Abstract
Purpose
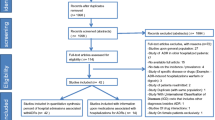
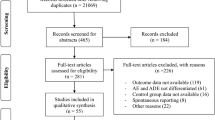
The study aimed to measure the percentage of preventable adverse drug reactions that lead to the hospitalization (PADRAd) and to explore the heterogeneity in its estimation through subgroup analysis of study characteristics.
Methods
Two investigators independently searched in electronic databases and related bibliography for prospective studies involving PADRAd. We excluded studies investigating medication errors and spontaneous and retrospective reporting. The primary outcome was PADRAd percentage. To explore the heterogeneity, we performed subgroup analysis based on study region, wards, age groups, adverse drug reaction (ADR) definitions, preventability assessment, ADR identification methods, study duration and sample size. We explored fatal PADRAd and causative drugs as a secondary outcome. We used the generic inverse variance method with random effect model to compute meta-analytic summary.
Results
Of the 68 full-text articles assessed, we included 22 studies. The mean PADRAd percentage was 45.11 % (95 % CI = 33.06–57.15; I 2 = 99 %). Studies including elderly (63.31 %) and all age groups (49.03 %) showed higher percentages than paediatric population (16.40 %). Studies examining all hospital populations showed higher percentages than specific wards. We observed high percentages in studies using Edwards and Aronson as an ADR definition and Hallas et al. as a preventability assessment tool. After age group adjustment, ADR detection methods did not show significant difference. The fatal PADRAd percentage was 1.58 % (95 % CI = −0.60 to 3.76; I 2 = 47 %). Paediatric and elderly studies showed a different causative drug pattern.
Conclusion
Variation in PADRAd across the studies can be explained by difference in study populations and data collection methods. Extrapolation of preventable reactions should be carried out considering all these factors with caution.





Similar content being viewed by others
References
Nivya K, Sri S, Ragoo N, Ayaprakash B, Sonal SM (2015) Systemic review on drug related hospital admissions—a PubMed based search. Saudi Pharm J 23(1):1–8
Brvar M, Fokter N, Bunc M, Mozina M (2009) The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol 9:8
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M (2009) Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One 4(2):e4439
Lazarou J, Pomeranz BH, Corey PN (1998) Prevalence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15):1200–1205
Patel H, Bell D, Molokhia M, Srishanmuganathan J, Patel M, Car J et al (2007) Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005. BMC Clin Pharmacol 7:9
Beijer HJM, De Blaey CJ (2002) Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 24(2):46–54
Leendertse AJ, Visser D, Egberts AC, van den Bemt PM (2010) The relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysis. Drug Saf 33(3):233–244
Hakkarainen KM, Hedna K, Petzold M, Hägg S (2012) Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions—a meta-analysis. PLoS One 7(3):e33236
Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U et al (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49(2):158–167
Moore N, Lecointre D, Noblet C, Mabille M (1998) Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol 45(3):301–308
Shultz M (2007) Comparing test searches in PubMed and Google Scholar. J Med Libr Assoc 95(4):442–445
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis and management. Lancet 356(9237):1255–1259
Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R et al (2012) Adverse drug reactions in children—a systematic review. PLoS One 7(3):e24061
Higgins JPT, Green S (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration.
Rücker G, Schwarzer G, Carpenter JR, Schumacher M (2008) Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 8(1):79
Karch FE, Lasagna L (1975) Adverse drug reactions—a critical review. JAMA 234(12):1236–1241
ATC/DDD index 2016. WHO Collaborating Centre for Drug Statistics Methodology. WHO collaborating centre, Oslo, Norway. Available from: http://www.whocc.no/atc_ddd_index/ [Last accessed on: 20th November, 2016].
