Abstract
Background
Intravenous iron is the preferred treatment for patients with iron deficiency anemia in a variety of clinical situations. Although uncommon, administration of modern IV iron formulations can result in hypersensitivity reactions (HSRs) and, rarely, anaphylactic or anaphylactoid reactions.
Aim
The objective of the present study was to systematically review the literature to identify and analyze data on the incidence of HSRs after administration of ferric derisomaltose (FDI) or ferric carboxymaltose (FCM).
Method
A prospectively-registered systematic literature review was conducted to identify prospective randomized controlled trials comparing FDI and FCM with other intravenous iron formulations or oral iron. Searches were conducted in PubMed (including MEDLINE), EMBASE, and the Cochrane Library in November 2020. The relative incidence of serious or severe HSRs occurring on the day or day after dosing of intravenous iron, recorded under the standardized Medical Dictionary for Regulatory Activities query for anaphylactic reaction.
Results
Data were obtained from seven randomized controlled trials of FCM (N = 2683) and ten of FDI (N = 3474) enrolling 10,467 patients in total. The number of patients experiencing any serious or severe HSR event was 29/2683 (1.08%) with FCM versus 5/3474 with FDI (0.14%). Bayesian inference of proportions showed the event rates to be significantly lower with FDI relative to FCM.
Conclusion
HSR events were uncommon with both intravenous iron formulations; however, the present study showed a significantly lower incidence of HSRs with FDI relative to FCM. Further large-scale, head-to-head trials of the iron formulations would be required to confirm this finding.
Similar content being viewed by others
Impact statements
-
Incidence of serious or severe hypersensitivity reactions was low with both ferric derisomaltose and ferric carboxymaltose, but the analysis showed a significantly lower incidence of hypersensitivity reactions with ferric derisomaltose versus ferric carboxymaltose.
-
The rarity of the events would mean that further, large-scale, head-to-head trials of the iron formulations would be required to confirm this finding.
Introduction
Intravenous (IV) iron is well established as a successful treatment for patients with iron deficiency (ID) and iron deficiency anaemia (IDA) associated with a diverse range of etiologies, including renal, gastroenterological, gynaecological, and oncological [1,2,3,4]. In recent years, the use IV iron treatment has expanded further, most notably in patients with heart failure [5,6,7,8,9] and in patients undergoing surgery where exogenous iron plays a key role in perioperative blood management programs [10,11,12,13].
The earliest IV iron formulations were associated with unacceptably high rates of adverse drug reactions caused by ‘labile’ or ‘rapid’ iron release [14, 15]. While the exact mechanism driving hypersensitivity reactions (HSRs) after infusion of IV iron is not currently understood, one hypothesis is that, in addition to reactions due to small amounts of labile iron, the iron-carbohydrate complexes can result in a complement-mediated pseudoallergic reaction (CARPA) [16, 17]. Modern IV iron formulations such as ferric derisomaltose (Monofer®/Monoferric®; Pharmacosmos A/S, Holbaek, Denmark; FDI; formerly known as iron isomaltoside) and ferric carboxymaltose (Ferinject®/Injectafer®; Vifor France, Paris, France; FCM) can be administered rapidly in high doses, differentiating them from other formulations such as iron sucrose and iron gluconate. The rapid, high-dose administration is facilitated by the small amounts of labile iron in both formulations compared to, e.g. iron sucrose [18].
The safety and efficacy of modern IV iron formulations have been characterized in large-scale systematic literature reviews and meta-analyses. A 2015 meta-analysis of 103 trials showed no increase in the incidence of severe adverse events in patients treated with IV iron (n = 10,390) relative to the control groups, including no iron, placebo, oral, or intramuscular iron (n = 8863) [19]. Other meta-analyses have been conducted in patients with specific etiologies of IDA, including chronic kidney disease (CKD) and IDA during pregnancy, with similar findings regarding safety [20, 21]. In patients with IDA during pregnancy, IV iron resulted in fewer medication reactions than oral iron (relative risk [RR] 0.34; 95% confidence interval [CI] 0.20–0.57), while the risk of serious adverse events was not significantly different between IV iron and oral iron in patients with CKD (RR 1.06, 95% CI 0.88–1.28), nor was the risk of infection (RR 1.31, 95% CI 0.89–1.92).
Studies have also attempted to indirectly compare the relative incidence of HSRs after administration of different IV irons using databases of spontaneous reporting of adverse events. Such studies are inherently limited, however, with underreporting and differential reporting of spontaneous adverse reactions potentially resulting in biased estimates of the relative incidence of HSRs [22,23,24]. Furthermore, such studies rely on distinct sources of data, such as market share or sales data, to inform total exposure to each IV iron formulation. This use of different data sources for the numerator and denominator is fraught with challenges which, when combined with the spontaneous nature of the event reporting, is unlikely to accurately estimate the relative incidence of HSRs. Given the shortfalls of analyses based on adverse events and pharmacovigilance data, estimates of the relative incidence of HSRs with different IV iron formulations should be conducted using data from studies with fewer intrinsic sources of bias, while simultaneously ensuring that the definition of HSRs and timing of events relative to the IV iron infusion are standardized.
