Abstract
Phytochemical studies on the roots, twigs and leaves of Meliaceae and Rutaceae family plants have revealed the presence of non-complex terpenes derived from limonoid fragmentation. The occurrence and chemical structure of these degraded limonoids isolated from 1930 to March 2022 are reported in this review. Particular attention is given to the degradation levels in these compounds and their absolute configuration to discover presumable deconstruction pathways from more complex limonoids. Plausible intermediates have been postulated for most of them that would explain their origin from limonoids. The total or semi-synthesis of the most isolated degraded limonoids or analogues remains undescribed. This review focuses on the bioactivity of these fragmented limonoids and their synthesized analogues. Based on pharmacological and agrochemical studies, degraded limonoids appear to be excellent structural leads to consider for the total or semi-synthesis of more potent derivatives with the aim of discovering new hits and clarifying their modes of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Limonoids are C-26 terpenoids (related to limonin) which may arise from the tetracyclic triterpene apoeuphol (Rao et al. 1968; Siddiqui et al. 1991). They feature a basic terpenic skeleton with 4,4,8-trimethyl-17-furyl substituents (Fig. 1). According to the connectivity of the parent core, naturally occurring limonoids are classified in ring-intact limonoids, ring-seco limonoids, highly decorated limonoids and degraded limonoids (Fu and Liu 2020). At least 35 bond patterns are observed including the following types: chukrasone, mexicanolide, havenensin, hortiolide, neotecleanin, azadirachtine, meliacarpin, andirobin, phragmalin, irobin, secomahoganin, salannin, among others (Tan and Luo 2011; Fang et al. 2011). Different cyclisation patterns of the ring backbones stand out in the basic limonoid structure (Li et al. 2016a).
Degraded limonoids are catabolic products resulting from the decomposition of limonoids. Several limonoid reviews have focused on the chemistry, biology and pharmacology of these natural products (Tundis et al. 2014; Lv et al. 2015a; Gualdani et al. 2016; Zhang et al. 2017; Sun et al. 2018; Shi et al. 2020). However, to the best of our knowledge, no review has fully covered the structures and bioactivities of degraded limonoids.
The number of isolated degraded limonoids has increased significantly in recent decades. In 1997, Okamura and co-workers reported that only five naturally occurring degraded limonoids were known (Okamura et al. 1997). These were fraxinellone (Pailer et al. 1965; Coggon et al. 1969), fraxinellonone (Boustie et al. 1990), isofraxinellone (Blaise and Winternitz 1985), dictamdiol (Hu et al. 1989) and calodendrolide (Cassady and Liu 1972). The number of degraded limonoids isolated from Meliaceae and Rutaceae plants increased to 12 in 2005 (Biavatti et al. 2005). Since then, that number has quadrupled.
Although simple in terms of their chemical structure, degraded limonoids have been less studied in comparison to other natural products and, in some cases, their absolute configuration incorrectly assigned. It is worth noting that the numbering of carbon atoms to describe the structures of degraded limonoids can sometimes be confusing due to the lack of consensus among authors. Most authors prefer to keep the original numbering corresponding to intact limonoids while others use the carbon atom numbering of the degraded limonoids themselves (Fig. 2). Due to these numbering discrepancies, some structures have been named in different ways depending on the numbering criterion chosen. This is the case of dasycarpol (Yang et al. 2005), which has been named as 9β-hydroxyfraxinellone (D’Ambrosio and Guerriero 2002) and also as 6β-hydroxyfraxinellone (Zhao et al. 1997, Fig. 2b).
In this review, the original carbon numbering established for the limonoid in question is used to describe the degraded limonoid. Furthermore, the C, D and E rings belonging to the intact limonoids are re-named as A, B and C rings, respectively for the degraded limonoids (Fig. 2a).
Moreover, the term degraded limonoid includes different structures with different degrees of degradation from the precursor limonoid (Fig. 3). To the best of our knowledge, no distinction is made in the literature between these levels of decomposition. In this review, we have drawn a distinction between highly-degraded and slightly-degraded limonoids. In the first group we include plant-derived products that have two or three fused-rings in their core while those tetracyclic compounds that have suffered degradation of the furan ring are referred as slightly-degraded limonoids.
Despite their much lower structural complexity and therefore easier synthetic access to these structures, very few syntheses have been described in the literature for these fascinating carbon scaffolds. Moreover, degraded limonoids are particularly unknown in terms of bioactivity as they have not been thoroughly studied. The interesting biological activities exhibited by some degraded limonoids, and their structural similarity to other more complex related natural products, could shed light on the structural features necessary for the effects observed for some of these compounds. These analyses could guide the design of a new set of biologically active molecules with promising uses in agriculture and medicine.
This review describes the extent to which all naturally occurring degraded limonoids discovered up to March 2022 have been studied, with emphasis on their structure, isolation, bioactivity, chemical and biological transformation and deconstruction pathways.
Natural sources of degraded limonoids
The chief sources of limonoids are the Meliaceae and Rutaceae plant families (Da Silva et al. 1984; Champagne et al. 1992; Roy and Saraf 2006; Liao et al. 2009; Fang et al. 2011; Sun et al. 2018; Sartor et al. 2019; Cui et al. 2021; Luo et al. 2022) but they also can be found very occasionally in Cneoracea and Ptaeroxylaceae families and Harrisonia spp. of Simaroubaceae (Ourison et al. 1964; Connolly 1983; Da Silva et al. 1987; Tundis et al. 2014; Amit and Shailendra 2006; Tan and Luo 2011).
Limonoids from Rutaceae plants feature fewer structural changes than the ones observed in metabolites isolated from the Meliaceae family (Amit and Shailendra 2006; Jolanta et al. 2014). Limonoids from Meliaceae are characterized by a high degree of oxidation and some feature rearrangements when compared to basic limonoid structures (Jolanta et al. 2014).
The chemical structure and occurrence of those compounds is found in Tables 1, 2, 3 and 4 and Figs. 4, 5, 6 and 7.
Highly-degraded limonoids.
Certain degraded limonoids contain an unsaturated δ-
Most of the highly-degraded limonoids are characterized by a fused-bicyclic substituted lactone with a 17α-furyl group and a 13α-methyl group at the ring junction position which could come from the C,D-rings of the precursor limonoids (Heasley 2011, Figs. 4 and 5). Based on the lactone motif size present in their structures, we have classified them into two groups: Limonoids bearing a δ-valero- or a γ-butyrolactone as a B ring. It would be reasonable to suggest a possible bio-relationship between the γ-butyrolactone and the δ-valerolactone rings due to in some cases degraded limonoids bearing a δ-valerolactone were isolated together with compounds that bear a γ-butyrolactone. For instance, azedaralide (19) which contains a δ-valerolactone was isolated from the root bark of Melia azedarach together with the degraded limonoids 24, 25 and 32. These latter compounds bear a γ-butyrolactone instead (Nakatani et al. 1998). Moreover, compounds 2–4, 7 and 11 (bearing a 6-membered lactone) were isolated from the root bark of Dictamnus dasycarpus along with compounds 24–26 and 35 that contain a 5-membered lactone (Gao et al. 2021). However, the bio-relationship between both lactone motif sizes remains unclear.
Additionally, a very small number of the highly degraded limonoids isolated (known as melazolides) keep the gem-dimethyl unit typically present in the A ring and have been hypothesised to derive from the left-side of the original limonoids (Fig. 6).
Degraded limonoids bearing a δ-valerolactone as B ring
Degraded limonoids 1–23 contain a δ-valerolactone substituted by a furyl group and a methyl group at the ring junction position and in most cases are oriented toward the same face of the molecule (Fig. 4). These compounds were isolated from both Rutaceae and Meliaceae families (Table 1).
Calodendrolide (1) was first isolated from seeds of Calodendrum capense (Cassady and Liu 1972) and was later identified in Dictamnus dasycarpus (Zhao et al. 1997; Yoon et al. 2008 and 2010), Euodiae fructus (Yin et al. 2016), Phellodendron amurense Rupr. (Lee et al. 2005; Kiprop et al. 2007) and Tetradium trichotomum (Quader et al. 1990). Structurally, calodendrolide (1) is characterized by an epoxidized δ-valerolactone ring and its structure and absolute stereochemistry was confirmed by chemical transformation into pyroangolensolide (18) (Kiprop et al. 2007). Compounds 2–11 contain an α-hydroxy-δ-valerolactone with either a hydroxyl or ether group attached at the C-9 position and were isolated from the genus Dictamnus. Dictamdiol (2) was described together with dictamdiols A and B (3–4) with α-oriented furyl and methyl groups based on biosynthetic assumptions (Hu et al. 1989; Yoon et al. 2008). However, in spite of all the limonoid precursors isolated to date feature both groups oriented toward the α-face, Zhao et al. assigned its absolute configuration as β-17-furyl-β-13-methyl by crystal X-ray diffraction (Zhao et al. 2007, 2008). Similarly, β-13-methyl and β-17-furyl configurations were established for isodictamdiol (5) (Zhao et al. 2007, 2008) and dictamdiol A (6) (Sun et al. 2013). The total synthesis of these compounds has not yet been described in the literature. Therefore, efforts toward the synthesis of these compounds it should be advisable to clarify their unusual absolute configurations. Ghao et al. isolated isodictamdiol C (7) from the root bark of Dictamnus dasycarpus (Ghao et al. 2021). The NMR data for 7 were almost identical to those of dictamdiol (2) (Jiang et al. 2006) but its absolute configuration was stablished as α-17-furyl- α-13-methyl by ECD spectrum. Dictamlimonols C-E (8–10) were isolated from the root bark of Dictamnus dasycarpus and are structurally related to 3 and 4 but bear a β- or α-oriented methoxy or ethoxy group attached at the C-9 position. Their absolute configurations were established by ECD experiments in combination with quantum chemical calculations (Chen et al. 2020). Although compound 10 was isolated from the root bark of the perennial herb Dictamnus dasycarpus and named dictamlimonol E by Chen et al. in 2020, a few months later it was described as a new degraded limonoid from the ripe fruit of Evodia rutaecarpa (Juss.) Benth. (Qin et al. 2021). Compound 10 was re-named 9α-methoxyl dictamdiol but the two structures are identical. α,β-Dihydroxy-δ-valerolactone dictamlimonol F (12) was also isolated and elucidated in that same study (Chen et al. 2020). Dasycarinone A (11) was isolated from the root bark of Dictamnus dasycarpus and it bears an extra ethoxy group in comparison to isodictamdiol C (7) (Ghao et al. 2021). Dictamnusine (13) was isolated from D. dasycarpus (Yoon et al. 2008) whereas fagaropsine (14) was identified in Fagaropsis glabra (Boustie et al. 1995). Both compounds share a common skeleton bearing an unsaturated δ-valerolactone with two oxygenated functions and differentiated from the position of the D-glucose moiety attached at the C-9 or C-15 positions, respectively. Fagaropsine (14) differs from other reported β-D-limonoid glycosides in the D-ring glycosylation pattern. Dysodensiols A-C (15–17) were isolated from Dysoxylum densiflorum, a species belonging to the Meliaceae family (Xie et al. 2008; Naini et al. 2022). They have an epoxylactone with either a hydroxyl or keto group at C-11 and their relative configurations were deduced from ROESY experiments (Xie et al. 2008). Pyroangolensolide (18) and azedaralide (19) are unsaturated δ-valerolactones isolated from the roots of Melia azedarach. The structure of pyroangolensolide (18) was determined long ago by NMR data and circular dichroism from pyrolysis of methyl angolensate but was not isolated from a natural source until 2002 (D’Ambrosio and Guerriero 2002). Trichiconnarins A-B (20–21) are rare degraded limonoids discovered in Trichilia connaroides bearing a contracted five-membered ring-A. This ring is believed to be an artefact produced from the isolation process by an aldol reaction of trichiconnarin A (20) with acetone followed by dehydration. The absolute configuration of these compounds is assumed to be the same as their biogenetic precursor trijugic C (Wang et al. 2008). Odoratin (22) was reported long ago from Cedrela odorata L. (Chan et al. 1966; Farnswoth et al. 1980; Nogueira et al. 2020) and is an extensive decomposition of the intact limonoids. Its fused-tricyclic skeleton was established by NMR experiments and supported by a mass-spectral fragmentation pattern and is the only degraded limonoid isolated with these features. Moreover, its absolute configuration was deduced from circular dichroism and was identical to other limonoids (Dreyer 1968). The name odoratin could be a mistake since it has also been ascribed to other natural products with different structures including a flavonoid (Sun et al. 2015a; Habbu et al. 2020) and even a pseudoguaianolide (Ortega et al. 1968; Hoffmann et al. 1978). Recently, a new degraded limonoid bearing a fused-tricyclic skeleton, named siamensinolide (23), was isolated from the twigs of Chalcas siamensis Tanaka (Boonyaketgoson et al. 2020).
Degraded limonoids bearing a γ-butyrolactone as B ring
Degraded-limonoids 24–38 contain a γ-butyrolactone ring belonging to a substituted bicyclooctanolide core (Fig. 5 and Table 2). Fraxinellone (24) and its related derivatives 25–33 have been isolated from both the Meliaceae and Rutaceae families. They have an unsaturated γ-butyrolactone ring in common, differing only in the substitution pattern at C-9, C-12 and C-30 positions. Furthermore, the botanical lactone fraxinellone (24) was encountered for the first time in Pluchea indica, a plant belonging to the Asteraceae family (Ruan et al. 2018). Recently, it has been also isolated from Pulsatilla Cernua (Thunb.) Bercht. ex J. Presl which belongs to the Ranunculaceae family. This is the first time a compound with a limonoid skeleton has been isolated from this family (Yan et al. 2021).Fraxinellone (24), together with fraxinellonone (25), have also been isolated from whole plants of Gaultheria nummularioides (Ericaceae family) (Yang et al. 2007; Liu et al. 2013a). Moreover, compounds 29 and 30 are two epimeric degraded limonoids bearing a β-D-glucose moiety at the C-9 position that were discovered from Dictamnus dasycarpus (Tou and Chen 2012; Chen et al. 2020). 8,14-epoxyfraxinellone (34) was isolated from the hexane extracts of Raulinoa echinata roots and its absolute configuration was confirmed by single crystal X-ray diffraction (Biavatti et al. 2005), whereas isofraxinellone (35) exhibited a cis-ring juncture and was originally identified from the trunk bark of Fagaropsis glabra (Blaise and Winternitz 1985) and later from the root bark of D. dasycarpus (Miyazawa et al. 1995). Lastly, compounds 36–38 share many similarities with fraxinellone (24), the main difference being the presence of a substituted α,β-unsaturated butenolide moiety instead of the furyl group. These compounds were also isolated from the root bark of D. dasycarpus (Miyazawa et al. 1995; Wang et al. 2014; Chen et al. 2020).
Melazolides
Melazolides 39–42 are non-furanoid compounds discovered in Melia azedarach roots which have been hypothesized to result from the decomposition of the right-side of the precursor limonoid (D’Ambrosio and Guerriero 2002, Fig. 6 and Table 3). Their absolute configurations were established by CD techniques.
Slightly-degraded limonoids.
