYP (Daneshmand et al. 2016), indicating potential beneficial effects on synapse function. Furthermore, abnormal processing of amyloid-β precursor protein (APP) from presenilin 1 (Psen1), the catalytic subunit of γ-secretase, promotes APP accumulation, leading to an increase in Aβ plaques; thus, an increase in Psen1 gene expression is seen in subjects with defective γ-secretase. Accordingly, a decreased concentration of Psen1 in mice hippocampus was noted in rt-qPCR analysis when mice models were treated with a combined extract containing UD (Daneshmand et al. 2016). Therefore, both Psen1 and Syp are markers for Aβ accumulation. The inflammatory process in AD occurs as the Aβ peptide produced by APP processing forms aggregates that activate microglia through TLRs and receptors for advanced glycation endproducts (RAGE). These, in turn, activate NF-κB and AP-1 transcription factors, which induce ROS production and the expression of inflammatory cytokines (e.g., IL-1, IL-6, and TNF). These inflammatory factors directly act on neurons and stimulate astrocytes, which amplify pro-inflammatory signals, inducing a neurotoxic effect. The inflammatory mediators are generated by resident CNS cells, which induce the production of adhesion molecules and chemokines, leading to the recruitment of peripheral immune cells (Meraz-Ríos et al. 2013). This combined extract mixture had already shown positive effects on the pro-inflammatory status in other models for neurodegenerative diseases (Mahmoodpoor et al. 2010; Mohseni-Salehi-Monfared et al. 2010; Mohammadirad et al. 2011; Bazazzadegan et al. 2017), as UD has an anti-inflammatory action through different pathways. According to these findings, the combined mixture has potential to prevent Aβ accumulation and modulate neuronal physiology, potentially mitigating the impact of AD. However, whether the anti-inflammatory effect seen in Aβ plaques is exclusive of UD or synergizes with the other extracts is not here a matter of discussion, but UD certainly contributes to the anti-inflammatory effect responsible for decreasing the impact of AD.
Musculoskeletal inflammatory diseases
One study evaluated the anti-inflammatory potential of UD leaves along with their toxicological proprieties by treating indomethacin-induced mice models with different extract solvents (Dar et al. 2013). The decrease in inflammation was evaluated through measurement of paw edema, and analysis of test results revealed that only the hexane fraction had an observable and significant effect. Paw swelling is characteristic of this model of inflammation and it occurs through distinct phases: an initial phase mediated by histamine and serotonin; an intermediate phase involving the activity of kinins; and a third phase with prostanoid synthesis induced by COXs (Di Rosa et al. 1971; Pérez G. 1996; McKoy et al. 2002). Initially, the crude ethanolic extract, and then one of the subfractions, significantly reduced the inflammatory state of paws from the moment of administration to the last measurement, suggesting that UD may exert its effect through inhibition on the synthesis and liberation of histamine and prostaglandin mediators. Prostaglandins are produced by COXs from arachidonic acid and COX-2 is present at sites of inflammation induced by cytokines and other mediators, being synthesized by cells involved in the inflammatory process such as monocytes, macrophages, and synoviocytes. A sequence of inflammatory mediators of cellular and plasma origin initiate and maintain vascular permeability and edema in the inflammatory cascade followed by infiltration of leukocytes (Olivo et al. 2007). Prostaglandins are in high concentrations at inflamed sites, contributing to local increases in blood flow, edema formation, and pain sensitization, being COXs leukocyte’s the enzymes which catalyze their synthesis (Giuliano and Warner 2002). COX inhibitors have an inhibitory action on edema formation and selective COX-2 inhibitors have been shown to actively reduce edema formation in mice models (Perretti et al. 1996; Bressan et al. 2003), with the peak of edema formation and PGE2 release being correlated with a marked expression of COX-2 in paw tissue (Olivo et al. 2007). Moreover, COX-2 dependency in paw-edema has been verified by studies, showing that pre-treating mice with a selective COX inhibitor decreases inflammation (Gupta et al. 2002; Nishikori et al. 2002). The inhibitory effect of UD on prostaglandin production has previously been confirmed, as a study reporting the anti-inflammatory activities of hydroalcoholic UD extracts that targeted receptors for allergic rhinitis showed that nettle administration inhibited COX-1 and 2 productions in mixed samples following immunoassay analysis (Roschek et al. 2009). As so, through direct inhibition of prostaglandins or inhibition of its mediators, a reverse in the inflammatory state and edema can be achieved. Although the exact anti-inflammatory role of UD on edema is still up to debate, its effect on decreasing COX, particularly COX-2, is thus seen. Another possible mechanism for the anti-inflammatory effect besides direct COX inhibition could be the inhibition of phospholipase A2, preventing the formation of arachidonic acid, thus blocking the inflammatory cascade. Additionally, in the same study (Dar et al. 2013), a phytochemical analysis was conducted to characterize the compounds in the extract that showed marked anti-inflammatory activity and a higher decrease in paw edema, and it revealed that UD contained a high content in fatty acid esters, namely, heptadecyl ester, hexyl octyl ester, butyl tetradecyl ester, and 1,2-benzenedicarboxylicacid, which are reported to exhibit anti-inflammatory activity (Li et al. 2004). Altogether, these findings favor the anti-inflammatory role of UD.
Inflammatory pain
Nettle exhibits anti-inflammatory activity coupled with an analgesic effect in animal models. Hajhashemi et.al. (Hajhashemi and Klooshani 2013) used two distinct mice models to evaluate the previously reported nettle antinociceptive and anti-inflammatory activities using writhing and formalin tests together with a paw edema test, respectively. Anti-inflammatory effects were observed in a dose-dependent manner, with only the highest dose being effective in reducing paw edema after 4 h in the treated groups. Together with these results, sensitization to pain was evaluated in another animal model. For testing analgesic capacity, a writhing test was performed, with UD being effective at all doses and in a dose-dependent manner. Accordingly, when mice were subjected to UD treatment before formalin injection, only the highest doses of UD were able to decrease licking in both the acute and chronic phases (Hajhashemi and Klooshani 2013). Pain is an important symptom that serves as a warning sign of a pathological condition, and recently the relationship between proinflammatory biomarkers and pain has been examined in patients in whom inflammation is a key pathological feature (Üçeyler et al. 2007, 2011; Iannuccelli et al. 2010; White et al. 2010; Lin et al. 2012; Parkitny et al. 2013). Following tissue damage, cytokines, and chemokines are released in the local environment of nerve endings contributing to the activation of pain nociceptors and the development of hyperalgesia (Angst et al. 2008). Edema is a clinical sign of inflammation caused by a transvascular influx of protein-rich fluid into the interstitial medium as a result of the action of histamine, bradykinin, leukotrienes, complement components, substance P, and platelet-activating factor (PAF) (Sherwood and Toliver-Kinsky 2004), with the carrageenan test being a widely used method for evaluating inflammation (Winter et al. 1962). Following tissue damage, intracellular components are released, and migrant and local cells, such as mast cells and basophils, release vasoactive amines (serotonin and histamine), increasing blood vessel permeability and vasodilatation, causing liquid leakage into the vascular compartment and pressure alterations (Majno and Palade 1961; Benly P 2015; Abdulkhaleq et al. 2018). Martin et al. (Martin et al. 1988) showed a vascular increase in rat models following IL-1 and interferon gamma (IFN-γ) induction. IL-1β is another approached molecule in a mechanism involving nociception and sensitivity to pain (Ren and Torres 2009), and an increase is seen together with TNF-α, inducing arachidonic acid metabolites (Feng et al. 1995). As so, the increase in cytokine concentration inducts arachidonic acid metabolites such as prostaglandins, leukotrienes, prostacyclin, and thromboxane, which mediate vascular changes (Okiji et al. 1989) and are involved in the modulation of pain (Williams and Morley 1973; Kawabata 2011). Consequently, the pain that rat models feel following the carrageenan test occurs as paw swelling leads to the recruitment of several proinflammatory cytokines, inducing prostaglandin production, which modulate nociception through activation of receptors involved in the mechanism of pain. Results from nettle administration on different mice models revealed that UD dose-dependently decreased inflammation, with the highest dose having the most notorious effect; showed analgesic activity through licking behavior; and reduced writhing (Hajhashemi and Klooshani 2013), which supports the idea of an anti-inflammatory activity from extracts. However, the exact mechanism by which pain and inflammation decrease in models of inflammatory pain requires further investigation. Phytochemicals from other plants, namely flavonoids and polyphenolic compounds, have been reported to exert anti-inflammatory and antinociceptive effects. As UD has been reported to have a multiverse and chemically rich content with the presence of these same molecules, it may act through modulation of histamine release from mast cells and regulation of the arachidonic acid metabolism, as well as through an antioxidant effect.
Colitis
Colonic serum analysis of rat models with induced colitis revealed that treatment with UD seed oil reverses the inflammatory state (Genc et al. 2011). Serum levels of TNF-α, IL-1β, and IL-6 increased in the colitic model, which were subsequently reversed by UD administration. Proinflammatory cytokines have a major role in inflammation and are frequently elevated in colitis. UD has been shown to possess strong anti-inflammatory activity, with NF-κB inhibition leading to a decrease in expression in proinflammatory markers (Riehemann et al. 1999). Therefore, it is likely that the administration of UD might be responsible for the decrease in inflammatory markers observed in colitis. Moreover, the absorbance reading of sialic acid (SA) exhibited a decrease in expression following UD treatment. Since increased SA concentration has been associated with the inflammatory process (Schauer 1982; Sillanaukee et al. 1999; Yida et al. 2015; Xue et al. 2018), UD may exert its action by reducing SA levels. Additionally, to assess the severity of the inflammatory state, evaluate recovery from colitis injury, and confirm molecular expression findings, macroscopic and microscopic analyses were performed, including an assessment of tissue damage scores and collagen content. UD proved to have a positive impact as it improved colonic lesions, with treated animals having decreased collagen fiber density and leukocyte count. Leukocytes produce proinflammatory mediators and are a major source of ROS in the inflamed colon mucosa, as observed in colitis patients (Guo et al. 1999), and infiltration of leukocytes in the mucosa has been suggested to contribute to tissue apoptosis and mucosal dysfunction. For these reasons, the lower leukocyte count observed in treated animals might be attributed to the anti-inflammatory action of nettle oil.
