Abstract
Background
The area under the curve (AUC) to minimum inhibitory concentration (MIC) ratio is proposed as a therapeutic drug-monitoring parameter for dosing vancomycin continuous infusion in methicillin-resistant Staphylococcus aureus (MRSA) infection. Individualised pharmacokinetic–pharmacodynamic (PK/PD) calculation of AUC24 may better represent therapeutic dosing than current Therapeutic Drug Monitoring (TDM) practices, targeting a Steady State Concentration of 15–25 mg/L.
Aim
To compare real world TDM practice to theoretical, individualised, PK/PD target parameters utilising Bayesian predictions to steady state concentrations (Css) for outpatients on continuous vancomycin infusions.
Method
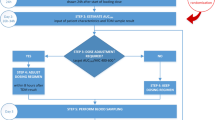
A retrospective single centre study was conducted at a tertiary hospital on adult patients, enrolled in an outpatient parenteral antimicrobial therapy (OPAT) program, receiving vancomycin infusions for MRSA infection. Retrospective Bayesian dosing was modelled to target PK/PD parameters and compared to real world data.
Results
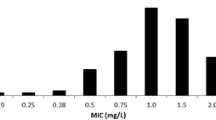
Fifteen patients were evaluated with 53% (8/15) achieved target CSS during hospitalisation, and 83% (13/15) as outpatient. Median Bayesian AUC/MIC was 613 mg.h/L with CSS 25 mg/L. Patients suffering an Acute Kidney Injury (33%) had higher AUC0–24/MIC values. Retrospective Bayesian modelling demonstrated on median 250 mg/24 h lower doses than that administered was required (R2 = 0.81) which achieved AUC24/MIC median 444.8 (range 405–460) mg.h/L and CSS 18.8 (range 16.8–20.4) mg/L.
Conclusion
Bayesian modelling could assist in obtaining more timely target parameters at lower doses for patients receiving continuous vancomycin infusion as part of an OPAT program, which may beget fewer adverse effects. Utilisation of personalised predictive modelling may optimise vancomycin prescribing, achieving earlier target concentrations as compared to empiric dosing regimens.


Similar content being viewed by others
References
Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312(13):1330–41.
Moise PA, Sakoulas G, Forrest A, et al. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(7):2582–6.
Verrall AJ, Llorin R, Tam VH, et al. Efficacy of continuous infusion of vancomycin for the outpatient treatment of methicillin-resistant Staphylococcus aureus infections. J Antimicrob Chemother. 2012;67(12):2970–3.
Thijs L, Quintens C, Vander Elst L, et al. Clinical efficacy and safety of vancomycin continuous infusion in patients treated at home in an outpatient parenteral antimicrobial therapy program. Antibiotics (Basel). 2022;11(5):702.
Seaton RA, Barr DA. Outpatient parenteral antibiotic therapy: principles and practice. Eur J Intern Med. 2013;24(7):617–23.
Voumard R, Gardiol C, André P, et al. Efficacy and safety of continuous infusions with elastomeric pumps for outpatient parenteral antimicrobial therapy (OPAT): an observational study. J Antimicrob Chemother. 2018;73(9):2540–5.
DiMondi VP, Rafferty K. Review of continuous-infusion vancomycin. Ann Pharmacother. 2013;47(2):219–27.
Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):10–1128.
Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):10–1128.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46(6):1127–53.
Therapeutic Guidelines Melbourne: Therapeutic Guidelines Limited; Available from: https://www.tg.org.au, Accessed 14 May 2023.
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98.
Wicha SG, Märtson AG, Nielsen EI, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021;109(4):928–41.
Holmes NE, Turnidge JD, Munckhof WJ, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57(4):1654–63.
Neely MN, Youn G, Jones B, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16.
Avent ML, Rogers BA. Optimising antimicrobial therapy through the use of Bayesian dosing programs. Int J Clin Pharm. 2019;41(5):1121–30.
Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64.
Pai MP, Neely M, Rodvold KA, et al. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–7.
Avent ML, Teoh J, Lees J, et al. Comparing 3 methods of monitoring gentamicin concentrations in patients with febrile neutropenia. Ther Drug Monit. 2011;33(5):592–601.
van Maarseveen EM, Gipmans S, Vasbinder E, et al. Switching from intermittent to continuous infusion of vancomycin in critically Ill patients: toward a more robust exposure. Ther Drug Monit. 2016;38(3):398–401.
Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 2001;45(9):2460–7.
Ingram PR, Lye DC, Fisher DA, et al. Nephrotoxicity of continuous versus intermittent infusion of vancomycin in outpatient parenteral antimicrobial therapy. Int J Antimicrob Agents. 2009;34(6):570–4.
Shakeraneh P, Fazili T, Wang D, et al. Nephrotoxicity risk and clinical effectiveness of continuous versus intermittent infusion vancomycin among patients in an outpatient parenteral antimicrobial therapy program. Pharmacotherapy. 2020;40(4):357–62.
Mahmood I, Duan J. Population pharmacokinetics with a very small sample size. Drug Metabol Drug Interact. 2009;24(2–4):259–74.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
Avent ML, Vaska VL, Rogers BA, et al. Vancomycin therapeutics and monitoring: a contemporary approach. Intern Med J. 2013;43(2):110–9.
Goti V, Chaturvedula A, Fossler MJ, et al. Hospitalized patients with and without hemodialysis have markedly different vancomycin pharmacokinetics: a population pharmacokinetic model-based analysis. Ther Drug Monit. 2018;40(2):212–21.
Legg A, Meagher N, Johnson SA, et al. Risk factors for nephrotoxicity in methicillin-resistant staphylococcus aureus bacteraemia: a post hoc analysis of the CAMERA2 trial. Clin Drug Investig. 2022;43:23–33.
Miano TA, Hennessy S, Yang W, et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144–55.
Elbarbry F. Vancomycin dosing and monitoring: critical evaluation of the current practice. Eur J Drug Metab Pharmacokinet. 2018;43(3):259–68.
Uster DW, Wicha SG. Optimized sampling to estimate vancomycin drug exposure: Comparison of pharmacometric and equation-based approaches in a simulation-estimation study. CPT Pharmacometrics Syst Pharmacol. 2022;11(6):711–20.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
A.F. is the developer of the Individually Designed Optimum Dosing Strategy (ID-ODS http://www.optimum-dosingstrategies.org/) which was the program utilised in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nolan, J., McCarthy, K., Farkas, A. et al. Feasibility of individualised patient modelling for continuous vancomycin infusions in outpatient antimicrobial therapy, a retrospective study. Int J Clin Pharm 45, 1444–1451 (2023). https://doi.org/10.1007/s11096-023-01618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01618-5