Abundant and varied slight fragmentations have been observed in diverse structural type limonoids:16-nor type limonoid backbone (Zhang et al. 2007a, 2008), 16,19-dinor degraded limonoid backbone (Liu et al. 2012) and some examples have been described where the furan ring is involved in the deconstruction of the common limonoid types (Zhang et al. 2008; Hu et al. 2013; Liu et al. 2014).
In this review, we have focused on those degraded limonoids where the main characteristic is the absence of the furanyl ring. Hence, we classified derivatives 43–49 as slightly-degraded limonoids (Fig. 7 and Table 4). These compounds have all been isolated from the Meliaceae family. Walsunoid A (43) is a cedrelone-type limonoid derivative isolated from the leaves of Walsura robusta. It has the same ring A − D pattern as 11β-hydroxycedrelone but the furyl group has been transformed into a 3-hydroxy-2-oxopropyl chain. The absolute configuration was assigned using the ECD technique (Wang et al. 2016). Compounds 44–45 are desfurano-azadiradione derivatives isolated from the fruit coats of Azadirachta indica (Siddiqui et al. 1992, 2003; Akihisa et al. 2009). Nimolactones 46–47 were isolated from the same plant and share many similarities with 44–45 but differ in the presence of an unsaturated γ-butyrolactone (Siddiqui et al. 1992; Kraus et al. 1994; Tariq et al. 2004; Akihisa et al. 2009; Kikuchi et al. 2011). Chuktabrin L (48) is the first example of a C16 degraded and C21-C15 cyclized 16-nor-phragmalin-type limonid which was isolated from the stems of Chukrasia tabularis A. Juss (Wang et al. 2019a). Cipaferen R (49) was isolated from the twigs and leaves of Cipadessa baccifera. This was the first degraded limonoid featuring an acetyl group at the C-17 position (Cao et al. 2020).
Nimbidiol, nimbisonol, nimbione and nimbionone were incorrectly listed as degraded limonoids (Manosroi et al. 2014; Akihisha el al. 2021). However, all these four compounds were originally described as diterpenoids (nimbidiol is a modified diterpenoid of the abiatane skeleton (Majumder et al. 1987); nimbisonol is a tricyclic diterpenoid (Ara et al. 1990); nimbione and nimbionone are two isomeric phenolic diterpenoids (Ara et al. 1988; Siddiqui et al. 1988)).
Biological activities of degraded limonoids
For the most part, the biological properties of degraded limonoids have not yet been described. However, several degraded limonoids such as calodendrolide (1), fraxinellone (24) and isofraxinellone (35) have proved to be active in diverse biological processes and this could be promising in terms of developing new active molecules. About 43% of the degraded limonoids described in this review have either not been studied for any biological activity or their negative results have not been described. Even in spite of the promising pharmacological activities showed by some degraded limonoids only a few studies have focused on the pharmacokinetics and bioavailability of these compounds (Chen et al. 2022). The bioactivity of the degraded limonoids tested to date are listed in Table 5.
Anticancer activity
Naturally occurring bioactive agents, including limonoids such as nimbolide, 29-deactylsendanin (A1542) and 29-isobutylsendanin (A1543), among others, are gaining in popularity over synthetic compounds in preventing and/or treating cancer (Lam et al. 1994; Tian et al. 2001; Ejaz et al. 2006; Bayazit et al. 2010; Bodduluru et al. 2014; Wang et al. 2019b; Shi et al. 2020; Yu et al. 2021). Some computational studies suggest nuclear receptors as the protein targets for limonoid-derived products (Nurlelasari et al. 2020).
Judging from the promising results shown in some studies, degraded limonoids could possess good anti-cancer therapeutic potential. Their biological targets and range of activity are listed in Table 6.
9α-Methoxyl dictamdiol (10) exhibited cytotoxic activity against the human tumor cell lines HL-60, Hela, HepG-2, A-549, AGS and, MDA-MA-468. Compound 10 significantly inhibited selective cytotoxic activity against HL-60 (IC50 = 5.8 μM) and Hela cells (IC50 = 8.1 μM) (Qin et al. 2021). In this study the compound 10 resulted to be more active than a set of limonoids including limonin and obacunone, among others which presented no cytotoxic effects against the mentioned cell lines (Qin et al. 2021). Moreover, a recent mini-review of the biological properties known for fraxinellone (24) includes anti-cancer activity (Bailly and Vergoten 2020). Fraxinellone (24) (together with matrine, dictamine, and maackiain) is one of the key active components of the chemopreventive agent of oral cancer antitumor B (ATB) (Bui et al. 2021). This natural product has been shown to inhibit the growth as well as the migration of osteosarcoma cells HOS and MG63. Studies show that its anti-cancer activity stems from promoting excessive autophagy flux (He et al. 2021). Fraxinellone (24) has also exhibited anticancer activity by inhibiting programmed cell death-ligand 1 (PD-L1) expression when it was assayed in in vivo studies (Xing et al. 2018). The PD-L1 plays a key role in tumorogenesis and compound 24 produced dose-dependent inhibition by reducing hypoxia-inducible factor-1α and STAT3. This study demonstrated that fraxinellone (24) inhibits proliferation and angiogenesis in cancer cells. Some patented products use fraxinellone (24) in the preparation of antitumor drugs because this compound is considered as a tumor cell inhibitor (for example, in leukemia and pancreas cancer cells) and is used to prevent and treat these types of cancer. This natural product did not exhibit any negative effects on normal pancreatic cells (Lee et al. 2017). Another patent identifies this compound as a new antitumor drug because of its tumor supressing effect (Yu et al. 2013; Yu and Zhu 2017). A novel strategy to treat pancreatic cancer has recently been developed involving fraxinellone-loaded CGKRK-modified nanoparticles (Frax-NP-CGKRK) that interfere with the oncogenic KRAS mutation. It was observed that fraxinellone-NP-CGKRK is able to attenuate the dense stroma barrier, reverse the activated cancer-associated fibroblasts and enhance tumor blood perfusion (Pei et al. 2019). Cytotoxic activity against lung lymphoma L1210 cell line has also been described for compound 24 (Kim et al. 1997). Other applications have also been developed for the preparation of fraxinellone-containing antitumor drugs (Yu et al. 2013, 2017). Lastly, dasycarpol (26) exhibited moderate anticancer activity against lung cancer cell line A549 (Yang et al. 2005).
Antiinflammatory activity
Many reports have described the presence of limonoid constituents from Meliaceae and Rutaceae plants which exhibit interesting anti-inflammation activities (Mahmoud et al. 2014a, b; Mireku et al. 2014; Kelley et al. 2015; Hilmayanti et al. 2022). Consequently, interest in these natural anti-inflammatory compounds (Chen et al. 2020; Luo et al. 2020; Yang et al. 2020a, b; Shi et al. 2021) and in the preparation of derived-limonoid compounds (Jia et al. 2020) is clearly on the rise for the treatment of various human ailments. The biological targets and the level of antiinflammatory activity of degraded limonoids are listed in Table 7.
The compounds 2–4, 7, 11, 24–26 and 35 were tested for anti-neuroinflammatory activities by suppressing the nitric oxide (NO) production in lipopolysaccharide (LPS) induced BV-2 cells. Fraxinellone (24) and isofraxinellone (35) exhibit similar IC50 values to the positive control (quercetin). Dasycarinone A (11), fraxinellonone (25) and dasycarpol (26) showed moderate anti-inflamatory activity (IC50 values: 46.9, 34.3 and 42.9 µM, respectively) (Gao et al. 2021). In this study not only degraded limonoids but also a set of non-degraded limonoids were evaluated. Their data pointed out that obacunone-class limonoids exhibited the strongest anti-inflammatory activity among the tested limonoids, followed by fraxinellone (24)-class products. Dictamdiol (2)-class compounds showed weak activity, while the limonin-class compounds almost had no effect (Gao et al. 2021). Some other reports have described fraxinellone (24) as a natural anti-inflammatory. It acts as an inhibitor of inflammasomes in a mouse model. Consequently, compound 24 could lead to the suppression of inflammatory cells during acute pancreatitis by inhibition of pro-inflammatory cytokines (Kim et al. 2019). The anti-inflammatory properties of fraxinellone (24) are connected to down-regulations of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) due to NF-κB inhibition. Fraxinellone (24) also inhibits LPS-induced nitric oxide and prostaglandin E2 production in RAW 264.7 cells and can reduce the LPS-induced expressions of iNOS (Kim et al. 2009). In a more recent study, fraxinellone (24) exhibited inhibitory activity on LPS-induced NO production at 40 µM when it was assayed in RAW 264.7 macrophages (Ruan et al. 2018). Regarding viral-induced neuroinflammation, this natural compound also inhibited double-stranded RNA-induced nitric oxide synthase expression which is related to viral-infected microglia (Lee et al. 2009). Inflammatory arthritis was analysed after treatment with fraxinellone (24) in mice and a clear mitigation effect was observed. Synovial inflammation and osteoclastogenesis was reduced. This process has been associated with the inhibition of cellular differentiation and activation (Jung et al. 2018). The aftereffect of fraxinellone (24) in colonic inflammation has also been studied in in vitro assays. The use of fraxinellone (24) in mice as animal models that mimic inflammatory bowel disease, specifically in dextran sulfate sodium-induced colitis, showed a decrease in the colonic levels of interleukin IL-1β, IL-6, IL-18 and tumor necrosis factor (TNF)-α (Wu et al. 2014). It has also recently been demonstrated that fraxinellone (24) alleviates inflammation and promotes osteogenic differentiation in lipopolysaccharide-stimulated periodontal ligament stem cells (PDLSCs) (Fu et al. 2021). Compounds 10, 24 and 36 exhibited stronger NO production inhibitory effects in RAW 264.7 cells than intact limonoid derivatives such as dictamlimonol A, dictamlimonoside B, limonin and obacunone. The levels of five proteins, specifically, TNF-α, IL-6, iNOS, NF-κB, and COX-2, in LPS-stimulated RAW 264.7 cells were also evaluated for these compounds. The reduced expression of these parameters, which are directly linked to inflammation, pointed to dasylactone A (36) as a beneficial compound in reducing inflammation (Chen et al. 2020).
Antimicrobial activity
Some limonoids isolated from medicinal plants have also attracted attention for their fungicidal (Adesogan and Taylor 1970; Adesisa et al. 1971; Champagne et al. 1992; Abdelgaleil et al. 2000, 2005) and bactericidal properties (Liu et al. 2013b; Zhang et al. 2007b; Rahman et al. 2009). The observed antimicrobial effects of degraded limonoids have been classified based on their antifungal or antibacterial activities (Tables 8 and 9).
Antifungal activity: Calodendrolide (1), fraxinellone (24), 9β-hydroxyfraxinellone (26) and isofraxinellone (35) exhibited fungistatic activity against the plant pathogenic fungus Cladosporium cucumerinum. Growth inhibition in a TLC bioassay was 5 µg for compounds 1, 24 and 26 and 2 µg for compound 35. The minimum inhibitory concentration observed for fraxinellone (24) was greater than 500 µg/mL (Zhao et al. 1997). It is remarkable that in spite of degraded limonoids 1, 24, 26 and 35 were isolated together with five limonoid compounds (limonin, obacunone, 7α-acetylobacunol, 7α-acetyldihydronomilin and limonin diosphenol) from the root bark of Dictamnus dasycarpus Turcz, these degraded limonoids exhibited fungistatic activity against C. cucumerinum while limonoids resulted to be inactive even when 100 µg were spotted on TLC plates. This fact suggests a positive response of the plants against potentially fungal attacks involving a biodegradation of limonoids into their degraded derivatives (Zhao et al. 1997). Compound 24 was also active against Alternaria longipes and Curvularia lunata (EC50 values of 64.2 μg/mL and 123.3 μg/mL, respectively) (Wang et al. 2006). Fraxinellone (24) was also tested against plant diseases and its inhibition rate against Rhizoctonia cerealis van der Hoeven apud. Boerema & Verhoeven was 100% (at 100 ppm) and against Colletotrichurm lagenarium & verhoeven was 72% (at 50 ppm) (Yuan et al. 2006). Additionally, molecular dynamics simulation identified fraxinellone (24) as a probable antifungal drug candidate against Fusarium graminearum (Joshi et al. 2021). Cipaferen R (49) was inactive in antifungal assays against Fusarium oxysporum f. sp. cubense and Ralstonia solanacearum (Cao et al. 2020).
Antibacterial activity: isodictamdiol (5) exhibited weak antibacterial activity against the Gram-( +) bacteria Bacillus subtilis (showing a growth inhibition zone diameter of 10–12 mm) and was not active against Staphylococcus aureus or Escherichia coli (Zhao et al. 2008). However, its stereoisomer dictamdiol (2) was inactive against these three bacteria (Zhao et al. 2008). Fraxinellone (24) exhibited no antibacterial inhibition in this study while fraxinellonone (25) exhibited weak antibacterial activity against E. coli (10–12 mm inhibition zone) (Zhao et al. 2008). In this antibacterial assay some intact limonoids were also evaluated. Limonin, obacunone and evodol were inactive against B. subtilis and E. coli (Zhao et al. 2008). Fraxinellone (24) showed antibacterial activity against S. aureus and B. megaterium with a 99.8% of inhibition rate at 50 ppm whereas 100% of inhibition rate was observed for E. coli (Yuan et al. 2006). Dasylactone B (38) was active against S. aureus with a MIC value of 160 g/mL (Wang et al. 2014). Nimolactones 46 and 47 exhibited antibacterial activity against Bacillus subtilis at doses of 10 µg (Kraus et al. 1994).
Insect-controlling activity
Plants have developed many defense strategies against insects producing an arsenal of phytochemical weapons resulting from the coevolution of plants and insects (Ehrlich and Raven 1964; Bentley et al. 1990). This is exemplified by the Meliaceae and Rutaceae plant families which are sources of many bio-pesticides including limonoids (Yang and Tang 1988; Akhtar et al. 2008; Morgan 2009). From among these active plant metabolites, the insecticidal activity of calodendrolide (1) and fraxinellone (24) has been evaluated in several insect-control studies (Lei et al. 2020). The insecticidal activities of degraded limonoids are listed in Table 10.
Calodendrolide (1) was active as a larvicidal compound against the dengue mosquito Aedes aegypti Linn showing a LC50 value of 13.1 µM, which is higher than the LC50 exhibited by pyroangolensolide (18) (16.6 µM) (Kiprop et al. 2005, 2007).
