Abstract
Alkaloids are major dietary components well known for their pharmacological activities. Herbal matrices require extensive processing due to their high level of complexity in order to isolate their alkaloidal contents. Molecularly imprinted polymers (MIPs) are tailored receptors with a highly specific molecular recognition, which is the most important function of receptors. They can hence be utilized in applications wherein selective binding activities are of significance, such as solid phase extraction (SPE), chromatographic separation and chemical sensors. This review presents on recent applications of MIPs to analyze alkaloids in plants and bio-fluid samples as well as herbal formulations. We discuss the development of nano-sized MIPs for various applications, particularly in biomimetic sensors for electrochemical detection of various alkaloids. Due to its easier phase separation compared to common MISPEs, magnetic MISPEs were also discussed in order to explore the potential benefits of this approach for further phytochemical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkaloids are one of the most important classes of secondary metabolites, with a wide range of biosynthetic pathways and structures including over 20,000 diverse molecules derived from approximately 20% of recognized tracheophytes (Yang and Stöckigt 2010). The alkaloid-rich plants have been utilized to improve human health for thousands of years. Beside their roles as foods and beverages they can also be used as stimulating agents, anti-inflammatory, analgesics, local anesthetic, pain alleviation, anti-cancer, anti-fungal, anti-bacterial, neuropharmacological, and for other pharmacological activities (Kaur 2015; Rajput et al. 2022). It should be noted that several alkaloids with promising medicinal properties can be found in other types of organisms viz. animals, insects, marine sources, bacteria and fungi (Willems et al. 2020; Zorrilla and Evidente 2022).The basis of the alkaloids constituents is dependent on their biosynthetic precursors and heterocyclic ring systems that include purine, quinolizidine, isoquinoline, tropane, imidazole, indole, piperidine, pyrrolizidine and pyrrolidine alkaloids (Roy 2017). Nicotine, atropine, morphine, codeine, ephedrine, caffeine, quinine, and papaverine are examples of the chief alkaloids that can have health benefits for humans (John-Africa et al. 2020; Pollastro et al. 2009).
Alkaloids are regularly found in plant tissues as salts of organic and inorganic acids. Because of their biological effects, several techniques have been employed to separate and purify alkaloids from their complicated matrices using thin layer chromatography (TLC) (Gadzikowska et al. 2005; Monforte-González et al. 1992), high performance liquid chromatography (HPLC) (S.-M. Kang et al. 2004; Min et al. 2007), gas chromatography (GC) (Lisko et al. 2013) and capillary electrophoresis (CE) (Song et al. 1999). However, when using these techniques even to determine alkaloids content in pharmaceutical preparations, several complicated pre-treatment protocols to extract, separate and pre-concentrate the analytes are often required. Although these pre-treatment procedures are necessary, they can be time-consuming and cannot achieve the required selectivity for recognition during the extraction process. Furthermore, the extraction and determination of alkaloids in real samples became more difficult owing to their low abundance, complex composition, and matrix interferences. Likewise, removal of alkaloids from dietary sources is typically included in the food industry as in case of decaffeinated coffee and tea and needed for many consumers. As a result, there is an urgent need to develop efficacious methodologies for extraction and isolation of alkaloids in usual plant matrices or dietary sources. The difficulty of extracting and separating the pharmacologically active components as in case of alkaloids is the main challenge in most phytochemical studies. In general, mixtures containing alkaloids must be dissolved in solvents with reagents and then alkaloids can be extracted from solutions using the proper extraction protocols. Finally, each alkaloid can be separated from the mixture and recovered in its purest form.
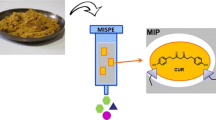
Within the last century, scientific research has effectively implemented the term “tailor made” at the molecular level. MIPs closely resemble the “lock and key” notion coined by Emil Fisher in the late 1800s, and represent a unique class of tailored synthetic receptors with the intent of selective binding (Mahony et al. 2005). The polymer is synthesized by arranging and crosslinking functional monomers around the target molecule, leaving a complementary structure and specific binding sites when the target is removed (Fig. 1). Coupling this custom-made cavity to the target molecule through hydrogen bonds, dipole–dipole, and ionic interactions allow for high efficiency, even in complex mixtures (Chen et al. 2016).
MIP application has become highly effective in sample preparation, which oftentimes is the most limiting step in an analytical procedure. Even with the use of highly selective detectors, such as mass spectrometry, whole sample extracts are still too complex for direct injection, prohibiting precise quantification, affecting detection limit especially of minor targeted analytes, and possibly risk instrument integrity. More importantly, analytes within natural products samples are often present at low concentrations even if active at such low concentrations and warranting for sensitive methods for its accurate detection and or quantification from such complex matrices (Enke and Nagels 2011). Therefore, pre-analytical approaches including solid-phase extraction (SPE), solid-phase microextraction (SPME) (Lisko et al. 2013), and more recently matrix solid-phase dispersion (MSPD) (Song et al. 1999), micro solid-phase extraction (MSPE) (Sereshti et al. 2014) or liquid-phase microextraction (LPME) (Wang et al. 2007) have been called upon for sample clean-up and for enriching a targeted analyte prior to its detection. However, these methods still lack selectivity, are tedious, and still leave matrix components. In contrast, using MIPs sorbent material instead of conventional sorbents used in extraction have allowed for analytical selectivity and automaticity, and are deemed molecularly imprinted solid-phase extractions (MISPEs) and molecularly imprinted solid-phase microextraction (MISPMEs). These systems absorb the target analyte, effectively concentrating it, from the complex plant matrix. This process is done in three sequential steps to include: sample loading, washing, and elution. The target analyte is first loaded onto the MISPE column through hydrophobic bonding, then, a washing solvent passes to elute residual interfering compounds. It is significant to highlight that the polarity of the washing solvent should be enough to affect nonspecifically bound compounds, and not to affect those of the target and MIP. Finally, an elution solvent is to be passed, destruct the MIP-target bond, and carries the concentrated target analyte to be analyzed. MIPs were previously prepared by bulk polymerization, but that caused both poor accessibility and low rebinding capacity of the target molecules due to the thick polymeric network (Renkecz and Horvath 2017). More complex polymerization techniques have now overcome these drawbacks, and MIPs can have many forms, including beads, monolithic layers, and coatings (Song et al. 2014).
Several reviews have reported on the different approaches applied for sample preparation for analyzing alkaloids in herbal matrices (Casado et al. 2022; Klein-Júnior et al. 2016; Zhang et al. 2023). However, these reviews provide general overview of many sample preparation techniques for alkaloids. In contrast, this review provides special emphasis, for the first time, of the various applications of molecular imprinting technologies (MITs) within the scope of sample clean-up and analysis for some medicinal important alkaloids. Significant advancements in MIP synthesis and application, as selective extraction media, have been discussed on a basis of separation and pre-concentration of some major classes of alkaloids entitled under pyridine, tropane, ephedra alkaloids, quinolizidine, nux vomica, opium, ergot and methylxanthine alkaloids. An overview of the different MIPs developed using these alkaloids as template, is presented in (Table 1). In addition, the chemical structures of the alkaloids described in this review is presented in (Fig. 2).
Applications MIPs for the extraction, isolation, purification and separation of alkaloids.
Pyridine alkaloids i.e., nicotine
Nicotine is the most plentiful alkaloid found in tobacco leaves and a major cause of tobacco dependence owing to its addictive properties (Chang et al. 2016). A cigarette contains ca. 7 to 24 mg of nicotine with average airborne nicotine concentrations ranging from 1 to 10 µg m−3, it has been reported that, approximately, 0.3 to 3.0 mg per cigarette will be inhaled or absorbed by the body skin (Yildiz 2004). Thus, analysis of nicotine in bio-fluid samples has become an important module of direct or passive exposure to tobacco smoke (Doctor et al. 2004).
Spectrophotometric approaches have several drawbacks related to the lower selectivity, which limits their application in complicated matrices like urine samples. Since then, sample pretreatment procedures have been developed to improve selectivity for spectroscopic analysis. Because of their beneficial selectivity, molecular imprinted polymers (MIPs) can combine successfully with spectroscopic analysis resulting in a simple, economical, selective, and rapid technique. In this way, an on-line flow injection (FI) MIP SPE manifold (FI–MIP–SPE) for spectrophotometric measurement of nicotine in smoker’s urine matrices was developed by Figueiredo et al. (2009). The non-covalent nicotine imprinted polymers (nicotine–MIPs) were fabricated utilizing a template molecule of nicotine, a functional monomer of methacrylic acid (MAA), a crosslinking reagent of ethylene glycol dimethacrylate (EGDMA) and 2,2-azobisisobutyronitrile (AIBN) as an initiator. This study was based on the spectrophotometric analysis of nicotine in alkaline medium through its reducing ability to convert manganese (VII) into manganese (VI) and monitoring the resulting derivative at 610 nm. A calibration curve using non-smoker urine samples spiked with nicotine standard ranging from 1.1 to 60 µmol L−1 showed good linearity with a lower limit of detection (LOD) of 1.1 µmol L−1. A SPE approach using MIP as a selective sorbent (MIP–SPE) has been described by Yang et al. (2007) and employed to measure hairy nicotine levels among smokers and non-smokers, as well as evaluating exposures to environmental tobacco smoke (ETS). Nicotine was used as a template molecule, and MAA, EGDMA and AIBN were utilized as the functional monomer, crosslinker and initiator, respectively to fabricate the nicotine–MIPs. With a LOD of 0.2 ng mL−1 and a lower limit of quantification (LOQ) of 0.5 ng mL−1. Nicotine-MIP–SPE approach exhibited intrinsic selectivity and sensitivity towards nicotine measurement in hairy samples. The linearity of nicotine spiked in drug-free hair samples was evaluated using HPLC technique over a concentration range of 0.5 to 80 ng mL−1, with LOD and LOQ of 0.2 ng mL−1 and 0.5 ng mL−1, respectively. Nicotine levels in smokers and non-smokers' hair ranged from 5.1 to 69.5 and 0.50 to 9.3 ng mg−1 hair, respectively. Liu et al. (2003) fabricated MIPs as sorbent materials displaying specific affinity for nicotine, using nicotine as the template, MAA as the functional monomer, EGDMA as the crosslinker and chloroform as the porogen. Nicotine–MIP showed enhanced binding affinity for nicotine with an imprinting factor of ca.3.0 compared to the reference polymer. Furthermore, it exhibited good capacity (90 μmol (nicotine)/g polymer) and is adequate for SPE comprising molecular imprinting mechanism. The nicotine-MIP SPE column was loaded with tobacco smoke resulting from smoking 0.75 g of purchased cigarette. After that, an elution solvent consisting of methanol, water, and acetic acid in a ratio of 90:5:5 (v/v/v) was used to extract nicotine followed by analysis using HPLC. The obtained results revealed that nicotine-MIP was a substantially more effective tool than commercial filter tip in removing nicotine from tobacco smoke.
E-cigarettes (ECs) were first launched to reduce the harmful effects of traditional cigarettes. Although these ECs are designed to reduce consumption and addiction, some e-liquids contain more nicotine than regular cigarettes. Due to numerous instances of mislabeling and harmful or unexplained ingredients, measurement of nicotine and other substances in ECs to investigate the authenticity and potential hazards of using nicotine-containing ECs seems warranted (DeVito and Krishnan-Sarin 2017). For the measurement of nicotine in the zero-level refill liquids of ECs, a surface molecularly imprinted polymer (SMIP) was developed by Xie et al. (2018). Nicotine was used as a template, MAA as a functional monomer, and EGDMA as a cross-linker in a toluene solution to synthesize SMIP on the surface of vinyl-SiO2 nanospheres. The fabricated SMIPs exhibited high adsorption capacity (247.0 µmol g−1), an outstanding imprinting factor (4.40), and high nicotine selectivity with coefficient greater than 2.9. For the measurement of nicotine, SMIP was employed as a SPE cartridge (SMIP–SPE) coupled to GC and mass spectrometry (GC–MS). Within the range of 2.00 to 40.00 µg mL−1, the developed method showed good linearity, suggesting that it could provide an alternative approach for EC products QC analysis.