In another study, the role of a commercially available UD extract, IDS30, used as adjuvant therapy in rheumatic diseases, was also investigated for its anti-inflammatory potential on chronic colitis, focusing on its downregulatory effect on proinflammatory markers (Konrad et al. 2005). ELISA examination of supernatants from the inflamed mucosa of treated animals revealed a decrease in the secretion of both TNF-α and IL-1β (Konrad et al. 2005). This effect is mediated by a lower maturation of DCs and expression of CD83 and CD86, leading to a downregulatory effect on T-cell response. IDS30 acts through several pathways for providing its anti-inflammatory effect on chronic inflammatory diseases, including the suppression of cytokines through downregulation of NF-κB expression (Riehemann et al. 1999), as in animal models of colitis inhibition of NF-κB or TNF-α has already been proved as a therapeutic strategy, leading to an improvement in disease prognostic (Neurath et al. 1996; Myers et al. 2003). Another mechanism might be the decreased secretion of TNF-α from the inflamed intestinal mucosa. Mucosal microflora triggers Th1 production, and nettle has been shown to provide a positive effect on immune-induced colitis (Madsen et al. 1999). Accordingly, as an imbalance between Th1 and Th2 reaction with a preponderance of Th1 is an accepted pathogenic concept in colitis, the immune response might take a vital role in inflammation. Moreover, anti-TNF-α therapy has been shown to highly improve disease prognosis (Schreiber et al. 2001; D’Haens 2003), and UD extracts have been validated to downregulate Th1 response, together with a decrease in IL-2 and IFN-α protein production (Klingelhoefer et al. 1999). Altogether, nettle may inhibit the Th1-triggered immune response, which correlates with a downregulation in the inflammatory cascade mediated via TNF-α, as observed in this study. Moreover, mononuclear cells from UD-treated mice proliferated less than those from water-treated animals upon LPS stimulation (Konrad et al. 2005). TNF-α and IL-1 secretion from mononuclear cells have a high correlation with colitis (Schreiber et al. 1999), therefore, IDS30 might suppress the immune response, decreasing the release of proinflammatory markers. The data suggest that nettle extracts have a suppressive effect on TNF-α, with a more pronounced effect observed in chronic treatment as compared to acute phase models that did not yield satisfactory results. This occurs as acute colitis is independent of lymphocytes, and is mainly triggered by toxic effects on epithelial cells of the colon, whereas chronic colitis involves immunologic mechanisms (Dieleman et al. 1994). As so, UD extracts exert their action on chronic colitis via an anti-inflammatory effect mediated through the downregulation of immune-induced proinflammatory markers.
Conclusion
This literature review aimed to investigate the anti-inflammatory potential of Urtica dioica in a variety of diseases with inflammatory origin, namely, MS, asthma, dermal wounds, BPH, acne, arthritis, rhinitis, diabetes mellitus, and neurodegenerative and musculoskeletal diseases. Discussion from findings regarding the existing literature surrounding this topic indicate that UD acts through different inflammatory pathways according to the type of disease and its extent. Further research showed that the type of extract solvent, plant part, and bioactive components have a deep role in counteracting the inflammatory response. This paper may contribute to further develop existing research surrounding this topic by reviewing evidence from distinct in vivo and in vitro studies. Nevertheless, as reflected throughout this review, the specific metabolites by which UD exerts its anti-inflammatory action are yet to be fully described, which presents a gap in establishing its safety and pharmacological profile. Additionally, the amount of specific in vivo and in vitro experiments and clinical trials using plant-based preparations or isolated phytochemicals from UD does not allow for a complete and certain description of its anti-inflammatory activity. To overcome the limitations of this study, additional screening for specific molecules, as well as more comprehensive in vivo and in vitro experiments and clinical trials, are necessary to further elaborate on the anti-inflammatory role of UD and to identify which molecules can provide targets for inflammatory-related diseases. Moreover, the current review only focused on direct markers of inflammation, but inflammation itself is a complex series of interconnected physiological events, necessitating further comparative studies and a deeper analysis of existing literature to support the aforementioned findings. Despite these challenges, verdicts from this review indicate that, given the current and historical significance of UD, nettle may serve as an anti-inflammatory agent through various pathways, namely, the inhibition of NF-κB, modulation of inflammatory cascades, and inhibition of cytokines and other pro-inflammatory genes. Nevertheless, among its diverse phytochemical content, quercetin was one molecule with noticeable anti-inflammatory activity. In conclusion, Urtica dioica, among the Urtica species, emerges as the most extensively studied and reported plant, offering a rich source of molecules with the potential for developing effective anti-inflammatory treatment approaches. Nonetheless, further research and well-designed trials are required to fully exploit the therapeutic potential of UD in combating inflammatory diseases.
References
Abdulkhaleq LA, Assi MA, Abdullah R et al (2018) The crucial roles of inflammatory mediators in inflammation: a review. Vet World 11:627–635. https://doi.org/10.14202/VETWORLD.2018.627-635
Ait A, Said H, Sbai I et al (2015) Highlights on nutritional and therapeutic value of Stinging nettle (Urtica dioica). Int J Pharm Pharm Sci 7:8–14
Akbay P, Basaran AA, Undeger U, Basaran N (2003) In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother Res 17:34–37. https://doi.org/10.1002/PTR.1068
Angst MS, Clark JD, Carvalho B et al (2008) Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain 139:15–27. https://doi.org/10.1016/J.PAIN.2008.02.028
Anosike CA, Obidoa O, Ezeanyika LU (2012) Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J Pharm Sci 20:1–7. https://doi.org/10.1186/2008-2231-20-76
Ansar W, Ghosh S (2016) Inflammation and inflammatory diseases markers and mediators: role of CRP in some inflammatory diseases. Biology of C reactive protein in health and disease, 1st edn. Springer, New Delhi, pp 67–107
Aquino DA, Capello E, Weisstein J et al (1997) Multiple sclerosis: altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol 56:664–672. https://doi.org/10.1097/00005072-199756060-00004
Aquino DA, Klipfel AA, JcF B, Norton WT (1993) The 70-kDa heat shock cognate protein (HSC70) is a major constituent of the central nervous system and is up-regulated only at the mRNA level in acute experimental autoimmune encephalomyelitis. J Neurochem 61:1340–1348. https://doi.org/10.1111/J.1471-4159.1993.TB13627.X
Arnold E, Benz T, Zapp C, Wink M (2015) Inhibition of cytosolic phospholipase A2α (cPLA2α) by medicinal plants in relation to their phenolic content. Molecules 20:15033–15048. https://doi.org/10.3390/molecules200815033
Asahara H, Fujisawa K, Kobata T et al (1997) Direct evidence of high DNA binding activity of transcription factor AP-1 in rheumatoid arthritis synovium. Arthritis Rheum 40:912–918. https://doi.org/10.1002/ART.1780400520
Asea A, Kraeft SK, Kurt-Jones EA et al (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442. https://doi.org/10.1038/74697
Asea A, Rehli M, Kabingu E et al (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034. https://doi.org/10.1074/JBC.M200497200
Attia EAS, Seada LS, El-Sayed MH, El-Shiemy SM (2010) Study of telomerase reverse transcriptase (hTERT) expression in normal, aged, and photo-aged skin. Int J Dermatol 49:886–893. https://doi.org/10.1111/J.1365-4632.2009.04374.X
Aziz N, Kim M, Cho J (2018) Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol 225:342–358. https://doi.org/10.1016/j.jep.2018.05.019
Bacchi S, Palumbo P, Sponta A, Coppolino MF (2012) Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem 11:52–64. https://doi.org/10.2174/187152312803476255
Baeuerle PA, Baichwal VR (1997) NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol 65:111–137
Baeuerle PA, Henkel T (1994) Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–179. https://doi.org/10.1146/ANNUREV.IY.12.040194.001041
Baltaci S, Orhan D, Gögüs Ç et al (2001) Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int 88:100–103. https://doi.org/10.1046/J.1464-410X.2001.02231.X
Barnum CJ, Chen X, Chung J et al (2014) Peripheral administration of the selective inhibitor of soluble tumor necrosis factor (TNF) XPro®1595 attenuates nigral cell loss and glial activation in 6-OHDA hemiparkinsonian rats. J Parkinsons Dis 4:349–360. https://doi.org/10.3233/JPD-140410
Basu S, Binder RJ, Suto R et al (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol 12:1539–1546. https://doi.org/10.1093/intimm/12.11.1539
Bazazzadegan N, Shasaltaneh MD, Saliminejad K et al (2017) Effects of herbal compound (IMOD) on behavior and expression of Alzheimer’s disease related genes in streptozotocin-rat model of sporadic Alzheimer’s disease. Adv Pharm Bull 7:491–494. https://doi.org/10.15171/APB.2017.060
Beere HM (2004) “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651. https://doi.org/10.1242/jcs.01284
Begley L, Monteleon C, Shah RB et al (2005) CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell 4:291–298. https://doi.org/10.1111/J.1474-9726.2005.00173.X