Considering recent RCTs of IV irons that include serious or severe HSR as an endpoint, a systematic review with meta-analysis is needed to demonstrate any potential difference in the rate of HSRs between different IV iron preparations. Further to this requirement, the rigorous classification of serious or severe HSRs is critical to improve comparisons of the safety of different IV irons. One such approach is available in the form of standardized Medical Dictionary for Regulatory Activities (MedDRA) queries (SMQs) [25]. SMQs are validated, predetermined sets of MedDRA terms, grouped together after extensive review, testing, analysis, and expert discussion (www.meddra.org). Using the Anaphylactic Reaction SMQ to evaluate serious or severe hypersensitivity is in line with the approach used by the Center for Drug Evaluation and Research (CDER; a division of the US Food and Drug Administration [FDA]) when they evaluated FCM for a New Drug Application in the US in 2013 [26].
Aim
The objective of the present study was to systematically review the literature to identify and analyze data on the incidence of hypersensitivity reactions (HSRs) after administration of ferric derisomaltose (FDI) or ferric carboxymaltose (FCM).
Method
Literature search strategy
The literature search protocol was prospectively registered in PROSPERO, the international prospective register of systematic reviews, with ID CRD42020215727 [27]. Literature searches were developed using free-text terms and Medical Subject Heading (MeSH) terms. Separate searches were conducted to identify studies of FDI versus any oral or IV iron formulation, and FCM versus any oral or IV iron formulation (including FDI; Supplementary Tables 1 and 2). Studies were retrieved from PubMed (including MEDLINE), EMBASE, and the Cochrane Library and imported into Sourcerer (Covalence Research Ltd, Harpenden, UK). Websites of regulatory authorities were also searched, including the European Medicines Agency (EMA), the FDA, and the Medicines and Healthcare products Regulatory Agency. After retrieval, duplicates were automatically removed, leaving two corpora of unique publications (FDI and FCM). Two reviewers (RFP and JP) independently conducted first-round title and abstract screening against the pre-specified exclusion criteria (Table 1). Inter-reviewer discrepancies after title and abstract screening were resolved by discussion, and the full-text articles of the included studies were obtained and screened independently by the same two reviewers against the same exclusion criteria (RFP and JP).
Studies eligible for inclusion were limited to prospectively-designed, active comparator, randomized controlled trials (RCTs) of either FCM or FDI compared with any other IV or oral iron formulation for the treatment of patients with IDA. Extension studies were excluded.
In line with the study protocol, where trials otherwise meeting the review inclusion/exclusion criteria were identified that did not report the specific hypersensitivity endpoint, corresponding authors were contacted with a view to obtaining data on file. The correspondence included a link to the PROSPERO protocol, which in turn included details of the proposed research methodology, source of funding for the research, and plan for dissemination of the findings.
Data extraction
We searched the included trials for the proportions of patients experiencing a serious or severe hypersensitivity reaction on the day or day after dosing of IV iron, categorized using the SMQ for anaphylactic reaction, and reported under SMQ groups A–D (Supplementary Table 3). All studies were assessed for bias using the Cochrane risk-of-bias tool version 2 (RoB2) for randomized trials [28].
Data analysis
The a priori data analysis plan was informed in part by a 2020 study that focused specifically on evaluating and comparing different methods of indirect comparison of the incidence of HSRs after administration of IV iron [29]. The analysis plan was expanded for the present study on the grounds that additional data may be made available from contacts with the study corresponding authors. Three analysis scenarios were outlined, in which the searches yielded clinically and statistically homogeneous data with or without closed loops in the evidence network, and in which the searches yielded either clinically and statistically heterogeneous data or data that precluded the derivation of appropriate arm-level weightings. Ultimately the latter scenario was employed as not all data could be obtained in a format that enabled study arm-level weightings to be calculated. Two distinct statistical methods proposed in the latter scenario are detailed below.
Bayesian analysis
We performed analyses of the naively-pooled data on the incidence of serious or severe HSRs on the day or day after administration of each of the IV iron formulations in SMQ groups and group combinations using Bayesian inference of proportions. To capture the uncertainty around the relative incidence with each of the IV iron formulations, we employed flat, uninformative priors, based on beta distributions parameterized with shape and scale parameters set to 1.