The utility of MIT in the development of innovative drug delivery systems and devices that are beneficial in closely related disciplines, such as diagnostic sensors or biological traps, is gaining increased attention. Polymeric materials synthesized by MITs can recognize certain bioactive compounds and have a sorption/release behavior that is sensitive to the properties of the surrounding medium. Enhancement of MIP affinity for certain medications and physiological substances has already been demonstrated to be a fascinating and versatile process of increasing drug loading and controlled release characteristics (Alvarez-Lorenzo & Concheiro 2004). The feasibility of MIPs as excipients for the regulated transdermal delivery of nicotine has been reported by Ruela et al. (2014). MIPs were fabricated by a free radical polymerization approach with nicotine acting as a template, MAA as the monomer and EGDMA as the cross-linker. Non-covalent drug–polymer interactions resulted in preferential drug adsorption within the polymer matrix, which could alter drug release and skin permeation profiles. MIPs might thus be considered a promising technology for transdermal nicotine delivery systems used in nicotine quitting aid as well as a functional excipient for increasing medication stability and control drug release. To accomplish this goal, Ruela and Pereira (2016) created MIPs using bulk polymerization and nicotine as a template and tested them as functional excipients for nicotine transdermal delivery. Because of its hydrophobic potential, mineral oil was found to be the most suitable vehicle for nicotine molecule recognition in MIP particles. Following Higuchi kinetics, mineral oil-based MIP- formulations were capable to regulate permeation flux for up to 24 h (Ruela et al. 2018) also improved the adsorption capability and selectivity of MIPs by adopting a precipitation polymerization process to build MIPs using the hydrophilic monomer 2-hydroxyethyl methacrylate (HEMA). The MIPs made with MAA and HEMA as functional monomers, EGDMA as a crosslinker, and 1,1 ′ azobis(cyclohexanecarbonitrile) (ABCN) as an initiator achieved better adsorption capacity and selectivity to modulate drug release for up to 48 h.
Tropane based alkaloids
Tropane is an important class of alkaloids found in plants around the world with a myriad of health effects. These compounds are indeed one of the oldest known drugs having significant biological activities. Tropane alkaloids are found primarily in Solanaceae plants and to include the anticholinergic drugs such as atropine, scopolamine and hyocyamine in addition to the anesthetic cocaine (Grynkiewicz & Gadzikowska 2008). Atropine is often used in pharmaceutical preparations to treat disorders like cardiomyopathy and Parkinson's disease (Okuda et al. 1991). It occurs naturally in Solanaceae family plants such as Atropa belladonna and Datura stramonium (Zárate et al. 1997). A high dose of atropine might cause altered renal functions, dry mouth, thirst, difficulty swallowing, tachycardia, fever, dilated pupils, delirium and stupor (Azizi et al. 2013; Hoefnagel 1961). Therefore, atropine dose levels in pharmaceutical preparations should be kept to a minimum, and sensitive methods for measuring its level in biological fluids should be applied to assess its toxicity (Crapnell and Banks 2021; Fonger et al. 2014).
Due to the lower atropine levels in bio-fluid samples, fluorometry detection methods are the most useful approaches for their analysis. In this regard, high-luminescent quantum dots (QDs) are gaining widespread attention due to their unique features such as photo-stability, wide absorption and narrow emission spectra, great quantum efficiency, and large stokes shift. MIP capped high fluorescent graphene quantum dots (GQDs) (MIP–GQDs) were synthesized (Fig. 3) through the co-polymerization interaction between a functional monomer of 3-(3-aminopropyl) triethoxysilane (APTES) and a crosslinking of tetraethyl orthosilicate (TEOS) (Khataee et al. 2018). Atropine was used as a template for this reaction. The developed blue luminescent MIP–GQDs composite, which exhibited high affinity for adsorbing atropine from sample solution, could result in significant fluorescence quenching. The quenching effect in the emission intensity of nearby GQDs against the corresponding atropine concentration resulted in a calibration graph ranging from 0.5 to 230 ng mL−1 with a LOD of 0.22 ng mL−1. Analyses of human plasma and urine samples were carried out using the developed MIP as SPE for atropine extraction and purification. The recoveries were within the acceptable range (98.2–102.9%) indicating the reliability of the proposed method for quantifying atropine in real samples. SPE tools with a predetermined selectivity for a particular analyte or group of structurally related compounds pose MIP as an ideal material for extraction, purification and separation processes of tropane alkaloids. (Zuo et al. 2019) fabricated a monoethyl fumarate (MFMA)–based MIP (MFMA–MIP) by precipitation polymerization using scopolamine as a template, MFMA as the functional monomer, EGDMA as the crosslinker, AIBN as an initiator and acetonitrile as the porogenic solvent. The prepared (MFMA–MIP) showed high selectivity (3.5.) for scopolamine with a high adsorption capacity (49.75 mg g−1). MFMA–MIP was applied as a SPE cartridge (MFMA–MIP–SPE) for extracting scopolamine from Datura indium, Belladonna, and Hyoscyamus Niger L alcohol extracts, followed by liquid chromatographic analysis of the eluted components. The recoveries of scopolamine in the three samples ranged from 96.0 to106.0%, with RSDs of less than 4.1%. A selectivity study showed that, MFMA–MIP exhibited high recognition ability toward scopolamine compared to its analogues, atropine and anisodamine. Furthermore, MFMA–MIP-SPE design demonstrated excellent regeneration and reusability. Such MFMA–MIP-SPE assembly may be a preferable choice for separating scopolamine from other tropane alkaloids. The monodispersed class-specific MIP in the micro-spherical bead format has been fabricated by a precipitation polymerization method utilizing anisodine as template, MAA as the functional monomer, trimethylolpropane trimethacrylate (TRIM) as the crosslinker and acetonitrile as the porogen (Zeng et al. 2015). The purpose of this MIP was to provide a simple and effective purification, extraction and pre-concentration technology for analysis of atropine, scopolamine, anisodamine, and anisodine in traditional Chinese medicine.
The strong peroxidase-like activity of zinc and cobalt bi-metal metal–organic frameworks (ZnCo–MOFs) has been described by Bagheri et al. (2019). ZnCo–MOFs have been applied for the sensitive colorimetric and fluorometric determination of atropine. MIP-modified magnetic graphene oxide (GO) (M–GO) (MIP–M–GO) was fabricated by locating SiO2-modified magnetic Fe3O4 nanoparticles (MNPs) on the surface of the GO sheet (Fig. 4A). Next, a MIP layer was created on the surface of the Fe3O4 @ SiO2–GO composite by the self-assembling reaction of APTES and TEOS monomers in the presence of atropine as a template (Fig. 4B). Firstly, a sample preparation step was applied for protein precipitation prior to atropine measurement in serum samples to improve selectivity. The supernatant was subjected to a treatment process based on magnetic SPE (MSPE) technique using (MIP–M–GO), as the adsorbent (Fig. 4C). The combination of high specific extraction of MIP–M–GO and high catalytic activity of ZnCo–MOF enabled ultra-sensitive and reliable measurement of atropine. The best correlation coefficient of the standard curve was achieved utilizing a fluorometric detection system for atropine concentrations ranging from 0.1 to 45 ng mL−1, with a LOD of 27 pg mL−1. The proposed method was evaluated for the analysis of atropine in bio-fluid samples with a recovery ranging from 95.90 to 103.57%.
Sun et al. (2021) fabricated a molecular imprinted electrochemical sensor to accurately determine atropine with high sensitivity. This method employed a hierarchical flower-like core–shell mesoporous silica nanosphere@zeoliteimidazolate framework-8 (MSN@ZIF-8) glassy carbon electrode (GCE). Then, an electropolymerization of golden nanoparticles (AuNPs) doped molecularly imprinted poly p-aminothiophenol (p-ATP) film on the modified GCE was carried out. The nanometer-sized ZIF-8 crystals provided easy access to their active sites. Moreover, the mesoporous channels of the silica facilitated mass transfer during electrochemical reaction. As a result, the composite exhibited a synergistic effect in terms of achieving higher adsorption performance, catalytic activity, and reaction rate. Meanwhile, the electropolymerizaiton of MIP film in the presence of AuNPs on the modified MSN@ZIF-8 electrode contributed to the achievement of a high selectivity for atropine capture. A standard curve with a good linearity between the peak current and the corresponding atropine concentration was achieved over the range from 5.0 nM to 9.5 μM with a LOD of 0.98 nM. Successful analyses of atropine in eye drops, serum, urine, beef, and cereals samples demonstrated the reliability of the developed sensor suggestive for its potential in various matrices and for pharmacokinetic studies in the future.
Molecularly imprinted photonic hydrogels (MIPHs), a self-reporting sensor platform for efficient detection of low concentration of atropine, has been developed (Fig. 5). The combination of colloidal-crystal with MIT was applied to provide atropine assay method based MIPHs through a label-free colorimetric chemosensor (Meng et al. 2013). This chemosensor showed high sensitivity and specificity, fast response, and good regenerating ability in aqueous environments, and was especially well suited for on-the-spot screening and semi-quantitative measurements of atropine. The smart atropine-MIPHs have been effectively employed for screening trace levels of atropine in urine, indicating that they are suited for forensic purposes.
A selective fluorescence sensor can be formed by combining the particular recognition properties of MIPs with the superior optical features of QDs. These core–shell materials have several advantages including a regular shape, a large specific surface area, high adsorption capability, and adjustability. Aqueous Mn-doped ZnS QDs with superior optical properties were prepared in an in situ method, using Na2S2O3 as precursor and L-cysteine as a catalyst as well as capping agent in water (Abbasifar & Samadi-Maybodi 2016). MIPs were then employed as selective materials for atropine recognition being fabricated on the surface of Mn-doped ZnS QDs (MIPs-Mn-doped ZnS QDs). Poly dopamine-coated molecularly imprinted Mn-doped ZnS quantum dots were synthesized using dopamine hydrochloride as a precursor. Finally, the prepared MIPs–Mn–doped ZnS QDs were employed for measuring atropine in pharmaceutical formulations. Assay linearity of atropine for the fluorescence emission intensities versus concentrations ranged from 2 × 10–8 to 7 × 10–6 M with an LOD of 3.2 nM.
To maintain a steady-state concentration of a specific medication in plasma, multiple daily dose regimens are typically required. As a result, designing a controlled release formulation to manage the continuous release and delivery of a medication throughout the body is a major challenge. Atropine sustained release medication delivery systems have undergone continuing technological improvements to increase their overall therapeutic efficacy (Deshpande et al. 2017). Biodegradable MIP microspheres were fabricated by (Yahui He et al. 2020) using thermally-initiated precipitation polymerization and functional MAA monomer β-cyclodextrin (MAA–β–CD).The synthesis of mono-dispersed MIP was carried out using atropine as a template molecule, MAA–β–CD as a monomer, trimethylolpropane trimethacrylate (TRIM) as a crosslinker, AIBN as an initiator and dimethylacetamide (DMAC) as a progen. Mono-dispersed MIP microspheres were applied as potential water-composites for in vitro controlled release of atropine. HPLC–MS/MS was used to investigate the release performance of atropine loaded microspheres in virtual gastric (pH 1.5) and intestinal fluids (pH 7.4) over the first 68 h in drug delivery system in which MIP appears as a very promising polymeric device for releasing and selecting atropine and potentially any target likewise alkaloid.
Ephedra and khat sympathomimetic alkaloids
Ephedra, also known as Ephedra alkaloids, is a class of sympathomimetic alkaloid produced from Ephedra plants (Shrubs). Ephedra alkaloids have a long history as a traditional folk remedy, especially among the Chinese. The main alkaloid, ephedrine, was used in its purest form in modern medicine as a bronchodilator, decongestant, and vasopressor (Roman 2004). Because of the health and legal consequences of using ephedra-containing products, advanced technologies that can precisely and reproducibly measure ephedrine and pseudoephedrine in herbal products and dietary supplements are warranted.