Fraxinellone (24) exhibited interesting pesticidal activities against many insects (Yang et al. 2018a). It was evaluated for it potential ovicidal activity against the insects Mythimna separata Walker and Bombyx mori Linaeus exhibiting selective activity against the eggs of M. separata. Eggshell size was reduced and the egg hatching inhibited. However, morphological changes were not observed in the eggshells of B. mori (Lv et al. 2020). In other studies, the antifeeding and mortality rates at 72 h against third-instar armyworm M. separata showed by fraxinellone (24) were 90.4% and 95.2%, respectively (Yuan et al. 2006; Yuan and Wang 2012). Using M. separata as a model insect, the insecticidal mechanism of this natural product on the peritrophic membrane of sixth instar larvae in both in vitro and in vivo treatments was studied. When fraxinellone (24) was applied at a concentration of 20 mg/mL, a decrease in the protein content and an increase in carbohydrate content were observed when 8 mg/mL was added. In the in vivo assay, an increase in carbohydrate content was also observed at a concentration of 20 mg/mL of fraxinellone. Hence, treatments with this compound modified the carbohydrate or protein content and also altered the structure of the peritrophic membrane compared to the control group (Lu et al. 2014). Recently, study of the interactions between three kinds of DNA (armyworm, salmon sperm and calf thymus) and natural product 24 showed only weak interaction. This theoretical study supports the possible application of this compound as a pesticide (Lei et al. 2020). Studies have also been conducted to determine antifeedant and larvicidal effects against four major pests, Mythimna separata, Agrotis ypsilon, Plutella xylostella and Culex pipiens pallens. Fraxinellone (24) acted as a feeding deterrent against the Lepidopteran larvae M. separata, A. ypsilon, and P. xylostella. It also proved toxic, with LC50 values of 15.95 mg/mL, against the third instar larvae of M. separata, 6.43 mg/mL against the second instar of P. xylostella and 3.60 × 10–2 mg/mL against the fourth instar larvae of C. pipiens. Pupation rate inhibition was also observed as was growth and egg hatching rate in M. separate (Lü et al. 2013). Fraxinellone (24) produces structural changes in the midgut epithelium of the lepidoptera M. separata Walker at a dosis of 20 mg/mL (Min et al. 2010). Compound 24 also exhibited antifeedant activity against the insects Tribolium castaneum and Sitophilus zeamais (EC50 values of 36.4 and 29.1 ppm for adults and larvae of T. castaneum, respectively, and EC50 value of 71.2 for S. zeamais adults) (Liu et al. 2002). When Ostrinia furnacalis (Asian corn borer) and Heliothis virescens (tobacco budworm) were fed with fraxinellone (24) at a concentration of 10 ppm (and above) and 4.31 × 10–5 mol/L (and above) respectively, growth and food consumption rate were reduced significantly (Liu et al. 2008, 2009). The larval midguts of both insects showed reduced activity of the proteins α-amilase and non-specific proteases, and showed higher activity of cytochrome P450s. Fraxinellone (24) acts as a digestive poison against M. separata, affecting the epithelium membrane in the midgut of this insect (Lü et al. 2010). It also produced changes in the levels of digestive and detoxification enzymes in 5th instar larvae of M. separata (α-amilase activity was inhibited whereas protease activity was increased (Lv et al. 2014). It was also toxic against the black cutworm Agrotis ypsilon (Lü et al. 2013). Fraxinellone (24) also exhibited feeding deterrent activity and reduced growth rate and food consumption in Tribolium castaneum at a concentration of 10 ppm for both adults and larvae (Liu et al. 2002).
Fraxinellonone (25) exhibited moderate insect-antifeedant activity against Spodoptera exigua (Okamura et al. 1997) and isofraxinellone (35) exhibited potent antifeedant activity against larvae of Spodoptera litura (4.43 μg/cm2) (Okuno et al. 2019). Insect antifeedant experiments against the third-instar larvae of Spodoptera littoralis showed that azedaralide (19), fraxinellone (24) and 12α-acetoxyfraxinellone (32) were active at 500 ppm (10 μg/cm2). However, fraxinellonone (25) did not exhibit any antifeedant activity (Nakatani et al. 1998). 8,14-Epoxy-fraxinellone (34) was evaluated against ant species Atta sexdens rubropilosa (also known as leaf-cutting ants) but was not toxic to them (Wang et al. 2016). Desfuranoazadiradione (44) and its derivative desfurano-6 α-hydroxyazadiradione (45) was toxic against Anopheles stephensi Liston at 37 and 43 ppm, respectively (Siddiqui et al. 2003). Both α-Nimolactone (46) and β-nimolactone (47) exhibited toxicity against Anopheles stephensi Liston, specifically against its 4th-instar larvae of the malaria vector mosquito with LC50 values of 60 and 45 ppm, respectively (Siddiqui et al. 2003; Tariq et al. 2004). Nimolactones 46 and 47 exhibited moderate antifeedant activity against Epilachna varivestis (EC50 values of 80 ppm) (Kraus et al. 1994).
Neuroprotective activity.
Some limonoids assayed for the treatment of neurodegenerative diseases showed notable neuroprotective activity (Abdelgaleil et al. 2000; Yoon et al. 2008; Sun et al. 2015b; Ouyang et al. 2016). Several degraded limonoids have also been evaluated for this activity. Their biological targets and the range of activity are listed in Table 11.
Calodendrolide (1) showed promising protection against glutamate-induced neurotoxicity when assayed in primary cultures of rat cortical cells at a concentration of 0.1 µM. It exhibited an even greater neuroprotective effect than the positive control dizocipline maleate (MK-801, a non-competitive NMDA receptor antagonist) l (Yoon et al. 2008). In the same toxicity assay, dictamdiol A (3), dictamnusine (13), fraxinellone (24) and dasycarpol (26) also proved active with EC50 values of 0.068, 0.042, 0.022 and 0.098, respectively at 0.1 µM whereas compound 29 possessed an EC50 value of 0.705 at 1.0 µM (Yoon et al. 2008). The neuroprotective mechanism involves the inhibition of calcium influx and the overproduction of reactive oxygen species and cellular nitric oxide. This all implies protection of neuronal cells against glutamate-induced oxidative stress (Yoon et al. 2010). Fraxinellone (24) and isofraxinellone (35) exhibited neuroprotective activity against H2O2-induced injury in SH-SY5Y cells (Wang et al. 2014).
Other biological activities
Degraded limonoids have also shown other interesting biological activities which are summarized in Table 12.
Calodendrolide (1) exhibited antigiogenic activity on internode blood vessels when assayed in Zebrafish at a concentration of 20 mg/L (Yin et al. 2016). Dictamdiol (2), isodictamdiol A (6) and fraxinellone (24) exhibited significant inhibitory effects against adenosine diphosphate-induced blood platelet aggregation at 250 μM. Compound 6 also exhibited anti-platelet aggregation activity (Sun et al. 2013). Fraxinellone (24) has displayed a broad range of other bioactivities. The vasorelaxing activity of fraxinellone (24) was studied on rats and proved to be an effective vasorelaxant. Fraxinellone (24) acts as a selective blocker of voltage-dependent Ca2+ channel (Yu et al. 1992). This natural product has also been added to medicines to treat allergic skin diseases as it induces Th1 type cells to produce IFN-γ (Lu et al. 2021). When fraxinellone (24) was administered to mice with passive skin allergy at a concentration of 100 mg/kg, the inhibition rate of skin mast cell degranulation reached 62.5% (Lu et al. 2021). Inhibitory effects on oxidative stress-senescence were also attributed to fraxinellone (24) where AMP-activated protein kinases seem to be involved in an autophagic mechanism associated to age-related decay (Han et al. 2018). The antifertility activity of fraxinellone (24) was apparently related to the inhibition of the implantation process in female Sprague–Dawley rats. Furthermore, it also exhibited oral toxicity in female rats with a LD50 value of 274 mg/kg (Woo et al. 1987). In addition to the previously described activities, compound 24 has been targeted as a potential natural product useful in the treatment of some liver disorders. Results show that fraxinellone (24) induces apoptosis in activated peripheral CD4+ T cells and results in less CD4+ activation and infiltration to the liver (Sun et al. 2009). Fraxinellone (24) has also been reported to be a potential hepatotoxic metabolite linked to a significant elevation of serum alanine aminotransferase and aspartate aminotransferase observed in experiments on mice (Zhou et al. 2020). On the basis of this natural compound has excellent antihepatic fribosis activity, Li et al. recently evaluated the capacity of cyclodextrin derivatives with fraxinellone (24) to increase solubility and find optimal oral fraxinellone formulations for anti-fibrotic therapy (Li et al. 2021). A recent study conducted on mice showed that fraxinellone (24) could alleviate folic acid-induced kidney fibrosis by inhibition of the activation of kidney fibroblasts and therefore it could potentially be a candidate as a new drug to treat chronic kidney disease (Zheng et al. 2021). Fraxinellone (24) is one of several drugs in a nano preparation for the treatment of hepatic fibrosis disorder (Jiang et al. 2018). The anti-fibrotic effects of a nanoemulsion formulation containing fraxinellone have recently been analysed in a tumor microenvironment (as it could stunt melanoma). Studies showed that the fraxinellone nanoemulsion combined with a tumor-specific peptide vaccine might remodel the tumor microenvironment (Hou et al. 2018). Subchronic toxicity studies using the colorimetric counting kit 8 assay (CCK-8) in HepG2 cells revealed that fraxinellone (24) decreased the cell viability (Fan et al. 2018). Fraxinellone (24) and fraxinellonone (25) also exhibited ichthyotoxic activity against the Japanese killifish Oryzias latipes at 10 and 50 ppm, respectively (Nakatani et al. 1998). Okamura et al. synthesized ( ±)-fraxinellone (( ±)-24) and ( ±)-isofraxinellone (( ±)-35) from fraxinellonone (( ±)-25) and these related compounds were then evaluated against killifish and all exhibited high levels of ichthyotoxicity at 50 ppm at 12 h (Okamura et al. 1997). The effects of fraxinellone (24) and isofraxinellone (35) on the mutagenicity of furylfuramide and Trp-P-1 were evaluated in the umu test and Ames test (Miyazawa et al. 1995). Fraxinellone (24) was found to be inactive in these tests. However, isofraxinellone (35) supressed the SOS-induction activity of furylfuramide at 0.86 µmol/mL in the umu test showing an ID50 value of 0.35 µmol/mL. The ID50 value was 0.50 µmol/mL when assayed with the mutagen Trp-P-1. In the Ames test using Salmonella typhimurium TA100, isofraxinellone (35) completely suppressed furylfuramide mutagenicity at 0.65 µmol/mL (ID50 of 0.24 µmol/mL), and at 0.73 µmol/mL with the mutagen Trp-P-1 (ID50 of 0.30 µmol/mL) (Miyazawa et al. 1995). Kinase Src is considered a target for drug development based on its established relationship with cancer and possible link to hypertension. Molecular dynamics simulation and molecular docking suggested that 9α-hydroxyfraxinellone-9-O-β-D-glucoside (9HFG, 29) has stable binding affinities with Src kinase and could have an inhibitory effect against this Src kinase by competitive binding against ATP. Also, compound 29 could be a potential ligand competing for the link to kinase Src (Tou and Chen 2012). Compounds 9α-hydroxyfraxinellone (27), 9α-hydroxy-12α-acetoxyfraxinellone (31) and 12α-hydroxyfraxinellone (33) were 100% lethal at 100 µM in the Brine Shrimp Lethality Test (against Artemia salina) (Fukuyama et al. 2006). (-)-8,14-Epoxyfraxinellone (34) exhibited weak activity against trypomastigote forms of Trypanosome cruzi, with an IC50 value of 246.8 µg/mL whereas other intact limonoid derivatives isolated from the same species such as limonin, limonexic acid, kihadalactone B and (-)-21-O-methyl-limonexic acid showed no activity (Biavatti et al. 2001). Desfuranoazadiradione (44), α-nimolactone (46) and β-nimolactone (47) were tested on melanogenesis in B16 melanoma cells. Compounds 44 and 46 exhibited a marked inhibitory effect in this assay. Compounds 44 and 46–47 also exhibited inhibitory effects against the Epstein-Barr virus early antigen activation induced by 12-O-tetradecanoylphorbol-13-acetate (Akihisa et al. 2009). Chuktabrin L (48) was evaluated for acetylcholinesterase inhibitory activity but was inactive (Wang et al. 2019a). Cipaferen R (49) was evaluated for nematicidal activity against Meloidogyne incognita but was inactive (Cao et al. 2020).
Deconstruction pathways of limonoids to degraded limonoids
The role of these compounds in the roots, twigs and leaves of plants has not yet been clearly ascertained. Nevertheless, their origins have been postulated in some reports searching for plausible intermediates in the conversion from limonoids to degraded limonoids. Within these, some highly degraded limonoids such as fraxinellone (24) and its derivatives have been described as degradation products from ring-intact or seco-ring limonoids (Tan and Luo 2011). Microbial systems may have evolved to break down limonoids in the environment but this remains unclear. Potential precursors of limonoids have been considered to shed light on the routes towards the degradation products described in this review.
Chan et al. (1966, 1972) speculated that odoratin (22) is biogenetically related to mexicanolides and carapin which are a bicyclononanolide group of limonoids. Transformations of these bicyclononanolides involving a reverse Michael reaction and a β-dicarbonyl cleavage could explain the C5-C10 and C2-C3 bonds cleavages to give odoratin (22) (Chan et al. 1972, Scheme 1).
Hypothesized degradation route towards odoratin (22) (Chan et al. 1972)
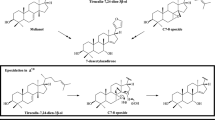
Coggon et al. proposed that fraxinellone (24) arises from the degradation of a limonoid bitter principle, limonin (also named dictamnolactone), which is a co-metabolite in Dictamnus albus extracts (Coggon et al. 1969, Scheme 2).
Limonin, hypothetic precursor of fraxinellone (24) (Coggon et al. 1969)
Cassady and Liu (1972) pointed out that calodendrolide (1) appears to derive from a limonoid by the loss of A and B rings and ensuing decarboxylation to give the corresponding lactol. Oxidation and double-bond isomerization would afford fraxinellone (24) (Cassady and Liu 1972, Scheme 3). The transformation of calodendrolide (1) into fraxinellone (24) has been postulated through isofraxinellone (35) by isomerization of the double bond (Cassady and Liu 1972).
Hypothesized route from calodendrolide (1) to fraxinellone (24) (Cassady and Liu 1972)
D’Ambrosio and Guerriero suggested hypothesized intermediates from limonin and andirobin-type limonoids to explain the deconstruction pathway to produce some degraded limonoids (D’Ambrosio and Guerriero 2002). Deoxyandirobin and methyl angolensate are two limonoids described as precursors of pyroangolensolide (18) and azedaralide (19) and the hypothesized intermediate I was postulated for the biogenesis of these compounds (Scheme 4a). In fact, limonoids methyl angolensate and 7-deacetyl-7-oxogedunin were also isolated from the root extract of M. azedarach as well as pyroangolensolide (18). These authors also suggested that calodendrolide (1), fraxinellone (24) and its derivatives fraxinellonone (25), 9β-hydroxyfraxinellone (26), 9α-hydroxyfraxinellone (27), 30-hydroxyfraxinellone (28) and isofraxinellone (35) are produced from the common intermediate II (Scheme 4b). 3-Teracrylmelazolides 39 and 40 are characterized by correspondence to the A-ring portion of an original limonoid skeleton such as deoxyandirobin or andirobin through a hypothetical intermediate III (Scheme 4c).
Hypothesized intermediates I–III leading to some degraded limonoids (D’Ambrosio and Guerriero 2002)
Dysodensiols A-C (15–17), isolated from Dysoxylum densiflorum, have also been proposed as deriving from a common B-seco-limonoid precursor (Xie et al. 2008, Scheme 5).
