Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and most common cause of dementia among older people. The main pathological hallmarks of AD are formation of insoluble amyloid beta senile plaques and paired helical filaments of neurofibrillary tangles. AD features gradual memory decline, mild to severe cognitive impairment, eventually total dependence of patients on caregivers. Currently available drugs have not been able to modify AD pathology. This has drawn increasing attention to plant food materials with high nutritional and bioactive constituents as potential complementary therapy for AD. Sorghum bicolor is a widely available cost-effective source of proteins, fats, crude fibres, biopeptides and polyphenols which are vital for human wellbeing. This review discussed the major mechanisms underlying AD pathology. The nutritional and bioactive constituents of Sorghum bicolor grains were extensively described. There is limited report on anti-AD activities of sorghum grains. Therefore, the pharmacological mechanisms of action including scavenging of reactive oxygen species, inhibition of oxidative stress, anti-acetylcholinesterase activity and modulation of mitophagy were only speculated. This comprehensive update suggests more robust innovative studies that will provide critical theoretical details necessary to promote utilization of sorghum grains as functional food or source of bioactive molecules for AD therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease is a multifactorial progressive neurodegenerative disorder, and most prevalent cause of dementia in older people (Prabakara et al. 2022). Alzheimer’s disease (AD) results from loss of neurones and brain atrophy, featuring gradual memory decline, mild to severe cognitive impairment, and total dependence of patients on caregivers (Mary et al. 2022). AD represents 70% of over 55 million cases of dementia globally, and the trend will reach 78 million and 139 million by 2030 and 2050, respectively (WHO 2022). In 2019, the global cumulative cost of dementia was US$1.3 trillion, and is projected to exceed US$ 2.8 trillion by 2030 (WHO 2022). In 2021, population of Australian aged 50 and above with AD was 153,888. This figure will rise by 73% to 266,144 by 2041 (Brown 2022), with an annual economic cost of $ 26.6 billion (Brown 2022). Till date, development of effective cure for AD still poses serious clinical challenge. However, several promising strategies for early diagnosis and treatments of AD are being investigated. This includes increasing focus on natural remedies.
Plant materials with high nutritional and bioactive contents have been utilized as nutraceuticals for managing numerous cardiovascular and neurodegenerative diseases (Zarifi et al. 2022), including AD. Besides, scientific evidence have shown that dietary intervention could minimise the risk of developing AD (Ngandu et al. 2015; Yurko-Mauro et al. 2010). This has resulted in remarkable increase in consumption and research on health benefits of nutritious and polyphenols-rich foods such as tea, cereals, fruits and vegetables (Saad et al. 2021; Thakur et al. 2020). Therefore, nutritional sources with potential to reduce AD pathologies are topical (Tournissac et al. 2018).
Sorghum grains are widely available cost-effective source of numerous dietary and bioactive components such as protein, minerals, fibre, bioactive peptides and polyphenols (Rashwan et al. 2021). Different classes of polyphenols have been identified in several genotypes of sorghum including flavonoids and phenolic acids. Polyphenol extracts from sorghum grains (PPES) present several biological properties including antioxidative, anti-inflammatory, anti-cancer, anti-diabetic, and anti-microbial properties (Chen et al. 2016a; Li et al. 2021a; Ofosu et al. 2021, 2020; Taylor et al. 2014; Taylor and Duodu 2015; Zhang et al. 2019). However, there is limited reports on the anti-AD activities of polyphenols and functional constituents from sorghum grains.
This article reviewed major pathological pathways of AD. We also presented an update on the nutritional contents, bioactive components, and antioxidant properties of sorghum grains. This review equally indicated that the extraction methods and solvents type impact the extracted compounds, and consequently bioactivity of sorghum grains. The identification and antioxidant mechanisms of sorghum polyphenol extracts were also highlighted. The potential pharmacological mechanisms of PPES against AD pathologies were discussed, and future perspectives extensively elaborated (Fig. 1).
Methodology
Literature search was done using electronic sources. Articles ranked in Scientific Citation Index (SCI) were pooled from Edith Cowan University world search, Pubmed, Springer, Scopus, and Google Scholar databases. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was used to filter articles based on the relevance. Recent original research articles were filtered using keywords “Sorghum bicolor”, “proximate composition”, “polyphenols”, “neurodegeneration”, “dementia”, and “Alzheimer’s disease. Abstracts, editorials, and bibliographies of articles not written in English language were excluded. Sorghum genotypes, proximate composition, phenolic profiles, methods of extraction, detection and neuroprotective properties including experimental models were highlighted in tables and figures.
Description of sorghum
Sorghum (Sorghum bicolor (L.) Moench) is the fifth most valuable grain, extensively cultivated in sub-Sahara Africa and Southeast Asia, with a global production of 61.4 million metric tonnes in 2021 (FAO 2022). In Africa and Asia, sorghum grain is a source of energy and nutrients for millions of people, but in countries such as Australia and United States of America, it served majorly as an animal feed or for biofuel production (Stefoska-Needham et al. 2015). In Australia, sorghum is a summer crop, and its production is uniformly distributed among northern New South Wales, southern Queensland, and central Queensland (GRDC 2017). Sorghum grains vary in size, shape and pigment including white, red, and different shades of brown.
Categorisation of sorghum
Sorghum is categorised using two major criteria, namely the extractable tannin content and the pigment type (Asquith et al. 1983). Based on the extractable tannin content, sorghum has been further grouped into three classes, comprising type I (low tannins extractable in 1% acidified methanol), type II (tannins extractable in 1% acidified methanol) and type III (tannins extractable in either methanol or acidified methanol) (Awika and Rooney 2004). Several sorghum genotypes have been identified with respect to pigments. The pigmented sorghum genotypes have a remarkably high concentration of phenolic compounds, total polyphenol content and antioxidant activity than the non-pigmented sorghums. Pericap pigment, pericarp thickness and testa availability influence the flavonoids and total flavonoids contents in sorghum (Taleon et al. 2012).
Nutritional profile of sorghum grains
Carbohydrates
Carbohydrates are the major macronutrient in sorghum averaging around 64–85% (Table 1). Carbohydrates are more abundant in the red, dark-brown, and white genotypes. In addition, the free sugar contents of sorghum varieties vary significantly, and glucose was the most abundant (2.22–2.50%), followed by fructose (0.72–1.00%), while raffinose and stachyose were the least (0.01–0.02%) (Juhaimi et al. 2019). Sorghum grains are, therefore, reservoir of nutrients with significant health benefits.
Dietary fibre (DF) was defined by the World Health Organization and Codex Alimentarius to include all carbohydrates that are not digested or absorbed in the small intestine, such as pectins, cellulose, hemicellulose, resistant starch and lignins (FAO 2010). The DF content of sorghum was up to 19.78% DW (Table 1) and compares with those of oat, maize, and wheat but greater than those of rice and millet (Kaur et al. 2021; P and Joye 2020). DF present prebiotic properties, therefore, its inclusion in human diets in high proportion could significantly enhance production of short-chain fatty acids in the large intestine, which enrich the gut microbiome, consequently attenuating metabolic, gastrointestinal, and neurodegenerative disorders (Gill et al. 2021).
Proteins and amino acids
Sorghum grain have a higher protein content compared to maize (Ape et al. 2016; Etuk et al. 2012). The protein content reported in sorghum grains was around 6.14–16.20% (Table 1). The protein in six sorghum varieties were between 10.06 and 12.93% (Treviño-Salinas et al. 2021). Pigmented sorghum of Chinese origin recorded highest protein contents between 13.0% and 16.20% (Rocchetti et al. 2020). The study showed that red and dark-brown pigments have the highest amounts (16.10–16.20%). Among 10 sorghum cultivars of Kenyan origin, Kari/Mtama-1 had the highest crude protein level (10.133%), while Seredo recorded the least value of 5.23% (Shinda et al. 2022). In addition, the main amino acid composition of red, white, and yellow pigmented sorghum includes glutamic acid, leucine, and proline (Table 2), while lysine, methionine, and threonine are the common limiting amino acids (Juhaimi et al. 2019; Qaku et al. 2020). A study involving a double-blind, randomized, placebo-controlled trial had indicated significant improvement in cognitive function in middle-aged and older adults after treatment with amino acids including leucine, lysine and valine (Suzuki et al. 2020).
Minerals
More than 65% of the world’s population lack one or more essential minerals (Punia and Kumar 2021). Sorghum contains significant amount of minerals that are essential for human nutrition and health. The major mineral constituents (mg/100 g dw) identified in seven varieties of sorghum include calcium (10.50–341.86), sodium (1.40–216.32), magnesium (39.22–1693), potassium (183.14–3566), iron (1.91–65.515) and zinc (0.433–28.43) (Juhaimi et al. 2019; Pontieri et al. 2022; Qaku et al. 2020). Zinc stabilizes protein structure, supports catalytic reactions and brain function in living organisms (Lei et al. 2021). Dietary calcium, potassium and magnesium are associated with reduced risk of dementia (Cherbuin et al. 2014).
Lipids and fatty acids
Lipids are majorly utilized for food, medicinal and other industrial applications. Lipids account for about 1.55–11.70% dried weight (DW) of different genotypes of sorghum grain from different countries (Table 1). Quality and digestibility of edible lipids depends on the composition and concentration of fatty acids (Punia and Kumar 2021). Fatty acids (FAs) act as both energy source and modulating agents for metabolism, exerting many biological functions and health benefits, especially polyunsaturated fatty acids (PUFAs). The FAs contents of sorghum have been reported in recent studies (Juhaimi et al. 2019; Pontieri et al. 2022, 2020; Treviño-Salinas et al. 2021; Zhang et al. 2019). The dominant FAs in sorghum include oleic acid, palmitic acid, stearic acid, and linoleic acid (Table 2). FAs were extracted from six sorghum varieties using boron trifluoride-methanol, followed gas chromatography analysis (Treviño-Salinas et al. 2021). Ten unsaturated and 15 saturated FAs were identified, including oleic acid and linoleic acid (omega 6 and omega 9). An earlier report had shown that in most of the sorghum varieties studied, polyunsaturated FAs were in higher concentration (45.0–51.0%) compared to monounsaturated fatty acids (36.0–40.0%) (Mehmood et al. 2008). A higher content of linoleic acid (43.75 g/100 g FAs) was also found in a newly developed red Ji Liang No. 1 sorghum genotype (Zhang et al. 2019). A similar trend was observed in white and black pigmented sorghum genotypes grown in Italy (Pontieri et al. 2022).