The conventional methods for determining (-)-ephedrine in ephedra herb (Ji-hong 2004; Li et al. 2001) often involve extensive procedures comprising multiple liquid–liquid extraction steps due to the complexity of herbal ingredients. It is thus desirable to synthesize MIP–SPE materials with high selectivity and affinity for the quantification of (-)-ephedrine in Chinese Ephedra. Dong et al. (2005) designed an MIP as a glass SPE cartridge (MIP–SPE) for the extraction of (-)-ephedrine using MAA as the functional monomer, EGDMA as a crosslinker, AIBN as an initiator and (-)-ephedrine as the template. An elution system of 5% trifluoroacetic acid in methanol was applied to elute (-)-ephedrine from the MIP–SPE, with eluate further collected and analyzed using HPLC. The chiral separation capacity of MIPs was also evaluated with the HPLC method utilizing MIP as the stationary phase. The experimental results elucidated that, (-)-ephedrine-MIP has the ability to recognize (-)-ephedrine from its stereoisomers: ( +)-pseudoephedrine and ( +)-ephedrine. In combination with supercritical fluid chromatography (SFC), MIPs for (-)-ephedrine- ((-)-ephedrine–MIPs) have been utilized as stationary phases to separate ( ±)-ephedrine enantiomers (Ansell et al. 2012). (-)-Ephedrine–MIPs were fabricated using MAA, EDMA and chloroform as functional monomer, crosslinker and porogenic solvent, respectively to exemplify the ease of MIPs as chromatographic stationary phases and the predictable elution order with the excellent mobile phase diffusivity as well as lower viscosity. A multi-step swelling and suspension polymerization process in an aqueous medium was applied to produce spherical (-)-ephedrine MIPs ((-)-ephedrine–SMIPs), employing MAA and EGDMA as functional monomer and cross linker, respectively (Li et al. 2006). The resulting (-)-ephedrine–SMIPs were used as HPLC stationary phases. When ( +)-ephedrine was employed as a competitive molecule, chromatographic analysis revealed that (-)-ephedrine–SMIP exhibited a unique selectivity for (-)-ephedrine the, more active form then the ( +)-isomer. Porous polymers are thought to have two classes of binding sites involved in SMIPs selectivity, one is hydrophilic and the other is hydrophobic. Both the stability and high loading capacities of SMIPs suggest that they have promising commercial values as HPLC stationary phases. It has been reported that, redox initiation system is preferable to photo-initiation technique by providing a stable interaction between the templates and monomers and improves analytes selectivity. Thus, MIPs fabricated by this approach have greater surface area and stronger affinity for template molecules. Liu and Dong fabricated (-)-ephedrine spherical MIPs ((-)-ephedrine–SMIPs) by a noncovalent imprinting procedure using (-)-ephedrine as template, MAA as the functional monomer, EGDMA as cross-linkers and benzoyl peroxide and N, N-dimethylaniline as initiators. The selectivity of (-)-ephedrine–SMIPs was assessed using HPLC assay, with separation performances of (-)-ephedrine–SMIPs under optimum conditions to show that both (+)-pseudoephedrine and (+)-ephedrine could be well separated from (-)-ephedrine (Liu et al. 2005a, b).
MIP for an analytical scale in capillary electrophoresis (CE) is becoming more successful than in LC due to its potential high separation efficiency and the requirement of minimum amounts of MIP templates. Mono-MIP column, with (-)-norepinephrine as a template was prepared as a solid stationary phase in capillary electrochromatography (CEC) for the recognition of a racemic mixture of norepinephrine, epinephrine and their analogues (Huang et al. 2011). A thermal non-covalent polymerization procedure was used to introduce itaconic acid into a pre-treated, salinized, fused-silica capillary, along with a mixture of EGDMA and AIBN in N,N-dimethylformamide. At 30°C, the related structures of the templates and their enantiomeric mixtures were effectively separated under optimum conditions.
Numerous studies have been conducted on the effects of magnetic fields on the chemical and physical characteristics of polymers (Kimura 2003). Polymers synthesized in the presence of a magnetic field often exhibit different properties and polymerization rates compared to polymers synthesized by conventional methods. Considering the unique effects of a magnetic field on the kinetics of polymerization processes as well as modification of polymer properties, the interest in developing this efficient polymerization technology is well-reported. When used as CSPs, MIPs provide both selectivity and elution predictability not commonly observed in commercial CSPs (Ansell 2005; Whitcombe et al. 2014). Application of magnetic field to improve the performance of MIPs has been investigated by Guerreiro et al. (2008). Polymer composition consisted of (-)-ephedrine as a template, HEMA as functional monomer, EGDMA as crosslinker, ABCN as initiator in chloroform was fabricated using UV initiation to improve the separation of ephedrine enantiomers. During the polymerization process, a continuous external magnetic field was applied, which could improve the ordering of the produced polymer's structure and the uniformity of its binding sites. Furthermore, when polymers were created under the force of a magnetic field, the high heterogeneity of the population of binding sites, an ordinary problem affecting MIPs, was minimized presenting an added value.
A unique technique based on immobilization of MIP for ephedrine (ephedrine–MIP) on an electro-synthesized polypyrrole (PPY) film has been applied by Mazzotta et al. (2008) to fabricate a voltammetry sensor for analysis of (-)-ephedrine. A three-electrode cell in "upside-down" (UD) configuration was used to prepare ephedrine–MIP/PPY–modified electrode. The electrolytic solution was placed on the surface of the GCE. Two platinum wires served as counter and quasi-reference electrodes were immersed in the drop on the GCE surface. In particular, ephedrine–MIP was applied after being deposited on the surface of the GCE. At 1.0 V, the PPY film grew at a constant potential. Following over oxidation of the PPY matrix, the sensor response was evaluated using cyclic voltammetry in the concentration range of 0.5–3 mM. The proposed sensor has a significant selectivity that can eliminate interferences from other compounds such as ascorbic acid, urea, glucose, glycine and sorbitol typically found in bio-fluid samples. Moreover, ephedrine–MIP/PPY sensor showed moderate stability as after 3 days its response decreased only by 6%.
Khat (Catha edulis Forsk) is a flowering big green shrub found in eastern and southern Africa, as well as the Arabian Peninsula presenting another source of sympathomimetic alkaloids. Leaves or short tops were habitually chewed for their stimulating effects. Phenylpropylamino alkaloids like ephedrine and pseudoephedrine are regarded to be the addictive and reinforcing molecules responsible for the continued chewing behavior, despite the fact that khat leaves contain more than 200 distinct compounds (Feyissa and Kelly 2008). Using (-)-norephedrine as the template, MAA as the functional monomer, EGDMA as the cross-linker, and chloroform as the porogen, (Atlabachew et al. 2015) prepared a (-)-norephedrine–MIP. The prepared MIP particles were slurry packed with acetonitrile into an empty SPE cartridge and used as MIP–SPE cartridge and applied to extract norephedrine and its analogs, cathinone and cathine from Khat leaf crude extract. Thereafter the alkaloids were eluted using acetic acid in methanol. The eluents were collected and analyzed by HPLC–DAD. The validated MIP–SPE–HPLC method achieved good recoveries ranging from 90.0 to 107% with high precision of 2.3 to 3.2%. According to a reusability study, the MIP–SPE cartridge can be reused at least four times.
Matrine-type (quinolizidine) alkaloids
Sophora root is the dried root of Sophora flavescens Aiton (Leguminosae), which contains matrine and oxymatrine, and is commonly used as a folk medicine in Japan and China (Chen et al. 2004). These alkaloids have a myriad of pharmacological effects including sedative, depressant, antipyretic, anti-tumor, cardiotonic as well as anti-virus (hepatitis B). Extraction using large amounts of organic solvents is the first step to get selected compounds from any natural product (Tang et al. 2017). Thus, there is growing interest in green and sustainable solvents capable of extracting and separating compounds with a wide range of polarities from natural products. Abbott et al. (2004) introduced deep eutectic solvents (DESs), which are promising alternatives to organic solvents. A two-step extraction procedure based on DES extraction and magnetic MIP (MMIP) (DES–MMIP) to selectively extract oxymatrine and matrine from Sophora flavescens root has been reported by Kang et al. (2021). MMIP was manufactured using oxymatrine, acrylic acid, EGDMA, AIBN, and acetonitrile-methylbenzene as template, functional monomer, crosslinker, initiator, and porogen, respectively. ChCl–based DESs were utilized as solvents for extracting oxymatrine and matrine from a real sample, with extraction yields of 21.0 and 1.53 mg g−1, respectively. Following that, MMIPs for secondary enrichment and purification of oxymatrine and matrine from DES extracts were applied. The recoveries for oxymatrine and matrine by MMIPs ranged from 80.21 to 89.15% and 85.33 to 95.28%, respectively. The proposed DES–MMIP extraction protocol seems to be an efficient approach to selectively extract specific components from complex plant samples.
A matrine-imprinted monolithic (Mon) (matrine–Mon–MIP) stationary phase was synthesized and employed as a SPE cartridge (matrine–Mon–MIP–SPE) to extract and purify matrine from Sophoraflavescens (Fu et al. 2011). Matrine–Mon–MIP was in situ polymerized with matrine as the template molecule, MAA as the function monomer, EGDMA as cross-linking agent and porogenic solvents of toluene and dodecanol. The SPE cartridge was used to assess the extraction and purification of matrine from Sophora flavescens followed by HPLC analysis. The workability of Mon–MIP–SPE cartridge was evaluated by extraction and purification of matrine from Sophoraflavescens crude extract. These results showed an enrichment factor of 29 and an extraction yield of 89.2% demonstrating good applicability of the Mon–MIP–SPE cartridge in the purification of matrine. Guo et al. (2011) used a functional monomer of melamine-urea–formaldehyde (MUF) and a template molecule of matrine to fabricate MIPs (matrine–MIPs) with unique molecular recognition properties. Matrine–MIPs were applied as an SPE sorbent (matrine–MIPs–SPE) for extraction and purification of martine in Sophora tonkinensis using acetone, water, and chloroform as eluents. GC/MS analysis revealed that MIPs had the best adsorption capacity after being treated with alkaline solution and using acetone as an elution solvent. Also, the extraction of martine from a real sample by matrine–MIPs–SPE is higher by 1.42-fold than that achieved using conventional LLE procedure. (Funaya and Haginaka 2012) fabricated MIPs for matrine and oxymartine, termed martine–MIP and oxymartine–MIP, using a precipitation polymerization method that produced spherical and monodispersed particles with high yields in a single step. Martine– and oxymartine–MIPs were prepared using martine and oxymartine as template molecules, MAA as a functional monomer, DVB as a cross-linker, and AIBN the initiator in a mixture of acetonitrile–toluene to yield monodispersed microspheres with diameters of 3.3 and 3.9 µm, respectively. Matrine–MIP–SPE column was employed to extract martine and sophocarpine (13,14-dehydromatrine), while oxymartine–MIP–SPE was used to extract oxymartine and oxysophocarpine (13,14-dehydrooxymatrine) from Sophora flavescens root. By graft polymerizing MAA on the surfaces of silica gel particles, grafted particle polymethacrylic acid (PMAA) (PMAA/SiO2) was created. By applying PMAA/SiO2 as matrix particles with cytisine as template and ethylene glycol diglycidyl ether as a crosslinking agent, MIP-PMAA/SiO2 was fabricated (Gao et al. 2010). Matrine and oxymatrine, two contrast alkaloids that coexist with cytisine in Sophoraalope curoides exhibit chemical structures that are comparable to cytisine to some extent, were used to explore the binding features of MIP–PMAA/SiO2 towards cytisine. The selectivity of MIP–PMAA/SiO2 for cytisine with regard to matrine or oxymatrine was significantly improved, reaching 12.08 folds, indicating that MIP–PMAA/SiO2 for cytisine has higher recognition selectivity. Likewise, improvement in adsorption affinity and selectivity of MIPPMAA/SiO2 towards cytosine was observed compared to the non-imprinted material PMAA/SiO2 (Gao et al. 2010). It has been mentioned that, MIPs synthesized with matrine as the single-template could extract matrine and sophocarpine from Sophora flavescens root, whereas MIPs prepared with oxymatrine as the single-template could retrieve oxymartine and oxysophocarpine (Gao et al. 2010; Guo et al. 2011; Lai et al. 2003). Considering the co-existence of martine, oxymartine, and sophocarpine in Sophoramoorcroftiana, developing SPE sorbents capable of simultaneously monitoring the three alkaloidal molecules is necessary. Because combination of two separate printing molecules, double– template, (DT) MIPs (DT–MIPs)) enables the production of more suitable hollows for analyte re-inclusion and enhanced diffusion pathways, DT–MIPs can add an additional degree of flexibility in material design (Dickert et al. 2001). An approach based DT–MIPs has been reported by Ma et al. (2018) for the molecular recognition of matrine-type alkaloids. DT–MIPs were fabricated by precipitation polymerization using the dual templates of martine and oxymartine, the crosslinker of EGDMA, the functional monomer of MAA, the initiator of AIBN and acetonitrile as a porogen. DT–MIP was applied as SPE tool (DT–MIP–SPE) for the simultaneous recognition, extraction, purification and enrichment of matrine, oxymatrine, and sophocarpine from extract of S.moorcroftiana leaves, stems, roots, and seeds. HPLC–MS/MS analysis of S.moorcroftiana samples spiked with matrine, oxymatrine, and sophocarpine in the concentration range of 50 to 1000 μg kg−1 showed good linear relationships (R2 > 0.99) for the three analytes under the developed DT–MIP–SPE protocol. Matrine, oxymatrine, and sophocarpine were successfully extracted and purified by DT–MIP–SPE as monitored by HPLC–MS/MS. The average recoveries ranged from 96.51–98.42, 80.34–92.48 and 73.25–76.02% with precision ranging from 2.15—6.82% for matrine, oxymatrine, and sophocarpine, respectively.