Plausible precursors of dysodensiols 15–17 (Xie et al. 2008)
Trichiconnarins A and B (20 and 21) feature a contracted five-membered ring-C. Trichiconnarin A (20) has been hypothesized as a product obtained by the cleavage of the C-2 and C-8 bonds of trijugin. Several trijugins have also been isolated from Trichilia connaroides which have been postulated to be derivatives of trijugin C. This natural product has also been suggested to be the precursor of trichiconnarin A (Wang et al. 2008, Scheme 6). Aldol reaction of trichiconnarin A (20) with acetone and subsequent dehydration could give rise to the formation of trichiconnarin B (21) (Wang et al. 2008; Tan and Luo 2011). However, the authors did point out that trichiconnarin B (21) could be an artefact produced during the isolation process (Wang et al. 2008).
Trijugin C, precursor of trichiconnarins A and B (Wang et al. 2008)
Walsunoid A (43) is considered to be a degradation product of cedrelone-type limonoids. A furanyl transformation mechanism underlines walsunoid A (43) formation (Wang et al. 2016, Scheme 7).
Walsunoid A (43) from cedrelone-type precursors (Wang et al. 2016)
A biosynthetic pathway starting from phragmalin-type limonoids as precursors was proposed by Wang et al. to explain the formation of chuktabrin L (48) (Wang et al. 2019a, Scheme 8).
Plausible biosynthetic route for chuktabrin L (48) (Wang et al. 2019a)
Lastly, in a more recent work, Bailly and Vergoten (2020) proposed that degraded limonoids like calodendrolide (1) and fraxinellone (24) could derive from tetra or pentacyclic limonoids such obacunone, kihadanins A-D or limonin (Bailly and Vergoten 2020, Scheme 9).
Hypothesized precursors leading to calodendrolide (1) and fraxinellone (24) (Bailly and Vergoten 2020)
Syntheses of degraded limonoids and analogues
Structurally complex natural limonoids as well as their analogues have been a profitable source to identify new bioactive agents (Furiassi et al. 2021; Mulani et al. 2022; Sun et al. 2022). However, degraded limonoids are also interesting biologically active entities that have attracted the attention of some chemists because they can be used as valuable building blocks for the total synthesis of intact limonoids. In 2011, Heasley published an excellent review on progress made in limonoid synthesis including degraded limonoids.At that time, only total or formal syntheses had been performed on calendrolide (1), pyroangolensolide (18), azedaralide (19), fraxinellone (24), fraxinellonone (25) and isofraxinellone (35) (Fukuyama et al. 1972, 1973; Drewes et al. 1985; Tokoroyama et al. 1990; Fernández-Mateos et al. 1991; Rajab et al. 1994; Okamura et al. 1997; Trudeau et al. 2005; Baker et al. 2006).
A year later, Guo et al. developed a stereoselective method to synthezise 9α-hydroxyfraxinellone (27) from fraxinellonone (25) (Guo et al. 2012a).It is worth mentioning that the authors named this molecule 4α-hydroxyfraxinellone because they used different carbon atom numbering for fraxinellone (C-9 re-named as C-4). Consequently, they described 27 as 4α-hydroxyfraxinellone (Scheme 10).
Synthesis of 9α-hydroxyfraxinellone (27) (Guo et al. 2012a)
A new review summarizing the main efforts made to synthesize limonoids was published by Fu and Liu (2020). They highlight the total enantioselective syntheses of these compounds covering from January 2011 to March 2020. Surprisingly, no new degraded limonoid syntheses were reported in this review. Recently, the total synthesis of (-)-melazolide B (42) was reported through a six-step sequence involving a preparation of a keto-lactone intermediate 50 derived from α-ionone. Chemoselective dehydrogenation of 50, epoxidation and Wharton reduction afforded (-)-melazolide B (42) (Scheme 11). The synthesis of C3-epi-melazolide B was also achieved by a similar synthetic route from compound 50 (Liu et al. 2022).
Syntheses of fraxinellone-based esters modified at C-15 or C-17. (Guo et al. 2013)
To the best of our knowledge, no total synthesis or semi-synthesis of the remainder of degraded limonoids listed in the present review has been performed. A simple approach to a set of degraded limonoids analogs has been carried out for the antibacterial (Ferrera-Suanzes et al. 2020) and antifeedant (Bentley et al. 1990) evaluation. In fact, two simplified models based on the C and D rings of limonin showed a higher antifeedant activity against L. decemlineata Larvae than limonin (Bentley et al. 1990). Additionally, the number of fraxinellone (24)-based molecules synthesized in the last decade for the purpose of obtaining new natural products-based pesticides is remarkable. In fact, fraxinellone (24) has been used as a platform where numerous structural modifications have been performed for evaluation as potentially more potent insecticides than traditional ones such as toosendanin (a natural limonoid which is a well-known digestive tract parasiticide (Shi et al. 2007) with a notable growth inhibition effect and potent antifeedant activity against armyworm M. separata Walker and other insects (Shi et al. 1986; Chen et al. 1995)) (Fig. 8).
A small number of these chemical structures of the synthetic fraxinellone analogues were listed in a recent mini review by Bailly and Vergoten in June 2020, but that review did not include the preparation or the insecticidal activity range of values of all the most potent derivatives. In order to list all the semi syntheses and clarify structure–activity relationships, in this review we present the structural modifications that have been performed in the A or B rings (Schemes 14, 15 and 16) and in the C-ring of fraxinellone (Schemes 17, 18, 19, 20 and 21), and a thorough analysis of the growth inhibition activity of the most active derivatives in terms of mortality rates versus the mortality rates observed in the control toosendanin and the precursor 24 (Table 13).
Synthesis of esters of fraxinellone C9/C30 oxime (Li et al. 2016b)
Halogenation and acylation reactions on the furyl-ring of fraxinellone (Guo et al. 2016)
Synthesis of N-phenylpyrazole ring derivatives (Yang et al. 2018a)
Structural modifications of A or B-rings of fraxinellone
Guo et al. studied the allylic oxidation of fraxinellone (24) (Guo et al. 2012a, b) and obtained either natural product 25 or compound 57 depending on the reaction conditions used (Scheme 12). Then, reduction of the generated carbonyl group at C-9 or C-30 and subsequent Steglich esterification afforded compounds 55 or 59, respectively (Guo et al. 2012a, Scheme 12b, d). Fraxinellone-based hidrazones modified at C-9 or C-30 were also prepared by Guo et al. (Guo et al. 2012b, Scheme 12c, e). Some years later Guo et al. reported the preparation of the N-phenylpyrazole derivatives 54 (Guo et al. 2017a, Scheme 12a).
Guo et al. (2013) performed the reduction of fraxinellone (24) using sodium bis(2-methoxyethoxy) aluminium hydride (Red-Al) at low temperature which gave the mixture of compounds 61–63 (Scheme 13a). Esterification of 61 and 62 enabled the authors to prepare analogues 64–66 (Scheme 13b, c). Thionation of the carbonyl group from the lactone of 24 gave rise to thiocarbonyl derivative 67 in moderate reaction yield (Scheme 13d).
Analogues 25 and 57 were converted into the corresponding oximes at C-9 and C-30 positions (69 and 71) through a two-step reaction sequence, respectively, in high reaction yields (Li et al. 2016b, Scheme 14).
Structural modifications of the C-ring of fraxinellone.
Among the modifications of fraxinellone (24), transformations on the furan ring are abundant (Guo et al. 2016, 2017b, 2018; Dong et al. 2018a, b; Yang et al. 2018a, b, Schemes 15–19). The Friedel–Crafts acylation of the fraxinellone C-ring was performed by Guo et al. in low to moderate reaction yields (Guo et al. 2016, Scheme 15a). Moreover, they converted fraxinellone (24) into their mono-, di- and tetra-halogenated analogues (Scheme 15b, c, d).
The acyl derivatives of fraxinellone 72b and 73b were also treated with 2-mercapto-5-aryl-1,3,4-oxadiazoles to afford a set of thioethers 80 and 81 (Guo et al. 2017b, Scheme 16a, d). Analogues 72b and 73b were also transformed to obtain carboxamides 83 and 85 through a two-step reaction sequence (Guo et al. 2017b, Scheme 16b, e). Aryloxy groups were added to the furan ring by reaction with phenols (Yang et al. 2018b, Scheme 16c, f).
Starting from compounds 72a and 73a, Yang et al. prepared two series of N-phenylpyrazole derivatives (89 and 91) characterised by halogen atoms (F, Cl or Br) on the phenyl group (Yang et al. 2018a, Scheme 17).
Guo et al. described the furyl-ring bromination of fraxinellone (24) getting the dibrominated derivative in only 18% reaction yield (Guo et al. 2016, Scheme 15c). Later, Dong et al. optimized the reaction yield of this transformation (up to 85%) using 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) as the brominating reagent and a higher reaction temperature (Dong et al. 2018a, b, Scheme 18a). This latter compound was also obtained together with the C-21 monobrominated derivative when N-bromosuccinimide was used (Scheme 18b). Compound 63 was also transformed into the mono- and di-brominated furyl derivatives 93 and 94 (Scheme 18c). All these products were coupled to organoboron species or terminal alkynes through Suzuyi-Miyaura or Sonogashira couplings respectively, to get the new carbon–carbon single bonds containing derivatives 95–98 (Scheme 18d, e).
Monocyclopropanated derivatives of fraxinellone (24) were formed through the [2 + 1] addition between the C22-C23 double bond of the furyl-ring of fraxinellone and a set of α-carbonyldiazoesters (Yang et al. 2020a, b, Scheme 19).
Insecticidal activity of semi-synthesized fraxinellone analogues
The previously mentioned fraxinellone-based analogues were assayed as insecticidal agents against lepidopteran insect pests (Mythimna separata Walker and/or Plutella xylostella Linnaeus). Those that exhibited stronger growth inhibition activity than their parental counterpart 24 and toosendanin (T) are listed in Table 13.
Antifungal studies carried out by Zhao et al. showed that the furyl-ring in degraded limonoids seemed to be crucial for the growth inhibition effect (Zhao et al. 1997). Three cyclopropyl analogues at C-22/C-23 positions (compounds 99a-c) with an aromatic substituent were prepared by Yang et al. (2020a, b). Compound 99c, with a bromine atom on the aryl side chain, was the most active product (Table 13, entry 1). Mode of action has yet to be described but data suggest that this analogue might affect the insect molting hormone based on larval malformations of the oriental armyworm. Among the twenty-five N-(1,3-thiazol-2-yl) carboxamide derivatives synthesized, it is remarkable those bulkier products that contain a -CH2Cl side chain in the carboxamide unit (compounds 83 and 84) (Guo et al. 2019). When the carboxamide motif was introduced at C-21 position, the reaction product became more active than 24 and toosendanin against both M. separata. and P. xylostella showing with mortality rate percentages (MR (%)) of 58.6% and 69.0%, respectively (Table 13, entry 2). Replacement of the -CH2Cl by a p-nitrophenyl group led to the most active one of all against the armyworm with a mortality rate of 75.9%, but was less active than the precursor and toosendanin against P. xylostella (Table 13, entry 2). The presence of the -CH2Cl unit in the analogue modified 85 at C-23 gave a stronger insecticidal agent against P. xylostella but lower activity against M. separata in comparison to fraxinellone (24) and toosendanin (Table 13, entry 3). When an N-phenylpyrazole unit is introduced at C-21 or C-23 of the furan-site of fraxinellone (89 and 91), an increase in mortality rate is observed if the phenyl group contains a halogen atom both at the ortho- and para- positions (Yang et al. 2018a, Table 13, entries 4 and 5). The most active compounds of this series were those that possess a chlorine atom in the ortho position and a fluorine atom in the para position of the phenyl group (MR = 82.8% and 75.9% for the C-21 and C-23 substituted analogues, respectively, versus 55.2% for toosendanin when the growth of M. separata was evaluated). The introduction of other bulky chains such as aryloxy or aryl groups on the furan ring at C-21 also gave promising insecticidal compounds 86 and 95 against the armyworm (Yang et al. 2018b, Table 13, entries 6 and 7). Reduction of the double bond of the A-ring of fraxinellone afforded compound 61 that exhibited a fourfold increase in growth inhibition of M. separata compared to 24 (MR = 71.4% versus 17.4%) (Dong et al. 2018a, b, Table 13, entry 8). However, if this latter molecule is coupled to an alkyl chain on the C-21 of the C-ring, a dramatic decrease in the inhibition rate is observed (Table 13, entry 9). The acyl derivative 73 (Table 13, entry 10) and chlorinated analogues 74–75 (Guo et al. 2016, Table 13, entries 11–12) led only to slightly higher mortality rates. Transformations on the A-ring of fraxinellone at C-9 or C-30 in terms of oxidation on the C-9 allylic position, transformation in an oxime or related esters of C9/C30 oximes or the introduction of bulky motifs (aromatic-ring containing esters or hydrazones at C-9/C-30) gave a set of higher potency natural-product-based derivatives (Table 13, entries 13–16 (Li et al. 2016b), entries 19–22 (Guo et al. 2012a, b)). Zhao et al. (1997) also indicated that the lactone group in limonoids did not appear to be critical for antifungal effects. Modifications of the B-ring to give compounds 61 and 63 did not result in significantly more potent insecticidal compounds (Guo et al. 2013, Table 13, entries 17–18) which is consistent with the Zhao’s assessment.
All these structural changes should be taken into account for further chemical modifications with a view to identifying promising insecticidal candidates. However, it is noteworthy a clear lack of consistency in the insecticidal data for the control compounds fraxinellone (24) and toosendanin (T). For example, it is described mortality rate values of 17.4% (Table 13, entries 1 and 7), around 42–45% (Table 13, entries 2–3, 6, 10–12) or even upper than 50% (Table 13, entries 21 and 22) when fraxinellone (24) was assayed against pre-third-instar larvae of M. separata Walker in the same concentration and 35-days assay. Some discrepancies in the mortality rate percentages are also observed for toosendanin (T). Variability in the data are also observed in the experiments against P. xylostella Linnaeus. For this reason, it would be inappropriated to conclude which of these fraxinellone (24)-analogs could be the most active of all of them.
Biotransformations of degraded limonoids
It is well known that conventional synthetic tools are tedious at times. Biotransformation of natural products could save time or eliminate undesired effects such as the sensitivity of some functional groups in the presence of specific reagents or steric hindrance. However, the literature contains only a few reports of biotransformation of limonoids highlighting hydroxylation on different carbons of the basic limonoid skeleton (Haldar et al. 2013, 2015)or rare modifications of the furan moiety (Haldar et al. 2014) among others (Madyastha and Venkatakrishnan 2000).
Only a handful of degraded limonoid biotransformations have been reported. In all of them, fraxinellone (24) or isofraxinellone (35) were modified using the fungi Aspergillus niger (Yang et al. 2005; Zhang et al. 2005).