Unsaturated FAs are essential for function and structure of biological membranes (Taylor et al. 2005). Regular intake of α-linoleic acid or eicosapentaenoic acid could promote longevity (Román et al. 2019). Higher consumption of omega 3 PUFAs are associated with reduced Aβ levels in AD regions on Pittsburgh compound B positron emission tomography (Mosconi et al. 2014). Preclinical and clinical studies have shown that omega 3 PUFAs support cognitive functions by acting on inflammatory mediators and oxidative stress (Giacobbe et al. 2020). Therefore, varieties of sorghum are potential sources of edible oil with high concentration of clinically important PUFAs.
Association of official analytical chemists, GC–MS: Gas chromatography mass spectrometry, GC-FID: Gas chromatography flame ionisation detector.
Bioactive constituents of sorghum
Alkaloids
Alkaloids in plant tissues are nitrogenous molecules, other than vitamin B, amino acids, and proteins (Gong et al. 2020). Separate studies used 5 g of sorghum powder in 50 ml 10% acetic acid for 4 h, followed by drop wise addition of NH4OH to precipitate the alkaloids (Hassan et al. 2019, 2020). The alkaloids content was approximately 0.051 mg/100 g. Pharmacokinetics studies have shown that Alkaloids are neuroprotective agents. Berberine, an isoquinoline alkaloid found in traditional Chinese herbs, inhibited oxidative stress, pathogenic enzymes, and neuroinflammation by activating autophagy and preventing apoptosis of heathy neuronal cells (Fan et al. 2019). Berberine also facilitate clearance of toxic misfolded and aggregated proteins in neurons by activating autophagy (Huang et al. 2017; Jiang et al. 2015). However, the alkaloids contents of sorghum grains have not been characterised.
Sterols
Plant-based foods are large reservoir of sterols, also known as phytosterols (Jiménez-Escrig 2012). Plant sterols are used as ingredient in human diet and are majorly present in margarines and oils. Sterols have numerous biological properties and health benefits (Herchi et al. 2009). Sterols have been extracted from sorghum, legumes, seeds, and vegetables (Hassan et al. 2019). The sterols contents of an unidentified sorghum variety obtained in USA was 0.655 mg CHE/g dw.
Bioactive peptides (Kafirins)
Bioactive peptides, including Kafirin, are inactive amino acid sequences within the precursor protein, comprising up to 20 amino acid units (Shahidi and Zhong 2019), and are thus an integral constituent of functional foods (e.g. Calpis, Evolus) health-promoting dietary supplements (e.g. Lactium, Vivinal Alpha), and pharmaceutical products (e.g. Vasotensin and drug delivery systems) (Garzón et al. 2022; Sarker 2022). Bioactive peptides exert antioxidant activities via several mechanisms including scavenging of free radicals, chelation of metal ions, and reduction of reactive oxygen and reactive nitrogen species (Elias et al. 2008).
Kafirin is a gluten-free major storage protein from sorghum grain. Kafirin represents highest proportion of total sorghum proteins and is the major by-product of industrial ethanol production and liquor brewing (Dai et al. 2022). The main processes of extracting and identifying Kafirin and other plant peptides include enzyme hydrolysis to form hydrolysate, followed by purification into antioxidant peptide using separation membrane, electrophoresis, and chromatography (Wen et al. 2020). A recent study isolated Kafirin via simulated gastrointestinal digestion model, followed by fractionation and detection with reversed-phase high-performance liquid chromatography (RP-HPLC) and high-resolution liquid chromatography-mass spectrometry (HRLC-MS). Three novel peptides were identified including LSICGEESFGTGSDHIR (PEP 1), SLGESLLQEDVEAHK (PEP2) and QLRDIVDK (PEP4) fractions with strong dipeptidyl peptidase IV (DPP-IV) inhibition IC50 values of 73.5, 82.5 and 8.55 µM, respectively (Majid et al. 2022). In addition, bioactive peptides have been innovatively isolated from white sorghum spent grain using neural protease-purazyme® and Sephadex G-25 (Garzón et al. 2022). The most active fractions (F2, F5 and F6) were analysed using liquid chromatography electrospray ionisation quadrupole time-of-flight mass spectrometry (LC-ESI-Q-TOF–MS). The fractions presented varying antioxidant, antidiabetic, and antimicrobial properties. The anti-AD properties of these peptide fractions are worth investigating.
Furthermore, effects of enzymes/solvent (E/S) ratio and reaction time on antioxidant properties of kafirin was analysed using alkalase enzyme (Xu et al. 2019a). An E/S of 0.4 at 4 h produced hydrolysates in < 1 kDa (47.98%), 1–3 kDa (14.97%), 3–5 kDa (14.98%), 5–10 kDa (16.58%) and > 10 kDa (5.48%). At a concentration of 10 mg/ml, the 5–10 kDa fraction presented the highest 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•) scavenging activities and Fe2+ chelating power of 74.74% and 22.78%, respectively. RP-HPLC and matrix-assisted laser desorption/ionisation time-of-flight/time-of-flight (MALDI-TOF/TOF) MS identified 26 peptide sequences including QWQQ and QQWQ, to which the authors attributed the antioxidant activities of the kafirin. The same authors had adopted similar techniques and papain in an earlier study (Xu et al. 2019b). The TPC (Total Phenolic Content), DPPH•, metal ion chelating and oxygen radical absorbance capacity (ORAC) of the 1–3 kDa fraction were 42.82 mg GAE/g, 6.23 IC50 mg/ml, 9.55% and 0.35 g TE/g, respectively (Xu et al. 2019b). The study identified 13 peptide fractions including LRQQ, YLRQ and WQPN. The strong antioxidant properties of these novel peptide fractions could translate to significant anti-AD activities.
In addition, the extraction of Kafirin from white sorghum (cv. Perla 101) was optimised by pre-treating with amyloglucosidase (Castro-Jácome et al. 2020). The optimum yield of 4.12% was obtained at 0.1% E/S ratio after 60 min, and Nano-LC/MSMS was used to identify four peptide fractions α1-KAF, α2-KAF, β-KAF, and γ-KAF. In silico analysis using BIOPEP-UWM Database demonstrated strong Angiotensin converting enzymes-inhibitory, antioxidative and hypotensive activities. The study identified 31 novel peptide sequences with potential biological activities. In silico and in vitro techniques have also been employed to obtain a purified DPP-IV inhibiting oligopeptides from sorghum (cv. Longmiliang No. 1) kafirin using papain and trypsin (Dai et al. 2022). The peptides sequence identified using nano-LC–ESI–MS/MS system include LPFYPQGV (LP8), LPFYPQ (LP6) and GPVTPPILG (GP9). The in-silico absorption, distribution, metabolism, extraction, and toxicity model indicated that the oligopeptides have good transmembrane absorption while molecular docking showed that the peptides LP6, LP8 and GP9 strongly inactivated DPP-IV by binding to its active sites.
It has been reported that food-derived bioactive peptides could inhibit AD by activating endogenous antioxidant enzymes, preventing mitochondria dysfunction, promoting cholinergic system, modulating autophagy, and minimising amyloidogenic processing of amyloid precursor protein (APP) in the central nervous system (Zhao et al. 2022). However, despite the identified numerous health benefits of sorghum Kafirin, the anti-AD potential is yet to be investigated.
Sorghum polyphenols
Description
Polyphenols are plant secondary metabolites with aromatic chemical structure that comprises at least two phenyl rings and one or more hydroxyl group (Ayad and Akkal 2019). Polyphenols have been broadly classified into two, namely phenolic acids and flavonoids which vary significantly in different food sources. Currently, over 8000 polyphenols have been identified, and 4000 belong to the class of flavonoids (Belščak-Cvitanović et al. 2018). Polyphenols are the most prevalent natural antioxidants in human diets, especially whole grains, red wine, tea, and olive oil, fruits, and vegetables. Sorghum grains have abundant reserve of flavonoids (e.g. luteolin, kaempferol, quercetin, catechin) and phenolic acids (e.g. ferulic acid, caffeic acid, vanillic acid, p-coumaric acid), as reviewed in (Rashwan et al. 2021; Rezaee et al. 2021). Studies have demonstrated that presence of polyphenols along with other molecules such as vitamin C, vitamin E or carotenoids, is responsible for the health benefits of fruits and vegetables.
Extraction and antioxidant properties of sorghum polyphenols
Several techniques have been employed to extract and characterise polyphenols from sorghum grains (Table 3). Until recently, most of the techniques were focused on the free polyphenol contents, while the bound polyphenols were discarded in extraction residue. This suggests that the polyphenol contents of sorghum grains may have been previously underestimated.
Recently, free and bound polyphenols were extracted from seven Australian sorghum genotypes, including Shawaya Short Black 1 (Xiong et al. 2020a, 2020b). The bound polyphenols were released from the residue using 2 M HCl, and the crude extracts were purified with ethyl acetate followed by characterisation using HPLC–DAD-ESI-QTOF-MS/MS and HPLC–DAD. About 114 polyphenols were identified in the free and bound extracts including kaempferol, narigenin and caffeic acid (Xiong et al. 2020a, 2020b). The comprehensive study indicated that Shawaya Short black 1 had the highest polyphenols constituents. Another study extracted phenolic acids, flavonoids and bound polyphenols using 80% methanol and 0.01 N HCl, and the fractions were characterised using RP-HPLC-DAD (Speranza et al. 2021). The antioxidant properties vary within varieties. While the red varieties Armorik, Arsky and Huggo recorded the highest (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS•+) and DPPH• scavenging activities, the white Golden variety presented the strongest ferric reducing antioxidant power (Table 3). The identified compounds include 7-methoxy-apigenidin, 5-methoxy-luteolidin and protocatechuic acid. Furthermore, free and bound polyphenols have been extracted from white, black, and red pigmented Italian sorghum genotypes (Pontieri et al. 2021). The red variety had the highest Trolox equivalent antioxidant capacity (TEAC), while the white was the least with 0.06 and 0.19 IC50 of Trolox µg mL−1/IC50 of sample µg/ml−1, respectively. In addition, 48 phenolic compounds, including insoluble 3—deoxyanthocyanidins were identified in free and bound polyphenol extracts of 11 Chinese sorghum genotypes using UHPLC-Q-TOF-MS (Li et al. 2021a). The extracts presented strong ORAC value around 10.32–42.26 mg TE/g, the most active being Tong Za 117 variety. More so, 70% ethanol and UHPLC-ESI-QTOF-MS/MS were employed to extract and characterise phenolic compounds from eight brown Korean sorghum genotypes including SOR 01, SOR 03, SOR 08, SOR 11, SOR 17, SOR 21, SOR 24, and SOR 33 (Ofosu et al. 2021). In all, 18 polyphenols were identified, and flavones, dihydroflavonoids and isoflavones were most abundant flavonoids in the SOR 11, SOR 17 and SOR 33 genotypes.