Nux vomica derived indole alkaloids (Strychnine and Brucine)
Strychnine and brucine are alkaloids obtained from Strychnosnux-vomica (Loganiaceae). They stimulate all parts of the central nervous system, resulting in a distinct motor pattern. Strychnine is used in folk medicine at low doses for gastrointestinal disorders, eye disease, circulatory problems, and depression (Mishra et al. 2013), but it is extremely fatal, causing spasms and convulsions at high doses (Wang et al. 2006). Although brucine is related to strychnine, it is not as toxic. However, people who consume more than 2 mg of pure brucine will almost experience symptoms similar to strychnine poisoning (Teske et al. 2011). Therefore, Strychnine and brucine in body fluids and tissues must be detected and accurately measured for life-saving and forensic investigation.
The higher porosity and thus permeability, as well as great surface area, make the Mon matrix as being ideal for both small molecules and large biopolymers. Accordingly, Mon MIPs (Mon–MIPs) are anticipated to increase the separation and enable high-performance and high-speed for direct analysis. The Mon-MIP approach combines the properties of Mon material characteristics with molecularly imprinted selectivity of MIP. Mon–MIPs as stationary phases were fabricated through a one-step, in-situ, free-radical polymerization "moulding" process inside the chromatographic column, eliminating the need for time-consuming grinding, sieving, and column packing procedures (Yin et al. 2005). An in-situ polymerization of MAA as a functional monomer, EGDMA as a cross-linking agent, toluene and dodecanol as porogenic solvents, and AIBN as an initiator produced Mon-MIPs with specific recognition capacity for strychnine. The Mon-MIPs have been applied as a packed column chromatography to separate strychnine from other compounds such as indole, quinine and brucine using HPLC–UV technique (Zhang et al. 2005).
High sensitivity and a wide dynamic range are the two main advantages of the chemiluminescence (CL) technique (Bowie et al. 1996; Robards and Worsfold 1992). At the same time, because the CL response is often an oxidation–reduction reaction, the analyte adsorbed on the MIP packed column would be destroyed during this reaction. Meanwhile, as water flows down the MIP column, the derivatization products are easily washed out from the polymer. These properties pose CL technique as a viable detection approach for MIP-based sensors. Liu et al. (2005a, b) developed a MIP CL (MIP–CL) sensor for measuring brucine in urine. The brucine MIPs (brucine-MIPs) were fabricated utilizing brucine, MAA, EGDMA, AIBN and chloroform as a template, a functional monomer, a crosslinker, an initiator and a porogenic solvent, respectively. A column packed with the brucine-MIPs, was coupled to the CL flow system, and employed as the sensor's recognition element. CL signal was obtained when acidic permanganate flowed through the MIP column and reacted with pre-adsorbed brucine. The sensor's linear response was ranged from 5.0 × 10–9 to 1.0 × 10–6 g mL−1 brucine (r2 0.9981) with LOD of 2 × 10–9 g mL−1.
Reversible addition–fragmentation chain transfer (RAFT) precipitation polymerization has been applied by Zhao et al. (2014) to prepare surface imprinted polymers coated on MWCNTs (MWCNTs@MIPs). The MWCNTs@MIPs were synthesized via RAFT precipitation polymerization using brucine as a template, EGDMA as a cross linker, AIBN as an initiator and acetone as a solvent. With MWCNTs@MIP as the immobilized iniferter, RAFT polymerization was employed to graft MWCNTs@MIP with poly(glycerol monomethacrylate) (p(GMMA)) brushes. The polymer was grafted with hydrophilic p(GMMA) brushes to improve water compatibility. In a water media, the molecularly imprinted material demonstrated better accessibility to brucine with good selective recognition property. For the electrochemical analysis of brucine, the material was supported on an ionic liquid functionalized graphene coated GCE, and the resulting electrochemical sensor exhibited good analytical performance. Following optimal conditions, the detector response was linear to the corresponding brucine concentration (μM) over the range from 0.006to 0.6 with sensitivity of 5.4 μA/μM mm2.
Nakamura et al. (2016) used two polymerization strategies to fabricate monodisperse MIPs for selective strychnine extraction: precipitation polymerization and multistep swelling and polymerization (MSSP). MAA, DVB, AIBN and toluene were utilized as functional monomer, crosslinker, initiator and porogen solvent in precipitation polymerization, respectively, whereas MAA, EGDMA, 2,2′-azobis(2,4-dimethylvaleronitrile) (ADVN), and toluene were used as functional monomer, crosslinker, initiator and porogen solvent in precipitation polymerization, respectively in the MSSP. The MIPs were employed as SPE columns for the extraction and purification of strychnine from nux-vomica crude extract in combination with HPLC to evaluate their retention and molecular-recognition properties. Based on the retention and imprinting factors of strychnine, precipitation polymerization appeared most suitable for the fabrication of MIPs.
Brucine and strychnine are frequently found together in strychnos seeds. Thus, highly selective methods should be devoted for the analysis of brucine owing to its interference with strychnine. A brucine imprinted poly-o-phenylenediamine (PoPD) voltammetric sensor based on integration of MIP with SWNTs has been fabricated by Liu et al. (2012). The applicability of the sensor was evaluated by analysis of brucine in human serum samples after protein precipitation with recovery ranging from 99.5 to 103.2%.
Opium alkaloids
Opium alkaloids are chemicals produced from the ripe capsules of Papaver somniferum L. plant, either naturally or in the laboratory (Behbahani et al. 2014; D’Aurelio et al. 2021; Fard et al. 2019; Guha et al. 2020; Hassanzadeh et al. 2016; Yunhua He et al. 2005; Kolaei et al. 2016; Lowdon et al. 2020; Manbohi and Ahmadi 2015; Nemati et al. 2021; Rahmani et al. 2017; Ratautaite et al. 2015; Rezaei et al. 2016; Sahebi et al. 2020). Raw Papaver somniferum L. plants contain opium alkaloids such as morphine, noscapine, codeine, thebaine, and papaverine. Among these opiates, morphine is the most abundant used to treat severe pain. Measurement of these drugs is an important concern in clinical studies considering their toxicities. Dispersion of solid phases in the sample solutions, dispersive SPE, is an attractive method for sample preparation compared to traditional SPE. The analyte's interaction with the adsorbent particles is accelerated by the dispersion effect leading to a reduction in the extraction time. Magnetic nanoparticles of Fe3O4 (Fe3O4–MNPs) are the most common adsorbents in dispersive SPE (Sahebi et al. 2020). Owing to the strong chemical activity of bare MNPs, oxidation in air may result in dispersibility and magnetism loss. To ensure their stability, the surface should be adequately (Fard et al. 2019). The short diffusion routes, which provide improved extraction dynamics, large surface area and thus high extraction efficiency, are the main advantages making dispersive SPE materials suitable as carriers for MIPs. The simple and fast isolation of MNPs from sample solutions, and the ease of functionalization/modification of surfaces add more benefits to this approach (Behbahani et al. 2014; Manbohi et al. 2015). Nemati et al. (2021) designed MIPs modified Fe3O4–MNPs (MIP-Fe3O4–MNPs) and evaluated its feasibility in dispersive SPE. The applicability of MIP-Fe3O4 NPs was evaluated as an excellent micro-disperse SPE for opium alkaloids derived from water samples in various pharmaceutical fields. The MIPs were prepared using morphine as a template molecule, MAA as a functional monomer, EGDMA as a crosslinker, AIBN as an initiator and acetonitrile as a porogenic solvent. MMIP nanoparticles were then coated with synthesized MIPs to fabricate MIP@MNPs of MIP @ MAA@Fe3O4 nanoparticles. The possibility of using MMIP sorbents as a dispersive SPE for the simultaneous extraction and enrichment of opium alkaloids from aqueous samples was evaluated. Recyclability studies have shown that MMIP is reusable, stable and selective for five selected alkaloids, so that it is of potential value for practical applications. Good linearity was achieved over the concentration range of 0.03–100 mg L−1 with LOD of 0.007, 0.007, 0.004, 0.003 and 0.003 mg L−1 for morphine, codeine, thebaine, noscapine and papaverine, respectively. Applying the developed method to real water samples showed quantitative extraction recoveries ranging from 79 to 87% with an enrichment factor of 200. Rezaei et al. (2016) prepared an electrochemical sensor coated with functionalized (f-MWCNTs) and a thin film of MIPs as well as deposited AuNPs to analyze papaverine hydrochloride in serum and urine samples. Using cyclic voltammetry, the sensing layer was constructed on a treated electrode surface of pencil graphite (PGE). Methyl trimethoxysilane (MTMOS) as a monomer, TEOS as a cross-linker, HCl as a catalyst, methanol as a homogenizer and papaverine as a template were utilized to fabricate MIP. MIP-sol–gel/f-MWCNT/PGE sensor was prepared by electrochemical formation of the imprinted sol–gel on the surface off-MWCNT/PGE to fabricate an AuNPs modified electrode (AuNP/MIP-sol–gel/f-MWCNT/PGE). The sensor's calibration curve was plotted in two linear ranges: 0.001 to 0.1 and 0.1 to 5.0 µmol L−1, respectively, with a LOD of 0.4 nmol L−1. Kolaei et al. (2016) prepared MWCNTs which were magnetized with Fe3O4 nanoparticles (MWCNTs–Fe3O4–NPs) and then coated with vinyl (phenyltrimethoxysilane) end groups. By using a surface imprinting polymerization process, MWCNT–Fe3O4–NPs were used as support for morphine MIP (MWCNT–Fe3O4–NPs@morphine-MIP). Ultrasonic-assisted magnetic (UAM) using MWCNT-Fe3O4–NPs@MO-MIP as SPE tool followed by UV/Vis detection (UAM–SPE-V/Vis) were investigated for the analysis of morphine. The calibration curve was linear at morphine levels ranging from 0.8 to 8.7 mg L−1, with a LOD of 0.18 mg L−1. The applicability of UAM–SPE–UV/Vis method was evaluated by analyzing morphine in wastewater and urine samples and showed a recovery range of 96.40–105.6%.