Begley LA, Kasina S, MacDonald J, Macoska JA (2008) The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 43:194–199. https://doi.org/10.1016/J.CYTO.2008.05.012
Benly P (2015) Role of histamine in acute inflammation. J Pharm Sci Res 7:373–376
Benn SC, Woolf CJ (2004) Adult neuron survival strategies-slamming on the brakes. Nat Rev Neurosci 5:686–700. https://doi.org/10.1038/NRN1477
Bennett JM, Reeves G, Billman GE, Sturmberg JP (2018) Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front Med (lausanne) 5:316. https://doi.org/10.3389/FMED.2018.00316
Beschia M, Leonte A, Oancea I (1982) Phenolic components with biological activity in vegetable. Tehnologia Si Chimia Produselor Alimentare 6:59–63
Bhusal KK, Magar SK, Thapa R et al (2022) Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): a review. Heliyon 8:e09717. https://doi.org/10.1016/j.heliyon.2022.e09717
Bindu S, Mazumder S, Bandyopadhyay U (2020) Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 180:114147. https://doi.org/10.1016/J.BCP.2020.114147
Birnbaum G, Kotilinek L, Miller SD et al (1998) Heat shock proteins and experimental autoimmune encephalomyelitis II: environmental infection and extra-neuraxial inflammation alter the course of chronic relapsing encephalomyelitis. J Neuroimmunol 90:149–161. https://doi.org/10.1016/S0165-5728(98)00141-6
Bisht R, Joshi BC, Kalia AN, Prakash A (2017) Antioxidant-rich fraction of Urtica dioica mediated rescue of striatal mito-oxidative damage in MPTP-induced behavioral, cellular, and neurochemical alterations in rats. Mol Neurobiol 54:5632–5645. https://doi.org/10.1007/s12035-016-0084-z
Bluteau G, Conrozier T, Mathieu P, Vignon E, Herbage D, Mallein-Gerin F (2001) Matrix metalloproteinase-1,-3,-13 and aggrecanase-1 and-2 are differentially expressed in experimental osteoarthritis. Biochim Biophys Acta (BBA) Gen Subj 1526(2):147–58. https://doi.org/10.1016/S0304-4165(01)00122-2
Bousquet J, Jeffery PK, Busse WW et al (2000) Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161:1720–1745. https://doi.org/10.1164/AJRCCM.161.5.9903102
Bradding P (2008) Asthma: eosinophil disease, mast cell disease, or both? Allergy Asthma Clin Immunol 4:84. https://doi.org/10.1186/1710-1492-4-2-84
Bradding P, Holgate ST (1999) Immunopathology and human mast cell cytokines. Crit Rev Oncol Hematol 31:119–133. https://doi.org/10.1016/S1040-8428(99)00010-4
Bradding P, Walls AF, Holgate ST (2006) The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol 117:1277–1284. https://doi.org/10.1016/j.jaci.2006.02.039
Bressan E, Farges RC, Ferrara P, Tonussi CR (2003) Comparison of two PBR ligands with classical antiinflammatory drugs in LPS-induced arthritis in rats. Life Sci 72:2591–2601. https://doi.org/10.1016/S0024-3205(03)00171-1
Broer J, Behnke B (2002) Immunosuppressant effect of IDS 30, a stinging nettle leaf extract, on myeloid dendritic cells in vitro. J Rheumatol 29:659–666
Brown TJ, Hooper L, Elliott RA et al (2006) A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: A systematic review with economic modelling. Health Technol Assess (Rockv) 10:1–183. https://doi.org/10.3310/hta10380
Bsibsi M, Ravid R, Gveric D, Van Noort JM (2002) Broad expression of toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013–1021. https://doi.org/10.1093/jnen/61.11.1013
Calderwood SK, Mambula SS, Gray PJ (2007) Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci 1113:28–39. https://doi.org/10.1196/annals.1391.019
Callahan LM, Vaules WA, Coleman PD (1999) Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. J Neuropathol Exp Neurol 58:275–287. https://doi.org/10.1097/00005072-199903000-00007
Cameron M, Gagnier JJ, Little CV et al (2009) Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part i: Osteoarthritis Phytother Res 23:1497–1515. https://doi.org/10.1002/PTR.3007
Cao L, Lee V, Adams ME et al (1999) β1-Integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol 18:343–355. https://doi.org/10.1016/S0945-053X(99)00027-X
Carvalho AR, Costa G, Figueirinha A et al (2017) Urtica spp.: phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res Int 99:485–494. https://doi.org/10.1016/j.foodres.2017.06.008
Castro P, Xia C, Gomez L et al (2004) Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate 60:153–159. https://doi.org/10.1002/pros.20051
Cawston TE (1996) Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther 70:163–182. https://doi.org/10.1016/0163-7258(96)00015-0
Chabas D, Baranzini SE, Mitchell D et al (2001) (2001) The influence of the proinflammatory cytokine, osteopontin, on autoimmue demyelinating disease. Science 294:1731–1735. https://doi.org/10.1126/science.1062960
Chaurasia N, Wichtl M (1986) Phenylpropane und lignane aus der wurzel von Urtica dioica L. Deutsche Apoth Zeitung 126:1559–1563
Chaurasia N, Wichtl M (1987) Flavonol Glycosides from Urtica dioica. Planta Med 53:432–434. https://doi.org/10.1055/S-2006-962765
Chen CH, Lin KC, Yu DTY et al (2006) Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology 45:414–420. https://doi.org/10.1093/rheumatology/kei208
Chen YF, Jobanputra P, Barton P et al (2008) Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 12:1–278. https://doi.org/10.3310/hta12110
Chikanza IC, Fernandes L (2000) Novel strategies for the treatment of osteoarthritis. Expert Opin Investig Drugs 9:1499–1510. https://doi.org/10.1517/13543784.9.7.1499
Chira A, Rekik I, Rahmouni F et al (2022) Phytochemical composition of Urtica dioica essential oil with antioxidant and anti-inflammatory properties: In vitro and in vivo studies. Curr Pharm Biotechnol. https://doi.org/10.2174/1389201023666220829104541
Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik SA (2007) A comprehensive review on nettle effect and efficacy profiles, Part I: Herba urticae. Phytomedicine 14:423–435. https://doi.org/10.1016/j.phymed.2007.03.004
Cicero AFG, Allkanjari O, Vitalone A et al (2019) Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Archivio Italiano Di Urologia e Andrologia 91:139–152. https://doi.org/10.4081/aiua.2019.3.139
Clark JD, Lin LL, Kriz RW et al (1991) A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65:1043–1051. https://doi.org/10.1016/0092-8674(91)90556-E
Council of Europe C of E on FS (2008) Natural sources of flavourings. In: Council of Europe C of E on FS (ed) Natural Sources of Flavorings. Council of Europe Pub, Belgium, p 208
Criado PR, Criado RFJ, Valente NYS et al (2006) The inflammatory response in drug-induced acute urticaria: ultrastructural study of the dermal microvascular unit. J Eur Acad Dermatol Venereol 20:1095–1099. https://doi.org/10.1111/j.1468-3083.2006.01744.x
Cwiklinska H, Cichalewska-Studzinska M, Selmaj KW, Mycko MP (2020) The heat shock protein HSP70 promotes Th17 genes’ expression via specific regulation of microRNA. Int J Mol Sci 21:2823. https://doi.org/10.3390/IJMS21082823
Daneshmand P, Saliminejad K, Dehghan Shasaltaneh M et al (2016) Neuroprotective effects of herbal extract (Rosa canina, Tanacetum vulgare and Urtica dioica) on rat model of sporadic Alzheimer’s disease. Avicenna J Med Biotechnol 8:120–125
Dar SA, Ganai FA, Yousuf AR et al (2013) Pharmacological and toxicological evaluation of Urtica dioica. Pharm Biol 51:170–180. https://doi.org/10.3109/13880209.2012.715172
De Nunzio C, Presicce F, Tubaro A (2016) Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol 10(13):613–626. https://doi.org/10.1038/nrurol.2016.168
De Vito R, Alisi A, Masotti A et al (2012) Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med 30:49–56. https://doi.org/10.3892/IJMM.2012.965
Dean DD, Martel-Pelletier J, Pelletier JP et al (1989) Evidence for metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Investig 84:678–685. https://doi.org/10.1172/JCI114215
Dennis EA, Cao J, Hsu YH et al (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111:6130–6185. https://doi.org/10.1021/CR200085W
Denzler KL, Waters R, Jacobs BL et al (2010) Regulation of inflammatory gene expression in PBMCs by immunostimulatory botanicals. PLoS ONE 5:e12561. https://doi.org/10.1371/journal.pone.0012561
Devkota HP, Paudel KR, Khanal S et al (2022) Stinging nettle (Urtica dioica L.): nutritional composition, bioactive compounds, and food functional properties. Molecules 27:5219. https://doi.org/10.3390/molecules27165219
D’Haens G (2003) Anti-TNF therapy for Crohn’s disease. Curr Pharm Des 9:289–294. https://doi.org/10.2174/1381612033391982
Di Lorenzo C, Dell’agli M, Badea M, et al (2013) Plant food supplements with anti-inflammatory properties: a systematic review (II). Crit Rev Food Sci Nutr 53:507–516. https://doi.org/10.1080/10408398.2012.691916
Di Rosa M, Giroud JP, Willoughby DA (1971) Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104:15–29. https://doi.org/10.1002/PATH.1711040103
Dieleman LA, Ridwan BU, Tennyson GS et al (1994) Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107:1643–1652. https://doi.org/10.1016/0016-5085(94)90803-6
Đurović S, Pavlić B, Šorgić S et al (2017) Chemical composition of stinging nettle leaves obtained by different analytical approaches. J Funct Foods 32:18–26. https://doi.org/10.1016/J.JFF.2017.02.019
Durović S, Zeković Z, Šorgić S et al (2018) Fatty acid profile of stinging nettle leaves: application of modern analytical procedures for sample preparation and analysis. Anal Methods 10:1080–1087. https://doi.org/10.1039/C7AY02559A
Ellnain-Wojtaszek M, Bylka W, Kowalewski Z (1986) Flavanoids compounds in Urtica dioica L. Herba Polonica 32:131–137
Farombi EO, Awogbindin IO, Farombi TH et al (2019) Neuroprotective role of kolaviron in striatal redo-inflammation associated with rotenone model of Parkinson’s disease. Neurotoxicology 73:132–141. https://doi.org/10.1016/J.NEURO.2019.03.005
