Abstract
Background
Algorithms for causality assessment of adverse drug reactions (ADRs) in a neonatal intensive care unit (NICU) are important in the management of adverse events, however, it is inconclusive which tool best suits pharmacovigilance in neonates.
Aim
To compare the performance of the algorithms of Du and Naranjo in determining causality in cases of ADRs in neonates in a NICU.
Method
This observational and prospective study was conducted in a NICU of a Brazilian maternity school between January 2019 and December 2020. Independently, three clinical pharmacists used the algorithms of Naranjo and Du in 79 cases of ADRs in 57 neonates. The algorithms were evaluated for inter-rater and inter-tool agreement using Cohen's kappa coefficient (k).
Results
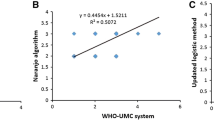
The Du algorithm showed greater ability to identify definite ADRs (≈ 60%), but had low reproducibility (overall k = 0.108; 95% CI 0.064–0.149). In contrast, the Naranjo algorithm showed a lower proportion of definite ADRs (< 4%), but had good reproducibility (overall k = 0.402; 95% CI 0.379–0.429). The tools showed no significant correlation regarding ADR causality classification (overall k = − 0.031; 95% CI − 0.049 to 0.065).
Conclusion
Although the Du algorithm has a lower reproducibility compared to the Naranjo, this tool showed good sensitivity for classifying ADRs as definite, proving to be a more suitable tool for neonatal clinical routine.

Similar content being viewed by others
References
Aranda JV, Portuguez-Malavasi A, Collinge JM, et al. Epidemiology of adverse drug reactions in the newborn. Dev Pharmacol Ther. 1982;5(3–4):173–84.
Belén Rivas A, Arruza L, Pacheco E, et al. Adverse drug reactions in neonates: a prospective study. Arch Dis Child. 2016;101(4):371–6.
Roberts EK, Hawcutt DB, Turner MA. Prospective identification and causality evaluation of suspected adverse drug reactions in neonates. Br J Clin Pharmacol. 2021;87(3):1541–6.
Salaslas RD, Díaz-Agudelo D. Adverse drug reactions in neonates hospitalized in neonatal intensive care units in Barranquilla, Colombia. Biomedica. 2017;37:33–42.
van den Anker J, Reed MD, Allegaert K, et al. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl 10):S10-25.
Allegaert K, van den Anker JN. Adverse drug reactions in neonates and infants: a population-tailored approach is needed. Br J Clin Pharmacol. 2015;80(4):788–95.
Costa HTML, Florencio AP, Bezerra PKV, et al. Comparative assessment of off-label and unlicensed drug prescription in neonatal intensive care: FDA versus Brazilian guidelines. An Pediatr (Engl Ed). 2021;94(3):153–60.
Pratico AD, Longo L, Mansueto S, et al. Off-label use of drugs and adverse drug reactions in pediatric units: a prospective. Multicenter Study. Curr Drug Saf. 2018;13(3):200–7.
Fabiano V, Mameli C, Zuccotti GV. Adverse drug reactions in newborns, infants and toddlers: pediatric pharmacovigilance between present and future. Expert Opin Drug Saf. 2012;11(1):95–105.
Allegaert K, van den Anker J. Dose-related adverse drug events in neonates: recognition and assessment. J Clin Pharmacol. 2021;61(Suppl 1):S152–60.
Hutchinson TA, Lane DA. Assessing methods for causality assessment of suspected adverse drug reactions. J Clin Epidemiol. 1989;42(1):5–16.
Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977;21(3):247–54.
Agbabiaka TB, Savović J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31(1):21–37.
Gallagher RM, Kirkham JJ, Mason JR, et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE. 2011;6(12): e28096.
Du W, Lehr VT, Lieh-Lai M, et al. An algorithm to detect adverse drug reactions in the neonatal intensive care unit. J Clin Pharmacol. 2013;53(1):87–95.
ICH Guideline E2D. Post-approval safety data management: definitions and standards for expedited reporting. 2004. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2D/Step4/E2D_Guideline.pdf. Accessed 20 Apr 2022.
Griffin FA, Resar RK. IHI global trigger tool for measuring adverse events (Second Edition). IHI Innovation Series white paper. Institute for Healthcare Improvement, Cambridge; 2009.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Khan LM, Al-Harthi SE, Osman AMM, et al. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm J. 2016;24(4):485–93.
Bégaud B, Jones JK. Assessing causality from case reports. In: Textbook of Pharmacoepidemiology, 3rd edn, 2021; p. 246–56.
Murali M, Suppes SL, Feldman K, et al. Utilization of the Naranjo scale to evaluate adverse drug reactions at a free-standing children’s hospital. PLoS ONE. 2021;16(1): e0245368.
Kane-Gill SL, Kirisci L, Pathak DS. Are the Naranjo criteria reliable and valid for determination of adverse drug reactions in the intensive care unit? Ann Pharmacother novembro de. 2005;39(11):1823–7.
Acknowledgements
We are grateful to all the members of the Neonatal Intensive Care Unit and the pharmacy of the Januario Cicco Maternity School for providing us with electronic medical records and other patient records that were important for the development of this work.
Funding
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leopoldino, R.W.D., de Oliveira, L.V.S., Fernandes, F.E.M. et al. Causality assessment of adverse drug reactions in neonates: a comparative study between Naranjo's algorithm and Du's tool. Int J Clin Pharm 45, 1007–1013 (2023). https://doi.org/10.1007/s11096-023-01595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01595-9