The selectivity of the CL approach is obviously poor but can be improved by using MIP-targeting molecules with good recognition capabilities. Yunhua He et al. (2005) developed a MIP-CL analytical procedure for measuring morphine utilizing morphine-MIPs as recognition moieties and a CL flow system of morphine permanganate as the detection mode. The morphine-MIPs were synthesized utilizing MAA as a functional monomer, EGDMA as a crosslinker, AIBN as an initiator and acetonitrile as a porogenic solvent. Connecting a morphine-MIP mini-column to the described CL flow system and taking advantage of its fine recognition and capture capabilities to target a certain molecule and isolate it from other coexisting molecules greatly improves the selectivity of the CL method. Under optimum conditions, this method showed a linear detection response over the concentration range of 5.0 × 10−9– 1.0 × 10−6 g mL−1 morphine with high correlation coefficient (0.9981) and LOD of 2 × 10 −9 g mL−1. The method is also highly selective and has been successfully applied to detect morphine in the urine of heroin abusers.
Electrochemical impedance spectrometry (EIS) and quartz crystal microbalance (QCM) sensing platforms are frequently utilized for characterization and development of sensors to detect small molecules (Guha et al. 2020; Ratautaite et al. 2015). MIP–based sensors are a potential strategy for overcoming the fragility of antibody-based sensors and other natural receptors (e.g., aptamers) and can be applied in severe environments (e.g., temperature fluctuation, denaturing agents) (Lowdon et al. 2020). D’Aurelio et al. (2021) synthesized MIP NPs (MIPNPs) with an affinity for morphine (morphine–MIPNPs) or codeine (codeine-MIPNPs) to be attached with EIS and QCM sensors. The EIS sensor has a LOD of 0.11 ng mL−1, three orders of magnitude lower than QCM sensor's (0.19 µg mL−1). Although both EIS and QCM sensors were shown to be capable of detecting morphine in a direct assay set-up, conjugation of AuNPs to morphine (MO-AuNPs) or codeine (CO-AuNPs) in case of QCM was necessary to improve sensitivity and reach LOD in the µg mL−1 range. Using the surface imprint technique, (Rahmani et al. 2017) fabricated MMIP with a homogeneous core–shell structure for the analysis of morphine in biological samples. Fe3O4 NPs were synthesized by coprecipitation, and then SiO2-NH2 was coupled to Fe3O4 via aminosilicate groups using APTES. MIP was applied to the surface of Fe3O4/SiO2-NH2 by copolymerization of terminal amino groups with MMA as a functional monomer, EGDMA as a crosslinking agent, AIBN as an initiator, and morphine as a template molecule. The MMIP was used as a dispersive SPE for the extraction of morphine selectively from plasma and urine. The calibration curves for spiked plasma and urine samples showed linearity over concentration range 0.1 to 30 μg mL−1 with LOD and LOQ of 0.03 and 0.08 μg mL−1, respectively. The recoveries ranged from 84.9 to 105.5% and 94.9 to 102.8% for plasma and urine, respectively.
Hassanzadeh et al. (2016) investigated chitosan-based (CS) MIP (CS–MIP) as a controlled drug delivery vehicle to deliver morphine more efficiently, thereby prolonging the analgesic effect of morphine after injection in mice. CS–MIP nanogel for morphine (morphine–CS–MIP) was fabricated using maleic anhydride-functionalized chitosan as a functional monomer, EGDMA as a cross-linker, AIBN as an initiator, and morphine as a template. The results showed that employing morphine-CS–MIP as a morphine carrier could reduce the number of injections and prolong the bioavailability of morphine, thereby improving its analgesic effect.
Ergot alkaloids
Ergot alkaloids are mycotoxins mainly produced by various Claviceps species of fungi, likely found in contaminated cereals and presenting health hazards warranting for the monitor of their levels. Ergot alkaloids have been classified into three categories, namely, clavines, lysergic acid amides and ergopeptines (Shahid et al. 2020). Ergotism, an ergot poisoning caused by alkaloid ingestion by humans and pets, leads to hallucinations, gangrene, and even death. To control human and animal exposure to such mycotoxins, contamination levels of ergot alkaloids must be assessed. Thus, purification procedures to limit the impact of the sample matrix can greatly improve the accuracy and precision of analysis. Among several sample cleanup procedures, a SPE protocol is gaining prominence with the advent of MIPs due to their selectivity for the targeted analytes. Thus, it’s significant to utilize an adsorbent material that can effectively interact with ergot alkaloids and provide a way to separate such contaminants, either for isolation, concentration, or measurement purposes, or to try to decrease their bioavailability and mitigate their toxicity. Lenain et al. (2012) fabricated MIPs toward various ergot alkaloids including ergometrine, ergosine, ergotamine, ergocornine, ergocryptine, and ergocristine, as well as their epimers. Metergoline was employed as a template molecule in suspension polymerization, along with MAA and EGDMA monomers. This method yielded spherical beads with a narrow size distribution, which was employed as a sorbent in a SPE cartridge for barley samples cleanup prior to LC–MS/MS analysis. This MIP-SPE allowed simple and cost-efficient extraction procedure. Lysergic acid diethylamide (LSD) is a psychoactive drug derived from ergot alkaloid named lysergic acid. A MIP, fabricated by a noncovalent imprinting technique to extract LSD from bio-fluid samples and hair for forensic applications, was applied as SPE sorbent for the offline extraction of the targeted compound prior to HPLC–MS analysis (Hugon-Chapuis et al. 2009). The MIP was synthesized using LSD as a template, MAA as a functional monomer, EGDMA as a crosslinker AIBN as an initiator and acetone as a porogen solvent. The concentrations of 0.1 and 0.5 ng mg−1 of LSD were effectively detected in hair and urine with extraction recoveries of ca. 82 83%. The highly specific and efficient ergotamine MIPs (ergotamine–MIPs) has been fabricated to provide an effective approach of extracting and isolating different ergot alkaloids from a complicated feed matrix. By self-assembly bulk polymerization, (Kudupoje et al. 2021) fabricated MIPs using ergotamine as template, styrene and HEMA as functional monomers, EGDMA as cross-linker, AIBN as the initiator, and toluene as the porogen. Kudupoje suggested that ergotamine-MIPs could be applied as SPE sorbents for purification and isolation of ergotamine in affinity chromatography and could be employed as biosensors for monitoring ergot poisoning in clinical samples i.e., bio-fluids.
Methylxanthine alkaloids in coffee and tea
Purine alkaloids, such as methylxanthines are commonly found in coffee, tea, and some foods represented mainly by caffeine, theobromine, and theophylline as one of the most common alkaloids in dietary sources considering coffee and tea large consumption worldwide (Damm et al. 2016; Mehari et al. 2016). Pharmaceuticals and personal care products PPCPs are emerging chemical pollutants that come in organic, inorganic, biodegradable, and non-biodegradable forms, all of which pose a hazard to aquatic ecosystems and human health. Caffeine is a PPCP that enters the environment mostly through pharmaceutical waste, colas, tea, energy drinks, coffee beans, and pharmaceuticals (Archana et al. 2016; Sophia et al. 2016).
HPLC (Cuervo et al. 2017) and HPLC–MS/MS (López-García et al. 2018) are the current most applied methodologies for measuring caffeine in complex matrices. These methods are labor-intensive and time-consuming, and necessitate significant quantities of hazardous organic solvents. SERS is a molecular spectroscopic measurement technique that provides sensitive and rapid analysis in a wide range of applications. One of the most significant drawbacks of SERS analysis though lies in its lower affinity and selectivity toward the target compounds (Li et al. 2017a, b). Furthermore, the presence of interferences in complex matrices hampered the application of SERS for the targeted analysis of molecules in these samples (de Albuquerque et al. 2018). As a result, accurate separation, and pre-concentration of the analytes in complex matrices is a major problem for SERS applications. To improve SERS selectivity and sensitivity, coupling of MIP technology with SERS (MIP–SERS) was introduced to target molecules in complex matrices. This coupling approach integrates the specific recognition characteristics of MIP with the signal amplification characteristics of SERS. A rapid and single step MIP–SERS nanosensor to detect caffeine in an aquatic matrix has been reported by Hu et al. (2018). The MIP particles were loaded with Ag nanoparticles (AgNPs) using in situ reduction approach via precipitation polymerized to fabricate AgNPs@MIP nanocomposites as an active SERS substrate. Because of the structural similarity to caffeine and its availability, theophylline was used as a dummy template molecule in the fabrication process in addition to MAA as the functional monomer, EGDMA as the crosslinker and AIBN as the initiator. For the selective separation and enrichment of caffeine, AgNPs@MIP nanocomposites were applied as adsorbents through a SPE cartridge. The LOD by the AgNPs@MIP nanocomposites as an extraction tool was 100 ng L−1, which is less than caffeine working concentrations in various studies.
DESs based on choline chloride and ionic liquids (ILs) based on 1-methylimidazole were used to modify the surface of Fe3O4–MIPs to obtain DESs–Fe3O4–MIPs and ILs-Fe3O4–MIPs, respectively (Li et al. 2017). The designed nanoparticles were utilized as a magnetic-SPE cartridge for extracting and purifying theobromine and theophylline, from the crude extract of tea. In comparison to the ILs–Fe3O4/MIPs, the DESs–Fe3O4/MIPs revealed stronger recognition with higher recoveries of theophylline and theobromine from green tea. DESs–Fe3O4/MIPs as magnetic–SPE materials for the purification of targeted compounds were evaluated by HPLC and showed good linearity for theobromine and theophylline within the range, 5–100.0 µg mL−1. Recoveries were at 92.27 and 87.51%, with real extraction amounts of 4.87 and 5.07 mg g−1 of theobromine and theophylline in green tea, respectively. Biphasic solvent system design coupled with MIP-SPE cartridge has been described by Rajabi Khorrami and Rashidpur (2009) for the selective purification of theophylline from serum samples. This method is based on simultaneous forward extraction of theophylline from an aqueous sample into an organic solvent and back extraction into a MIP solid phase. This approach is termed solvent extraction (SE) MIP SPE (SE–MIP–SPE). The results showed that the proposed cartridge significantly separated THP from other structurally related methylxanthines such as theobromine and caffeine. HPLC–UV was used to evaluate the performance of the SE–MIP–SPE for extracting theophylline from human serum samples. Under optimum conditions, a linear plot of peak areas versus theophylline concentrations in the range of 0.5–30 µg mL−1 was achieved (r2 = 0.9974) with LOD and LOQ of 0.09 and 0.3 µg mL−1, respectively.
Conclusion
Because of their outstanding potential as adaptable receptors for precise recognition of target analytes, MIPs applications for extraction, isolation, purification and separation of natural components have been on the rise. The current review provides a comprehensive overview of the existing status of MIPs and highlights their synthesis and applications for the efficient analysis of some medically important alkaloids in plants and other bio-fluid samples. The preparation of various MIPs that can be adopted in modern sample extraction approaches have been validated by a wide range of scientific and technical experts and the results showed good potential for future studies in these areas. MIPs can be included into cutting-edge pattern extraction strategies without affecting their inherent selectivity and stability by making clever (and comparatively simple) modifications to the polymerization procedures. The procedures developed to manufacture MIP-based extraction devices are simple and reliable, and can be performed in any laboratory with the required equipment. Although MISPEs have received increasing attention as excellent tools for selective extraction of various alkaloids, the organic solvents utilized in the extraction procedures, on the other hand, limit their applications. Furthermore, these MISPE tools have the following drawbacks. (1) MISPE cartridges or columns must be packed; this adds to the extraction process' complexity and necessitates the use of significant amounts of hazardous organic solvents for SPE. (2) Dead adsorption occurs in the cartridges or columns, resulting in sample loss.