Ahern F, Sahm LJ, Lynch D, McCarthy S (2014) Determining the frequency and preventability of adverse drug reaction-related admissions to an Irish university hospital: a cross-sectional study. Emerg Med J 31(1):24–29
Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M et al (2008) Adverse drug reactions as a cause of hospital admissions: a 6-month experience in a single centre in Greece. Eur J Intern Med 19(7):505–510
Al-Arifi M, Abu-Hashem H, Al-Meziny M, Said R, Aljadhey H (2014) Emergency department visits and admissions due to drug related problems at Riyadh military hospital (RMH), Saudi Arabia. Saudi Pharm J 22(1):17–25
Chan M, Nicklason F, Vial JH (2001) Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 31(4):199–205
Davies EC, Green CF, Mottram DR, Rowe PH, Pirmohamed M (2010) Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol 70(5):749–755
Dormann H, Criegee-Rieck M, Neubert A, Egger T, Geise A, Krebs S et al (2003) Lack of awareness of community-acquired adverse drug reactions upon hospital admission dimensions and consequences of a dilemma. Drug Saf 26(5):353–362
Dormann H, Neubert A, Criegee-Rieck M, Egger T, Radespiel-Troger M, Azaz-Livshits T et al (2004) Readmissions and adverse drug reactions in internal medicine: the economic impact. J Intern Med 255(6):653–663
Doshi MS, Patel PP, Shah SP, Dikshit RK (2012) Intensive monitoring of adverse drug reactions in hospitalized patients of two medical units at a tertiary care teaching hospital. J Pharmacol Pharmacother 3(4):308–313
Easton KL, Chapman CB, Brien JA (2004) Frequency and characteristics of hospital admissions associated with drug-related problems in paediatrics. Br J Clin Pharmacol 57(5):611–615
Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G et al (2008) Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit a prospective study of 1756 patients. Drug Saf 31(6):545–556
Gallagher RM, Bird KA, Mason JR, Peak M, Williamson PR, Nunn AJ et al (2011) Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clin Pharm Ther 36(2):194–199
Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR et al (2012) Adverse drug reactions causing admission to a paediatric hospital. PLoS One 7(12):e50127
Grenouillet-Delacre M, Verdoux H, Moore N, Haramburu F, Miremont-Salamé G, Etienne G et al (2007) Life-threatening adverse drug reactions at admission to medical intensive care: a prospective study in a teaching hospital. Intensive Care Med 33(12):2150–2157
Hopf Y, Watson M, Williams D (2008) Adverse-drug-reaction related admissions to a hospital in Scotland. Pharm World Sci 30(6):854–862
Benard-Laribierea A, Miremont-Salamea G, Perault-Pochatc MC, Noizea P, Haramburua F (2015) EMIR Study Group on behalf of the French network of pharmacovigilance centres. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol 29(1):106–111
Olivier P, Boulbés O, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2002) Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice a study in a French emergency department. Drug Saf 25(14):1035–1044
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ et al (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ 329(7456):15–19
Posthumus AAG, Alingh CCW, Zwaan CCM, van Grootheest KK, Hanff LL, Witjes BB et al (2012) Adverse drug reaction related admissions in paediatrics, a prospective single-centre study. BMJ Open 2(4):e000934
Rottenkolber D, Schmiedl S, Rottenkolber M, Farker K, Saljé K, Mueller S et al (2011) Adverse drug reactions in Germany: direct costs of internal medicine hospitalizations. Pharmacoepidemiol Drug Saf 20(6):626–634
Rivkin A (2007) Admissions to a medical intensive care unit related to adverse drug reactions. Am J Health-Syst Pharm 64(17):1840–1843
Ruiz B, García M, Aguirre U, Aguirre C (2008) Factors predicting hospital readmissions related to adverse drug reactions. Eur J Clin Pharmacol 64(7):715–722
Wasserfallen J, Livio F, Buclin T, Tillet L, Yersin B, Biollaz J (2001) Rate, type, and cost of adverse drug reactions in emergency department admissions. Eur J Intern Med 12(5):442–447