Feldmann M, Brennan FM, Maini RN (1996) Rheumatoid arthritis. Cell 85:307–310. https://doi.org/10.1016/S0092-8674(00)81109-5
Feng L, Xia Y, Garcia GE et al (1995) Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest 95:1669–1675. https://doi.org/10.1172/JCI117842
Fibbi B, Penna G, Morelli A et al (2010) Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl 33:475–488. https://doi.org/10.1111/J.1365-2605.2009.00972.X
Fisher P, Ward A (1994) Complementary medicine in Europe. BMJ 309:107. https://doi.org/10.1136/BMJ.309.6947.107
FitzGerald GA (2003) COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov 2:879–890. https://doi.org/10.1038/NRD1225
Fleming TP, Watkins AJ, Velazquez MA et al (2018) Origins of lifetime health around the time of conception: causes and consequences. Lancet 391:1842–1852. https://doi.org/10.1016/S0140-6736(18)30312-X
Fleshner M, Johnson JD (2005) Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia 21:457–471. https://doi.org/10.1080/02656730500088211
Flood-Page P, Swenson C, Faiferman I et al (2007) A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 176:1062–1071. https://doi.org/10.1164/RCCM.200701-085OC
Foster JA, Brown IR (1997) Differential induction of heat shock mRNA in oligodendrocytes, microglia, and astrocytes following hyperthermia. Mol Brain Res 45:207–218. https://doi.org/10.1016/S0169-328X(96)00138-6
Foxwell B, Browne K, Bondeson J et al (1998) Efficient adenoviral infection with IκBα reveals that macrophage tumor necrosis factor α production in rheumatoid arthritis is NF-κB dependent. Proc Natl Acad Sci U S A 95:8211. https://doi.org/10.1073/PNAS.95.14.8211
Francišković M, Gonzalez-Pérez R, Orčić D et al (2017) Chemical composition and immuno-modulatory effects of Urtica dioica L. (Stinging Nettle) extracts. Phytother Res 31:1183–1191. https://doi.org/10.1002/PTR.5836
Freemont AJ, Hampson V, Tilman R et al (1997) Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis 56:542–549. https://doi.org/10.1136/ARD.56.9.542
Fujisawa K, Aono H, Hasunuma T et al (1996) Activation of transcription factor NF-κB in human synovial cells in response to tumor necrosis factor α. Arthritis Rheum 39:197–203. https://doi.org/10.1002/ART.1780390205
Furman D, Campisi J, Verdin E et al (2019) (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25(12):1822–1832. https://doi.org/10.1038/s41591-019-0675-0
Gandaglia G, Briganti A, Gontero P et al (2013) The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int 112:432–441. https://doi.org/10.1111/BJU.12118
Gao YL, Brosnan CF, Raine CS (1995) Experimental autoimmune encephalomyelitis. Qualitative and semiquantitative differences in heat shock protein 60 expression in the central nervous system. J Immunol 154:3548–3556. https://doi.org/10.4049/jimmunol.154.7.3548
Genc Z, Yarat A, Tunali-Akbay T et al (2011) The effect of stinging nettle (Urtica dioica) seed oil on experimental colitis in rats. J Med Food 14:1554–1561. https://doi.org/10.1089/jmf.2011.0028
Germolec DR, Shipkowski KA, Frawley RP, Evans E (2018) Markers of Inflammation. Methods Mol Biol 1803:57–79. https://doi.org/10.1007/978-1-4939-8549-4_5
Ghasemi S, Moradzadeh M, Hosseini M et al (2019) Beneficial effects of Urtica dioica on scopolamine-induced memory impairment in rats: protection against acetylcholinesterase activity and neuronal oxidative damage. Drug Chem Toxicol 42:167–175. https://doi.org/10.1080/01480545.2018.1463238
Ghorbanibirgani A (2012) Efficacy of quercetin in treatment of benign prostatic hyperplasia in a double-blind randomized clinical trial in Iran—2011. Contraception 85:321. https://doi.org/10.1016/j.contraception.2011.11.038
Giri D, Ittmann M (2001) Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am J Pathol 159:139–147. https://doi.org/10.1016/S0002-9440(10)61681-1
Giuliano F, Warner TD (2002) Origins of prostaglandin E2: involvements of cyclooxygenase (COX)−1 and COX−2 in human and rat systems. J Pharmacol Exp Ther 303:1001–1006. https://doi.org/10.1124/JPET.102.041244
Goldring MB (2000) The role of the chondrocyte in osteoarthritis. Arthritis Rheum 43:1916–1926. https://doi.org/10.1002/1529-0131(200009)43:9%3c1916::AID-ANR2%3e3.0.CO;2-I
Goldring MB (1999) The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res 40:1–11. https://doi.org/10.3109/03008209909005273
Gong G, Guan Y, Zhang Z, Rahman K, Wang S, Zhou S, Luan X, Zhang H (2020) Isorhamnetin: a review of pharmacological effects. Biomed Pharmacother 128:110301. https://doi.org/10.1016/j.biopha.2020.110301
Gopalakrishnan A, Ram M, Kumawat S et al (2016) Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-β1. Indian J Exp Biol 54:187–195
Gowen M, Wood DD, Ihrie EJ et al (1984) Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta 797:186–193. https://doi.org/10.1016/0304-4165(84)90121-1
Gradini R, Realacci M, Ginepri A et al (1999) Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology. J Pathol 189:224–229
Grauso L, de Falco B, Lanzotti V, Motti R (2020) Stinging nettle, Urtica dioica L.: botanical, phytochemical and pharmacological overview. Phytochem Rev 19:1341–1377. https://doi.org/10.1007/S11101-020-09680-X/TABLES/2
Grzanna R, Lindmark L, Frondoza CG (2005) Ginger–an herbal medicinal product with broad anti-inflammatory actions. J Med Food 8:125–132. https://doi.org/10.1089/JMF.2005.8.125
Guil-Guerrero JL, Rebolloso-Fuentes MM, Torija Isasa ME (2003) Fatty acids and carotenoids from stinging nettle (Urtica dioica L.). J Food Compos Anal 16:111–119. https://doi.org/10.1016/S0889-1575(02)00172-2
Gül S, Emirci B, Başer KH et al (2012) Chemical composition and in vitro cytotoxic, genotoxic effects of essential oil from Urtica dioica L. Bull Environ Contam Toxicol 88:666–671. https://doi.org/10.1007/S00128-012-0535-9
Gulsel MK (2003) Urtica: therapeutic and nutritional aspects of stinging nettles, 1st edn. CRC Press, London
Guo X, Wang WP, Ko JKS, Cho CH (1999) Involvement of neutrophils and free radicals in the potentiating effects of passive cigarette smoking on inflammatory bowel disease in rats. Gastroenterology 117:884–892. https://doi.org/10.1016/S0016-5085(99)70347-1
Gupta SK, Bansal P, Bhardwaj RK et al (2002) Comparison of analgesic and anti-inflammatory activity of meloxicam gel with diclofenac and piroxicam gels in animal models: pharmacokinetic parameters after topical application. Skin Pharmacol Appl Skin Physiol 15:105–111. https://doi.org/10.1159/000049397
Hajhashemi V, Klooshani V (2013) Antinociceptive and anti-inflammatory effects of Urtica dioica leaf extract in animal models. Avicenna J Phytomed 3:193–200
Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q (2011) Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol 44:127–133. https://doi.org/10.1165/RCMB.2010-0027TR
Handel ML, Mcmorrow LB, Gravallese EM (1995) Nuclear factor–kB in rheumatoid synovium. Localization of P50 and P65. Arthritis Rheum 38:1762–1770. https://doi.org/10.1002/ART.1780381209
Harms AS, Barnum CJ, Ruhn KA et al (2011) Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson’s disease. Mol Ther 19:46–52. https://doi.org/10.1038/MT.2010.217
Harms AS, Ferreira SA, Romero-Ramos M (2021) Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol 141:527–545. https://doi.org/10.1007/S00401-021-02268-5
Henderson B, Pockley AG (2010) Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol 88:445–462. https://doi.org/10.1189/JLB.1209779
Hewitt AT, Varner HH, Silver MH et al (1982) The isolation and partial characterization of chondronectin, an attachment factor for chondrocytes. J Biol Chem 257:2330–2334. https://doi.org/10.1016/S0021-9258(18)34926-3
Hodroj MH, Al Bast NAH, Taleb RI et al (2020) Nettle tea inhibits growth of acute myeloid leukemia cells in vitro by promoting apoptosis. Nutrients 12:1–18. https://doi.org/10.3390/nu12092629
Holzmann I, Da Silva LM, Corrêa Da Silva JA et al (2015) Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol Biochem Behav 136:55–63. https://doi.org/10.1016/J.PBB.2015.07.003
Hopkin SJ, Lewis JW, Krautter F et al (2019) Triggering the resolution of immune mediated inflammatory diseases: can targeting leukocyte migration be the answer? Front Pharmacol 10:184. https://doi.org/10.3389/FPHAR.2019.00184/BIBTEX
Horton WE, Udo I, Precht P et al (1998) Cytokine inducible matrix metalloproteinase expression in immortalized rat chondrocytes is independent of nitric oxide stimulation. In Vitro Cell Dev Biol Anim 34:378–384. https://doi.org/10.1007/S11626-998-0019-8
Hwang M, Berceli SA, Tran-Son-Tay R (2010) Modeling and role of leukocytes in inflammation. In: Marc G, Barbara Lee B, Christophe C et al (eds) Computational Surgery and Dual Training, 1st edn. Springer, New York, pp 221–232
Iannuccelli C, Di Franco M, Alessandri C et al (2010) Pathophysiology of fibromyalgia: a comparison with the tension-type headache, a localized pain syndrome. Ann N Y Acad Sci 1193:78–83. https://doi.org/10.1111/J.1749-6632.2009.05365.X
Irani M, Choopani R, Esmaeili S et al (2020) Effect of nettle seed on immune response in a murine model of allergic asthma. Rev Fr Allergol 60:417–422. https://doi.org/10.1016/J.REVAL.2020.03.007
Irgin C, Çörekçi B, Ozan F et al (2016) Does stinging nettle (Urtica dioica) have an effect on bone formation in the expanded inter-premaxillary suture? Arch Oral Biol 69:13–18. https://doi.org/10.1016/j.archoralbio.2016.05.003
Jacquet A, Girodet PO, Pariente A et al (2009) Phytalgic®, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther 11:R192. https://doi.org/10.1186/AR2891
Jaiswal V, Lee H-J (2022) Antioxidant Activity of Urtica dioica: An important property contributing to multiple biological activities. Antioxidants (basel) 11:2494. https://doi.org/10.3390/antiox11122494