Magnetic MIPs (MMIPs) have recently aroused the interest of researchers in order to explore the potential benefits of this approach due to the use of an external magnet which can attain various advantages over common MISPEs. For this approach, MIPs are combined with magnetic nanoparticles (MNPs) to offer a magnetic characteristic to improve the selectivity of adsorption while avoiding the time-consuming centrifugal and filtration steps in the whole separation process. The aforementioned drawbacks of MISPEs can thus be efficiently resolved by MMISPE. Because MMIPs eliminate the need to pack cartridges or columns for adsorption testing, dead adsorption is reduced, and the whole extraction procedure is simplified. Furthermore, magnetism makes it easier to separate MMIPs from solutions using external magnets, therefore MMISPE only requires a little amount of organic solvent.
MIPs have recently emerged as promising option for the selective and sensitive electrochemical detection of alkaloids in complex matrices due to incorporation of nanoparticles into their polymeric structures. As a result, some of their properties can be enhanced and new capabilities can be achieved. Because of the enormous number of nanoparticles available, choosing the appropriate nanostructured MIPs design for sensors is much easier. The nanomaterials' catalytic and electrical conductivity, along with the MIP's improved selectivity, have made these sensors efficient instruments for electrochemical detection of various types of alkaloids.
Notwithstanding the prominent achievements of MIT, there are still some important developmental challenges that need to be addressed. In order for MIPs to take their area in the analytical marketplace, novel imprinting strategies are urgently needed to produce imprinting materials with excessive capability, selectivity and homogeneity of binding affinity. In addition, large-scale production techniques should be explored to facilitate the manufacture of MIP products. A rational strategy such as molecular modeling will support the efficient design and selection of novel functional and cross-linking monomers, thus reducing the development time and accelerating the synthetic process when manufacturing of MIPs is difficult and expensive. The use of new monomers with responsive functions and the introduction of new polymerization procedures will lead to the fabrication of new sensors for alkaloids detection. The integration of different disciplines is important to make contributions to MITs. In particular, integrating the knowledge of combinatorial chemistry and molecular modeling might provide possibilities for the prediction of imprinted material activities and advancing imprinting technologies. Therefore, more efforts should be devoted to the continuous improvement of the current methods of preparing MIPs by introducing new preparation strategies and adopting advanced functional materials. Also, the application of MIP to extend drug release is a novel approach that has yet to be explored more for clinical applications for alkaloids.
Abbreviations
- ABCN:
-
1,1 ′ Azobis(cyclohexanecarbonitrile)
- ADVN:
-
2,2 ′-Azobis(2,4-dimethylvaleronitrile)
- AIBN:
-
2,2-Azobisisobutyronitrile
- APTES:
-
3-(3-Aminopropyl) triethoxysilane
- CE:
-
Capillary electrophoresis
- CO-AuNPs:
-
Cocaine conjugated gold nanoparticles
- DMAC:
-
Dimethylacetamide
- DT:
-
Double-templeted
- EGDMA:
-
Ethylene glycol dimethacrylate
- ETS:
-
Environmental tobacco smoke
- FI:
-
Flow injection
- GC:
-
Gas chromatography
- GCE:
-
Glassy carbonelectrode
- GQDs:
-
High fluorescent graphene quantum dots
- HEMA:
-
2-Hydroxyethyl methacrylate
- HPLC:
-
High performance liquid chromatography
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- LPME:
-
Liquid-phase microextraction
- MFMA–MIP:
-
Monoethyl fumarate (MFMA)–based MIP
- MIPs:
-
Molecular imprinted polymer
- MISPE:
-
Molecular imprinted solid-phase extraction
- MISPMEs:
-
Molecularly imprinted solid-phase microextraction
- MITs:
-
Molecular imprinting technologies
- MMISPE:
-
Magnetic molecular imprinted solid-phase extraction
- MO-AuNPs:
-
Morphine conjugate gold nanoparticles
- MSPD:
-
Matrix solid-phase dispersion
- MSPE:
-
Micro solid-phase extraction
- p-ATP:
-
P-Aminothiophenol
- PMAA:
-
Polymethacrylic acid
- PoPD:
-
Poly-o-phenylenediamine
- QCM:
-
Quartz crystal microbalance
- QDs:
-
High-luminescent quantum dots
- SPE:
-
Solid phase extraction
- SPME:
-
Solid-phase microextraction
- TEOS:
-
Tetraethyl orthosilicate
- TLC:
-
Thin layer chromatography
- TRIM:
-
Trimethylolpropane trimethacrylate
References
Abbasifar J, Samadi-Maybodi A (2016) Selective determination of atropine using poly dopamine-coated molecularly imprinted Mn-Doped ZnS quantum dots. J Fluoresc 26(5):1645–1652. https://doi.org/10.1007/s10895-016-1853-9
Abbott A, Boothby D, Capper G, Davies D, Rasheed R (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147. https://doi.org/10.1021/ja048266j
Alvarez-Lorenzo C, Concheiro A (2004) Molecularly imprinted polymers for drug delivery. J Chromatogr B 804(1):231–245. https://doi.org/10.1016/j.jchromb.2003.12.032
Ansell RJ (2005) Molecularly imprinted polymers for the enantioseparation of chiral drugs. Adv Drug Deliv Rev 57(12):1809–1835. https://doi.org/10.1016/j.addr.2005.07.014
Ansell RJ, Kuah JKL, Wang D, Jackson CE, Bartle KD, Clifford AA (2012) Imprinted polymers for chiral resolution of (±)-ephedrine, 4: packed column supercritical fluid chromatography using molecularly imprinted chiral stationary phases. J Chromatogr A 1264:117–123. https://doi.org/10.1016/j.chroma.2012.09.069
Archana G, Dhodapkar R, Kumar A (2016) Offline solid-phase extraction for preconcentration of pharmaceuticals and personal care products in environmental water and their simultaneous determination using the reversed phase high-performance liquid chromatography method. Environ Monit Assess. https://doi.org/10.1007/s10661-016-5510-1
Atlabachew M, Torto N, Chandravanshi B, Redi M, Chigome S, Mothibedi K, Combrinck S (2015) A (-) norephedrine-based molecularly imprinted polymer for the solid-phase extraction of psychoactive phenylpropylamino alkaloids from Khat (Catha edulis Vahl. Endl.) chewing leaves. Biomed Chromatogr BMC. https://doi.org/10.1002/bmc.3643
Azizi SN, Chaichi MJ, Shakeri P, Bekhradnia A (2013) Determination of atropine using Mn-doped ZnS quantum dots as novel luminescent sensitizers. J Lumin 144:34–40. https://doi.org/10.1016/j.jlumin.2013.05.054
Bagheri N, Habibi B, Khataee A, Hasanzadeh J (2019) Application of surface molecular imprinted magnetic graphene oxide and high performance mimetic behavior of bi-metal ZnCo MOF for determination of atropine in human serum. Talanta. https://doi.org/10.1016/j.talanta.2019.04.023
Behbahani M, Bagheri S, Amini MM, Sadeghi Abandansari H, Reza Moazami H, Bagheri A (2014) Application of a magnetic molecularly imprinted polymer for the selective extraction and trace detection of lamotrigine in urine and plasma samples. J Sep Sci 37(13):1610–1616. https://doi.org/10.1002/jssc.201400188
Bowie AR, Sanders MG, Worsfold PJ (1996) Analytical applications of liquid phase chemiluminescence reactions — a review. J Biolumin Chemilumin 11(2):61–90. https://doi.org/10.1002/(SICI)1099-1271(199603)11:2%3c61::AID-BIO406%3e3.0.CO;2-O
Casado N, Morante-Zarcero S, Sierra I (2022) Application of the QuEChERS strategy as a useful sample preparation tool for the multiresidue determination of pyrrolizidine alkaloids in food and feed samples: a critical overview. Appl Sci 12(9):4325
Chang C, Edwards S, Arab A, Valle-Pinero A, Yang L, Hatsukami D (2016) Biomarkers of tobacco exposure: summary of an FDA-sponsored public workshop. Cancer Epidemiol Biomark Prevent. https://doi.org/10.1158/1055-9965.EPI-16-0675
Chen L, Wang X, Lu W, Wu X, Li J (2016) Molecular imprinting: perspectives and applications. Chem Soc Rev 2016:2137–2211. https://doi.org/10.1039/C6CS00061D
Chen X, Yi C, Yang X, Wang X (2004) Liquid chromatography of active principles in Sophora flavescens root. J Chromatogr B 812(1):149–163. https://doi.org/10.1016/j.jchromb.2004.08.032
Crapnell RD, Banks CE (2021) Electroanalytical overview: the detection of the molecule of murder atropine. Talanta Open 4:100073. https://doi.org/10.1016/j.talo.2021.100073
Cuervo S, Cubides L, Perez C (2017) Optimization and validation of a simple and fast RP-HPLC method for simultaneous determination of acetaminophen and caffeine in tablets. Indian J Pharmaceut Sci. https://doi.org/10.4172/pharmaceutical-sciences.1000286
D’Aurelio R, Tothill IE, Salbini M, Calò F, Mazzotta E, Malitesta C, Chianella I (2021) A comparison of EIS and QCM NanoMIP-based sensors for morphine. Nanomaterials (Vol. 11)
Damm I, Enger E, Chrubasik S, Schieber A, Zimmermann B (2016) Fast and comprehensive analysis of secondary metabolites in cocoa products using ultra high performance liquid chromatography directly after pressurized liquid extraction. J Sep Sci. https://doi.org/10.1002/jssc.201600422
de Albuquerque CDL, Sobral-Filho RG, Poppi RJ, Brolo AG (2018) Digital protocol for chemical analysis at ultralow concentrations by surface-enhanced raman scattering. Anal Chem 90(2):1248–1254. https://doi.org/10.1021/acs.analchem.7b03968
Deshpande S, Sharma S, Koul V, Singh N (2017) Core-shell nanoparticles as an efficient, sustained, and triggered drug-delivery system. ACS Omega 2(10):6455–6463. https://doi.org/10.1021/acsomega.7b01016
DeVito E, Krishnan-Sarin S (2017) E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. https://doi.org/10.2174/1570159X15666171016164430
Dickert FL, Achatz P, Halikias K (2001) Double molecular imprinting – a new sensor concept for improving selectivity in the detection of polycyclic aromatic hydrocarbons (PAHs) in water. Fresenius J Anal Chem 371(1):11–15. https://doi.org/10.1007/s002160100955
Doctor P, Gokani V, Kulkarni PK, Parikh J, Saiyed H (2004) Determination of nicotine and cotinine in tobacco harvesters’ urine by solid-phase extraction and liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci 802:323–328. https://doi.org/10.1016/j.jchromb.2003.12.013
Dong X, Wang W, Ma S, Sun H, Li Y, Guo J (2005) Molecularly imprinted solid-phase extraction of (−)-ephedrine from Chinese Ephedra. J Chromatogr A 1070(1):125–130. https://doi.org/10.1016/j.chroma.2005.03.017
Enke CG, Nagels LJ (2011) Undetected components in natural mixtures: how many? What concentrations? Do they account for chemical noise? What is needed to detect them? Anal Chem 83(7):2539–2546. https://doi.org/10.1021/ac102818a