Goettler M, Schneeweiss S, Hasford J (1997) Adverse drug reaction monitoring—cost and benefit considerations. Part II: cost and preventability of adverse drug reactions leading to hospital admission. Pharmacoepidemiol Drug Saf Suppl 3:S79–S90
Winterstein AG, Sauer BC, Hepler CD, Poole C (2002) Preventable drug-related hospital admissions. Ann Pharmacother 36(7–8):1238–1248
Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P et al (2007) Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 63(2):136–147
Kongkaew C, Noyce PR, Ashcroft DM (2008) Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 42(7):1017–1025
Taché SV, Sönnichsen A, Ashcroft DM (2011) Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother 45(7–8):977–989
Passarelli MC, Jacob-Filho W, Figueras A (2005) Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging 22(9):767–777
Miguel A, Azevedo LF, Araújo M, Pereira AC (2012) Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 21(11):1139–1154
Hakkarainen KM, Andersson Sundell K, Petzold M, Hägg S (2012) Methods for assessing the preventability of adverse drug events: a systematic review. Drug Saf 35(2):105–126
Hallas J, Harvald B, Gram LF, Grodum E, Brosen K, Haghfelt T et al (1990) Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 228(2):83–90
Olivier P, Caron J, Haramburu F, Imbs JL, Jonville-Béra AP, Lagier G et al (2005) Validation of a measurement scale: example of a French Adverse Drug Reactions Preventability Scale. Therapie 60(1):39–45
Schumock GT, Thornton JP (1992) Focusing on the preventability of adverse drug reactions. Hosp Pharm 27(6):538
Field TS, Gurwitz JH, Harrold LR, Rothschild JM, Debellis K, Seger AC et al (2004) Strategies for detecting adverse drug events among older persons in the ambulatory setting. J Am Med Inform Assoc 11(6):429–498
Pardo Cabello AJ, González Contreras LG, ManzanoGamero MV, Gómez Jiménez FJ, Puche Cañas E (2009) Prevalence of fatal adverse drug reactions in hospitalized patients. Int J Clin Pharmacol Ther 47(10):596–602
Wester K, Jönsson AK, Spigset O, Druid H, HäggS (2008) Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol 65(4):573–579.
Jönsson AK, Hakkarainen KM, Spigset O, Druid H, Hiselius A, HäggS (2010) Preventable drug related mortality in a Swedish population. Pharmacoepidemiol Drug Saf 9(2):211–215.
Zoppi M, Braunschweig S, Kuenzi UP, Maibach R, Hoigné R (2000) Incidence of lethal adverse drug reactions in the comprehensive hospital drug monitoring, a 20-year survey, 1974-1993, based on the data of Berne/St. Gallen Eur J Clin Pharmacol 56(5):427–430
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
Supplementary file 1
(DOCX 13 kb)
Supplementary file 2
(DOCX 13 kb)
Supplementary file 3
(DOCX 41 kb)
Supplementary file 4
(DOCX 11 kb)
Supplementary Fig. 1
Meta-analytic summary of prevalence of PADRAd. Abbreviation is as follows: PADR Ad preventable ADRs resulting in hospital admission. (JPEG 685 kb)
Supplementary Fig. 2
Meta-analytic summary of subgroup analysis of percentage of PADRAd in paediatric patients for ADR definition and preventability assessment tool. Abbreviation is as follows: PADR Ad preventable ADRs resulting in hospital admission. (JPEG 592 kb)
Supplementary Fig. 3
Meta-analytic summary of subgroup analysis of percentage of PADRAd according to study region. Abbreviation is as follows: PADR Ad preventable ADRs resulting in hospital admission. (JPEG 3014 kb)
Supplementary Fig. 4
Meta-analytic summary of subgroup analysis of percentage of PADRAd according to ADR definition. Abbreviation is as follows: PADR Ad preventable ADRs resulting in hospital admission. (JPEG 2978 kb)
Supplementary Fig. 5
Meta-analytic summary of subgroup analysis of percentage of PADRAd according to ADR identification method. Abbreviation is as follows: PADR Ad preventable ADRs resulting in hospital admission. (JPEG 2797 kb)
Supplementary Fig. 6
Meta-analytic summary of fatal ADRAd and preventable fatal ADRAd. (JPEG 1169 kb)
Rights and permissions
About this article
Cite this article
Patel, N.S., Patel, T.K., Patel, P.B. et al. Hospitalizations due to preventable adverse reactions—a systematic review. Eur J Clin Pharmacol 73, 385–398 (2017). https://doi.org/10.1007/s00228-016-2170-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2170-6