The strong antioxidant activities discussed above are related to the anti-cancer, anti-diabetic, anti-inflammatory and anti-microbial properties of sorghum polyphenol extracts (Rashwan et al. 2021; Rezaee et al. 2021). Polyphenols initiates protective mechanisms by activating endogenous antioxidant defence system and various cell signalling pathways (Punia and Kumar 2021). Consequently, the polyphenol extracts from sorghum grain could inhibit AD pathology, which will vary with sorghum varieties, origin, and extraction techniques.
Pathogenesis of Alzheimer’s disease (AD)
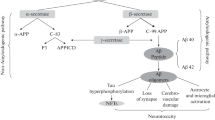
As explained earlier, AD is the most predominant progressive neurodegenerative disorder that causes dementia majorly in old people. The main pathological hallmark of AD are accumulation of amyloid beta (Aβ) and formation of tau aggregates, followed by neuronal loss, and brain atrophy (Li et al. 2021b). Amyloid pathogenesis begins when APP, a protein in the plasma membrane, is sequentially cleaved by β-secretase (BACE1) and γ-secretase to produce Aβ deposits. The Aβ then form oligomers, which polymerises into senile plaques that diffuses into synaptic clefts and disrupts synaptic signalling. The senile plaques also cause activation of kinases, leading to hyperphosphorylation of microtubule-associated tau protein, which also polymerises into insoluble neurofibrillary tangles (NFTs) (Tiwari et al. 2019). The Aβ plaques and tau tangles trigger activation of microglia, astrocytes, cellular inflammatory response, and neurotoxicity (Selkoe 2021). The pathological pathways of AD are elaborated below.
Amyloid beta (Aβ)
APP is a transmembrane protein that is expressed in the brain and central nervous system (CNS). APP is cleaved by proteases (α-, β- and γ-secretase) to produce several derivatives with distinct biological functions including sAPPα, sAPPβ and Aβ peptides (Coronel et al. 2018), through either amyloidogenic pathway or non-amyloidogenic pathway. In physiological condition, cleavage of APP follows the non-amyloidogenic pathway whereby APP is cleaved by α-secretase to form soluble extracellular sAPPα and C83 fragment, which is then cut by γ-secretase similar to length of 99 amino acids (Mary et al. 2022). This pathway does not release Aβ, rather a short protein fragment p3 is formed. However, in the amyloidogenic pathway, APP is first cleaved by β-secretase producing C-terminal fragment of the length of C99 and a chain. The C99 is then cleaved by γ-secretase to produce Aβ monomer which contain 38–43 amino acids residues and the intracellular C-terminal domain (Mary et al. 2022). The resulting Aβ monomers aggregate into neurotoxic hydrophobic oligomer, insoluble fibrils, and senile plaques in the CNS (Hardy and Selkoe 2002).
The main forms of Aβ that have been identified in the cerebrospinal fluid (CSF) include Aβ38, Aβ40 and Aβ42, as well as minor concentrations of forms 15,16,17,34,37, and 39 amino acids in length (Portelius et al. 2012). Although production of Aβ40 is more abundant, Aβ42 is highly hydrophobic, neurotoxic, and more prone to aggregation (Mary et al. 2022), and it is the predominant species in plaques characteristically observed in brains of AD patients (Hardy and Selkoe 2002). The amyloid plaques in the brain trigger formation of NFTs, followed by cell loss, vascular damage and eventually dementia. Numerous therapeutic strategies have targeted Aβ plaques without success (Selkoe 2021). Recently, Aducanumab and Lecanemab were approved by the United States Food and Drug Authority (USFDA) for AD therapy. Both drugs are IgG1 monoclonal antibodies and have been shown to clear neurotoxic Aβ plaques along with moderate reduction in cognitive decline in early stages of AD (McDade et al. 2022; Parmar. 2022 Jan; van Dyck et al. 2022). However, there is no information whether either of these drugs could reverse damage caused by Aβ plaques such as mitochondrial dysfunction and defective mitophagy. Other drugs targeting Aβ plaques are in the pipeline including Donanemab, Gantenerumab and Simufilam (Cummings et al. 2022).
Neurofibrillary tangles (NFTs)
NFTs are abnormal accumulation of tau protein that deposits inside the neurons. In healthy neurons, internal structures called microtubules support transport of nutrients and molecules from cell to the axon and dendrites. Tau binds to the microtubules and supports neuronal plasticity, intracellular transport network and stability of axons (Naseri et al. 2019). In AD, however, extracellular deposits of aggregated Aβ induce kinase/phosphate activities that cause hyperphosphorylation of tau in more than 40 sites (Naseri et al. 2019). This cause tau to detach from microtubules, stick together and then accumulates to form NFTs, misfolded tau proteins inside the neurons. The NFTs trigger impairment of neuronal transport system, skeleton, and mitochondrial integrity, leading to reduced synaptic communication between neurons (Chen et al. 2017). Studies have indicated that levels of neocortical NFTs have a stronger correlation with ante-mortem cognitive status than Aβ plaques (Nelson et al. 2012). In addition, data from longitudinal studies indicated significant correlations between plasma tau levels and progressing cognitive decline, including increases in atrophy examined by magnetic resonance imaging (MRI) and in hypermetabolism assessed by fluorodeoxyglucose positron emission tomography (FDG-PET) during follow-up (Mattsson et al. 2016). The intraneuronal NFT which comprises majorly tau protein, is therefore another strong target in anti-Alzheimer’s research. Specific drugs aimed at clearing NFTs are still being investigated. TRx0237 and Nilotinib BE are in phase 3 clinical trial for this purpose (Cummings et al. 2022).
Oxidative stress
Oxidative stress occurs when production of reactive oxygen species (ROS) overwhelms the endogenous antioxidant defence system. The central nervous system is prone to oxidative stress due to low antioxidant potential, oxygen metabolism and high lipid content (Ojo et al. 2018). Oxidative stress has been implicated in the early stage and drive pathogenesis of AD (Butterfield and Halliwell 2019). Mitochondria are the major source of intracellular ROS (Guo et al. 2020). In AD, Aβ deposits bind to mitochondrial membrane and disrupts their functions, resulting in over production of ROS (Xu et al. 2021). The ROS overload could further trigger increased production of Aβ, tau phosphorylation, and oxidative stress by initiating a series of damaging events, including higher intracellular calcium level, mitochondrial dysfunction, apoptosis, and damages to DNA, membrane, and neurons (Tönnies and Trushina 2017). Therefore, prevention of oxidative stress is a potential therapeutic target to attenuate AD progression. Blarcamesine is a major synthetic drug being developed to target oxidative stress. Satisfactory outcome is being awaited at clinical trial phase 3.
Mitochondrial dysfunction
Mitochondria are cell organelles which perform important functions such as adenosine triphosphate (ATP) production, respiration, and energy conversion, as well as other cellular processes including proliferation, redox homeostasis, and apoptosis (Reddy and Beal 2005; Xie et al. 2022). Studies have associated defects in these critical mitochondrial functions with AD pathogenesis. In AD, Aβ and p-Tau deposit within the mitochondria and interact with the outer membrane proteins (Camara et al. 2017), followed by alteration of mitochondrial structure, reduced mitochondrial respiratory function and ATP production, declined mitochondrial dynamics, and increased mitochondria-associated oxidative stress (Guo et al. 2020; Reiss et al. 2022). Intramitochondrial Aβ also disrupt electron transport chain (ETC), and trigger formation of ROS (Pagani and Eckert 2011). Aβ has been found in mitochondria in the brain of AD mouse models and AD patients, where its levels correlate with impairment in mitochondrial structure and function (Guo et al. 2020). Exposure of mitochondria to Aβ also results in enrichment of proteins associated with elevated mitochondrial fission, decreased mitochondrial fusion (Wang et al. 2008), and impaired biogenesis and mitophagy (Rai et al. 2020; Reddy and Oliver 2019).
Mitochondria fission, fusion, and biogenesis
Mitochondrial function is maintained through a balance between mitochondrial dynamics (mitochondrial fission and fusion) and biogenesis (Calkins et al. 2011), which in turn determine neuronal health. A mitochondrion undergo fission to produce two separate mitochondria (Zorzano and Claret 2015), which concurrently undergo fusion whereby both the inner and outer membranes collapse and rejoin to form a single mitochondrion (Pradeepkiran et al. 2022). A balance between the fission and fusion processes determines mitochondrial shape and, are dependent on proteolysis and posttranslational changes in certain proteins (Wang et al. 2009).
The proteins associated with mitochondrial fission are fission 1 (Fis1) and dynamic-1-like protein (Drp1), while mitochondrial fusion is dependent on mitofuscin-1 (Mfn1), mitofuscin-2 (Mfn2) and optic atrophy 1(Opa1) (Tran and Reddy 2021). In physiological condition, Drp1 participates in mitochondrial division, distribution, and synaptic functions. However, in AD, interactions of Drp1 with Aβ and P-tau promotes GTPase activity of Drp1, which elevates mitochondrial fragmentation and impair mitochondrial dynamics, resulting in defective mitophagy and synaptic loss (Ross et al. 2015). Drp1, Aβ and P-tau also interact with mitochondrial outer membrane proteins such as PARKIN and phosphatase and tensin homologue (PTEN)-induced putative kinase 1 (PINK1), causing defective mitophagy, which eventually inhibit normal turnover of damaged or old mitochondria (Cai and Jeong 2020). Impaired biogenesis of new mitochondria observed in AD neurons is also associated with significant reduction in expression of nuclear respiratory factor 1 (Nrf1), nuclear respiratory factor 2 (Nrf2), peroxi (PGC-1α) and TEAM (Liu et al. 2020). Drugs in the pipeline to promote mitochondrial functions include Tricaptilin and Blarcamesine (Cummings et al. 2022).