Fard S, Ahmadi S, Hajimahmoodi M, Fazaeli R, Amini M (2019) Preparation of magnetic iron oxide nanoparticles modified with imidazolium-based ionic liquids as a sorbent for extraction of eight phthalate acid esters in water samples followed by UPLC-MS/MS analysis: experimental design methodology. Anal Methods. https://doi.org/10.1039/C9AY02073J
Feyissa A, Kelly J (2008) A review of the neuropharmacological properties of khat. Progr Neuro Psychopharmacol Biol Psychiatry. Prog Neuropsychopharmacol Biol Psychiat 32:1147–1166. https://doi.org/10.1016/j.pnpbp.2007.12.033
Figueiredo EC, de Oliveira DM, de Siqueira ME, Arruda MAZJA, c. a. (2009) On-line molecularly imprinted solid-phase extraction for the selective spectrophotometric determination of nicotine in the urine of smokers. Anal Chimica Acta 635(1):102–107
Fonger G, Hakkinen P, Jordan S, Publicker S (2014) The National Library of Medicine’s (NLM) hazardous substances data bank (HSDB): background recent enhancements and future plans. Toxicology. https://doi.org/10.1016/j.tox.2014.09.003
Fu Q, Fang Q, Feng B, Sun S, Du W, Amut E, Chang C (2011) Matrine-imprinted monolithic stationary phase for extraction and purification of matrine from Sophorae flavescentis Ait. J Chromatogr B 879(13):894–900. https://doi.org/10.1016/j.jchromb.2011.02.041
Funaya N, Haginaka J (2012) Matrine- and oxymatrine-imprinted monodisperse polymers prepared by precipitation polymerization and their applications for the selective extraction of matrine-type alkaloids from Sophora flavescens Aiton. J Chromatogr A 1248:18–23. https://doi.org/10.1016/j.chroma.2012.05.081
Gadzikowska M, Petruczynik A, Waksmundzka-Hajnos M, Hawrył M, Jóźwiak G (2005) Two-dimensional planar chromatography of tropane alkaloids from Datura innoxia Mill. Jpc-J Planar Chromatogr Mod Tlc 18:127–131. https://doi.org/10.1556/JPC.18.2005.2.7
Gao B, Niu Q, Du RJJOSS (2010) Preparation and recognition performance of cytisine alkaloid-imprinted material prepared using novel surface molecular imprinting technique. J Sep Sci 33(9):1338–1348
Grynkiewicz G, Gadzikowska M (2008) Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol Rep 60:439–463
Guerreiro AR, Korkhov V, Mijangos I, Piletska EV, Rodins J, Turner APF, Piletsky SA (2008) Influence of continuous magnetic field on the separation of ephedrine enantiomers by molecularly imprinted polymers. Biosens Bioelectron 23(7):1189–1194. https://doi.org/10.1016/j.bios.2007.09.009
Guha A, Ahmad OS, Guerreiro A, Karim K, Sandström N, Ostanin VP, Ghosh SK (2020) Direct detection of small molecules using a nano-molecular imprinted polymer receptor and a quartz crystal resonator driven at a fixed frequency and amplitude. Biosens Bioelectron 158:112176. https://doi.org/10.1016/j.bios.2020.112176
Guo Z, Zhang L, Song C, Zhang X (2011) Molecularly imprinted solid-phase extraction of matrine from radix Sophorae tonkinensis. Analyst 136(14):3016–3022. https://doi.org/10.1039/C1AN15281E
Hassanzadeh M, Ghaemy M, Ahmadi S (2016) Extending time profile of morphine-induced analgesia using a chitosan-based molecular imprinted polymer nanogel. Macromol Biosci. https://doi.org/10.1002/mabi.201600177
He Y, Lu J, Liu M, Du J, Nie F (2005) Determination of morphine by molecular imprinting-chemiluminescence method. J Anal Toxicol 29(6):528–532. https://doi.org/10.1093/jat/29.6.528
He Y, Zeng S, Abd El-Aty AM, Hacımüftüoğlu A, Kalekristos Yohannes W, Khan M, She Y (2020) Development of water-compatible molecularly imprinted polymers based on functionalized β-cyclodextrin for controlled release of atropine. Polymers 12(1):130
Hoefnagel DJNEJ, o. M. (1961) Toxic effects of atropine and homatropine eyedrops in children. New Engl J Med 264(4):168–171
Hu R, Tang R, Xu J, Lu F (2018) Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal Chim Acta 1034:176–183. https://doi.org/10.1016/j.aca.2018.06.012
Huang B-Y, Chen Y-C, Wang G-R, Liu C-Y (2011) Preparation and evaluation of a monolithic molecularly imprinted polymer for the chiral separation of neurotransmitters and their analogues by capillary electrochromatography. J Chromatogr A 1218(6):849–855. https://doi.org/10.1016/j.chroma.2010.12.054
Hugon-Chapuis F, Cruz-Vera M, Savane R, Hadj Ali W, Valcarcel M, Deveaux M, Pichon V (2009) Selective sample pretreatment by molecularly imprinted polymer for the determination of LSD in biological fluids. J Sep Sci 32:3301–3309. https://doi.org/10.1002/jssc.200900247
Ji-hong FJJOXU (2004) Comparative research on extraction method in herba ephedrae
John-Africa L, Danborno A, Miriam G, Nazizi K (2020) Anti-nociceptive effects of the hydroethanolic extract of Alysicarpus ovalifolius in rodents. J Med Plants Res 14:195–201. https://doi.org/10.5897/JMPR2019.6769
Kang S-M, Jung H-Y, Kang Y-M, Yun D-J, Bahk J-D, Yang J-K, Choi M-S (2004) Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci 166(3):745–751. https://doi.org/10.1016/j.plantsci.2003.11.022
Kang X, Deng L, Quan T, Gao M, Zhang K, Xia Z, Gao D (2021) Selective extraction of quinolizidine alkaloids from Sophora flavescens Aiton root using tailor-made deep eutectic solvents and magnetic molecularly imprinted polymers. Sep Purif Technol 261:118282. https://doi.org/10.1016/j.seppur.2020.118282
Kaur R (2015) Alkaloids – important therapeutic secondary metabolites of plants origin. J Crit Rev 2:1–8
Khataee A, Hasanzadeh J, Kohan E (2018) Specific quantification of atropine using molecularly imprinted polymer on graphene quantum dots. Spectrochimica Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2018.07.088
Kimura T (2003) Study on the effect of magnetic fields on polymeric materials and its application. Polym J 35(11):823–843. https://doi.org/10.1295/polymj.35.823
Klein-Júnior LC, Vander Heyden Y, Henriques AT (2016) Enlarging the bottleneck in the analysis of alkaloids: a review on sample preparation in herbal matrices. TrAC, Trends Anal Chem 80:66–82. https://doi.org/10.1016/j.trac.2016.02.021
Kolaei M, Dashtian K, Rafiee Z, Ghaedi M (2016) Ultrasonic-assisted magnetic solid phase extraction of morphine in urine samples by new imprinted polymer-supported on MWCNT-Fe3O4-NPs: Central composite design optimization. Ultrason Sonochem 33:240–248. https://doi.org/10.1016/j.ultsonch.2016.05.003
Kudupoje M, Vanzant E, McLeod K, Yiannikouris A (2021) Synthesis, evaluation, and characterization of an ergotamine imprinted styrene-based polymer for potential use as an ergot alkaloid selective adsorbent. ACS Omega. https://doi.org/10.1021/acsomega.1c02158
Lai J-P, He X-W, Jiang Y, Chen F (2003) Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal Bioanal Chem 375(2):264–269. https://doi.org/10.1007/s00216-002-1675-2
Lenain P, Diana Di Mavungu J, Dubruel P, Robbens J, Saeger S (2012) Development of suspension polymerized molecularly imprinted beads with metergoline as template and application in a solid-phase extraction procedure toward ergot alkaloids. Anal Chem. https://doi.org/10.1021/ac302671h
Li G, Wang X, Row KH (2017) Magnetic solid-phase extraction with Fe3O4/molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids for the rapid purification of alkaloid isomers (theobromine and theophylline) from green tea. Molecules 22(7):1061
Li H, Ding M, Lü K, Yu J (2001) Separation and determination of ephedrine alkaloids and 2,3,5,6-tetramethyl pyrazine in Ephedra herba by HPLC. Chinese J Chromatogr 19:161–163
Li W, Zhao X, Yi Z, Glushenkov AM, Kong L (2017b) Plasmonic substrates for surface enhanced Raman scattering. Anal Chim Acta 984:19–41. https://doi.org/10.1016/j.aca.2017.06.002
Li Y, Fu Q, Zhang Q, He L (2006) Preparation and evaluation of uniform-size (-)-ephedrine-imprinted polymeric microspheres by multi-step swelling and suspension polymerization. Anal Sci 22(10):1355–1360
Lisko JG, Stanfill SB, Duncan BW, Watson CH (2013) Application of GC-MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species. Anal Chem 85(6):3380–3384. https://doi.org/10.1021/ac400077e
Liu M, Lu J, He Y, Du J (2005a) Molecular imprinting–chemiluminescence sensor for the determination of brucine. Anal Chim Acta 541(1):97–102. https://doi.org/10.1016/j.aca.2004.11.003
Liu P, Zhang X, Xu W, Guo C, Wang S (2012) Electrochemical sensor for the determination of brucine in human serum based on molecularly imprinted poly-o-phenylenediamine/SWNTs composite film. Sens Actuators, B Chem 163(1):84–89. https://doi.org/10.1016/j.snb.2012.01.011
Liu S, Dong X, Li F (2005b) Evaluation of the (−)-ephedrine imprinted polymers with high affinity for template molecule synthesized using redox initiation system. Anal Lett 38(2):227–236. https://doi.org/10.1081/AL-200045128
Liu Y, Liu X, Wang P (2003) Molecularly imprinted solid-phase extraction sorbent for removal of nicotine from tobacco smoke. Anal Lett 36(8):1631–1645. https://doi.org/10.1081/AL-120021554
López-García E, Mastroianni N, Postigo C, Valcárcel Y, González-Alonso S, Barceló D, López de Alda M (2018) Simultaneous LC–MS/MS determination of 40 legal and illegal psychoactive drugs in breast and bovine milk. Food Chem 245:159–167. https://doi.org/10.1016/j.foodchem.2017.10.005
Lowdon JW, Diliën H, Singla P, Peeters M, Cleij TJ, van Grinsven B, Eersels K (2020) MIPs for commercial application in low-cost sensors and assays – An overview of the current status quo. Sens Actuators, B Chem 325:128973. https://doi.org/10.1016/j.snb.2020.128973
Ma X, Lin H, Zhang J, She Y, Zhou X, Li X, Shao Y (2018) Extraction and identification of matrine-type alkaloids from Sophora moorcroftiana using double-templated molecularly imprinted polymers with HPLC–MS/MS. J Sep Sci 41(7):1691–1703. https://doi.org/10.1002/jssc.201701133
Mahony JO, Nolan K, Smyth MR, Mizaikoff B (2005) Molecularly imprinted polymers—potential and challenges in analytical chemistry. Anal Chim Acta 534(1):31–39. https://doi.org/10.1016/j.aca.2004.07.043
Manbohi A, Ahmadi SH (2015) In-tube magnetic solid phase microextraction of some fluoroquinolones based on the use of sodium dodecyl sulfate coated Fe3O4 nanoparticles packed tube. Anal Chim Acta 885:114–121. https://doi.org/10.1016/j.aca.2015.05.030
Mazzotta E, Picca R, Malitesta C, Piletsky S, Piletska E (2008) Development of a sensor prepared by entrapment of MIP particles in electrosynthesised polymer films for electrochemical detection of ephedrine. Biosens Bioelectron 23:1152–1156. https://doi.org/10.1016/j.bios.2007.09.020
Mehari B, Redi M, Chandravanshi B, Atlabachew M, Combrinck S, McCrindle R (2016) Simultaneous determination of alkaloids in green coffee beans from ethiopia: chemometric evaluation of geographical origin. Food Anal Methods. https://doi.org/10.1007/s12161-015-0340-2
Meng L, Meng P, Tang B, Zhang Q, Wang Y (2013) Molecularly imprinted photonic hydrogels for fast screening of atropine in biological samples with high sensitivity. Forensic Sci Int 231(1):6–12. https://doi.org/10.1016/j.forsciint.2013.04.008