A fermentation culture of A. niger to which fraxinellone (24) was added led to the isolation and purification of a new compound known as fraxinigerllone (100) (Scheme 20a). This compound also exhibits antitumor activity (Zhang et al. 2005). In other microbial transformation studies, fraxinellone (24) was modified by Aspergillus niger (AS 3.421) and dasycarpol (9β-hydroxyfraxinellone, 26) giving rise to fraxinigerllone (100) as the biotransformation product (Yang et al. 2005, Scheme 20b). In this study, dasycarpol (26) and fraxinigerllone (100) were also evaluated against EC9706 and A549 cell lines. Both products exhibited moderate cytotoxicity against the A549 cell line. Dasycarpol (26) showed an LC50 value of 20 µg/mL while fraxinigerllone (100) showed an inhibition rate of 32% at a concentration of 87 µg/mL.
The cytotoxic activities of 1–3were tested in vitro against EC9706 and A549 cell lines.
The bioconversion of ( +)-isofraxinellone (( +)-35) was achieved through incubation with the fungi Aspergillus niger leading to (4S)-4-hydroxyisofraxinellone (101) (Miyazawa et al. 2009; Okuno et al. 2019; Scheme 21). Both the starting material (( +)-35) and the biotransformation product 101 exhibited antifeedant activity against Spodoptera litura, with ED50 values of 3.91 and 4.43 µg/cm2, respectively.
Conclusions and future outlook
In summary, this comprehensive review covered the isolation of slightly- and highly-degraded limonoids for the first time. From 1930 to March 2022, 49 degraded limonoids were reported, mostly from Meliaceae and Rutaceae plants.
Although common hypothesized intermediates have been postulated to explain the fragmentation routes towards degraded limonoids from more complex limonoid precursors, in-depth knowledge about how limonoid biodegradation occurs in the environment remain unavailable. Surprisingly, in most cases the furanyl ring remains intact in the decomposition pathways in both Meliaceae and Rutaceae families. In this review, the bioactivities of the isolated degraded limonoids were also enhanced in search of insecticidal, anticancer, anti-inflammatory, antimicrobial and neuroprotective activity. Nevertheless, approximately 43% of these compounds have not been tested in any biological evaluation to date or have turned out to be inactive. The most degraded limonoids have yet to be synthesized and their preparation could shed light on some stereochemical discrepancies. Moreover, chemical or microbial transformations could lead to new limonoid-derived analogues which could then be explored for potential biological activities. That is why we believe that these compounds could display yet to be discovered and potentially excellent pharmacological activities, which would spark more interest in them, prompt further studies and get more chemists interested in the total or semi syntheses of degraded limonoids. We anticipate that these compounds, and their more potent analogues, which to date remain under-explored, could be used in the study of protein-natural product interactions with the aim of designing new agrochemicals and therapeutic agents.
References
Abdelgaleil SAM, Okamura H, Iwagawa T, Doe M, Natakani M (2000) Novel rings B, D-secolimonoids from the stem bark of Khaya senegalensis. Heterocycles 53:2233–2240
Abdelgaleil SAM, Hashinaga F, Nakatani M (2005) Antifungal activity of limonoids from Khaya ivorensis. Pest Manag Sci 61:186–190. https://doi.org/10.1002/ps.978
Adesisa GA, Adesogan EK, Okorie DA, Taylor DAH, Styles BT (1971) The limonoid chemistry of the genus Khaya (Meliaceae). Phytochemistry 10:1845–1853. https://doi.org/10.1016/s0031-9422(00)86448-1
Adesogan EK, Taylor DAH (1970) Limonoid extractives from Khaya ivorensis. J Chem Soc. https://doi.org/10.1039/j39700001710
Akhmedzhanova VI, Bessonova IA, Yunusov SY (1978) Study of dictamnus angustifolius roots. Khim Prir Soedin 4:476–478
Akhtar Y, Yeoung YR, Isman MB (2008) Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, trichoplusia ni and pseudaletia unipuncta. Phytochem Rev 7:77–88. https://doi.org/10.1007/s11101-006-9048-7
Akihisa T, Noto T, Takahashi A, Fujita Y, Banno N, Tokuda H, Koike K, Suzuki T, Yasukawa K, Kimura Y (2009) Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci 58:581–594. https://doi.org/10.5650/jos.58.581
Akihisa T, Zhang J, Manosroi A, Kikuchi T, Manosroi J, Abe M (2021) Limonoids and other secondary metabolites of Azadirachta indica (neem) and Azadirachta indica var. siamensis (Siamese neem), and their bioactivities. Stud Nat Prod Chem 68:29–65. https://doi.org/10.1016/B978-0-12-819485-0.00013-X
Amit R, Shailendra S (2006) Limonoids: overview of significant bioactive triterpenes distributed in plant kingdom. Biol Pharm Bull 29:191–201. https://doi.org/10.1248/bpb.29.191
Ara I, Siddiqui BS, Faizi S, Siddiqui S (1988) Terpenoids from the stem bark of Azadirachta indica. Phytochemistry 27:1801–1804. https://doi.org/10.1016/0031-9422(88)80447-3
Ara I, Siddiqui BS, Faizi S, Siddiqui S (1990) Three new diterpenoids from the stem bark of Azadirachta indica. J Nat Prod 53:816–820. https://doi.org/10.1021/np50070a006
Arene EO, Bevan CWL, Powell JW, Taylor DAH (1965) West African timbers Part XI the structure of carapin, an extractive from Carapa procera. Chem Commun. https://doi.org/10.1039/c19650000302
Bai Y, Jin X, Jia X, Tang W, Wang X, Zhao Y (2014a) Two new apotirucallane-type isomeric triterpenoids from the root bark of Dictamnus dasycarpus with their anti-proliferative activity. Phytochem Lett 10:118–122. https://doi.org/10.1016/j.phytol.2014.06.017
Bai YY, Tang WZ, Wang XJ (2014b) Chemical constituents from root bark of Dictamnus dasycarpus. J Chin Med Mater 37(2):263–265
Bailly C, Vergoten G (2020) Fraxinellone: From pesticidal control to cancer treatment. Pestic Biochem Physiol 168:104624. https://doi.org/10.1016/j.pestbp.2020.104624
Baker LA, Williams CM, Bernhardt PV, Yanik GW (2006) Azedaralide: total synthesis, relative and absolute stereochemical assignment. Tetrahedron 62:7355–7360. https://doi.org/10.1016/j.tet.2006.05.030
Bayazit V, Vahit K (2010) Biochemical and physiological evaluations of limonoids as potential cancer destroyers. J Anim Vet Adv 9:1099–1107. https://doi.org/10.3923/javaa.2010.1099.1107
Bentley MD, Rajab MS, Mendel MJ, Alford AR (1990) Limonoid model insect antifeedants. J Agric Food Chem 38:1400–1403. https://doi.org/10.1021/jf00096a022
Biavatti MW, Vieira PC, da Silva MFGF, Fernandes JB, Albuquerque S (2001) Limonoids from the Endemic Brazilian Species Raulinoa echinata. Z Naturforsch 56:570–574. https://doi.org/10.1515/znc-2001-7-815
Biavatti MW, Westerlon R, Vieira PC, Fatima M, da Silva GF, Fernandes JB, Penaflor MFGV, Bueno OC, Ellena J (2005) Leaf-cutting ants toxicity of limonexic acid and degraded limonoids from Raulinoa echinata. X-ray structure of epoxy-fraxinellone. J Braz Chem Soc 16:1443–1447. https://doi.org/10.1590/s0103-50532005000800025
Blaise AJ, Wintemitz F (1985) Isofraxinellone, a limonoid lactone from the bark of Fagaropsis glabra. Phytochemistry 24:2379–2381. https://doi.org/10.1016/S0031-9422(00)83045-9
Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R (2014) Chemopreventive and therapeutic effects of nimbolide in cancer: the underlying mechanisms. Toxicol Vitro 28:1026–1035. https://doi.org/10.1016/j.tiv.2014.04.011
Boonyaketgoson S, Rukachaisirikul V, Phongpaichit S, Trisuwan K (2020) Limonoids and carbazole alkaloids from the twigs of Chalcas siamensis Tanaka. Nat Prod Res 36:122–129. https://doi.org/10.1080/14786419.2020.1768084
Boustie J, Moulis C, Gleye J, Fouraste I, Servin P, Bon M (1990) A degraded limonoid from Fagaropsis glabra. Phytochemistry 29:1699–1701. https://doi.org/10.1016/0031-9422(90)80152-7
Boustie J, Gleye J, Blaise A, Fouraste I (1992) Limonoids of fagaropsis glabra. Planta Med 58:228. https://doi.org/10.1055/s-2006-961440
Boustie J, Respaud M-J, Moulis C, Lavaud C, Gleye J, Fouraste I (1995) Fagaropsin, a degraded limonoid glucoside from Fagaropsis glabra. Phytochemistry 38:217–219. https://doi.org/10.1016/0031-9422(94)00605-S
Bui D, Yin T, Duan S, Wei B, Yang P, Wong SJ, You M, Singh R, Hu M (2021) Pharmacokinetic characterization and bioavailability barrier for the key active components of botanical drug antitumor B (ATB) in mice for chemoprevention of oral cancer. J Nat Prod 84:2486–2495. https://doi.org/10.1021/acs.jnatprod.1c00501
Cao D-H, Liao S-G, Sun P, Xiao Y-D, Xiao C-F, Hu H-B, Weckwerth W, Xu Y-K (2020) Mexicanolide-type limonoids from the twigs and leaves of Cipadessa baccifera. Phytochemistry 177:112449. https://doi.org/10.1016/j.phytochem.2020.112449
Cassady JM, Liu C-S (1972) Structure of calodendrolide, a terpenoid from Calodendrum capense. J Chem Soc Chem Commun 2:86–87. https://doi.org/10.1039/C39720000086
Champagne DE, Koul O, Isman MB, Scudder GGE, Towers GHN (1992) Biological activity of limonoids from the Rutales. Phytochemistry 31:377–394. https://doi.org/10.1016/0031-9422(92)90003-9
Chan WR, Taylor DR, Aplin RT (1966) Odoratin, an undecanortriterpenoid from Cedrela odorata. Chem Commun. https://doi.org/10.1039/C19660000576
Chan WR, Taylor DR, Aplin RT (1972) Extracts of Cedrela odorata. IV. structure of odoratin, an undecanortriterpene. Tetrahedron 28:431–437. https://doi.org/10.1016/0040-4020(72)84006-7
Chen W, Isman MB, Chiu SF (1995) Antifeedant and growth-inhibitory efects of the limonoid toosendanin and Melia-Toosendan extracts on the variegated cutworm, Peridroma-Saucia (Lep, Noctuidae). J Appl Entomol 119:367–370. https://doi.org/10.1111/j.1439-0418.1995.tb01302.x
Chen J, Tang JS, Tian J, Wang YP, Wu FE (2000) Dasycarine, a new quinoline alkaloid from Dictamnus dasycarpus. Chin Chem Lett 11:707–708
Chen Y, Ruan J, Sun F, Wang H, Yang S, Zhang Y, Yan J, Yu H, Guo Y, Zhang Y, Wang T (2020) Anti-inflammatory limonoids from Cortex Dictamni. Front Chem 8:73. https://doi.org/10.3389/fchem.2020.00073
Chen M, Shen X, Yang X, Yin Q, Tian D, Li L, Lu C, Ye CJ-N, Chen Y, Yan L, Wang F (2022) A methodology for quantitation of dictamnine and fraxinellone and its application to study pharmacokinetics and bioavailability in rats via oral and intravenous administration. J Chromatogr Sci. https://doi.org/10.1093/chromsci/bmac053
Coggon P, McPhall AT, Storer R, Young DW (1969) Structure and absolute configuration of fraxinellone, a biogenetically intriguing terpenoid from Dictamnus albus. J Chem Soc d Chem Commun 14:828. https://doi.org/10.1039/C29690000828
Connolly JD (1983) Chemistry of the limonoids of the Meliaceae and Cneoraceae. Proc Phytochem Soc Eur 22:175–213
Connolly JD, McCrindle R, Overton KH (1968) Tetranortriterpenoids. IV. Bicyclononanolides II constitution and stereochemistry of mexicanolide. Tetrahedron 24:1489–1495
Cui Z-R, Li Y, Zhao M-L, Xu R, Chen M-H, Li S, An F-L, Zhang P-P, Kong L-Y, Luo J (2021) MS diagnostic model and rapid distinguishing of bioactive limonoids in fruits of Melia toosendan using solid-phase extraction coupled with LC-MS/MS. Phytochem Anal 32:308–317. https://doi.org/10.1002/pca.2977
D’Ambrosio M, Guerriero A (2002) Degraded limonoids from Melia azedarach and biogenetic implications. Phytochemistry 60:419–424. https://doi.org/10.1016/S0031-9422(02)00107-3
Da Silva MFDGF, Gottlieb OR, Dreyer DL (1984) Plant chemosystematics and phylogeny. part XXVI. evolution of limonoids in the Meliaceae. Biochem Syst Ecol 12:299–310. https://doi.org/10.1016/0305-1978(84)90053-X
Da Silva MF, Das GF, Gottlieb OR (1987) Evolution of quassinoids and limonoids in the Rutales. Biochem Syst Ecol 15:85–103
Dong Q-M, Dong S, Shen C, Cao Q-H, Song M-Y, He Q-R, Wang X-L, Yang X-J, Tang J-J, Gao J-M (2018a) Furan-site bromination and transformations of fraxinellone as insecticidal agents against Mythimna separata Walker. Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-26747-0
Dong W, Chang J, Kou J (2018b) Preparation method of fraxinellone, dictamnine and obakunone. China, CN108690041 A 2018b–10–23
Drewes SE, Grieco PA, Huffman JC (1985) A short synthesis of dl-epi-pyroangolensolide and dl-pyroangolensolide: confirmation of the structures of pyroangolensolide and calodendrolide. J Org Chem 50:1309–1311. https://doi.org/10.1021/jo00208a035
Dreyer DL (1968) Citrus bitter principles. VIII. Application of optical rotatory dispersion and circular dichroism to stereochemical problems. Tetrahedron 24:3273–3283