Mitophagy
Mitophagy is the process by which superfluous or damaged mitochondria are targeted into the lysosome for degradation and recycling (Fang et al. 2019). During mitophagy, damaged mitochondria are selectively engulfed by a double membrane phagophore to form mitophagosome, which then fuse with a lysosome to form mitophagolysosome (Mary et al. 2022). Then, hydrolases in the lysosome degrade and recycle the engulfed mitochondria. Balance between mitophagy and biogenesis of functional mitochondria ensures physiological homeostasis of mitochondria. Mitophagy regulates mitochondrial quality and quantity, in addition to inhibition of accumulation of dysfunctional mitochondria that can promote cellular degeneration commonly observed in AD (Pradeepkiran et al. 2022). Studies have reported two types of mitophagy namely, PINK1/Parkin-dependent pathway and PINK1/Parkin-independent pathway. Both types of mitophagy are induced by several stimuli and proteins mentioned earlier.
PINK1/Parkin-dependent mitophagy
This type of mitophagy is driven by reduced mitochondrial membrane potential (Δψmit), resulting in stabilization of PINK1 at the outer mitochondrial membrane (OMM), and recruitment of E3 Ubiquitin ligase Parkin (Pradeepkiran et al. 2022). This generates polyphospho-ubiquitinated chains (p-S65-Ub) on the OMM proteins that create a ‘degrade me’ alert for damaged mitochondria (Mary et al. 2022). Proteins carrying p-S65-Ub are attracted and bind to mitophagy receptors such as NDP52, NBR1, OPTN, p62 or TAX1BP1. Then, phagosomes are recruited through their LC-3 interacting region (LIR) motif present at the surface of the phagosome. The phagosome engulfs damaged mitochondria to form mitophagosome. Lysosome fuses with mitophagosomes to form mitophagolysosomes, followed by degradation of the damaged mitochondria by acidic hydrolases (Mary et al. 2022).
PINK1/Parkin-independent mitophagy
The PINK1/Parkin-independent mitophagy is activated by proteins in the outer OMM during hypoxic condition. Hypoxia causes transcriptional upregulation of BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) and NIX by HIF 1/2 transcription factors, followed by subsequent mitophagy process (Pradeepkiran et al. 2022). BNIP3 and NIX are single-pass transmembrane proteins targeted to mitochondria and have cytosolic tails with LIR motifs. Phosphorylation at the Ser34 and 35 residues, and the Ser17 and 24 residues around the BNIP3 and NIX LIR motifs enhance interaction with LC3-II (Pradeepkiran et al. 2022). Then, the LC3-II is identified by mitochondrial receptor protein such as BNIP, NIX, and FUNDCI to promote completion of the phagophore, resulting in encapsulation of the mitochondrion (Chen et al. 2016b). These proteins play vital role in the mitophagy process. NIX accumulates on the mitochondrial membrane to flag mitochondria for mitophagic degradation; BNIP3 binds to phagophore LC3-II to induce mitophagy through Opa1, alongside Drp1 and Fis1 which activate fission (van der Bliek et al. 2013); and FUN14 domain containing 1 (FUNDC1) receptor supports mitochondrial clearance, interacts with the ER and recruits Drp1 for mitochondrial fragmentation (Pradeepkiran et al. 2022).
Mitophagy dysfunction
Dysfunctional mitophagy, which is reflected through defective biogenesis and accumulation of damaged mitochondria, has been associated with AD (Pradeepkiran et al. 2022). Results from studies on cellular and animal models of AD mimicking familial type of AD and in the brains of AD patients, have shown that dysfunctional mitophagy is a critical aspect of the disease, and could occur at both upstream and downstream of Aβ and Tau in a self-propagating vicious cycle that cause synaptic dysfunctions and cognitive deficits (Kerr et al. 2017). An increase of PINK1 at early stage of AD and of Parkin at late stage of AD, along with an elevated mitochondrial content markers at both stages have been reported in sporadic AD hippocampi (Martín-Maestro et al. 2017). This is due to decreased mitophagic flux associated with a defect in the activation of PINK1/Parkin cascade. The same study found that in AD brains there was downregulation of the expression of autophagy and mitophagy-dependent proteins such as Beclin-1, ULK1, ATG5, ATG12, and VCP/P97 (Martín-Maestro et al. 2017). Another study reported 30–50% reduction in basal levels of mitophagy in post-mortem hippocampal brain samples from AD patients compared with healthy controls (Fang et al. 2019). The study indicated accumulation of functionally and structurally damaged mitochondria (decrease size, scattered cristae and low ATP production), reduced LC3-II recruitment to mitochondria and dysregulated AMP-activated protein kinase (AMPK) cascade. Mitophagy/autophagy inducing therapy include Trehalose and Nilotinib BE are at phase 1 and phase 3 clinical trial, respectively (Cummings et al. 2022).
The above are only a few of the therapy for managing and treating AD under clinical trial. Readers are referred to (Cummings et al. 2022) for comprehensive details. However, none of these medications have shown ability to completely inhibit AD pathology, acting only on minimal symptoms progression in addition to adverse effects such as blood clothing and headache. The multifactorial nature of AD has been further complicated by the complex interlink between the pathologies (Fig. 2). Therefore, there is increasing need for efficacious, well-tolerated complementary therapy that could minimise cognitive decline in AD. In response to current therapeutic limitations, there is increasing interest in the application of cost-effective, widely available and multi-targets plant materials as nutraceuticals for AD therapy (Cacabelos 2018; Manju Singh et al. 2021; Shankar et al. 2021). Moreover, plant-derived polyphenol extracts are less expensive and pose minimal side effects than synthetic drugs (Manju Singh et al. 2021; Shankar et al. 2021).
Sorghum grains as potential complimentary therapy for AD
Multiple lines of evidence have demonstrated anti-AD properties of plant derived polyphenols. Dietary polyphenols, particularly resveratrol, curcumin, and flavonoids could inhibit cognitive decline that precedes onset of AD (Yang et al. 2021). Epigallocatechin-3-gallate, tannic acid, curcumin, wine, or olive tree derived polyphenols prevent Aβ aggregation and disrupt β-sheets (Toni et al. 2017; Velander et al. 2017). Rosmarinic acid block fibrillization of β sheets of tau protein associated with AD (Cornejo et al. 2017). However, isolated individual compounds have been shown to present reduced bioactivity or may react differently as when the compound is ingested within the whole food matrix (Kapinova et al. 2018).
Plants polyphenol extracts and biopeptide fractions have inhibited Aβ1-42 aggregation in different AD models (Alghazwi et al. 2020; Bhattacharyya et al. 2020; Malta et al. 2022; Ochiishi et al. 2021; Romero-Márquez et al. 2022). Anthocyanins-rich elderberry juice promoted mitochondrial function (Yiannopoulou and Papageorgiou 2020). Grape seed extract inhibited taupathy in TMHT mouse model in dose and time-dependent manner by disintegrating the paired helical filaments (Guéroux et al. 2017). A comprehensive optimal combination of dietary polyphenols associated with risk of AD was studied in a large cohort of 1329 older adults (Lefèvre-Arbogast et al. 2018). The participants were subjected to intake of diets rich in 26 dietary polyphenols subclasses with proper follow up for 12 years for dementia/AD. The study demonstrated that lower risk of dementia/AD was associated with the polyphenol-rich diets including olive oil, berries, citrus, soy, leafy vegetables, and cereals. Interestingly, whole polyphenols extract from sorghum grains comprise phenolic acids, flavonoids, including uniquely high level of 3-DXA (Xiong et al. 2020a, 2020b) that could act synergistically against AD.
Evidence of mechanistic studies with animal models, several cross-sectional and epidemiological human studies indicated that dietary components are vital for maintaining brain functions and improving cognitive performance (Fernando et al. 2019). Emerging evidence has associated decreased risk of AD with regular intake of heart-healthy diet such as chicken, fish, legumes, olive oil, fruits, vegetables, and whole grains. Diets rich in vitamins, protein, fibre or polyphenols can improve human memory and reduce the risk of AD by inhibiting amyloid beta (Dong et al. 2020). Higher intake of potassium, calcium and magnesium were associated with reduced risk of developing all form of dementia in a 17-follow-up study of over 1000 older adults (Ozawa et al. 2012). Medium chain fatty acids from coconut oil could be converted into ketone bodies, which has been associated with reduction of oxidation and enhancement of mitochondrial function (Yuan et al. 2017). A phase 3 clinical trial on the effects of coconut oil on mild to moderate AD was terminated in 2017 due to insufficient funding and poor enrolment (Yuan et al. 2017).
In addition, our cross-sectional study of well-characterised cohort of cognitively normal older adults have shown that higher dietary protein intake reduced the likelihood of high Aβ level in the brain (Fernando et al. 2018). We have also reported that individuals who consume a diet rich in fibre have a reduced risk of developing AD compared to those who consume a low-fibre diet (Fernando et al. 2018; Martins and Fernando 2014). Recent studies have also indicated that regular intake of whole grains decreased the risk of AD and cognitive decline (Wang et al. 2022; Wang and Zhou 2023). Consequently, whole sorghum grains comprising macro- and micro-nutrients, polyphenols and other bioactive constituents may present additional synergistic benefits against AD. To this end, our research group have developed an interest on the potential anti-AD properties of 3-deoxyantocyanidins-rich sorghum genotypes. Preliminary findings indicated that the whole polyphenol extracts inhibited mitochondrial dysfunction, Aβ and tau toxicities (data not included). However, the mechanisms underlying the potential anti-AD of sorghum desire robust investigation.