Min J, Jung H, Kang S, Kim Y, Kang Y, Park D, Choi M (2007) Production of tropane alkaloids by small-scale bubble column bioreactor cultures of Scopolia parviflora adventitious roots. Bioresource Technol 98:1748–1753. https://doi.org/10.1016/j.biortech.2006.07.033
Mishra S, Devasish B, Shukla S, Durgabanshi A, Esteve-Romero J (2013) Monitoring strychnine and brucine in biochemical samples using direct injection micellar liquid chromatography. Anal Methods 5:1747–1754. https://doi.org/10.1039/C3AY26014C
Monforte-González M, Ayora-Talavera T, Maldonado-Mendoza IE, Loyola-Vargas VM (1992) Quantitative analysis of serpentine and ajmalicine in plant tissues of Catharanthus roseus and hyoscyamine and scopolamine in root tissues of Datura stramonium by thin layer chromatography-densitometry. Phytochem Anal 3(3):117–121. https://doi.org/10.1002/pca.2800030305
Nakamura Y, Matsunaga H, Haginaka J (2016) Preparation of molecularly imprinted polymers for strychnine by precipitation polymerization and multistep swelling and polymerization and their application for the selective extraction of strychnine from nux-vomica extract powder. J Sep Sci 39(8):1542–1550. https://doi.org/10.1002/jssc.201600027
Nemati N, Ahmadi SH, Tabar heydar, K. (2021) Simultaneous determination of five opium alkaloids in underground waters using molecularly imprinted polymer-modified magnetic nanoparticle based dispersive micro-solid phase extraction followed by high performance liquid chromatography. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1940161
Okuda T, Nishida M, Sameshima I, Kyoyama K, Hiramatsu K, Takehara Y, Kohriyama K (1991) Determination of atropine in biological specimens by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 567(1):141–149. https://doi.org/10.1016/0378-4347(91)80318-7
Pollastro F, Fontana G, Appendino G (2009). In comprehensive natural products II chemistry and biology, pp 205–236
Rahmani M, Ansari Dogaheh M, Kazemipour M, Nateghi M (2017) Selective extraction of morphine from biological fluids by magnetic molecularly imprinted polymers and determination using UHPLC with diode array detection. J Sep Sci. https://doi.org/10.1002/jssc.201701018
Rajabi Khorrami A, Rashidpur A (2009) Design of a new cartridge for selective solid phase extraction using molecularly imprinted polymers: Selective extraction of theophylline from human serum samples. Biosens Bioelectron 25:647–651. https://doi.org/10.1016/j.bios.2008.11.033
Rajput A, Sharma R, Bharti R (2022) Pharmacological activities and toxicities of alkaloids on human health. Mater Today Proc 48:1407–1415. https://doi.org/10.1016/j.matpr.2021.09.189
Ratautaite V, Plausinaitis D, Baleviciute I, Mikoliunaite L, Ramanaviciene A, Ramanavicius A (2015) Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens Actuators B Chem 212:63–71. https://doi.org/10.1016/j.snb.2015.01.109
Renkecz T, Horvath V (2017) Preparation of molecularly imprinted microspheres by precipitation polymerization. In: Tiller T (ed) Synthetic antibodies: methods and protocols. Springer, New York, pp 341–352
Rezaei B, Esfahani M, Ensafi AA (2016) Modified au nanoparticles/imprinted sol-gel/multiwall carbon nanotubes pencil graphite electrode as a selective electrochemical sensor for papaverine determination. IEEE Sens J 16:1–1. https://doi.org/10.1109/JSEN.2016.2598381
Robards K, Worsfold PJ (1992) Analytical applications of liquid-phase chemiluminescence. Anal Chim Acta 266(2):147–173. https://doi.org/10.1016/0003-2670(92)85040-D
Roman MC et al (2004) Determination of ephedrine alkaloids in botanicals and dietary supplements by HPLC-UV: collaborative study. J AOAC Int 87(1):1–14
Roy A (2017) A review on the alkaloids an important therapeutic compound from plants. Int J Plant Biotechnol 3:1–9
Ruela ALM, Pereira GR (2017) Design and evaluation of molecularly imprinted polymers as drug delivery systems. Advanced molecularly imprinting materials. Beverly: Wiley-Scrivener, 413-454
Ruela ALM, de Figueiredo EC, Carvalho FC, de Araújo MB, Pereira GR (2018) Adsorption and release of nicotine from imprinted particles synthesised by precipitation polymerisation: Optimising transdermal formulations. Eur Polymer J 100:67–76. https://doi.org/10.1016/j.eurpolymj.2018.01.021
Ruela ALM, Figueiredo EC, Pereira GR (2014) Molecularly imprinted polymers as nicotine transdermal delivery systems. Chem Eng J 248:1–8. https://doi.org/10.1016/j.cej.2013.12.106
Sahebi H, Konoz E, Ezabadi A, Niazi A, Ahmadi SH (2020) Simultaneous determination of five penicillins in milk using a new ionic liquid-modified magnetic nanoparticle based dispersive micro-solid phase extraction followed by ultra-performance liquid chromatography-tandem mass spectrometry. Microchem J 154:104605. https://doi.org/10.1016/j.microc.2020.104605
Sereshti H, Khosraviani M, Samadi S, Amini-Fazl M (2014) Simultaneous determination of theophylline, theobromine and caffeine in different tea beverages by graphene-oxide based ultrasonic-assisted dispersive micro solid-phase extraction combined with HPLC-UV. RSC Adv. https://doi.org/10.1039/C4RA06412G
Shahid M, Nadeem M, Gulzar A, Saleem M, Ur Rehman H, Ghafoor G, Nelofer R (2020) Novel ergot alkaloids production from penicillium citrinum employing response surface methodology technique. Toxins 12:427. https://doi.org/10.3390/toxins12070427
Song J-Z, Xu H-X, Tian S-J, But PP-H (1999) Determination of quinolizidine alkaloids in traditional Chinese herbal drugs by nonaqueous capillary electrophoresis. J Chromatogr A 857(1):303–311. https://doi.org/10.1016/S0021-9673(99)00758-X
Song X, Xu S, Chen L, Wei Y, Xiong H (2014) Recent advances in molecularly imprinted polymers in food analysis. J Appl Polym Sci 2014:40766. https://doi.org/10.1002/app.40766
Sophia C, Lima E, Allaudeen N, Rajan S (2016) Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater- a review. Desalin Water Treat 57:1–14. https://doi.org/10.1080/19443994.2016.1172989
Sun J, Zhang L, Liu X, Zhao A, Hu C, Gan T, Liu Y (2021) Rational design of a mesoporous silica@ZIF-8 based molecularly imprinted electrochemical sensor with high sensitivity and selectivity for atropine monitoring. J Electroanal Chem 903:115843. https://doi.org/10.1016/j.jelechem.2021.115843
Tang X, Zhu D, Huai W, Zhang W, Fu C, Xie X, Fan H (2017) Simultaneous extraction and separation of flavonoids and alkaloids from Crotalaria sessiliflora L. by microwave-assisted cloud-point extraction. Sep Purif Technol 175:266–273. https://doi.org/10.1016/j.seppur.2016.11.038
Teske J, Weller J-P, Albrecht U-V, Fieguth A (2011) Fatal Intoxication Due to Brucine. J Anal Toxicol 35(4):248–253. https://doi.org/10.1093/anatox/35.4.248
Wang C, Han D, Wang Z, Zang X, Wu Q (2006) Analysis of Strychnos alkaloids in traditional Chinese medicines with improved sensitivity by sweeping micellar electrokinetic chromatography. Anal Chim Acta 572(2):190–196. https://doi.org/10.1016/j.aca.2006.05.030
Wang C, Li C, Zang X, Han D, Liu Z, Wang Z (2007) Hollow fiber-based liquid-phase microextraction combined with on-line sweeping for trace analysis of Strychnos alkaloids in urine by micellar electrokinetic chromatography. J Chromatogr A 1143(1):270–275. https://doi.org/10.1016/j.chroma.2007.01.027
Whitcombe M, Kirsch N, Nicholls I (2014) Molecular imprinting science and technology: a survey of the literature for the years 2004–2011. J Mol Recognit. https://doi.org/10.1002/jmr.2347
Willems T, De Mol ML, De Bruycker A, De Maeseneire SL, Soetaert WK (2020) Alkaloids from marine fungi: promising antimicrobials. Antibiotics 9(6):340
Xie J, Zhou B, Zhang T, Zeng X, Yang M, Wang W, Yang J (2018) Preparation of nicotine surface molecularly imprinted polymers for selective solid-phase extraction of nicotine from zero-level refill liquids of electronic cigarettes. Anal Methods. https://doi.org/10.1039/C8AY00616D
Yang J, Hu Y, Cai JB, Zhu XL, Su QD, Hu YQ, Liang FX (2007) Selective hair analysis of nicotine by molecular imprinted solid-phase extraction: An application for evaluating tobacco smoke exposure. Food Chem Toxicol 45(6):896–903. https://doi.org/10.1016/j.fct.2006.11.010
Yang L, Stöckigt J (2010) Trends for diverse production strategies of plant medicinal alkaloids. Nat Prod Rep 27(10):1469–1479. https://doi.org/10.1039/C005378C
Yildiz D (2004) Nicotine, its metabolism and an overview of its biological effects. Toxicon 43(6):619–632. https://doi.org/10.1016/j.toxicon.2004.01.017
Yin J, Yang G, Chen Y (2005) Rapid and efficient chiral separation of nateglinide and its l-enantiomer on monolithic molecularly imprinted polymers. J Chromatogr A 1090(1):68–75. https://doi.org/10.1016/j.chroma.2005.06.078
Zárate R, Hermosin B, Cantos M, Troncoso A (1997) Tropane alkaloid distribution in atropa baetica plants. J Chem Ecol 23(8):2059–2066. https://doi.org/10.1023/B:JOEC.0000006489.76006.cb
Zeng S, She Y, Jiao B, Liu G, Wang J, Su X, Wang S (2015) Molecularly imprinted polymer for selective extraction and simultaneous determination of four tropane alkaloids from Przewalskia tangutica Maxim fruit extracts using LC-MS/MS. RSC Adv 5(115):94997–95006. https://doi.org/10.1039/C5RA18608K
Zhang J, He L, Fu Q (2005) Chromatographic features and molecular recognition mechanism of a strychnine monolithic molecularly imprinted polymer. Chromatographia 62(5):319–323. https://doi.org/10.1365/s10337-005-0614-8
Zhang L, Yu H, Chen H, Huang Y, Bakunina I, de Sousa DP, Zhang J (2023) Application of molecular imprinting polymers in separation of active compounds from plants. Fitoterapia 164:105383. https://doi.org/10.1016/j.fitote.2022.105383
Zhao L, Zhao F, Zeng B (2014) Preparation of surface-imprinted polymer grafted with water-compatible external layer via RAFT precipitation polymerization for highly selective and sensitive electrochemical determination of brucine. Biosens Bioelectron 60:71–76. https://doi.org/10.1016/j.bios.2014.03.069
Zorrilla JG, Evidente A (2022) Structures and biological activities of alkaloids produced by mushrooms, a fungal subgroup. Biomolecules 12(8):1025
Zuo J, Zhang X, Li X, Li Z, Li Z, Li H, Zhang W (2019) Preparation of monoethyl fumarate-based molecularly imprinted polymers and their application as a solid-phase extraction sorbent for the separation of scopolamine from tropane alkaloids. RSC Adv 9:19712–19719. https://doi.org/10.1039/C9RA03542G
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emara, S., Kamal, M., Sallam, I.E. et al. Application of molecular imprinting approach for alkaloids analysis in food and nutraceuticals: review and perspective. Phytochem Rev 23, 459–483 (2024). https://doi.org/10.1007/s11101-023-09893-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-023-09893-w