Jan KN, Zarafshan K, Singh S (2017) Stinging nettle (Urtica dioica L.): a reservoir of nutrition and bioactive components with great functional potential. J Food Meas Charact 11:423–433. https://doi.org/10.1007/S11694-016-9410-4/TABLES/3
Jangra A, Sriram CS, Lahkar M (2016) Lipopolysaccharide-induced behavioral alterations are alleviated by sodium phenylbutyrate via attenuation of oxidative stress and neuroinflammatory cascade. Inflammation 39:1441–1452. https://doi.org/10.1007/S10753-016-0376-5
Jawale A, Datusalia AK, Bishnoi M, Sharma SS (2016) Reversal of diabetes-induced behavioral and neurochemical deficits by cinnamaldehyde. Phytomedicine 23:923–930. https://doi.org/10.1016/J.PHYMED.2016.04.008
Jeremy AHT, Holland DB, Roberts SG et al (2003) Inflammatory events are involved in acne lesion initiation. J Invest Dermatol 121:20–27. https://doi.org/10.1046/J.1523-1747.2003.12321.X
Jie F, Yang F, Yang B, Liu Y, Wu L, Lu B (2022) Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed Pharmacother 153:113317. https://doi.org/10.1016/j.biopha.2022.113317
Mengshol JA, Vincenti MP, Coon CI et al (2001) Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-jun N-terminal kinase, and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheumatol 43:801–811. https://doi.org/10.1002/1529-0131(200004)43:4%3c801::AID-ANR10%3e3.0.CO;2-4
Johnson TA, Sohn J, Inman WD et al (2013) Lipophilic stinging nettle extracts possess potent anti-inflammatory activity, are not cytotoxic and may be superior to traditional tinctures for treating inflammatory disorders. Phytomedicine 20:143–147. https://doi.org/10.1016/j.phymed.2012.09.016
Jonas WB, Rapoza CP, Blair WF (1996) The effect of niacinamide on osteoarthritis: a pilot study. Inflamm Res 45:330–334. https://doi.org/10.1007/BF02252945
Josiah SS, Famusiwa CD, Crown OO et al (2022) Neuroprotective effects of catechin and quercetin in experimental Parkinsonism through modulation of dopamine metabolism and expression of IL-1β, TNF-α, NF-κB, IκKB, and p53 genes in male Wistar rats. Neurotoxicology 90:158–171. https://doi.org/10.1016/J.NEURO.2022.03.004
Jung UJ, Kim SR (2018) Beneficial effects of flavonoids against Parkinson’s disease. J Med Food 21:421–432. https://doi.org/10.1089/JMF.2017.4078
Karami AA, Sheikhsoleimani M, Memarzadeh MR et al (2020) Urtica dioica root extract on clinical and biochemical parameters in patients with benign prostatic hyperplasia, randomized controlled trial. Pak J Biol Sci 23:1338–1344. https://doi.org/10.3923/pjbs.2020.1338.1344
Karin M, Liu ZG, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9:240–246. https://doi.org/10.1016/S0955-0674(97)80068-3
Karpenko MN, Vasilishina AA, Gromova EA et al (2018) Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell Immunol 327:77–82. https://doi.org/10.1016/J.CELLIMM.2018.02.011
Karuppagounder SS, Madathil SK, Pandey M et al (2013) Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 236:136–148. https://doi.org/10.1016/J.NEUROSCIENCE.2013.01.032
Kasouni AI, Chatzimitakos TG, Stalikas CD et al (2021) The unexplored wound healing activity of Urtica dioica L. extract: an in vitro and in vivo study. Molecules 26:6248. https://doi.org/10.3390/molecules26206248
Kawabata A (2011) Prostaglandin E2 and pain—an update. Biol Pharm Bull 34:1170–1173. https://doi.org/10.1248/bpb.34.1170
Kaya İ, Gülabi D, Yilmaz M et al (2016) Intraarticular Ankaferd blood stopper application increases cartilage degeneration: an experimental study. Turk J Med Sci 46:236–240. https://doi.org/10.3906/sag-1406-5
Keyszer G, Lambiri I, Nagel R et al (1999) Circulating levels of matrix metalloproteinases MMP-3 and MMP-1, tissue inhibitor of metalloproteinases 1 (TIMP-1), and MMP-1/TIMP-1 complex in rheumatic disease. Correlation with clinical activity of rheumatoid arthritis versus other surrogate markers. J Rheumatol 26:251–258
Kim J, Ochoa M-T, Krutzik SR et al (2002) Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 169:1535–1541. https://doi.org/10.4049/JIMMUNOL.169.3.1535
Kim S, Oh S, Noh HB et al (2018) In vitro antioxidant and anti-Propionibacterium acnes activities of cold water, hot water, and methanol extracts, and their respective ethyl acetate fractions, from Sanguisorba officinalis L. roots. Molecules 23:3001. https://doi.org/10.3390/molecules23113001
Kips JC, O’Connor BJ, Langley SJ et al (2003) Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med 167:1655–1659. https://doi.org/10.1164/RCCM.200206-525OC
Kirtikar KR, Basu BD (2008) Indian Medicinal Plants, 2nd edn. International book distributors, Dehradun
Kılıç S, Okullu SÖ, Kurt Ö et al (2019) Efficacy of two plant extracts against acne vulgaris: initial results of microbiological tests and cell culture studies. J Cosmet Dermatol 18:1061–1065. https://doi.org/10.1111/jocd.12814
Kk J, Sg M, Jn M et al (2015) Protective effects of Urtica dioica and cimetidine® on liver function following acetaminophen induced hepatotoxicity in mice. J Dev Drugs 4:130. https://doi.org/10.4172/2329-6631.1000130
Klingelhoefer S, Obertreis B, Quast S, Behnke B (1999) Antirheumatic effect of IDS 23, a stinging nettle leaf extract, on in vitro expression of T helper cytokines. J Rheumatol 26:2517–2522
Kolkenbrock H, Hecker-Kia A, Orgel D et al (1993) A trypsin sensitive stromelysin isolated from rheumatoid synovial fluid is an activator for matrix metalloproteinases. Eur J Clin Chem Clin Biochem 31:625–632. https://doi.org/10.1515/CCLM.1993.31.10.625
Konrad A, Mähler M, Arni S et al (2005) Ameliorative effect of IDS 30, a stinging nettle leaf extract, on chronic colitis. Int J Colorectal Dis 20:9–17. https://doi.org/10.1007/s00384-004-0619-z
Kosher RA, Church RL (1975) Stimulation of in vitro somite chondrogenesis by procollagen and collagen. Nature 258:327–330. https://doi.org/10.1038/258327A0
Kosher RA, Lash JW, Minor RR (1973) Environmental enhancement of in vitro chondrogenesis. IV. Stimulation of somite chondrogenesis by exogenous chondromucoprotein. Dev Biol 35:210–220. https://doi.org/10.1016/0012-1606(73)90018-3
Kramer G, Marberger M (2006) Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol 16:25–29. https://doi.org/10.1097/01.MOU.0000193368.91823.1B
Kramer G, Steiner GE, Handisurya A et al (2002) Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate 52:43–58. https://doi.org/10.1002/PROS.10084
Kraus R, Spiteller G (1991) Gas chromatography/mass spectrometry of trimethylsilylated phenolic glucosides from roots of Urtica dioica. Biol Mass Spectrom 20:53–60. https://doi.org/10.1002/BMS.1200200203
Kregiel D, Pawlikowska E, Antolak H (2018) Urtica spp.: ordinary plants with extraordinary properties. Molecules 23:1664. https://doi.org/10.3390/MOLECULES23071664
Kudritsata SE, Filman GM, Zagorodskaya LM, Chikovanii DM (1987) Carotenoids of Urtica dioica. Chem Nat Compd 22:604–605. https://doi.org/10.1007/BF00599278/METRICS
Kyriakis JM (1999) Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr 7:217–231
Labib DA, Ashmawy I, Elmazny A et al (2022) Toll-like receptors 2 and 4 expression on peripheral blood lymphocytes and neutrophils of Egyptian multiple sclerosis patients. Int J Neurosci 132:323–327. https://doi.org/10.1080/00207454.2020.1812601
Lanneau D, de Thonel A, Maurel S et al (2007) Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion 1:53–60. https://doi.org/10.4161/pri.1.1.4059
Leckie MJ, Ten Brinke A, Khan J et al (2000) Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356:2144–2148. https://doi.org/10.1016/S0140-6736(00)03496-6
Lee WR, Kim KH, An HJ et al (2014) The protective effects of melittin on Propionibacterium acnes–induced inflammatory responses In Vitro and In Vivo. J Investig Dermatol 134:1922–1930. https://doi.org/10.1038/JID.2014.75
Li RW, Leach DN, Myers SP et al (2004) Anti-inflammatory activity, cytotoxicity and active compounds of Tinospora smilacina Benth. Phytother Res 18:78–83. https://doi.org/10.1002/PTR.1373
Li XH, Tu XY, Zhang DX et al (2009) Effects of wuwei dilong decoction on inflammatory cells and cytokines in asthma model guinea pigs. J Tradit Chin Med 29:220–223. https://doi.org/10.1016/S0254-6272(09)60070-4
Li Y, Yao J, Han C et al (2016) Quercetin, inflammation and immunity. Nutrients 8:167. https://doi.org/10.3390/NU8030167
Liao H, Ye J, Gao L, Liu Y (2021) The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: a comprehensive review. Biomed Pharmacother 133:110917. https://doi.org/10.1016/j.biopha.2020.110917
Lim HJ, Kang SH, Song YJ et al (2021) Inhibitory effect of quercetin on Propionibacterium acnes-induced skin inflammation. Int Immunopharmacol 96:107557. https://doi.org/10.1016/j.intimp.2021.107557
Lin S, Yokoyama H, Rac VE, Brooks SC (2012) Novel biomarkers in diagnosing cardiac ischemia in the emergency department: a systematic review. Resuscitation 83:684–691. https://doi.org/10.1016/J.RESUSCITATION.2011.12.015
Liu JH, Zschocke S, Reininger E, Bauer R (1998) Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med 64:525–529. https://doi.org/10.1055/S-2006-957507
Liu L, Orozco IJ, Planel E et al (2008) A transgenic rat that develops Alzheimer’s disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol Dis 31:46–57. https://doi.org/10.1016/J.NBD.2008.03.005
Liu P, Zou D, Yi L et al (2015) Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restor Neurol Neurosci 33:143–157. https://doi.org/10.3233/RNN-140446
Madsen KL, Doyle JS, Jewell LD et al (1999) Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116:1107–1114. https://doi.org/10.1016/S0016-5085(99)70013-2
Magalingam KB, Radhakrishnan AK, Haleagrahara N (2015) Protective mechanisms of flavonoids in Parkinson’s disease. Oxid Med Cell Longev 2015:314560. https://doi.org/10.1155/2015/314560