Du C, Yang X, Tu P (2005) Studies on chemical constituents in bark of Dictamnus dasycarpus. Zhongguo Zhongyao Zazhi China J Chin Mater Med 30:1663–1666
Ehrlich PR, Raven AH (1964) Evolution butterflies and plants: a study in coevolution. Evolution 18:586–608. https://doi.org/10.2307/2406212
Ejaz S, Ejaz A, Matsuda K, Lim CW (2006) Limonoids as cancer chemopreventive agents. J Sci Food Agric 86:339–345. https://doi.org/10.1002/jsfa.2396
Ekong DEU, Fakunle CO, Fasina AK, Okogun JI (1969) Meliacins (limonoids). Nimbolin A and B, two new meliacin cinnamates from Azadirachta indica and Melia azedarach. J Chem Soc d Chem Commun 20:1166–1167. https://doi.org/10.1039/C29690001166
Fan Q, Zhao B, Wang C, Zhang J, Wu J, Wang T, Xu A (2018) Subchronic toxicity studies of cortex Dictamni extracts in mice and its potential hepatotoxicity mechanisms in vitro. Molecules. https://doi.org/10.3390/molecules23102486
Fang X, Di YT, Hao XJ (2011) The advances in the limonoid chemistry of the Meliaceae family. Curr Org Chem 15:1363–1391. https://doi.org/10.2174/138527211795378254
Farnsworth NR, Cordell GA, Kaas CJ (1980) What is odoratin? J Pharm Sci 69:1107. https://doi.org/10.1002/jps.2600690935
Fernández Mateos A, De la Fuente Blanco JA (1991) Synthesis of limonoid model insect antifeedants through stereoselective aldol addition reactions. J Org Chem 56:7084–7092. https://doi.org/10.1021/jo00025a025
Ferrera-Suanzes M, Prieto V, Medina-Olivera AJ, Botubol-Ares JM, Galán-Sánchez F, Rodríguez-Iglesias MA, Hernández-Galán R, Durán-Peña MJ (2020) Synthesis of degraded limonoid analogs as new antibacterial scaffolds against Staphylococcus aureus. Antibiotics 9:488. https://doi.org/10.3390/antibiotics9080488
Fu S, Liu B (2020) Recent progress in the synthesis of limonoids and limonoid-like natural products. Org Chem Front 7:1903–1947. https://doi.org/10.1039/d0qo00203h
Fu Z, Wang X, Li B, Tang Y (2021) Fraxinellone alleviates inflammation and promotes osteogenic differentiation in lipopolysaccharide-stimulated periodontal ligament stem cells by regulating the bone morphogenetic protein 2/Smad pathway. Arch Oral Biol 121:104927. https://doi.org/10.1016/j.archoralbio.2020.104927
Fukuyama Y, Tokoroyama T, Kubota T (1972) Total synthesis of fraxinellone. Tetrahedron Lett. https://doi.org/10.1016/s0040-4039(01)94055-9
Fukuyama Y, Tokoroyama T, Kubota T (1973) Synthetic studies on terpenoid compounds III total synthesis of pyroangolensolide. Tetrahedron Lett. https://doi.org/10.1016/s0040-4039(01)87359-7
Fukuyama Y, Nakaoka M, Yamamoto T, Takahashi H, Minami H (2006) Degraded and oxetane-bearing limonoids from the roots of Melia azedarach. Chem Pharm Bull 8:1219–1222. https://doi.org/10.1248/cpb.54.1219
Furiassi L, Tonogai EJ, Hergenrother PJ (2021) Limonin as a starting point for the construction of compounds with high scaffold diversity. Angew Chem Int Ed 60:16119–21612. https://doi.org/10.1002/anie.202104228
Gao P, Wang L, Zhao L, Lu Y-Y, Zeng K-w, Zhao M-B, Jiang Y, Tu P-F, Guo X-Y (2021) Rapid identification, isolation, and evaluation on anti-neuroinflammatory activity of limonoids derivatives from the root bark of Dictamnus dasycarpus. J Pharm Biomed Anal 200:114079. https://doi.org/10.1016/j.jpba.2021.114079
Gualdani R, Cavalluzzi MM, Lentini G, Habtemariam S (2016) The chemistry and pharmacology of citrus limonoids. Molecules. https://doi.org/10.3390/molecules21111530
Guo Y, Yan Y, Yu X, Wang Y, Zhi X-Y, Hu Y, Xu H (2012a) Synthesis and insecticidal activity of some novel fraxinellone-based esters. J Agric Food Chem 60:7016–7021. https://doi.org/10.1021/jf301734h
Guo Y, Yan Y-Y, Yang C, Yu X, Zhi X-Y, Xu H (2012b) Regioselective synthesis of fraxinellone-based hydrazone derivatives as insecticidal agents. Bioorg Med Chem Lett 22:5384–5387. https://doi.org/10.1016/j.bmcl.2012.07.058
Guo Y, Qu H, Zhi X, Yu X, Yang C, Xu H (2013) Semisynthesis and insecticidal activity of some fraxinellone derivatives modified in the b ring. J Agric Food Chem 61:11937–11944. https://doi.org/10.1021/jf404195m
Guo Y, Yang R, Xu H (2016) New insecticidal agents from halogenation/acylation of the furyl-ring of fraxinellone. Sci Rep 6:35321. https://doi.org/10.1038/srep35321
Guo Y, Wang X, Fan J, Zhang Q, Wang Y, Zhao Y, Huang M, Ding M, Zhang Y (2017a) Semisynthesis and insecticidal activity of some novel fraxinellone-based thioethers containing 1,3,4-oxadiazole moiety. R Soc Open Sci. https://doi.org/10.1098/rsos.171053
Guo Y, Wang X, Qu L, Xu S, Zhao Y, Xie R, Huang M, Zhang Y (2017b) Design, synthesis, antibacterial and insecticidal activities of novel N-phenylpyrazole fraxinellone hybrid compounds. RSC Adv 7:11796–11802. https://doi.org/10.1039/C6RA28064A
Guo X, Zhao LN, Wang J, Bi QR, Wang Z, Tan NH, Liu S (2018) Chemical constituents from root barks of Dictamnus dasycarpus and their cytotoxic activities. J Chin Mater Med 24:4869–4877
Guo Y, Fan J, Zhang Q, Bao C, Liu Z, Yang R (2019) Turning natural products into insecticide candidates: Design and semisynthesis of novel fraxinellone-based N-(1,3-thiazol-2-yl)carboxamides against two crop-threatening insect pests. Bioorg Med Chem Lett 29:179–184. https://doi.org/10.1016/j.bmcl.2018.12.002
Habbu PV, Miskin N, Kulkarni VH, Bhat P, Joshi A, Bhandarkar A, Kulkarni S, Dixit SR, Vaijanathappa J (2020) Isolation, characterization, and cytotoxic studies of secondary metabolites from the leaves of Bauhinia foveolata Dalzell: an endemic tree from the Western Ghats, India. J Appl Pharm Sci 10:135–148. https://doi.org/10.1016/10.7324/japs.2020.103018
Haldar S, Kolet SP, Thulasiram HV (2013) Biocatalysis: fungi mediated novel and selective 12β- or 17β-hydroxylation on the basic limonoid skeleton. Green Chem 15:1311–1317. https://doi.org/10.1039/c3gc40193f
Haldar S, Kolet SP, Dandekar DS, Kale BS, Gonnade RG, Thulasiram HV (2014) Biocatalyst mediated functionalization of salannin, an insecticidal limonoid. RSC Adv 4:27661–27664. https://doi.org/10.1039/c4ra04652h
Haldar S, Mulani FA, Aarthy T, Thulasiram HV (2015) Whole-cell mediated 11β-hydroxylation on the basic limonoid skeleton by Cunninghamella echinulata. J Org Chem 80:6490–6495. https://doi.org/10.1021/acs.joc.5b00417
Han X, Chen H, Zhou J, Tai H, Gong H, Wang X, Huang N, Qin J, Fang T, Wang F, Xiao H (2018) The inhibitory effect in Fraxinellone on oxidative stress-induced senescence correlates with AMP-activated protein kinase-dependent autophagy restoration. J Cell Physiol 233:3945–3954. https://doi.org/10.1002/jcp.26169
He B, Zhang W, He J (2021) Fraxinellone has anticancer activity by inducing osteosarcoma cell apoptosis via promoting excessive autophagy flux. Front Pharmacol 12:653212. https://doi.org/10.3389/fphar.2021.653212
Heasley B (2011) Synthesis of limonoid natural products. Eur J Org Chem 1:19–46. https://doi.org/10.1002/ejoc.201001218
Hilmayanti E, Nurlelasari SU, Kabayama K, Shimoyama A, Fukase K (2022) Limonoids with anti-inflammatory activity: a review. Phytochemistry 204:113469. https://doi.org/10.1016/j.phytochem.2022.113469
Hoffmann JJ, Jolad SD, Torrance SJ, Luzbetak DJ, Wiedhopf RM, Cole JR (1978) Odoratin and paucin: cytotoxic sesquiterpene lactones from Baileya pauciradiata (Compositae). J Pharm Sci 67:1633–1634. https://doi.org/10.1002/jps.2600671136
Hou L, Liu Q, Shen L, Liu Y, Zhang X, Chen F, Huang L (2018) Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics 8:3781–3796. https://doi.org/10.7150/thno.24821
Hu K, Liu J-Q, Li X-n, Chen J-C, Zhang W-M, Li Y, Li L-q, Guo L-l, Ma W-g, Qiu M-H (2013) Chukfuransins A-D, four new phragmalin limonoids with β-furan ring involved in skeleton reconstruction from Chukrasia tabularis. Org Lett 15:3902–3905. https://doi.org/10.1021/ol401650m
Hu C, Han J, Zhan J, Song G, Li Y, Yin D (1989) Limonoids from Dictamnus angustifolius. Zhiwu Xuebao 31:453-458
Jia C, Hu B, Ji Y, Su Y, Gong G, Zhu Q, Xu Y (2020) Synthesis of limonin derivatives with improved anti-inflammatory and analgesic properties. Lett Drug Des Discov 17:285–299. https://doi.org/10.2174/1570180816666181113102359
Jiang Y, Tu P-F, Zhang N (2006) Assignment of 1H and 13C chemical shifts of dictamdiol. Bopuxue Zazhi 23:327–332
Jiang H, Xing L, Fan Q (2018) Nano drug carrier for loading anti-fibrosis drugs and its application in preparing drugs for treating hepatic fibrosis. China, CN108273061 A 2018–07–13
Jolanta P, Jan SD, Agata K, Stanislaw L (2014) Variability of biological activities of limonoids derived from plant sources. Mini Rev Org Chem 11:269–279. https://doi.org/10.2174/1570193x1103140915105445
Joshi T, Joshi T, Sharma P, Pundir H, Chandra S (2021) In silico identification of natural fungicide from Melia azedarach against isocitrate lyase of Fusarium graminearum. J Biomol Struct Dyn 39:4816–4834. https://doi.org/10.1080/07391102.2020.1780941
Jung SM, Lee J, Baek SY, Lee J, Jang SG, Hong SM, Park JS, Cho ML, Park SH, Kwok SK (2018) Fraxinellone attenuates rheumatoid inflammation in mice. Int J Mol Sci. https://doi.org/10.3390/ijms19030829
Kaku T, Ri H (1935) Lactones from Dictamnus albus L. grown in Korea. Yakugaku Zasshi 55:219–221
Kelley DS, Adkins YC, Zunino SJ, Woodhouse LR, Bonnel EL, Breksa AP, Manners GD, Mackey BE (2015) Citrus limonin glucoside supplementation decreased biomarkers of liver disease and inflammation in overweight human adults. J Funct Foods 12:271–281. https://doi.org/10.1016/j.jff.2014.11.026
Kikuchi T, Ishii K, Noto T, Takahashi A, Tabata K, Suzuki T, Akihisa T (2011) Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (Neem). J Nat Prod 74:866–870. https://doi.org/10.1021/np100783k
Kim S-W, Yeo W-H, Ko Y-S, Kim S-K (1997) Isolation and characterization of antitumor agents from Dictamnus albus. Saengyak Hakhoechi 4:209–214
Kim JH, Park YM, Shin JS, Park SJ, Choi JH, Jung HJ, Park HJ, Lee KT (2009) Fraxinellone inhibits lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by negatively regulating nuclear factor-kappa B in RAW 264.7 macrophages cells. Biol Pharm Bull 32:1062–1068. https://doi.org/10.1248/bpb.32.1062
Kim MJ, Bae GS, Jo IJ, Choi SB, Kim DG, Jung HJ, Song HJ, Park SJ (2019) Fraxinellone inhibits inflammatory cell infiltration during acute pancreatitis by suppressing inflammasome activation. Int Immunopharmacol 69:169–177. https://doi.org/10.1016/j.intimp.2019.01.043
Kiprop AK, Rajab MS, Wanjala FME (2005) Isolation and characterization of larvicidal components against mosquito larvae (Aedes aegypti Linn.) from Calodendrum capense Thunb. Bull Chem Soc Ethiop 1:145–148
Kiprop AK, Kiprono PC, Rajab MS, Kosgei MK (2007) Limonoids as larvicidal components against mosquito larvae (Aedes aegypti Linn.). Z Naturforsch C J Biosci 62:826–828. https://doi.org/10.1515/znc-2007-11-1209
Kraus W, Koll-Weber M, Maile R, Wunder T, Vogler B (1994) Biologically active constituents of tropical and subtropical plants. Pure Appl Chem 66:2347–2352. https://doi.org/10.1351/pac199466102347
Lam LKT, Zhang J, Hasegawa S (1994) Citrus limonoid reduction of chemically induced tumorigenesis. Food Technol 48:104–108
Lee JH, Lee BW, Moon YH, Yang MS, Jang KC, Park KH (2005) Phytochemical constituents from the stem bark of Phellodendron amurense Rupr. Agric Chem Biotechnol 48:93–96
Lee CS, Won C, Yoo H, Yi EH, Cho Y, Maeng JW, Sung SH, Ye SK, Chung MH (2009) Inhibition of double-stranded RNA-induced inducible nitric oxide synthase expression by fraxinellone and sauchinone in murine microglia. Biol Pharm Bull 32:1870–1874. https://doi.org/10.1248/bpb.32.1870
Lee EJ, Jung HJ, Kim MJ, Kim HY (2017) Adjuvant composition for anti-pancreatic cancer agent using fraxinellone. Repub Korea KR 1756688(B1):20170711
Lei J, Gou X, Wei S, Zhou J, Li Z (2020) Spectroscopy, thermodynamics and molecular docking of fraxinellone with DNA. Bull Environ Contam Toxicol 104:864–870. https://doi.org/10.1007/s00128-020-02860-7
Li X, Tang H, Gou X, Tu J (2008) Study on chemical constituents of the root of Dictamnus dasycarpus. Zhongyaocai 31:1816–1819
Li Q, Huang X, Li S, Ma J, Lv M, Xu H (2016a) Semisynthesis of esters of fraxinellone C4/10-oxime and their pesticidal activities. J Agric Food Chem 64:5472–5478. https://doi.org/10.1021/acs.jafc.6b01995
Li W, Shen L, Bruhn T, Pedpradab P, Wu J, Bringmann G (2016b) Trangmolins A-F with an unprecedented structural plasticity of the rings A and B: new insight into limonoid biosynthesis. Chem Eur J 22:11719–11727. https://doi.org/10.1002/chem.201602230