Potential mechanisms underlying the anti-Alzheimer's effects of sorghum polyphenol
Sorghum polyphenol extracts could inhibit AD pathogenesis by scavenging free radicals, inhibiting oxidative stress, modulating neurotransmitters, mitophagy and numerous other proteins associated with AD (Fig. 3).
Potential mechanisms underlying effects of Sorghum polyphenols on Alzheimer’s disease pathologies. Sorghum polyphenol regulated mitophage by increased JC-1 mitochondrial membrane potential FBX07, and GATA2 expression. Scavenging of free radicals, especially reactive oxygen species, will significantly inhibit oxidative stress which preceeds amyloid beta accumulation
Inhibition of oxidative stress
Neuronal tissues generate reactive oxygen species (ROS) due to metabolism of amino acids and synthesis of neurotransmitters (Ward et al. 2015). Excessive reactive oxygen species (ROS) and other free radicals trigger oxidative stress. The human brain is susceptible to oxidative stress (OS) due to its high concentration of fatty acids, metal ions and limited antioxidant power (Ademosun and Oboh 2012; Li et al. 2021b). Prolonged oxidative stress overwhelms the endogenous antioxidant systems which can potentially lead to progressive molecular damage in cell components including lipid peroxidation, protein oxidation, DNA damage, and cellular aging (Li et al. 2021b; Salazar-López et al. 2020). Sorghum polyphenols present numerous antioxidant mechanisms (Table 3) which could prove significantly effective in minimising neuronal oxidative stress that initiates AD.
Inhibition of acetylcholinesterase (AChE) in the central nervous system
Acetylcholine (ACh) is an important neurotransmitter for memory and learning (Huang et al. 2022). AChE, a cholinergic enzyme naturally present at postsynaptic neuromuscular junctions in muscles and nerves, hydrolyses ACh into acetic acid and choline (Nikjoo 2014). People with AD have reduced levels of ACh in the synaptic cleft, hence cognitive impairment (Marucci et al. 2021). Inhibiting activities of AChE ensures availability of more ACh for brain function and reduced clinical symptoms of AD. Therefore, AChE inhibitors are clinical therapeutic strategy for AD. Some commercial cereals including rice, millet and sorghum were screened for their anti-dementia potential (Song et al. 2010). The result indicated that 50 µg/ml Sorghum grain methanol extract recorded the highest AChE inhibitory activities of 63.4%, suggesting its potential utilization to enhance learning, cognitive function, and memory (Song et al. 2010).
Maintenance of mitochondria and mitophagy
As discussed in previous section, mitochondria produce ATP for optimal functions of neurons, and damaged mitochondria has been linked to AD (Kerr et al. 2017; Scheibye-Knudsen et al. 2015). Clearance of damaged mitochondria through mitophagy pathway is dependent on several genes including Parkin, Pink1 and Fbx07 (Huang et al. 2020). Apart from mitophagy, Fbx07 modulates cell growth and proteosome activity (Li et al. 2021b). Fbxo7 supports mitochondrial maintenance by interacting with PINK1 and Parkin (Burchell et al. 2013). Sorghum grain polyphenol extract (10 µg/ml) enhanced Fbx07 transcription, replenish mitochondria membrane potential and increased proteasome activity in MPP+-induced Parkinson’s disease model of SH-SY5Y cells (Chen et al. 2016a). Interestingly, PD shares some pathological pathways with AD such as oxidative stress, inflammation, mitophagy and mitochondrial dysfunction (Van Bulck et al. 2019). Although this remains the only study, we could speculate that sorghum polyphenols will effectively activate mitophagy and related pathways to attenuate pathogenesis of AD.
It is important to note that the mechanisms discussed above, and several others are still being investigated. In view of limited study, more extensive studies are necessary to fully understand the pharmacological mechanisms underlying the anti-AD effects of sorghum grain polyphenol extracts.
Conclusion and future perspectives
The efficacy of available anti-AD therapeutics is weakened by the complex link between amyloid beta, tau, oxidative stress, mitochondrial dysfunction and defective mitophagy. There is urgent need for complementary therapy that can potentially target the multiple pathways. As highlighted in this review, sorghum grains are rich in dietary fibre, protein, fatty acids, biopeptides as well as polyphenols which have strong antioxidant capacity to attenuate AD pathogenesis through several mechanisms such as inhibition of oxidative stress, anti-AChE activities, and modulation of mitophagy. These activities could be associated with the uniquely high concentration of 3-DXA in pigmented sorghum genotypes. The studies examined in this review suggest that sorghum grains could find application in prevention and management of AD. To this end, we have highlighted below a logical sequence of study to achieve the desired outcome:
-
i.
In silico study involving molecular docking, molecular dynamics simulation and quantum mechanics/molecular mechanics to understand the interactions between sorghum polyphenols and Aβ42/Tau proteins.
-
ii.
In vitro and cell culture assays to identify sorghum genotypes with strongcapacity to neutralise and inhibit aggregation of neurotoxic Aβ42 and or Tau oligomer species.
-
iii.
In vivo experiments with simple physiologically relevant animal model such as Caenorhabditis elegans. The genes that respond to Aβ in C. elegans are homologs of genes involved in human AD (Godini et al. 2019). AD C. elegans models are low cost and can answer some of the same research questions as mice at a fraction of the cost and could prove to be a productive starting point.
-
iv.
Pharmacokinetic studies in AD mice models to determine effective doses, biotransformation, bioavailability, and blood brain barrier permeability of sorghum grain polyphenols.
-
v.
Recovery and fabrication of biodegradable and biocompatible low-cost drug delivery systems, such as microcrystalline cellulose (MCC) or cellulose microcrystal (CMC), from the sorghum polyphenol extraction residue. CMC and MCC are ingredients for drug delivery systems with strong adhesion to the mucus, potential pharmaceutical excipients, or intranasal deliverable stimuli responsive hydrogel, to achieve nose-brain drug delivery to overcome both mucociliary clearance and screening of drug molecules by the blood brain barrier.
-
vi.
The potential anti-AD effects of sorghum kafirin deserve extensive study. This could contribute to dietary-based therapeutic options for people experiencing AD.
References
Ademosun AO, Oboh G (2012) Inhibition of acetylcholinesterase activity and Fe2+-induced lipid peroxidation in rat brain in vitro by some citrus fruit juices. J Med Food 15(5):428–434. https://doi.org/10.1089/jmf.2011.0226
Aducanumab (2022) StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK573062/ Accessed 2022 Jan
Alghazwi M, Charoensiddhi S, Smid S, Zhang W (2020) Impact of Ecklonia radiata extracts on the neuroprotective activities against amyloid beta (Aβ1-42) toxicity and aggregation. J Funct Foods 68:103893. https://doi.org/10.1016/j.jff.2020.103893
Alzheimer's disease facts and figures (2022) Alzheimers dement 18(4):700–789 https://doi.org/10.1002/alz.12638
Ape D, Nwogu N, Uwakwe E, Ikedinobi C (2016) Comparative proximate analysis of maize and sorghum bought from Ogbete main market of Enugu State, Nigeria. Greener J Agric Sci 6:272–275. https://doi.org/10.15580/GJAS.2016.9.101516167
Asquith TN, Izuno CC, Butler LG (1983) Characterization of the condensed tannin (proanthocyanidin) from a group II sorghum. J Agric Food Chem 31(6):1299–1303
Awika JM, Rooney LW (2004) Sorghum phytochemicals and their potential impact on human health. Phytochemistry 65(9):1199–1221. https://doi.org/10.1016/j.phytochem.2004.04.001
Ayad R, Akkal S (2019) Chapter 12 - Phytochemistry and biological activities of algerian Centaurea and related genera. In: Atta ur R (ed.) Studies in natural products chemistry. vol 63. Elsevier, pp 357–414
Belščak-Cvitanović A, Durgo K, Huđek Turković A, Bacun-Druzina V, Komes D (2018) Overview of polyphenols and their properties. p 3–44
Bhattacharyya R, Bhattacharjee S, Pathak BK, Sengupta J (2020) Heptameric peptide interferes with amyloid-β aggregation by structural reorganization of the toxic oligomers. ACS Omega 5(26):16128–16138. https://doi.org/10.1021/acsomega.0c01730
Brown LJ (2022) The economic and societal cost of Alzheimer’s disease in Australia, 2021–2041. University of Canberra, Canberra, NATSEM
Burchell VS, Nelson DE, Sanchez-Martinez A et al (2013) The Parkinson’s disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat Neurosci 16(9):1257–1265. https://doi.org/10.1038/nn.3489
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160. https://doi.org/10.1038/s41583-019-0132-6
Cacabelos R (2018) Have there been improvements in Alzheimer’s disease drug discovery over the past 5 years? Exp Opin Drug Discov 13(6):523–538. https://doi.org/10.1080/17460441.2018.1457645
Cai Q, Jeong YY (2020) Mitophagy in Alzheimer’s disease and other age-related neurodegenerative diseases. Cells. https://doi.org/10.3390/cells9010150
Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH (2011) Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet 20(23):4515–4529. https://doi.org/10.1093/hmg/ddr381
Camara AKS, Zhou Y, Wen P-C, Tajkhorshid E, Kwok W-M (2017) Mitochondrial VDAC1: A key gatekeeper as potential therapeutic target. Front Physiol. https://doi.org/10.3389/fphys.2017.00460