Mahmoodpoor A, Mojtahedzadeh M, Najafi A et al (2010) Examination of Setarud (IMOD™) in the management of patients with severe sepsis. Daru 18:23–28
Majno G, Palade GE (1961) Studies on inflammation: I. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol 11:571. https://doi.org/10.1083/JCB.11.3.571
Mamta S, Preeti K (2014) Urtica Dioica (Stinging Nettle): a review of its chemical, pharmacological, toxicological and ethnomedical properties. Int J Pharm 4:270–277
Manicourt DH, Fujimoto N, Obata KI, Thonar EJ (1995) Levels of circulating collagenase, stromelysin-1, and tissue inhibitor of matrix metalloproteinases 1 in patients with rheumatoid arthritis: relationship to serum levels of antigenic keratan sulfate and systemic parameters of inflammation. Arthritis Rheum 38(8):1031–9
Mannila E, Marti-Quijal FJ, Selma-Royo M et al (2022) In Vitro bioactivities of food grade extracts from yarrow (Achillea millefolium L.) and stinging nettle (Urtica dioica L.) leaves. Plant Foods Hum Nutr 78:132–138. https://doi.org/10.1007/s11130-022-01020-y
Marok R, Winyard PG, Coumbe A et al (1996) Activation of the transcription factor nuclear factor-κB in human inflamed synovial tissue. Arthritis Rheum 39:583–591. https://doi.org/10.1002/ART.1780390407
Martel-Pelletier J (1998) Pathophysiology of osteoarthritis. Osteoarthritis Cartilage 6:374–376. https://doi.org/10.1053/JOCA.1998.0140
Martel-Pelletier J, McCollum R, Fujimoto N et al (1994) Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest 70:807–815
Martin S, Maruta K, Burkart V et al (1988) IL-1 and IFN-gamma increase vascular permeability. Immunology 64:301–305
Matsuda H, Tomohiro N, Ido Y, Kubo M (2002) Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol Pharm Bull 25:809–812. https://doi.org/10.1248/BPB.25.809
McCoy MK, Martinez TN, Ruhn KA et al (2006) Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci 26:9365–9375. https://doi.org/10.1523/JNEUROSCI.1504-06.2006
McCoy MK, Ruhn KA, Martinez TN et al (2008) Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther 16:1572–1579. https://doi.org/10.1038/MT.2008.146
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919. https://doi.org/10.1038/ni1507
McIntyre KW, Shuster DJ, Gillooly KM et al (2003) A highly selective inhibitor of IκB kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum 48:2652–2659. https://doi.org/10.1002/art.11131
McKoy MLG, Thomas EA, Simon OR (2002) Preliminary investigation of the anti-inflammatory properties of an aqueous extract from Morinda citrifolia (Noni). Proc West Pharmacol Soc 45:76–78
Mengus C, Le Magnen C, Trella E et al (2011) Elevated levels of circulating IL-7 and IL-15 in patients with early stage prostate cancer. J Transl Med 9:162. https://doi.org/10.1186/1479-5876-9-162
Meraz-Ríos MA, Toral-Rios D, Franco-Bocanegra D et al (2013) Inflammatory process in Alzheimer’s disease. Front Integr Neurosci 7:59. https://doi.org/10.3389/FNINT.2013.00059
Merzoug S, Toumi ML, Tahraoui A (2014) Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedebergs Arch Pharmacol 387:921–933. https://doi.org/10.1007/S00210-014-1008-Y
Mi Y, Zhong L, Lu S et al (2022) Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J Ethnopharmacol 290:115066. https://doi.org/10.1016/J.JEP.2022.115066
Mohammadirad A, Khorram-Khorshid HR, Gharibdoost F, Abdollahi M (2011) Setarud (IMOD™) as a Multiherbal drug with promising benefits in animal and human studies: a comprehensive review of biochemical and cellular evidences. Asian J Anim Vet Adv 6:1185–1192. https://doi.org/10.3923/AJAVA.2011.1185.1192
Mohseni-Salehi-Monfared SS, Habibollahzadeh E, Sadeghi H et al (2010) Efficacy of setarud (IMOD™), a novel electromagnetically-treated multi-herbal compound, in mouse immunogenic type-1 diabetes. Arch Med Sci 6:663–669. https://doi.org/10.5114/AOMS.2010.17078
Moparthi L, Koch S (2019) Wnt signaling in intestinal inflammation. Differentiation 108:24–32. https://doi.org/10.1016/j.diff.2019.01.002
Moroi Y, Mayhew M, Trcka J et al (2000) Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci U S A 97:3485–3490. https://doi.org/10.1073/PNAS.97.7.3485
Mosser DD, Morimoto RI (2004) Molecular chaperones and the stress of oncogenesis. Oncogene 23:2907–2918. https://doi.org/10.1038/sj.onc.1207529
Murakami S, Lefebvre V, De Crombrugghe B (2000) Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem 275:3687–3692. https://doi.org/10.1074/JBC.275.5.3687
Murphy G, Gavrilovic J (1999) Proteolysis and cell migration: creating a path? Curr Opin Cell Biol 11:614–621. https://doi.org/10.1016/S0955-0674(99)00022-8
Mutoh M, Takahashi M, Fukuda K et al (2000) Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 21:959–963. https://doi.org/10.1093/CARCIN/21.5.959
Myers KJ, Murthy S, Flanigan A et al (2003) Antisense oligonucleotide blockade of tumor necrosis factor-alpha in two murine models of colitis. J Pharmacol Exp Ther 304:411–424. https://doi.org/10.1124/JPET.102.040329
Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Cytokines in Parkinson’s disease. J Neural Transm Suppl. https://doi.org/10.1007/978-3-7091-6284-2_12
Namazi F, Bordbar E, Bakhshaei F, Nazifi S (2022) The effect of Urtica dioica extract on oxidative stress, heat shock proteins, and brain histopathology in multiple sclerosis model. Physiol Rep 10:e15404. https://doi.org/10.14814/phy2.15404
Namazi N, Esfanjani AT, Heshmati J, Bahrami A (2011) The effect of hydro alcoholic nettle (Urtica dioica) extracts on insulin sensitivity and some inflammatory indicators in patients with type 2 diabetes: a randomized double-blind control trial. Pak J Biol Sci 14:775–779. https://doi.org/10.3923/pjbs.2011.775.779
Nantel F, Fong C, Lamontagne S et al (2004) Expression of prostaglandin D synthase and the prostaglandin D2 receptors DP and CRTH2 in human nasal mucosa. Prostaglandins Other Lipid Mediat 73:87–101. https://doi.org/10.1016/J.PROSTAGLANDINS.2003.12.002
Nazir S, Ahmad I, Mobashar A, Sharif A, Shabbir A, Chaudhary W (2024) Mechanistic evaluation of antiarthritic and anti-inflammatory effect of campesterol ester derivatives in complete Freund’s adjuvant-induced arthritic rats. Front Pharmacol 14:1346054. https://doi.org/10.3389/fphar.2023.1346054
Nédélec E, Abid A, Cipolletta C et al (2001) Stimulation of cyclooxygenase-2-activity by nitric oxide-derived species in rat chondrocyte: lack of contribution to loss of cartilage anabolism. Biochem Pharmacol 61:965–978. https://doi.org/10.1016/S0006-2952(01)00559-7
Netea MG, Balkwill F, Chonchol M et al (2017) A guiding map for inflammation. Nat Immunol 18:826–831. https://doi.org/10.1038/NI.3790
Neurath MF, Pettersson S, Meyer Zum Buschenfelde KH, Strober W (1996) Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med 2:998–1004. https://doi.org/10.1038/NM0996-998
Nishikori T, Irie K, Suganuma T et al (2002) Anti-inflammatory potency of FR167653, a p38 mitogen-activated protein kinase inhibitor, in mouse models of acute inflammation. Eur J Pharmacol 451:327–333. https://doi.org/10.1016/S0014-2999(02)02238-0
Obertreis B, Giller K, Teucher T et al (1996a) Anti-inflammatory effect of Urtica dioica folia extract in comparison to caffeic malic acid. Arzneimittelforschung 46:52–56
Obertreis B, Ruttkowski T, Teucher T et al (1996b) Ex-vivo in-vitro inhibition of lipopolysaccharide stimulated tumor necrosis factor-alpha and interleukin-1 beta secretion in human whole blood by extractum urticae dioicae foliorum. Arzneimittelforschung 46:389–394
O’Byrne PM (2006) Cytokines or their antagonists for the treatment of asthma. Chest 130:244–250. https://doi.org/10.1378/CHEST.130.1.244
Oguz S, Kanter M, Erboga M, Ibis C (2013) Protective effect of Urtica dioica on liver damage induced by biliary obstruction in rats. Toxicol Ind Health 29:838–845. https://doi.org/10.1177/0748233712445045
Okiji T, Morita I, Sunada I, Murota S (1989) Involvement of arachidonic acid metabolites in increases in vascular permeability in experimental dental pulpal inflammation in the rat. Arch Oral Biol 34:523–528. https://doi.org/10.1016/0003-9969(89)90090-3
Olivo RD, Teixeira CF, Wallace JL, Gutierrez JM, Zamuner SR (2007) Role of cyclooxygenases in oedema-forming activity of bothropic venoms. Toxicon 49(5):670–7. https://doi.org/10.1016/J.TOXICON.2006.11.006
Orcic D, Franciškovic M, Bekvalac K et al (2014) Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem 143:48–53. https://doi.org/10.1016/J.FOODCHEM.2013.07.097
Osman NI, Sidik NJ, Awal A et al (2016) In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J Intercult Ethnopharmacol 5:343. https://doi.org/10.5455/JICE.20160731025522
Otles S, Yalcin B (2012) Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci World J 2012:564367. https://doi.org/10.1100/2012/564367
Pajonk F, Riedisser A, Henke M et al (2006) The effects of tea extracts on proinflammatory signaling. BMC Med 4:28. https://doi.org/10.1186/1741-7015-4-28
Palmer WR, Balacescu A (1977) A multi-centre trial of pollen-tyrosine adsorbate. Acta Allergol 32:44–57. https://doi.org/10.1111/J.1398-9995.1977.TB02703.X
Parkitny L, McAuley JH, Di Pietro F et al (2013) Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology 80:106–117. https://doi.org/10.1212/WNL.0b013e31827b1aa1
Patel SS, Gupta S, Udayabanu M (2016) Urtica dioica modulates hippocampal insulin signaling and recognition memory deficit in streptozotocin induced diabetic mice. Metab Brain Dis 31:601–611. https://doi.org/10.1007/s11011-016-9791-4
Patel SS, Ray RS, Sharma A et al (2018) Antidepressant and anxiolytic like effects of Urtica dioica leaves in streptozotocin induced diabetic mice. Metab Brain Dis 33:1281–1292. https://doi.org/10.1007/s11011-018-0243-1
Patel SS, Udayabanu M (2014) Urtica dioica extract attenuates depressive like behavior and associative memory dysfunction in dexamethasone induced diabetic mice. Metab Brain Dis 29:121–130. https://doi.org/10.1007/s11011-014-9480-0