Li J, Feng T, Yang W, Xu Y, Wang S, Cai H, Liu Z, Qiang H, Zhang J (2021) Rational formulation engineering of fraxinellone utilizing 6-O-α-D-maltosyl-β-cyclodextrin for enhanced oral bioavailability and hepatic fibrosis therapy. Drug Deliv 28:1890–1902. https://doi.org/10.1080/10717544.2021.1976310
Liao SG, Chen HD, Yue JM (2009) Plant orthoesters. Chem Rev 109:1092–1140. https://doi.org/10.1021/cr0782832
Liu ZL, Xu YJ, Wu J, Goh SH, Ho SH (2002) Feeding deterrents from Dictamnus dasycarpus Turcz against two stored-product insects. J Agric Food Chem 50:1447–1450. https://doi.org/10.1021/jf010838l
Liu ZL, Ho SH, Goh SH (2008) Effect of fraxinellone on growth and digestive physiology of Asian corn borer, Ostrinia furnacalis Guenee. Pestic Biochem Physiol 91:122–127. https://doi.org/10.1016/j.pestbp.2008.03.003
Liu ZL, Ho SH, Goh SH (2009) Modes of action of fraxinellone against the tobacco budworm, Heliothis virescens. Insect Sci 16:147–155. https://doi.org/10.1111/j.1744-7917.2009.00266.x
Liu SC, Bai H, Tang WZ, Ma T, Yao QQ (2011) Study on chemical constituents of Melia azedarach. Zhongguo Shiyan Fangjixue Zazhi 17:93–96
Liu HB, Zhang H, Li P, Gao ZB, Yue JM (2012) Chukrasones A and B: potential Kv1.2 potassium channel blockers with new skeletons from Chukrasia tabularis. Org Lett 14:4438–4441. https://doi.org/10.1021/ol301942v
Liu J-Q, Peng X-R, Zhang W-M, Shi L, Li X-Y, Chen J-C, Qiu M-H (2013a) Swietemahalactone, a rearranged phragmalin-type limonoid with anti-bacterial effect, from Swietenia mahagoni. RSC Adv 3:4890–4893. https://doi.org/10.1039/c3ra23401k
Liu W-R, Qiao W-L, Liu Z-Z, Wang X-H, Jiang R, Li S-Y, Shi R-B, She G-M (2013b) Gaultheria: phytochemical and pharmacological characteristics. Molecules 18:12071–12108. https://doi.org/10.3390/molecules181012071
Liu H-L, Chen X-L, Xiao W (2014) Guo Y-W (2014) Velutinalide C, a new polycyclic phragmalin limonoid from the leaves of Chukrasia tabularis var. velutina. Helv Chim Acta 97:1445–1451. https://doi.org/10.1002/hlca.201400117
Liu Y, Schuppe AW, Zhao Y, Lee J, Newhouse TR (2022) Synthesis of (-)-melazolide B, a degraded limonoid, from a natural terpene precursor. Tetrahedron Chem 1:100011. https://doi.org/10.1016/j.tchem.2022.100011
Lü M, Wu WJ, Liu HX (2010) Effects of fraxinellone on the midgut ultrastructural changes of Mythimna separata Walker. Pestic Biochem Physiol 98:263–268. https://doi.org/10.1016/j.pestbp.2010.06.017
Lü M, Wu W, Liu H (2013) Insecticidal and feeding deterrent effects of fraxinellone from Dictamnus dasycarpus against four major pests. Molecules 18:2754–2762. https://doi.org/10.3390/molecules18032754
Lu M, Chen SY, Han LB, Hu JY, Xu H, Wu WJ (2014) Effects of fraxinellone on the peritrophic membrane of Mythimna separata (Lepidoptera:Noctuidae). Kunchong Xuebao 57:1389–1394
Lu C, Zhao R, Liu L, Wu Y, Zhao Y (2021) Application of fraxinellone in preparation of medicines for treatment of allergic skin diseases. China CN113456628 A 2021–10–01
Luo X, Yu Z, Yue B, Ren J, Zhang J, Mani S, Wang Z, Dou W (2020) Obacunone reduces inflammatory signalling and tumour occurrence in mice with chronic inflammation-induced colorectal cancer. Pharm Biol 58:886–897. https://doi.org/10.1080/13880209.2020.1812673
Luo J, Sun Y, Li Q, Kong L (2022) Research progress of meliaceous limonoids from 2011 to 2021. Nat Prod Rep 39:1325–1365. https://doi.org/10.1039/d2np00015f
Lv M, Wu W, Liu H (2014) Effects of fraxinellone on the midgut enzyme activities of the 5th instar larvae of oriental armyworm, mythimna separata walker. Toxins 6:2708–2718. https://doi.org/10.3390/toxins6092708
Lv M, Tian Y, Zhang Z, Liang J, Xu F, Sun J (2015a) Plant metabolomics driven chemical and biological comparison of the root bark of Dictamnus dasycarpus and Dictamnus angustifolius. RSC Adv 5:15700–15708. https://doi.org/10.1039/C5RA00115C
Lv M-Y, Tang B-Q, Teng J, Liang J-Y, Xu F-G, Zhang Z-J, Sun J-B (2015b) Chemotaxonomic significance of limonoids and triterpenoids from Dictamnus angustifolius G. Don Ex Sweet Biochem Syst Ecol 59:311–313. https://doi.org/10.1016/j.bse.2015.02.015
Lv X, Zhao S, Ning Z, Zeng H, Shu Y, Tao O, Xiao C, Lu C, Liu Y (2015c) Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem Cent J. https://doi.org/10.1186/s13065-015-0145-9
Lv M, Chen S, Zhang S, Xu H (2020) Toxicology study of fraxinellone as ovicidal agents against Mythimna separata Walker and Bombyx mori Linaeus. Z Naturforsch C, J Biosci 75:291–295. https://doi.org/10.1515/znc-2020-0041
Madyastha KM, Venkatakrishnan K (2000) Structural flexibility in the biocatalyst-mediated functionalization of ring “A” in salannin, a tetranortriterpene from Azadirachta indica. Perkin 1:3055–3062
Mahmoud MF, Gamal S, El-Fayoumi HM (2014a) Limonin attenuates hepatocellular injury following liver ischemia and reperfusion in rats via toll like receptor dependent pathway. Eur J Pharmacol 740:676–682. https://doi.org/10.1016/j.ejphar.2014.06.010
Mahmoud MF, Hamdan DI, Wink M, El-Shazly AM (2014b) Hepatoprotective effect of limonin, a natural limonoid from the seed of Citrus aurantium var. bigaradia, on D-galactosamine-induced liver injury in rats. Naunyn Schmiedebergs Arch Pharmacol 387:251–261. https://doi.org/10.1007/s00210-013-0937-1
Majumder PL, Maiti DC, Kraus W, Bokel M (1987) Nimbidiol, a modified diterpenoid of the root-bark of Azadirachta indica. Phytochemistry 26:3021–3023. https://doi.org/10.1016/s0031-9422(00)84584-7
Manosroi A, Kitdamrongtham W, Ishii K, Shinozaki T, Tachi Y, Takagi M, Ebina K, Zhang J, Manosroi J, Akihisa R, Akihisa T (2014) Limonoids from Azadirachta indica var. siamensis extracts and their cytotoxic and melanogenesis-inhibitory activities. Chem Biodivers 11:505–531. https://doi.org/10.1002/cbdv.201300406
Min L, Wu W, Liu H (2010) Effects of fraxinellone on the midgut ultrastructural changes of Mythimna separata Walker. Pestic Biochem Physiol 98:263–268. https://doi.org/10.1016/j.pestbp.2010.06.017
Mireku EA, Mensah AY, Mensah MLK, Tocher DA, Habtemariam S (2014) Anti-inflammatory properties of the stem-bark of Anopyxis kalineana and its major constituent, methyl angolensate. Phytother Res 28:1855–1860. https://doi.org/10.1002/ptr.5212
Miyazawa M, Shimamura H, Nakamura S, Kameoka H (1995) Antimutagenic activity of isofraxinellone from Dictamnus dasycarpus. J Agric Food Chem 43:1428–1431. https://doi.org/10.1021/jf00054a003
Miyazawa M, Tsurumi J, Mori K (2009) Degraded limonoids, their preparations, insect antifeedants and compositions containing them, and insect control using the compositions. Jpn Kokai Tokkyo Koho JP 2009179590 A 20090813
Morgan ED (2009) Azadirachtin, a scientific gold mine. Bioorg Med Chem 17:4096–4105. https://doi.org/10.1016/j.bmc.2008.11.081
Mulani FA, Nandikol SS, Thulasiram, HV (2022) Chemistry and biology of novel Meliaceae limonoids. ChemRxiv https://doi.org/10.26434/chemrxiv-2022-2bpb9-v3
Naini AA, Mayanti T, Supratman U (2022) Triterpenoids from Dysoxylum genus and their biological activities. Arch Pharm Res 45:63–89. https://doi.org/10.1007/s12272-022-01371-9
Nakatani M, Huang RC, Okamura H, Iwagawa T, Tadera K (1998) Degraded limonoids from Melia azedarach. Phytochemistry 49:1773–1776. https://doi.org/10.1016/s0031-9422(98)00262-3
Nogueira TSR, Passos MDS, Nascimento LPS, Arantes MBDS, Monteiro NO, Boeno SIDS, de Carvalho Junior A, Azevedo ODA, Terra WDS, Vieira MGC, Braz-Filho R (2020) Chemical compounds and biologic activities: a review of Cedrela genus. Molecules 25(22):5401. https://doi.org/10.3390/molecules25225401
Nurlelasari N, Firdaus ARR, Harneti D, Indrayati N, Baroroh U, Yusuf M (2020) Computational study of the potential molecular target for antibreast cancer activity of limonoid derivatives from chisocheton sp. J App Pharm Sci 10:7–12. https://doi.org/10.7324/japs.2020.102002
Okamura H, Yamauchi K, Miyawaki K, Iwagawa T, Nakatani M (1997) Synthesis and biological activities of degraded limonoids, (±)-fraxinellonone and its related compounds. Tetrahedron Lett 38:263–266. https://doi.org/10.1016/s0040-4039(96)02277-0
Okuno Y, Marumoto S, Tsurumi J, Miyazawa M (2019) Biotransformation of (+)-isofraxinellone by Aspergillus niger and insect antifeedant activity. Nat Prod Res 33:1518–1521. https://doi.org/10.1080/14786419.2017.1422180
Ortega A, Romo-de-Vivar A, Romo J (1968) Odoratin: a new pseudoguaianolide isolated from Hymenoxus odorata. Can J Chem 46:1539–1541. https://doi.org/10.1139/v68-252
Ourison G, Grabbe P, Roding O (1964) Tetracyclic Triterpenes. Holden-Day Inc Publishers, San Francisco
Ouyang GQ, Li CJ, Yang JZ, Ma J, Li L, Peng Y, Wang XL, Zhang DM (2016) Limonoids with neuroprotective activity from the stems of Clausena emarginata. J Asian Nat Products Res 18:928–937
Pailer M, Shaden G, Spiteller G, Fenzl W (1965) Structure of fraxinellone from Dictamnus albus. Monatsh Chem 96:1324–1346
Pei Y, Chen L, Huang Y, Wang J, Feng J, Xu M, Chen Y, Song Q, Jiang G, Gu X, Zhang Q, Gao X, Chen J (2019) Sequential targeting TGF-β signaling and KRAS mutation increases therapeutic efficacy in pancreatic cancer. Small. https://doi.org/10.1002/smll.201900631
Qin J, Liao C-N, Chen W-W, Li H-Y, Su J, Wu X-D, He J-B, Zhang G-H (2021) New limonoids and quinolone alkaloids with cytotoxic and anti-platelet aggregation activities from Evodia rutaecarpa (Juss) Benth. Fitoterapia 152:104875. https://doi.org/10.1016/j.fitote.2021.104875
Quader A, Put PH, Gray AI, Hartley TG, Hu Y, Waterman PG (1990) Alkaloids and limonoids of Tetradium trichotomum: chemotaxonomic significance. Biochem Syst Ecol 18:251–252. https://doi.org/10.1016/0305-1978(90)90069-R
Rahman AKMS, Chowdhury AKA, Ali H-A, Raihan SZ, Ali MS, Nahar L, Sarker SD (2009) Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J Nat Med 63:41–45. https://doi.org/10.1007/s11418-008-0287-3
Rajab MS, Guyo MP (1994) A formal synthesis of (±)-calodendrolide from (±)-pyroangolensolide. Bull Chem Soc Ethiop 8:35–38
Rajab MS, Fronczek FR, Rugutt JK, Fischer N (1998) Calodendrolide, a degraded limonoid from Calodendrum capense. Acta Crystallogr Sec C Cryst Struct Commun C54:950–952. https://doi.org/10.1107/S0108270197015151
Rao MM (1968) Naturally occurring C26 terpenoids. Bull Natl Inst Sci India 37:205–221
Roy A, Saraf S (2006) Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull 29:191–201. https://doi.org/10.1248/bpb.29.191
Ruan J, Li Z, Yan J, Huang P, Yu H, Han L, Zhang Y, Wang T (2018) Bioactive constituents from the aerial parts of Pluchea indica less. Molecules. https://doi.org/10.3390/molecules23092104
Sartor CF, Lima MDP, da Silva MFG, Fernandes JB, Forim MR, Pirani JR (2019) New limonoids from Dictyoloma vandellianum and Sohnreyia excelsa: chemosystematic considerations. J Braz Chem Soc 30:2464–2476
Schuppe AW, Zhao Y, Liu Y, Newhouse TR (2019) Total Synthesis of (+)-Granatumine A and related bislactone limonoid alkaloids via a pyran to pyridine interconversion. J Am Chem Soc 141:9191–9196. https://doi.org/10.1021/jacs.9b04508
Shi YL, Li M-F (2007) Biological effects of toosendanin, a triterpenoid extracted from Chinese traditional medicine. Prog Neurob 82:1–10. https://doi.org/10.1016/j.pneurobio.2007.02.002
Shi YL, Wang WP, Liao CY, Chiu SF (1986) Effect of Toosendanin on the sensory inputs of chemoreceptors of the armyworm Mythimna separata larvae. Acta Entomol Sin 29:233–238
Shi Y-S, Zhang Y, Li H-T, Wu C-H, El-Seedi HR, Ye W-K, Wang Z-W, Li C-B, Zhang X-F, Kai G-Y (2020) Limonoids from citrus: Chemistry, anti-tumor potential, and other bioactivities. J Funct Foods 75:104213. https://doi.org/10.1016/j.jff.2020.104213
Shi Q-Q, Zhang X-J, Wang T-T, Zhang Y, Zeb MA, Zhang R-H, Li X-L, Xiao W-L (2021) Toonaones A-I, limonoids with NLRP3 inflammasome inhibitory activity from Toona ciliata M. Roem. Phytochem 184:112661. https://doi.org/10.1016/j.phytochem.2021.112661
Siddiqui S, Ara I, Faizi S, Mahmood T, Siddiqui BS (1988) Phenolic tricyclic diterpenoids from the bark of Azadirachta indica. Phytochemistry 27:3903–3907. https://doi.org/10.1016/0031-9422(88)83041-3
Siddiqui S, Siddiqui BS, Ghiasuddin FS (1991) Tetracyclic triterpenoids of the fruit coats of Azadirachta indica. J Nat Prod 54:408–415. https://doi.org/10.1021/np50074a010