Castro-Jácome TP, Alcántara-Quintana LE, Tovar-Pérez EG (2020) Optimization of sorghum kafirin extraction conditions and identification of potential bioactive peptides. Biores Open Access 9(1):198–208. https://doi.org/10.1089/biores.2020.0013
Chen CM, Chen IC, Chen YL et al (2016a) Medicinal herbs Oenanthe javanica (Blume) DC., Casuarina equisetifolia L. and Sorghum bicolor (L.) Moench protect human cells from MPP(+) damage via inducing FBXO7 expression. Phytomedicine 23(12):1422–1433. https://doi.org/10.1016/j.phymed.2016.08.004
Chen M, Chen Z, Wang Y et al (2016b) Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 12(4):689–702. https://doi.org/10.1080/15548627.2016.1151580
Chen G-f, Xu T-h, Yan Y et al (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38(9):1205–1235. https://doi.org/10.1038/aps.2017.28
Cherbuin N, Kumar R, Sachdev PS, Anstey KJ (2014) Dietary mineral intake and risk of mild cognitive impairment: the PATH through life project. Front Aging Neurosci 6:4. https://doi.org/10.3389/fnagi.2014.00004
Cornejo A, Aguilar Sandoval F, Caballero L et al (2017) Rosmarinic acid prevents fibrillization and diminishes vibrational modes associated to β sheet in tau protein linked to Alzheimer’s disease. J Enzyme Inhib Med Chem 32(1):945–953
Coronel R, Bernabeu-Zornoza A, Palmer C et al (2018) Role of amyloid precursor protein (APP) and Its derivatives in the biology and cell fate specification of neural stem cells. Mol Neurobiol 55(9):7107–7117. https://doi.org/10.1007/s12035-018-0914-2
Cummings J, Lee G, Nahed P et al (2022) Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dementia Transl Res Clin Interv 8(1):e12295. https://doi.org/10.1002/trc2.12295
Dai L, Kong L, Cai X et al (2022) Analysis of the structure and activity of dipeptidyl peptidase IV (DPP-IV) inhibitory oligopeptides from sorghum Kafirin. J Agric Food Chem 70(6):2010–2017. https://doi.org/10.1021/acs.jafc.1c04484
D’Almeida CTdS, Mameri H, Menezes NdS et al (2021) Effect of extrusion and turmeric addition on phenolic compounds and kafirin properties in tannin and tannin-free sorghum. Food Res Int 149:110663. https://doi.org/10.1016/j.foodres.2021.110663
de Oliveira LdL, de Oliveira GT, de Alencar ER, Queiroz VAV, de Alencar Figueiredo LF (2022) Physical, chemical, and antioxidant analysis of sorghum grain and flour from five hybrids to determine the drivers of liking of gluten-free sorghum breads. LWT 153:112407. https://doi.org/10.1016/j.lwt.2021.112407
Dong K, Fernando W, Jayasena V (2020) Dietary compounds and memory loss. Int J Food Sci Nutr 5:50–59
Elias RJ, Kellerby SS, Decker EA (2008) Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 48(5):430–441. https://doi.org/10.1080/10408390701425615
Etuk E, Ifeduba AV, Okata UE et al (2012) Nutrient composition and feeding value of sorghum for livestock and poultry. J Anim Sci Adv 2:510–524
Fan D, Liu L, Wu Z, Cao M (2019) Combating neurodegenerative diseases with the plant alkaloid Berberine: molecular mechanisms and therapeutic potential. Curr Neuropharmacol 17(6):563–579. https://doi.org/10.2174/1570159x16666180419141613
Fang EF, Hou Y, Palikaras K et al (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22(3):401–412. https://doi.org/10.1038/s41593-018-0332-9
FAO (2010) Food and agriculture organization/world health organization codex alimentarius commission
Fernando W, Rainey-Smith SR, Gardener SL et al (2018) Associations of dietary protein and fiber intake with brain and blood amyloid-β. J Alzheimers Dis 61(4):1589–1598. https://doi.org/10.3233/jad-170742
Fernando W, Gupta V, Jayasena V, Brennan C, Martins R (2019) Carbohydrate and protein metabolism: influences on cognition and Alzheimer's disease. p 149–187
Food and Agriculture Organization (2022) FAOSTAT—Crops and livestock products. Accessed on August 1st, 2022. https://www.fao.org/faostat/en/#data/QCL/visualize
Garzón AG, Veras FF, Brandelli A, Drago SR (2022) Purification, identification and in silico studies of antioxidant, antidiabetogenic and antibacterial peptides obtained from sorghum spent grain hydrolysate. LWT 153:112414. https://doi.org/10.1016/j.lwt.2021.112414
Giacobbe J, Benoiton B, Zunszain P, Pariante CM, Borsini A (2020) The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front Psychiatr 11:122. https://doi.org/10.3389/fpsyt.2020.00122
Gill SK, Rossi M, Bajka B, Whelan K (2021) Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol 18(2):101–116. https://doi.org/10.1038/s41575-020-00375-4
Godini R, Pocock R, Fallahi H (2019) Caenorhabditis elegans hub genes that respond to amyloid beta are homologs of genes involved in human Alzheimer’s disease. PLoS ONE 14(7):e0219486. https://doi.org/10.1371/journal.pone.0219486
Gong X, Li X, Xia Y et al (2020) Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci Technol 103:304–320. https://doi.org/10.1016/j.tifs.2020.07.026
GRDC (2017) Grains research and development corporation
Guéroux M, Fleau C, Slozeck M, Laguerre M, Pianet I (2017) Epigallocatechin 3-gallate as an inhibitor of tau phosphorylation and aggregation: a molecular and structural insight. J Prev Alzheimers Dis 4(4):218–225
Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y (2020) Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener 15(1):40. https://doi.org/10.1186/s13024-020-00391-7
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. https://doi.org/10.1126/science.1072994
Hassan S, Ahmad N, Ahmad T, Imran M, Xu C, Khan MK (2019) Microwave processing impact on the phytochemicals of sorghum seeds as food ingredient. J Food Process Preserv 43(5):e13924. https://doi.org/10.1111/jfpp.13924
Hassan S, Imran M, Ahmad MH et al (2020) Phytochemical characterization of ultrasound-processed sorghum sprouts for the use in functional foods. Int J Food Prop 23(1):853–863. https://doi.org/10.1080/10942912.2020.1762644
Herchi W, Harrabi S, Sebei K et al (2009) Phytosterols accumulation in the seeds of Linum usitatissimum L. Plant Physiol Biochem 47(10):880–885. https://doi.org/10.1016/j.plaphy.2009.07.001
Hong S, Pangloli P, Perumal R et al (2020) A comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolysaccharide-Induced RAW 264.7 Macrophages. Antioxidants (basel). https://doi.org/10.3390/antiox9121297
Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y (2017) Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp Gerontol 91:25–33. https://doi.org/10.1016/j.exger.2017.02.004
Huang T, Fang L, He R et al (2020) Fbxo7 and Pink1 play a reciprocal role in regulating their protein levels. Aging (albany NY) 13(1):77–88. https://doi.org/10.18632/aging.202236
Huang Q, Liao C, Ge F, Ao J, Liu T (2022) Acetylcholine bidirectionally regulates learning and memory. J Neurorestoratol 10(2):100002. https://doi.org/10.1016/j.jnrt.2022.100002
Jiang W, Wei W, Gaertig MA, Li S, Li X-J (2015) Therapeutic effect of Berberine on Huntington’s disease transgenic mouse model. PLoS ONE 10(7):e0134142. https://doi.org/10.1371/journal.pone.0134142
Jiménez-Escrig A (2012) Analysis of dietary phytochemicals needs to be applauded: glycosylated plant sterols. Eur J Lipid Sci Technol 114(6):615–616. https://doi.org/10.1002/ejlt.201200102
Juhaimi FA, Şimşek Ş, Ghafoor K et al (2019) Effect of varieties on bioactive properties and mineral contents of some sorghum, millet and lupin seeds. J Oleo Sci 68(11):1063–1071. https://doi.org/10.5650/jos.ess19113
Kapinova A, Kubatka P, Golubnitschaja O et al (2018) Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environ Health Prev Med 23(1):36. https://doi.org/10.1186/s12199-018-0724-1
Kaur H, Singh B, Singh A (2021) Comparison of dietary fibers obtained from seven Indian cereal grains. J Cereal Sci 102:103331. https://doi.org/10.1016/j.jcs.2021.103331
Kerr JS, Adriaanse BA, Greig NH et al (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3):151–166. https://doi.org/10.1016/j.tins.2017.01.002
Kumari PK, Umakanth AV, Narsaiah TB, Uma A (2021) Exploring anthocyanins, antioxidant capacity and α-glucosidase inhibition in bran and flour extracts of selected sorghum genotypes. Food Biosci 41:100979. https://doi.org/10.1016/j.fbio.2021.100979
Lee H-S, Santana ÁL, Peterson J et al (2022) Anti-adipogenic activity of high-phenolic sorghum brans in pre-adipocytes. Nutrients 14(7):1493
Lefèvre-Arbogast S, Gaudout D, Bensalem J et al (2018) Pattern of polyphenol intake and the long-term risk of dementia in older persons. Neurology 90(22):e1979–e1988. https://doi.org/10.1212/wnl.0000000000005607
Lei P, Ayton S, Bush AI (2021) The essential elements of Alzheimer’s disease. J Biol Chem 296:100105. https://doi.org/10.1074/jbc.REV120.008207
Li M, Xu T, Zheng W et al (2021a) Triacylglycerols compositions, soluble and bound phenolics of red sorghums, and their radical scavenging and anti-inflammatory activities. Food Chem 340:128123. https://doi.org/10.1016/j.foodchem.2020.128123
Li P, Feng D, Yang D et al (2021b) Protective effects of anthocyanins on neurodegenerative diseases. Trends Food Sci Technol 117:205–217. https://doi.org/10.1016/j.tifs.2021.05.005
Li Y, Li M, Liu J et al (2021c) Chemical composition profiling and biological activities of phenolic compounds in eleven red sorghums. J Agric Food Chem 69(32):9407–9418. https://doi.org/10.1021/acs.jafc.1c03115
Liu L, Liao X, Wu H, Li Y, Zhu Y, Chen Q (2020) Mitophagy and its contribution to metabolic and aging-associated disorders. Antioxid Redox Signal 32(12):906–927. https://doi.org/10.1089/ars.2019.8013