Paulauskienė A, Tarasevičienė Ž, Laukagalis V (2021) Influence of harvesting time on the chemical composition of wild stinging nettle (Urtica dioica L.). Plants (Basel) 10:686. https://doi.org/10.3390/PLANTS10040686
Penna G, Fibbi B, Amuchastegui S et al (2009) Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol 182:4056–4064. https://doi.org/10.4049/JIMMUNOL.0801875
Pérez GRM (1996) Anti-inflammatory activity of Ambrosia artemisiaefolia and Rhoeo spathacea. Phytomedicine 3:163–167. https://doi.org/10.1016/S0944-7113(96)80030-4
Perretti M, Ahluwalia A, Harris JG et al (1996) Acute inflammatory response in the mouse: exacerbation by immunoneutralization of lipocortin 1. Br J Pharmacol 117:1145–1154. https://doi.org/10.1111/J.1476-5381.1996.TB16709.X
Pigat N, Reyes-Gomez E, Boutillon F et al (2019) Combined Sabal and Urtica Extracts (WS® 1541) exert anti-proliferative and anti-inflammatory effects in a mouse model of benign prostate hyperplasia. Front Pharmacol 10:311. https://doi.org/10.3389/fphar.2019.00311
Pinelli P, Ieri F, Vignolini P et al (2008) Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J Agric Food Chem 56:9127–9132. https://doi.org/10.1021/JF801552D
Pogačnik L, Ota A, Ulrih NP (2020) An overview of crucial dietary substances and their modes of action for prevention of neurodegenerative diseases. Cells 9:576. https://doi.org/10.3390/CELLS9030576
Ponder A, Long MD (2013) A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol 5:237–247. https://doi.org/10.2147/CLEP.S33961
Proestos C, Boziaris IS, Nychas GJE, Komaitis M (2006) Analysis of flavonoids and phenolic acids in Greek aromatic plants: investigation of their antioxidant capacity and antimicrobial activity. Food Chem 95:664–671. https://doi.org/10.1016/J.FOODCHEM.2005.01.049
Quintana FJ, Cohen IR (2005) Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol 175:2777–2782. https://doi.org/10.4049/JIMMUNOL.175.5.2777
Quintana FJ, Cohen IR (2011) The HSP60 immune system network. Trends Immunol 32:89–95. https://doi.org/10.1016/J.IT.2010.11.001
Quintana FJ, Farez MF, Viglietta V et al (2008) Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A 105:18889–18894. https://doi.org/10.1073/pnas.0806310105
Ra J, Lee S, Kim HJ et al (2010) Bambusae caulis in taeniam extract reduces ovalbumin-induced airway inflammation and T helper 2 responses in mice. J Ethnopharmacol 128:241–247. https://doi.org/10.1016/J.JEP.2010.01.023
Rahmati M, Keshvari M, Xie W et al (2022) Resistance training and Urtica dioica increase neurotrophin levels and improve cognitive function by increasing age in the hippocampus of rats. Biomed Pharmacother 153:113306. https://doi.org/10.1016/j.biopha.2022.113306
Rainsford KD (2007) Anti-inflammatory drugs in the 21st century. Subcell Biochem 42:3–27. https://doi.org/10.1007/1-4020-5688-5_1
Randall C, Dickens A, White A et al (2008) Nettle sting for chronic knee pain: a randomised controlled pilot study. Complement Ther Med 16:66–72. https://doi.org/10.1016/J.CTIM.2007.01.012
Rayburn K, Fleischbein E, Song J et al (2009) Stinging nettle cream for osteoarthritis. Altern Ther Health Med 15:60–61
Ren K, Torres R (2009) Role of interleukin-1β during pain and inflammation. Brain Res Rev 60:57–64. https://doi.org/10.1016/j.brainresrev.2008.12.020
Renz H, Holt PG, Inouye M et al (2017) An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol 140:24–40. https://doi.org/10.1016/J.JACI.2017.05.015
Riehemann K, Behnke B, Schulze-Osthoff K (1999) Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-κB. FEBS Lett 442:89–94. https://doi.org/10.1016/S0014-5793(98)01622-6
Robbins JR, Thomas B, Tan L et al (2000) Immortalized human adult articular chondrocytes maintain cartilage-specific phenotype and responses to interleukin-1β. Arthritis Rheum 43:2189–2201. https://doi.org/10.1002/1529-0131(200010)43:10%3c2189::AID-ANR6%3e3.0.CO;2-S
Robert G, Descazeaud A, Nicolaïew N et al (2009) Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate 69:1774–1780. https://doi.org/10.1002/PROS.21027
Robert G, Smit F, Hessels D et al (2011) Biomarkers for the diagnosis of prostatic inflammation in benign prostatic hyperplasia. Prostate 71:1701–1709. https://doi.org/10.1002/pros.21387
Rodriguez-Nieves JA, Macoska JA (2013) Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol 10:546–550. https://doi.org/10.1038/nrurol.2013.149
Roelofs MF, Boelens WC, Joosten LAB et al (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176:7021–7027. https://doi.org/10.4049/JIMMUNOL.176.11.7021
Roschek B, Fink RC, McMichael M, Alberte RS (2009) Nettle extract (Urtica dioica) affects key receptors and enzymes associated with allergic rhinitis. Phytother Res 23:920–926. https://doi.org/10.1002/ptr.2763
Roth GA, Abate D, Abate KH et al (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1736. https://doi.org/10.1016/S0140-6736(18)32203-7
Royuela M, Ricote M, Parsons MS et al (2004) Immunohistochemical analysis of the IL-6 family of cytokines and their receptors in benign, hyperplasic, and malignant human prostate. J Pathol 202:41–49. https://doi.org/10.1002/PATH.1476
Adel M, Caipang CM, Dawood MA (2017) Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish shellfish immunol 71:230–8. https://doi.org/10.1016/J.FSI.2017.10.016
Saito S, Katoh M, Masumoto M et al (1998) Involvement of MMP-1 and MMP-3 in collagen degradation induced by IL-1 in rabbit cartilage explant culture. Life Sci 62:PL359–PL365. https://doi.org/10.1016/S0024-3205(98)00181-7
Sakthivel K, Vishnupriya S, Dharshini L, Rasmi R, Ramesh B (2022) Modulation of multiple cellular signalling pathways as targets for anti-inflammatory and anti-tumorigenesis action of scopoletin. J Pharm Pharmacol 74:147–161. https://doi.org/10.1093/jpp/rgab047
Saponaro M, Giacomini I, Morandin G et al (2020) Serenoa repens and Urtica dioica fixed combination: in-vitro validation of a therapy for benign prostatic hyperplasia (BPH). Int J Mol Sci 21:1–14. https://doi.org/10.3390/ijms21239178
Schauer IG, Ressler SJ, Tuxhorn JA et al (2008) Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology 72:205–213. https://doi.org/10.1016/j.urology.2007.11.083
Schauer R (1982) Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem 40:131–234. https://doi.org/10.1016/S0065-2318(08)60109-2
Schöttner M, Ganßer D, Spiteller G (1997a) Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Med 63:529–532. https://doi.org/10.1055/S-2006-957756/BIB
Schöttner M, Reiner J, Tayman FSK (1997b) (+)-neo-olivil from roots of Urtica dioica. Pergamon
Schreiber S, Campieri M, Colombel JF et al (2001) Use of anti-tumour necrosis factor agents in inflammatory bowel disease. European guidelines for 2001–2003. Int J Colorectal Dis 16:1–11. https://doi.org/10.1007/S003840100285
Schreiber S, Nikolaus S, Hampe J et al (1999) Tumour necrosis factor α and interleukin 1β in relapse of Crohn’s disease. The Lancet 353:459–461. https://doi.org/10.1016/S0140-6736(98)03339-X
Schulze-Tanzil G, De Souza P, Behnke B et al (2002) Effects of the antirheumatic remedy Hox alpha - a new stinging nettle leaf extract - on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol 17:477–485
Schulze-Tanzil G, De Souza P, Merker HJ, Shakibaei M (2001) Co-localization of integrins and matrix metalloproteinases in the extracellular matrix of chondrocyte cultures. Histol Histopathol 16:1081–1089. https://doi.org/10.14670/HH-16.1081
Sciarra A, Di Silverio F, Salciccia S et al (2007) Inflammation and chronic prostatic diseases: evidence for a link? Eur Urol 52:964–972. https://doi.org/10.1016/J.EURURO.2007.06.038
Selmaj K, Brosnan CF, Raine CS (1992) Expression of heat shock protein-65 by oligodendrocytes in vivo and in vitro: implications for multiple sclerosis. Neurology 42:795–800. https://doi.org/10.1212/WNL.42.4.795
Selmaj K, Brosnan CF, Raine CS (1991) Colocalization of lymphocytes bearing gamma delta T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci U S A 88:6452–6456. https://doi.org/10.1073/PNAS.88.15.6452
Shabir S, Yousuf S, Singh SK et al (2022) Ethnopharmacological effects of urtica dioica, Matricaria chamomilla, and Murraya koenigii on rotenone-exposed D. melanogaster: an attenuation of cellular, biochemical, and organismal markers. Antioxidants (Basel) 11:1623. https://doi.org/10.3390/antiox11081623
Shahzad N, Alzahrani AR, Ibrahim IAA et al (2021) In Vivo pharmacological testing of herbal drugs for anti-allergic and anti-asthmatic properties. J Pharm Bioallied Sci 13:380–386. https://doi.org/10.4103/jpbs.jpbs_454_21
Shakibaei M (1998) Inhibition of chondrogenesis by integrin antibody in vitro. Exp Cell Res 240:95–106. https://doi.org/10.1006/excr.1998.3933
Shakibaei M, Allaway D, Nebrich S, Mobasheri A (2012) Botanical extracts from rosehip (Rosa canina), willow bark (Salix alba), and nettle leaf (Urtica dioica) suppress IL-1β-induced NF-κB activation in canine articular chondrocytes. Evid Based Complement Alternat Med 2012:509383. https://doi.org/10.1155/2012/509383
Shakibaei M, John T, De Souza P et al (1999) Signal transduction by beta1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem J 342:615. https://doi.org/10.1042/0264-6021:3420615
Shakibaei M, Schulze-Tanzil G, De Souza P et al (2001) Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J Biol Chem 276:13289–13294. https://doi.org/10.1074/jbc.M010859200
Sherwood ER, Toliver-Kinsky T (2004) Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol 18:385–405. https://doi.org/10.1016/J.BPA.2003.12.002
Shibuya N, Goldstein IJ, Shafer JA et al (1986) Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch Biochem Biophys 249:215–224. https://doi.org/10.1016/0003-9861(86)90577-1