Siddiqui BS, Ghiasuddin FS, Siddiqui S (1992) Triterpenoids from the fresh fruit coats of Azadirachta indica. Phytochemistry 31:4275–4278. https://doi.org/10.1016/0031-9422(92)80457-p
Siddiqui BS, Rasheed M, Faizi S, Ali ST, Tariq RM, Naqvi SNUH (2003) Transformation of Azadiradione to Nimbocinol and 17β-Hydroxynimbocinol, and Structure Pesticidal-Activity Relationship of Triterpenoids isolated from Azadirachta indica A. Juss.(Neem). Helv Chim Acta 86(10):3342–3353. https://doi.org/10.1002/hlca.200390277
Storer R, Young DW (1973) Constituents of the root of Dictamnus albus. Tetrahedron 29:1217–1222. https://doi.org/10.1016/0040-4020(73)80105-x
Sun Y, Qin Y, Gong FY, Wu XF, Hua Z, Chen T, Xu Q (2009) Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice. Biochem Pharmacol 77:1717–1724. https://doi.org/10.1016/j.bcp.2009.03.002
Sun J-B, Qu W, Wang P, Wu F-H, Wang L-Y, Liang J-Y (2013) Degraded limonoids and quinoline alkaloids from Dictamnus angustifolius G. Don ex Sweet. and their anti-platelet aggregation activity. Fitoterapia 90:209–213. https://doi.org/10.1016/j.fitote.2013.07.023
Sun J-B, Jiang N, Lv M-Y, Wang P, Xu F-G, Liang J-Y, Qu W (2015a) Limonoids from the root bark of Dictamnus angustifolius: potent neuroprotective agents with biometal chelation and halting copper redox cycling properties. RSC Adv 5:24750–24757. https://doi.org/10.1039/c5ra00278h
Sun X-D, Fang S-M, Zang X-D, Yang C-X, Li H-R, Kitanaka S, Yang X-D (2015b) Isoflavonoids from caragana changduensis and their nitric oxide inhibitory activities. Zhongguo Zhongyao Zazhi 40:3220–3223. https://doi.org/10.4268/cjcmm20151619
Sun Y-P, Jin W-F, Wang Y-Y, Wang G, Morris-Natschke SL, Liu J-S, Wang G-K, Lee K-H (2018) Chemical structures and biological activities of limonoids from the genus Swietenia (Meliaceae). Molecules. https://doi.org/10.3390/molecules23071588
Sun Y, Li Q, Cui L, Tang P, Li Y, Kong L, Luo J (2022) Diverse ring-seco limonoids from Munronia unifoliolata and their biological activities. Chin J Chem 40:123–136. https://doi.org/10.1002/cjoc.202100717
Tan Q-G, Luo X-D (2011) Meliaceous limonoids: Chemistry and biological activities. Chem Rev 111:7437–7522. https://doi.org/10.1021/cr9004023
Tariq RM, Naqvi SNH, Siddiqui BS, Rasheed M, Faizi S, Aslam M, Zafar SMN (2004) Toxicity of sixteen pure compounds from the fruit-coat of neem tree (Azadirachta indica A. Juss.) against Anopheles stephensi Liston. Int J Biol Biotech 1:83–89
Thoms H, Dambergis C (1930) Constituents of Dictamnus albus. Arch Pharm Ber Dtsch Pharm Ges 268:39–48
Tian Q, Miller EG, Ahmad H, Tang L, Patil BS (2001) Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr Cancer 40:180–184. https://doi.org/10.1207/s15327914nc402_15
Tokoroyama T, Kotsuji Y, Matsuyama H, Shimura T, Yokotani K, Fukuyama Y (1990) Synthetic studies on terpenoid compounds part 27 total synthesis of calodendrolide. J Chem Soc Perkin Trans 1(6):1745–1752
Tou WI, Chen CYC (2012) In silico investigation of potential Src kinase ligands from traditional Chinese medicine. PLoS ONE. https://doi.org/10.1371/journal.pone.0033728
Trudeau S, Morken JP (2005) Short and efficient total synthesis of fraxinellone limonoids using the stereoselective Oshima-Utimoto reaction. Org Lett 7:5465–5468. https://doi.org/10.1021/ol0522687
Tundis R, Loizzo MR, Menichini F (2014) An overview on chemical aspects and potential health benefits of limonoids and their derivatives. Crit Rev Food Sci Nutr 54:225–250. https://doi.org/10.1080/10408398.2011.581400
Wang M, Zhang J, Qian Y, Li J, Ji Z (2006) The study on fungicidal activity of Dictamnus dasycarpus. Nongyao 45:739–741
Wang X-N, Fan C-Q, Yin S, Gan L-S, Yue J-M (2008) Structural elucidation of limonoids and steroids from Trichilia connaroides. Phytochemistry 69:1319–1327. https://doi.org/10.1016/j.phytochem.2008.01.018
Wang LL, Jiang CS, Fu Y, Chen FF, Lan LF, Zhang HY, Guo YW (2014) Two new limonoids from the root bark of chinese medicinal plant Dictamnus dasycarpus. Helv Chim Acta 97:1301–1306. https://doi.org/10.1002/hlca.201400027
Wang GC, Yu JH, Shen Y, Leng Y, Zhang H, Yue JM (2016) Limonoids and triterpenoids as 11β-HSD1 inhibitors from Walsura robusta. J Nat Prod 79:899–906. https://doi.org/10.1021/acs.jnatprod.5b00952
Wang N, Fan Y, Yuan CM, Song J, Yao Y, Liu W, Gajendran B, Zacksenhaus E, Li Y, Liu J, Hao XJ, Ben-David Y (2019a) Selective ERK1/2 agonists isolated from Melia azedarach with potent anti-leukemic activity. BMC Cancer 19:764. https://doi.org/10.1186/s12885-019-5914-8
Wang Y, Wang H, Mei W, Liu S, Kong F, Zhao Y, Dai H (2019b) Limonoids and triterpenoids from the stems of Chukrasia tabularis A. Juss Phytochem Lett 33:119–124. https://doi.org/10.1016/j.phytol.2019.08.009
Woo WS, Lee EB, Kang SS, Shin KH, Chi HJ (1987) Antifertility principle of Dictamnus albus root bark. Planta Med 53:399–401. https://doi.org/10.1055/s-2006-962756
Wu TS, Li CY, Leu YL, Hu CQ (1998) Limonoids and alkaloids of the root bark of Dictamnus angustifolius. Phytochemistry 50:509–512. https://doi.org/10.1016/s0031-9422(98)00519-6
Wu XF, Ouyang ZJ, Feng LL, Chen G, Guo WJ, Shen Y, Wu XD, Sun Y, Xu Q (2014) Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol Appl Pharmacol 281:146–156. https://doi.org/10.1016/j.taap.2014.10.002
Xiao H, Zheng R-r, Zhang J, Song M, Gao X-d, Zhang X-q, Ye W-c (2015) Chemical constituents from the twigs and leaves of Harrisonia perforate. Yaoxue Xuebao 50:1622–1624
Xie BJ, Yang SP, Yue JM (2008) Terpenoids from Dysoxylum densiflorum. Phytochemistry 17:2993–2997. https://doi.org/10.1016/j.phytochem.2008.09.017
Xing Y, Mi C, Wang Z, Zhang ZH, Li MY, Zuo HX, Wang JX, Jin X, Ma J (2018) Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1α and STAT3. Pharmacol Res 135:166–180. https://doi.org/10.1016/j.phrs.2018.08.004
Yahui Y, Liu X, Zhang L, Wang Y, Chen Q, Chen Z, Xu L, Liu T (2020) Chemical constituents from Dictamnus dasycarpus Turcz. Biochem Syst Ecol 93:104134. https://doi.org/10.1016/j.bse.2020.104134
Yan Y, Zhang L, Liu X, Zhu Q, Wang Y, Liang H, Chen Z, Yang Y, Xu L, Liu T (2021) Phytochemical and chemotaxonomic study of Pulsatilla cernua (Thunb.) Bercht.ex. J Presl Biochem Syst Ecol 97:104291. https://doi.org/10.1016/j.bse.2021.104291
Yang RZ, Tang CS (1988) Plants used for pest control in China: a literature review. Econ Bot 42:376–406. https://doi.org/10.1007/BF02860162
Yang RL, Jia TL, Zhang RQ (2005) Microbial transformation of fraxinellone by Aspergillus niger. J Asian Nat Prod Res 7:843–845. https://doi.org/10.1080/10286020310001653318
Yang MF, Li YY, Li BG, Zhang GL (2007) A novel alkaloid from Gaultheria nummularioides. J Asian Nat Prod Res 9:183–186. https://doi.org/10.1080/10286020412331286515
Yang JL, Liu LL, Shi YP (2011) Limonoids and quinoline alkaloids from Dictamnus dasycarpus. Planta Med 77:271–276. https://doi.org/10.1055/s-0030-1250344
Yang R, Xu T, Fan J, Zhang Q, Ding M, Huang M, Deng L, Lu Y, Guo Y (2018a) Natural products-based pesticides: design, synthesis and pesticidal activities of novel fraxinellone derivatives containing N-phenylpyrazole moiety. Ind Crop Prod 117:50–57. https://doi.org/10.1016/j.indcrop.2018.02.088
Yang R, Guo Y, Zhang Y, Xu H (2018b) Semisynthesis of new ethers from furyl-ring-based acylation derivatives of fraxinellone as insecticidal agents against Mythimna separata Walker in vivo. Chin Chem Lett 29:995–997. https://doi.org/10.1016/j.cclet.2017.10.018
Yang B-J, Fan S-R, Cai J-Y, Wang Y-T, Jing C-X, Guo J-J, Chen D-Z, Hao X-J (2020a) Aphananoid A is an anti-inflammatory limonoid with a new 5/6/5 fused ring featuring a C24 carbon skeleton from Aphanamixis polystachya. J Org Chem 85:8597–8602. https://doi.org/10.1021/acs.joc.0c00922
Yang X-J, Dong Q-M, Wang M-R, Tang J-J (2020b) Semi-synthesis of C-ring cyclopropyl analogues of fraxinellone and their insecticidal activity against Mythimna separata walker. Molecules 25:1109. https://doi.org/10.3390/molecules25051109
Yin Y-Y, Liu S-S, Han L-W, He Q-X, Zhang Q-W, Liu K-C, Yan L-H, Wang Z-M (2016) Chemical components of alkaloids from Euodiae Fructus and their anti-angiogenic activities. Chin J Exp Tradit Med Formulae 5:45–53
Yoon JS, Sung SH, Kim YC (2008) Neuroprotective limonoids of root bark of Dictamnus dasycarpus. J Nat Prod 71:208–211. https://doi.org/10.1021/np070588o
Yoon JS, Yang H, Kim SH, Sung SH, Kim YC (2010) Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J Mol Neurosci 42:9–16. https://doi.org/10.1007/s12031-010-9333-1
Yu SM, Ko FN, Su MJ, Wu TS, Wang ML, Huang TF, Teng CM (1992) Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers. Naunyn Schmiedebergs Arch Pharmacol 345:349–355. https://doi.org/10.1007/bf00168697
Yu F, Gajendran B, Wang N, Sample KM, Liu W, Wang C, Hu A, Zacksenhaus E, Hao X, Ben-David Y (2021) ERK activation via A1542/3 limonoids attenuates erythroleukemia through transcriptional stimulation of cholesterol biosynthesis genes. BMC Cancer 21:680. https://doi.org/10.1186/s12885-021-08402-6
Yu L, Zhu H (2017) Application of fraxinellone in preparing antitumor drug sensitizer. Faming Zhuanli Shenqing CN 106924243 A 20170707
Yu L, Tang L, Liu Z, Hu L (2013) Application of fraxinellone in preparing antitumor drugs. Faming Zhuanli Shenqing CN 103127057 A 20130605
Yuan CL, Wang XL (2012) Biological activity of Dictamnus dasycarpus against Mythimna separata. Baoji Wenli Xueyuan Xuebao Ziran Kexueban 32:5–7
Yuan C, Wang X, Yang D (2006) Antimicrobial and pesticidal activity of fraxinellone from Dictamnus dasycarpus. Chem J Internet 8:33–38
Yuan CM, Zhang Y, Tang GH, Li Y, He HP, Li SF, Hou L, Li XY, Di YT, Li SL, Hua HM, Hao XJ (2013) Cytotoxic limonoids from Melia azedarach. Planta Med 79:163–168. https://doi.org/10.1055/s-0032-1328069
Zhang Y, Xu H (2017) Recent progress in the chemistry and biology of limonoids. RSC Adv 7:35191–35220. https://doi.org/10.1039/C7RA04715K
Zhang CR, Yang SP, Liao SG, Fan CQ, Wu Y, Yue JM (2007a) Chuktabularins A-D, four new limonoids with unprecedented carbon skeletons from the stem bark of Chukrasia tabularis. Org Lett 9:3383–3386. https://doi.org/10.1021/ol701437h
Zhang Q, Shi Y, Liu X-T, Liang J-Y, Ip NY, Min Z-D (2007b) Minor limonoids from Melia toosendan and their antibacterial activity. Planta Med 73:1298–1303. https://doi.org/10.1055/s-2007-981618
Zhang CR, Fan CQ, Zhang L, Yang SP, Wu Y, Lu Y, Yue JM (2008) Chuktabrins A and B, two novel limonoids from the twigs and leaves of Chukrasia tabularis. Org Lett 10:3183–3186. https://doi.org/10.1021/ol800885h
Zhang R, Xie L, Yang R, Jia T, Wang H, Zheng G (2005) Production of fraxinigerllone, a novel antitumor metabolite from Aspergillus niger. Faming Zhuanli Shenqing Gongkai Shuomingshu, CN 1590554 A 20050309
Zhao W, Wolfender JL, Hostettmann K, Xu R, Qin G (1997) Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry 47:7–11. https://doi.org/10.1016/S0031-9422(97)00541-4
Zhao PH, Sun LM, Cao MA, Yuan CS (2007) Two limonoids from the root of Dictamnus radicis Cortex. Chem Lett 36:1200–1201. https://doi.org/10.1246/cl.2007.1200
Zhao PH, Sun LM, Liu XJ, Cao MA, Yuan CS (2008) Limonoids from the root of Dictamnus radicis Cortex. Chem Pharm Bull 56:102–104. https://doi.org/10.1248/cpb.56.102
Zheng B, Yuan M, Wang S, Tan Y, Xu Y, Ye J, Gao Y, Sun X, Wang T, Kong L, Wu X, Xu Q (2021) Fraxinellone alleviates kidney fibrosis by inhibiting CUG-binding protein 1-mediated fibroblast activation. Toxicol Appl Pharmacol 420:115530. https://doi.org/10.1016/j.taap.2021.115530
Zhou H, Liu Q, Zhang J, Yao J, Wang C, Zhang Y, Li Y, Zhang X, Zhang L (2020) Cytochrome P450-mediated bioactivation: implication for the liver injury induced by fraxinellone, a bioactive constituent from Dictamni Cortex. Chem Res Toxicol 33(7):1960–1968. https://doi.org/10.1021/acs.chemrestox.0c00141
Acknowledgements
We would like to thank the research programme ‘Programa de fomento e impulso de la investigación y la transferencia en la Universidad de Cádiz’ from the University of Cádiz for funding this project PR2017-046.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Durán-Peña, M.J., Botubol-Ares, J.M., Collado, I.G. et al. Degraded limonoids: biologically active limonoid fragments re-enhancing interest in Meliaceae and Rutaceae sources. Phytochem Rev 22, 695–741 (2023). https://doi.org/10.1007/s11101-023-09856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-023-09856-1