Luo M, Hou F, Dong L, Huang F, Zhang R, Su D (2020) Comparison of microwave and high-pressure processing on bound phenolic composition and antioxidant activities of sorghum hull. Int J Food Sci Technol 55(9):3190–3202. https://doi.org/10.1111/ijfs.14583
Majid A, Lakshmikanth M, Lokanath NK, Poornima Priyadarshini CG (2022) Generation, characterization and molecular binding mechanism of novel dipeptidyl peptidase-4 inhibitory peptides from sorghum bicolor seed protein. Food Chem 369:130888. https://doi.org/10.1016/j.foodchem.2021.130888
Malta SM, Batista LL, Silva HCG et al (2022) Identification of bioactive peptides from a Brazilian kefir sample, and their anti-Alzheimer potential in Drosophila melanogaster. Sci Rep 12(1):11065. https://doi.org/10.1038/s41598-022-15297-1
Manju Singh M, Arvind KS, Raosaheb K (2021) Dietary phytochemicals: as a natural source of antioxidants. In: Viduranga W (ed.) Antioxidants. IntechOpen, Rijeka, p 21
Martín-Maestro P, Gargini R, García E, Perry G, Avila J, García-Escudero V (2017) Slower dynamics and aged mitochondria in sporadic Alzheimer’s disease. Oxid Med Cell Longev 2017:9302761. https://doi.org/10.1155/2017/9302761
Martins IJ, Fernando WMADB (2014) High fibre diets and Alzheimer¡¯s disease. Food Nutr Sci. https://doi.org/10.4236/fns.2014.54049
Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F (2021) Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 190:108352. https://doi.org/10.1016/j.neuropharm.2020.108352
Mary A, Eysert F, Checler F, Chami M (2022) Mitophagy in Alzheimer’s disease: molecular defects and therapeutic approaches. Mol Psychiatr. https://doi.org/10.1038/s41380-022-01631-6
Mattsson N, Zetterberg H, Janelidze S et al (2016) Plasma tau in Alzheimer disease. Neurology 87(17):1827–1835. https://doi.org/10.1212/wnl.0000000000003246
McDade E, Cummings JL, Dhadda S et al (2022) Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther 14(1):191. https://doi.org/10.1186/s13195-022-01124-2
Mehmood S, Orhan I, Ahsan Z, Aslan S, Gulfraz M (2008) Fatty acid composition of seed oil of different Sorghum bicolor varieties. Food Chem 109(4):855–859. https://doi.org/10.1016/j.foodchem.2008.01.014
Mosconi L, Murray J, Davies M et al (2014) Nutrient intake and brain biomarkers of Alzheimer’s disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study. BMJ Open 4(6):e004850. https://doi.org/10.1136/bmjopen-2014-004850
Naseri NN, Wang H, Guo J, Sharma M, Luo W (2019) The complexity of tau in Alzheimer’s disease. Neurosci Lett 705:183–194. https://doi.org/10.1016/j.neulet.2019.04.022
Nelson PT, Alafuzoff I, Bigio EH et al (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71(5):362–381. https://doi.org/10.1097/NEN.0b013e31825018f7
Ngandu T, Lehtisalo J, Solomon A et al (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385(9984):2255–2263. https://doi.org/10.1016/s0140-6736(15)60461-5
Nikjoo D (2014) Design and synthesis of a small set of thiourea-based compounds as inhibitors of AChE1 from Mosquitoes
Npv P, Joye IJ (2020) Dietary fibre from whole grains and their benefits on metabolic health. Nutrients. https://doi.org/10.3390/nu12103045
Ochiishi T, Kaku M, Kajsongkram T, Thisayakorn K (2021) Mulberry fruit extract alleviates the intracellular amyloid-β oligomer-induced cognitive disturbance and oxidative stress in Alzheimer’s disease model mice. Genes Cells 26(11):861–873. https://doi.org/10.1111/gtc.12889
Ofosu FK, Elahi F, Daliri EB et al (2020) Flavonoids in decorticated sorghum grains exert antioxidant, antidiabetic and antiobesity activities. Molecules. https://doi.org/10.3390/molecules25122854
Ofosu FK, Elahi F, Daliri EB et al (2021) UHPLC-ESI-QTOF-MS/MS characterization, antioxidant and antidiabetic properties of sorghum grains. Food Chem 337:127788. https://doi.org/10.1016/j.foodchem.2020.127788
Ojo OA, Ojo AB, Ajiboye BO et al (2018) HPLC-DAD fingerprinting analysis, antioxidant activities of Tithonia diversifolia (Hemsl.) A. Gray leaves and its inhibition of key enzymes linked to Alzheimer’s disease. Toxicol Rep 5:585–592. https://doi.org/10.1016/j.toxrep.2018.05.003
Ozawa M, Ninomiya T, Ohara T et al (2012) Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the Hisayama Study. J Am Geriatr Soc 60(8):1515–1520. https://doi.org/10.1111/j.1532-5415.2012.04061.x
Pagani L, Eckert A (2011) Amyloid-Beta interaction with mitochondria. Int J Alzheimers Dis 2011:925050. https://doi.org/10.4061/2011/925050
Pontieri P, Troisi J, Romano R et al (2020) Nutritional composition of a selected white food-grade waxy sorghum variety grown in Mediterranean environment. Aust J Crop Sci 14:1525–1532. https://doi.org/10.21475/ajcs.20.14.09.p2783
Pontieri P, Pepe G, Campiglia P et al (2021) Comparison of content in phenolic compounds and antioxidant capacity in grains of white, red, and black sorghum varieties grown in the Mediterranean area. ACS Food Sci Technol 1(6):1109–1119. https://doi.org/10.1021/acsfoodscitech.1c00115
Pontieri P, Troisi J, Calcagnile M et al (2022) Chemical composition, fatty acid and mineral content of food-grade white, red and black sorghum varieties grown in the mediterranean environment. Foods
Portelius E, Zetterberg H, Dean RA et al (2012) Amyloid-β(1–15/16) as a marker for γ-secretase inhibition in Alzheimer’s disease. J Alzheimers Dis 31(2):335–341. https://doi.org/10.3233/jad-2012-120508
Prabakara A, Agrawal M, Dethe MR et al (2022) Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: current advancements and challenges. Expert Opin Drug Deliv 19(1):87–102. https://doi.org/10.1080/17425247.2022.2029845
Pradeepkiran JA, Hindle A, Kshirsagar S, Reddy PH (2022) Are mitophagy enhancers therapeutic targets for Alzheimer’s disease? Biomed Pharmacother 149:112918. https://doi.org/10.1016/j.biopha.2022.112918
Punia S, Kumar M (2021) Litchi (Litchi chinenis) seed: nutritional profile, bioactivities, and its industrial applications. Trends Food Sci Technol 108:58–70. https://doi.org/10.1016/j.tifs.2020.12.005
Punia H, Tokas J, Malik A, Sangwan S (2021) Characterization of phenolic compounds and antioxidant activity in sorghum [Sorghum bicolor (L.) Moench] grains. Cereal Res Commun 49:343–353
Qaku XW, Adetunji A, Dlamini BC (2020) Fermentability and nutritional characteristics of sorghum Mahewu supplemented with Bambara groundnut. J Food Sci 85(6):1661–1667. https://doi.org/10.1111/1750-3841.15154
Rai SN, Singh C, Singh A, Singh MP, Singh BK (2020) Mitochondrial dysfunction: a potential therapeutic target to treat Alzheimer’s disease. Mol Neurobiol 57(7):3075–3088. https://doi.org/10.1007/s12035-020-01945-y
Rashwan AK, Yones HA, Karim N, Taha EMM, Chen W (2021) Potential processing technologies for developing sorghum-based food products: an update and comprehensive review. Trends Food Sci Technol 110:168–182
Ravisankar S, Dizlek H, Awika JM (2021) Changes in extractable phenolic profile during natural fermentation of wheat, sorghum and teff. Food Res Int 145:110426. https://doi.org/10.1016/j.foodres.2021.110426
Reddy PH, Beal MF (2005) Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev 49(3):618–632. https://doi.org/10.1016/j.brainresrev.2005.03.004
Reddy PH, Oliver DM (2019) Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer’s disease. Cells. https://doi.org/10.3390/cells8050488
Reiss AB, Ahmed S, Dayaramani C et al (2022) The role of mitochondrial dysfunction in Alzheimer’s disease: a potential pathway to treatment. Exp Gerontol 164:111828. https://doi.org/10.1016/j.exger.2022.111828
Rezaee N, Fernando WMADB, Hone E et al (2021) Potential of sorghum polyphenols to prevent and treat Alzheimer’s disease: a review article. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2021.729949
Rocchetti G, Giuberti G, Busconi M, Marocco A, Trevisan M, Lucini L (2020) Pigmented sorghum polyphenols as potential inhibitors of starch digestibility: an in vitro study combining starch digestion and untargeted metabolomics. Food Chem 312:126077. https://doi.org/10.1016/j.foodchem.2019.126077
Román G, Jackson R, Gadhia R, Román A, Reis J (2019) Mediterranean diet: the role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Revue Neurologique 175(10):724–741
Romero-Márquez JM, Navarro-Hortal MD, Jiménez-Trigo V et al (2022) An olive-derived extract 20% rich in hydroxytyrosol prevents β-amyloid aggregation and oxidative stress, two features of Alzheimer disease, via SKN-1/NRF2 and HSP-16.2 in caenorhabditis elegans. Antioxidants 11(4):629
Ross JM, Olson L, Coppotelli G (2015) Mitochondrial and ubiquitin proteasome system dysfunction in ageing and disease: two sides of the same coin? Int J Mol Sci 16(8):19458–19476. https://doi.org/10.3390/ijms160819458
Saad B, Ghareeb B, Kmail A (2021) Metabolic and epigenetics action mechanisms of Antiobesity medicinal plants and phytochemicals. Evid Based Complement Altern Med 2021:1–19. https://doi.org/10.1155/2021/9995903
Salazar-López NJ, González-Aguilar GA, Rouzaud-Sández O, Loarca-Piña G, Gorinstein S, Robles-Sánchez M (2020) Sorghum bran supplementation ameliorates dyslipidemia, glucose dysregulation, inflammation and stress oxidative induced by a high-fat diet in rats. CyTA J Food 18(1):20–30. https://doi.org/10.1080/19476337.2019.1702105
Sarker A (2022) A review on the application of bioactive peptides as preservatives and functional ingredients in food model systems. J Food Process Preserv 46(8):e16800. https://doi.org/10.1111/jfpp.16800
Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM 3rd, Bohr VA (2015) Protecting the mitochondrial powerhouse. Trends Cell Biol 25(3):158–170. https://doi.org/10.1016/j.tcb.2014.11.002