Shiozawa S, Shimizu K, Tanaka K, Hino K (1997) Studies on the contribution of c-fos/AP-1 to arthritic joint destruction. J Clin Invest 99:1210–1216. https://doi.org/10.1172/JCI119277
Sillanaukee P, Pönniö M, Jääskeläinen IP (1999) Occurrence of sialic acids in healthy humans and different disorders. Eur J Clin Invest 29:413–425. https://doi.org/10.1046/J.1365-2362.1999.00485.X
Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20:175–193. https://doi.org/10.1038/S41580-018-0089-8
Sloane JA, Blitz D, Margolin Z, Vartanian T (2010) A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Med 12:149–163. https://doi.org/10.1007/s12017-009-8094-x
Soleymani S, Farzaei MH, Zargaran A et al (2020) Promising plant-derived secondary metabolites for treatment of acne vulgaris: a mechanistic review. Arch Dermatol Res 312:5–23. https://doi.org/10.1007/S00403-019-01968-Z
Steiner GE, Newman ME, Paikl D et al (2003) Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 56:171–182. https://doi.org/10.1002/PROS.10238
Stys PK, Zamponi GW, Van Minnen J, Geurts JJG (2012) Will the real multiple sclerosis please stand up? Nat Rev Neurosci 13:507–514. https://doi.org/10.1038/NRN3275
Taheri Y, Quispe C, Herrera-Bravo J et al (2022) Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid Based Complement Alternat Med 2022:4024331. https://doi.org/10.1155/2022/4024331
Tang ZJ, Zou W, Yuan J et al (2015) Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behav Pharmacol 26:427–435. https://doi.org/10.1097/FBP.0000000000000143
Tanghetti EA (2013) The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol 6:27
Tansey MG, Wallings RL, Houser MC et al (2022) Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 22:657–673. https://doi.org/10.1038/S41577-022-00684-6
Teucher T, Obertreis B, Ruttkowski T, Schmitz H (1996) Cytokine secretion in whole blood of healthy subjects following oral administration of Urtica dioica L. plant extract. Arzneimittelforschung 46:906–910
Theoharides TC, Alysandratos KD, Angelidou A et al (2012) Mast cells and inflammation. Biochim Biophys Acta 1822:21–23. https://doi.org/10.1016/J.BBADIS.2010.12.014
Theuns J, Van Broeckhoven C (2000) Transcriptional regulation of Alzheimer’s disease genes: implications for susceptibility. Hum Mol Genet 9:2383–2394. https://doi.org/10.1093/HMG/9.16.2383
Todhunter PG, Kincaid SA, Todhunter RJ et al (1996) Immunohistochemical analysis of an equine model of synovitis-induced arthritis. Am J Vet Res 57:1080–1093
Toldy A, Atalay M, Stadler K et al (2009) The beneficial effects of nettle supplementation and exercise on brain lesion and memory in rat. J Nutr Biochem 20:974–981. https://doi.org/10.1016/j.jnutbio.2008.09.001
Toldy A, Stadler K, Sasvári M et al (2005) The effect of exercise and nettle supplementation on oxidative stress markers in the rat brain. Brain Res Bull 65:487–493. https://doi.org/10.1016/j.brainresbull.2005.02.028
Toyoda M, Morohashi M (2001) Pathogenesis of acne. Med Electron Microsc 34:29–40. https://doi.org/10.1007/S007950100002
Traenckner EBM, Pahl HL, Henkel T et al (1995) Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J 14:2876–2883. https://doi.org/10.1002/J.1460-2075.1995.TB07287.X
Tseng HC, Wang MH, Chang KC et al (2020) Protective effect of (−)epigallocatechin-3-gallate on rotenone-induced parkinsonism-like symptoms in rats. Neurotox Res 37:669–682. https://doi.org/10.1007/S12640-019-00143-6
Tucakov J (1997) Lečenje biljem: fitoterapija. Lečenje biljem: fitoterapija. Rad, Belgrad, p 405
Turturici G, Tinnirello R, Sconzo G et al (2014) Positive or negative involvement of heat shock proteins in multiple sclerosis pathogenesis: an overview. J Neuropathol Exp Neurol 73:1092–1106. https://doi.org/10.1097/NEN.0000000000000136
Üçeyler N, Häuser W, Sommer C (2011) Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord 12:245. https://doi.org/10.1186/1471-2474-12-245
Üçeyler N, Rogausch JP, Toyka KV, Sommer C (2007) Differential expression of cytokines in painful and painless neuropathies. Neurology 69:42–49. https://doi.org/10.1212/01.wnl.0000265062.92340.a5
Uğur Y, Güzel A (2023) Determination of phytochemical content by LC-MS/MS, investigation of antioxidant capacity, and enzyme inhibition effects of nettle (Urtica dioica). Eur Rev Med Pharmacol Sci 27:1793–1800. https://doi.org/10.26355/eurrev_202303_31540
Upton R (2013) Stinging nettles leaf (Urtica dioica L.): extraordinary vegetable medicine. J Herb Med 3:9–38. https://doi.org/10.1016/J.HERMED.2012.11.001
Vafaee F, Zangiabadi N, Pour FM et al (2012) Neuroprotective effects of the immunomodulatory drug Setarud on cerebral ischemia in male rats. Neural Regen Res 7:2085–2091. https://doi.org/10.3969/j.issn.1673-5374.2012.27.001
Van Damme EJ, Broekaert WF, Peumans WJ (1988) The Urtica dioica Agglutinin is a complex mixture of isolectins. Plant Physiol 86:598–601. https://doi.org/10.1104/pp.86.2.598
Veiga M, Costa EM, Silva S, Pintado M (2020) Impact of plant extracts upon human health: a review. Crit Rev Food Sci Nutr 60:873–886. https://doi.org/10.1080/10408398.2018.1540969
Vignozzi L, Cellai I, Santi R et al (2012) Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol 214:31–43. https://doi.org/10.1530/JOE-12-0142
Vora J, Srivastava A, Modi H (2018) Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform Med Unlocked 13:128–132. https://doi.org/10.1016/J.IMU.2017.10.005
Wadibhasme PG, Ghaisas MM, Thakurdesai PA (2011) Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharm Biol 49:508–515. https://doi.org/10.3109/13880209.2010.521754
Wagner H, Willer F, Kreher B (1989) Biologically active compounds from the aqueous extract of Urtica dioica. Planta Med 55:452–454. https://doi.org/10.1055/S-2006-962062
Wallin RPA, Lundqvist A, Moré SH et al (2002) Heat-shock proteins as activators of the innate immune system. Trends Immunol 23:130–135. https://doi.org/10.1016/S1471-4906(01)02168-8
Walport M, Brennan FM, Maini RN, Feldmann M (1992) TNF alpha–a pivotal role in rheumatoid arthritis? Br J Rheumatol 31:293–298. https://doi.org/10.1093/RHEUMATOLOGY/31.5.293
Wang L, Yang JR, Yang LY, Liu ZT (2008) Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med Hypotheses 70(5):1021–3. https://doi.org/10.1016/J.MEHY.2007.08.022
Warger T, Hilf N, Rechtsteiner G et al (2006) Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J Biol Chem 281:22545–22553. https://doi.org/10.1074/JBC.M502900200
Warner TT, Schapira AH (2003) Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol 53(S3):S16-25. https://doi.org/10.1002/ANA.10487
White AT, Light AR, Hughen RW et al (2010) Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology 47:615–624. https://doi.org/10.1111/J.1469-8986.2010.00978.X
Williams TJ, Morley J (1973) Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature 246:215–217. https://doi.org/10.1038/246215A0
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111:544–547. https://doi.org/10.3181/00379727-111-27849
Xue Z, Zhao H, Zhu R et al (2018) On the use of abiotic sialic acids to attenuate cell inflammation. Sci Rep 8:17320. https://doi.org/10.1038/S41598-018-35477-2
Yang TLB, Chen Q, Deng JT et al (2017) Mutual reinforcement between telomere capping and canonical Wnt signalling in the intestinal stem cell niche. Nat Commun 8:14766. https://doi.org/10.1038/NCOMMS14766
MohdI Y, Gopalakrishnan A, Saxena A et al (2018) Anti-Inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—a review. Recent Pat Inflamm Allergy Drug Discov 12:39–58. https://doi.org/10.2174/1872213x12666180115153635
Yida Z, Imam MU, Ismail M et al (2015) High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats. J Biomed Sci 22:1–10. https://doi.org/10.1186/S12929-015-0211-6/FIGURES/7
Yilmaz B, Basar O, Aktas B et al (2014) Effects of urtica dioica extract on experimental acute pancreatitis model in rats. Int J Clin Exp Med 7:1313–1318
Yong VW, Krekoski CA, Forsyth PA et al (1998) Matrix and diseases of the CNS. Trends Neurosci 21:75–80. https://doi.org/10.1016/S0166-2236(97)01169-7
Yuk JE, Woo JS, Yun CY et al (2007) Effects of lactose-beta-sitosterol and beta-sitosterol on ovalbumin-induced lung inflammation in actively sensitized mice. Int Immunopharmacol 7:1517–1527. https://doi.org/10.1016/J.INTIMP.2007.07.026
Zemmouri H, Sekiou O, Ammar S et al (2017) Urtica dioica attenuates ovalbumin-induced inflammation and lipid peroxidation of lung tissues in rat asthma model. Pharm Biol 55:1561–1568. https://doi.org/10.1080/13880209.2017.1310905
Zhang H, Bai L, He J et al (2017) Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur J Med Chem 141:257–272. https://doi.org/10.1016/J.EJMECH.2017.09.068
Zhang H, Nie X, Shi X et al (2018) Regulatory mechanisms of the Wnt/β-catenin pathway in diabetic cutaneous ulcers. Front Pharmacol 9:1114. https://doi.org/10.3389/fphar.2018.01114
Zhang S, Liu M, Liu D, Shang Y, Du G, Wang Y (2022) Network pharmacology analysis and experimental validation of kaempferol in the treatment of ischemic stroke by inhibiting apoptosis and regulating neuroinflammation involving neutrophils. Int J Mol Sci 23(20):12694. https://doi.org/10.3390/ijms232012694
Zhang P, Liu N, Xue M, Zhang M, Liu W, Xu C, Fan Y, Meng Y, Zhang Q, Zhou Y (2023) Anti-Inflammatory and antioxidant properties of β-sitosterol in copper sulfate-induced inflammation in zebrafish (Danio rerio). Antioxidants 12:391. https://doi.org/10.3390/antiox12020391
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parente, R., Paiva-Santos, A.C., Cabral, C. et al. Comprehensive review of Urtica dioica L. (Urticaceae) phytochemistry and anti-inflammatory properties. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09980-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09980-6