Selkoe DJ (2021) Treatments for Alzheimer’s disease emerge. Science 373(6555):624–626. https://doi.org/10.1126/science.abi6401
Shahidi F, Zhong Y (2019) Bioactive peptides. J AOAC Int 91(4):914–931. https://doi.org/10.1093/jaoac/91.4.914
Shankar A, Saini D, Roy S, Bharati SJ, Mishra S, Singh P (2021) Role of complementary and alternative medicine in the management of cancer cachexia. Asia Pac J Oncol Nurs 8(5):539–546. https://doi.org/10.4103/apjon.apjon-2149
Shinda C, Nthakanio P, Gitari J, Runo S, Mukono S, Thaithi S (2022) Nutrient content of sorghum hybrid lines between Gadam and hard coat tannin sorghum cultivars. Food Sci Nutr. https://doi.org/10.1002/fsn3.2830
Song J-E, Song J-H, Cho S-M, Min G-H, Lee J-S (2010) Nutritional characteristics and physiological functionality of antidementia acetylcholinesterase inhibitor-containing methanol extract from sorghum bicolor. Korean J Food And Nutr 23
Speranza S, Knechtl R, Witlaczil R, Schönlechner R (2021) Reversed-phase HPLC characterization and quantification and antioxidant capacity of the phenolic acids and flavonoids extracted from eight varieties of sorghum grown in Austria. Front Plant Sci. https://doi.org/10.3389/fpls.2021.769151
Stefoska-Needham A, Beck EJ, Johnson SK, Tapsell LC (2015) Sorghum: an underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev Intl 31(4):401–437. https://doi.org/10.1080/87559129.2015.1022832
Suzuki H, Yamashiro D, Ogawa S et al (2020) Intake of seven essential amino acids improves cognitive function and psychological and social function in middle-aged and older adults: a double-blind, randomized. Placebo-Controll Trial Front Nutr 7:586166. https://doi.org/10.3389/fnut.2020.586166
Taleon V, Dykes L, Rooney WL, Rooney LW (2012) Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J Cereal Sci 56(2):470–475. https://doi.org/10.1016/j.jcs.2012.05.001
Tasie MM, Gebreyes BG (2020) Characterization of nutritional, antinutritional, and mineral contents of thirty-five sorghum varieties grown in Ethiopia. Int J Food Sci 2020:8243617. https://doi.org/10.1155/2020/8243617
Taylor JR, Duodu KG (2015) Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J Sci Food Agric 95(2):225–237. https://doi.org/10.1002/jsfa.6713
Taylor EN, Stampfer MJ, Curhan GC (2005) Fatty acid intake and incident nephrolithiasis. Am J Kidney Dis 45(2):267–274
Taylor JR, Belton PS, Beta T, Duodu KG (2014) Increasing the utilisation of sorghum, millets and pseudocereals: Developments in the science of their phenolic phytochemicals, biofortification and protein functionality. J Cereal Sci 59(3):257–275
Thakur M, Singh K, Khedkar R (2020) Phytochemicals. p 341–361
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomed 14:5541–5554. https://doi.org/10.2147/ijn.S200490
Toni M, Massimino ML, De Mario A, Angiulli E, Spisni E (2017) Metal dyshomeostasis and their pathological role in prion and prion-like diseases: the basis for a nutritional approach. Front Neurosci 11:3
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121. https://doi.org/10.3233/jad-161088
Tournissac M, Vandal M, Tremblay C et al (2018) Dietary intake of branched-chain amino acids in a mouse model of Alzheimer’s disease: effects on survival, behavior, and neuropathology. Alzheimers Dement (n Y) 4:677–687. https://doi.org/10.1016/j.trci.2018.10.005
Tran M, Reddy PH (2021) Defective autophagy and mitophagy in aging and Alzheimer’s disease. Front Neurosci. https://doi.org/10.3389/fnins.2020.612757
Treviño-Salinas M, Perales-Torres A, Castillo-Ruíz O et al (2021) Proximal analysis and profile of fatty acids on six varieties of white grain sorghum with potential use in human consumption. CyTA J Food 19(1):547–551. https://doi.org/10.1080/19476337.2021.1928757
Van Bulck M, Sierra-Magro A, Alarcon-Gil J, Perez-Castillo A, Morales-Garcia JA (2019) Novel approaches for the treatment of Alzheimer’s and Parkinson’s disease. Int J Mol Sci. https://doi.org/10.3390/ijms20030719
van der Bliek AM, Shen Q, Kawajiri S (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a011072
van Dyck CH, Swanson CJ, Aisen P et al (2022) Lecanemab in early Alzheimer’s disease. N Engl J Med 388(1):9–21. https://doi.org/10.1056/NEJMoa2212948
Velander P, Wu L, Henderson F, Zhang S, Bevan DR, Xu B (2017) Natural product-based amyloid inhibitors. Biochem Pharmacol 139:40–55
Wang K, Zhou Q (2023) Risk estimates of dementia and Alzheimer’s disease among different whole grain food consumption categories: a pilot study. J Prev Alzheimers Dis 10(1):133–136. https://doi.org/10.14283/jpad.2022.91
Wang X, Su B, Siedlak SL et al (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA 105(49):19318–19323. https://doi.org/10.1073/pnas.0804871105
Wang X, Su B, Lee HG et al (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103. https://doi.org/10.1523/jneurosci.1357-09.2009
Wang K, Tang W, Hao X, Liu H (2022) High consumption of whole grain foods decreases the risk of dementia and Alzheimer’s disease: Framingham offspring cohort. Psychiatry Clin Neurosci. https://doi.org/10.1111/pcn.13509
Ward RJ, Dexter DT, Crichton RR (2015) Ageing, neuroinflammation and neurodegeneration. Front Biosci (schol Ed) 7(1):189–204. https://doi.org/10.2741/s433
Wen C, Zhang J, Zhang H, Duan Y, Ma H (2020) Plant protein-derived antioxidant peptides: isolation, identification, mechanism of action and application in food systems: a review. Trends Food Sci Technol 105:308–322. https://doi.org/10.1016/j.tifs.2020.09.019
World Health Organisation. Dementia (2022) https://www.who.int/health-topics/dementia#tab=tab_1. Accessed August 1st, 2022.
Xie C, Zhuang X-X, Niu Z et al (2022) Amelioration of Alzheimer’s disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nat Biomed Eng 6(1):76–93. https://doi.org/10.1038/s41551-021-00819-5
Xiong Y, Zhang P, Warner RD, Shen S, Johnson S, Fang Z (2020a) Comprehensive profiling of phenolic compounds by HPLC-DAD-ESI-QTOF-MS/MS to reveal their location and form of presence in different sorghum grain genotypes. Food Res Int 137:109671. https://doi.org/10.1016/j.foodres.2020.109671
Xiong Y, Zhang P, Warner RD, Shen S, Johnson S, Fang Z (2020b) HPLC-DAD-ESI-QTOF-MS/MS qualitative analysis data and HPLC-DAD quantification data of phenolic compounds of grains from five Australian sorghum genotypes. Data Brief 33:106584. https://doi.org/10.1016/j.dib.2020.106584
Xu S, Shen Y, Li Y (2019a) Antioxidant activities of sorghum kafirin alcalase hydrolysates and membrane/gel filtrated fractions. Antioxidants (basel). https://doi.org/10.3390/antiox8050131
Xu S, Shen Y, Xu J et al (2019b) Antioxidant and anticancer effects in human hepatocarcinoma (HepG2) cells of papain-hydrolyzed sorghum kafirin hydrolysates. J Funct Foods 58:374–382. https://doi.org/10.1016/j.jff.2019.05.016
Xu H, Zhou Q, Liu B, Cheng KW, Chen F, Wang M (2021) Neuroprotective Potential of Mung Bean (Vigna radiata L.) polyphenols in Alzheimer’s disease: a review. J Agric Food Chem 69(39):11554–11571. https://doi.org/10.1021/acs.jafc.1c04049
Yang W, Cui K, Li X et al (2021) Effect of polyphenols on cognitive function: evidence from population-based studies and clinical trials. J Nutr Health Aging 25(10):1190–1204. https://doi.org/10.1007/s12603-021-1685-4
Yiannopoulou KG, Papageorgiou SG (2020) Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis 12:1179573520907397. https://doi.org/10.1177/1179573520907397
Yuan Q, Wang C-w, Shi J, Lin Z-x (2017) Effects of Ginkgo biloba on dementia: an overview of systematic reviews. J Ethnopharmacol 195:1–9. https://doi.org/10.1016/j.jep.2016.12.005
Yurko-Mauro K, McCarthy D, Rom D et al (2010) Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 6(6):456–464. https://doi.org/10.1016/j.jalz.2010.01.013
Zarifi SH, Bagherniya M, Banach M, Johnston TP, Sahebkar A (2022) Phytochemicals: a potential therapeutic intervention for the prevention and treatment of cachexia. Clin Nutr 41(12):2843–2857. https://doi.org/10.1016/j.clnu.2022.11.009
Zhang Y, Li M, Gao H et al (2019) Triacylglycerol, fatty acid, and phytochemical profiles in a new red sorghum variety (Ji Liang No 1) and its antioxidant and anti-inflammatory properties. Food Sci Nutr 7(3):949–958. https://doi.org/10.1002/fsn3.886
Zhao L, Li D, Qi X et al (2022) Potential of food-derived bioactive peptides in alleviation and prevention of Alzheimer’s disease. Food Funct 13(21):10851–10869. https://doi.org/10.1039/D2FO02278H
Zorzano A, Claret M (2015) Implications of mitochondrial dynamics on neurodegeneration and on hypothalamic dysfunction. Front Aging Neurosci 7:101. https://doi.org/10.3389/fnagi.2015.00101
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
RA: Conceptualisation, Methodology, Writing—Original draft preparation. RNM: Writing—Reviewing and Editing. PB: Writing—Reviewing and Editing. ZL: Writing—Reviewing and Editing. RC: Writing—Reviewing and Editing. SJ: Conceptualisation, Writing—Reviewing and Editing. WMADBF: Conceptualisation, Writing—Reviewing and Editing. This manuscript is being submitted for publication based on the Read and Published (R&P) Agreements between Edith Cowan University and SPRINGER NATURE.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationship that could influence the works reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdulraheem, R.A., Martins, R.N., Bharadwaj, P. et al. Nutrients and polyphenols-rich Sorghum bicolor genotypes as complementary therapy for Alzheimer’s disease. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09942-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09942-y