The Bayesian inference of proportions analyses were conducted using Just Another Gibbs Sampler (JAGS) using the RJAGS package [30]. Gelman-Rubin statistics and Gelman plots were used to establish whether the chains had converged [31]. The number of tuning and burn-in steps were set to 500 and 1000, respectively, at which point results were stable. Plots of the posterior distributions were generated, including regions of practical equivalent (ROPEs), which covered an odds ratio range of 0.9–1.1. Uncertainty around the mean odds ratio was summarized using 95% highest posterior density intervals (HDI), corresponding to the interval containing 95% of the posterior distribution of odds ratios.
Frequentist naïve pooling
We pooled data on the incidence of all serious or severe HSRs occurring on the day or day after administration of IV iron across all of the study arms for FDI and FCM, split by SMQ group. Odds ratios were then derived directly from the pooled numbers of patients experiencing events and the number of patients treated, with the significance of the results estimated using Fisher’s exact tests, and confidence intervals around the mean odds ratios derived using the Clopper-Pearson methodology [32].
Results
Literature searches were conducted on November 4, 2020. The FDI searches retrieved 301 records, of which 122 were duplicates across the databases, leaving 179 unique records for screening. The FCM searches retrieved 1,148 records, of which 474 were duplicates, leaving 674 unique records for screening. After title and abstract screening by two independent reviewers and resolution of inter-reviewer disagreements (N = 26 across both searches), 545 FCM records and 139 FDI records were definitively excluded. Of the remaining studies, 129 FCM records (corresponding to 77 unique trials) and 40 FDI records (corresponding to 19 unique trials) were included for full-text screening. Six of the 96 total studies across the two searches were head-to-head comparisons of FDI and FCM, leaving records pertaining to 90 unique studies for full-text review (Fig. 1).
None of the publications identified reported the incidence of serious or severe hypersensitivity reactions occurring on the day or the day after administration of IV iron using the preferred MedDRA terms falling under the Anaphylactic Reaction SMQ (groups A, B, C, or D). Of the 90 unique studies, four had been withdrawn or suspended, two did not meet the study inclusion criteria on examination of the full-text publication, and two did not include any author or sponsor contact details. Data requests were sent to corresponding authors of the remaining 82 studies describing the study objective (including the PROSPERO protocol), and enquiring as to whether data on file could be made available for analysis (Fig. 2).
As no data were ultimately received from Vifor (the marketing authorization holder for FCM), Luitpold Pharmaceuticals, Inc. or American Regent (the US licensees of FCM), the only source of data for FCM was therefore the 2013 FDA CDER Medical Review of FCM, which presented pooled data on the incidence of serious or severe HSRs across the four SMQ groups from five RCTs: REPAIR-IDA, 1VIT09031, 1VIT08019, 1VIT08020 and 1VIT08021 [33,34,35,36]. The REPAIR-IDA and 1VIT09031 studies were designed to assess the cardiovascular safety of the 750 mg dose of FCM, with the former being conducted in a population of patients with non-dialysis dependent CKD, and the latter being conducted in population with a mixture of IDA etiologies [33, 34]. The 1VIT08019, 1VIT08020, and 1VIT08021 studies also investigated the 750 mg dose of FCM [35, 36].
Ultimately, data from 15 RCTs were made available for analysis, seven of which compared FCM with other IV iron formulations or standard medical care, and ten of which compared FDI with other IV iron formulations (including two with FCM) or oral iron [33,34,35,36,37,38,39,40,41,42,43,45]. The studies included a total of 10,467 patients, of whom 6157 patients who had received either FDI or FCM; 3474 patients treated with FDI and 2683 patients treated with FCM (Table 2). Data for five of the studies of FCM were obtained from the FDA CDER Medical Review of FCM [26]. Across the five RCTs, 2,566 patients were treated with FCM, of whom 28 (1.1%) experienced HSRs across all groups in the SMQ for anaphylactic reactions [26]. Four studies were at low risk of bias, ten had some concerns, and one was classified as being at high risk of bias (Supplementary Fig. 1).
Data requests for serious or severe HSR incidence data were ultimately fulfilled for 10 RCTs sponsored by Pharmacosmos A/S via the contacts listed in the respective trial records on clinicaltrials.gov. Data for two RCTs included in the data request were not provided on the grounds that the final study manuscripts had not yet been published [46, 47]. Of the 10 studies for which data were available, four were conducted in patient populations with mixed etiologies of IDA, three in patients with CKD, and one each in patients with inflammatory bowel disease, postpartum hemorrhage, and non-myeloid malignancies receiving chemotherapy.
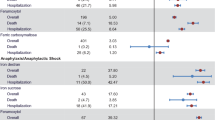
Bayesian analysis of proportions showed that FDI would result in a significant reduction in the incidence of HSRs across SMQ groups A + B + C + D relative to FCM, with a mean odds ratio of 0.16 (95% HDI: 0.05–0.33; Fig. 3) and 0% of the posterior distribution falling within the ROPE. The greatest reductions in odds occurred in SMQ groups B and D, with odds ratios of 0.14 and 0.19, respectively, while SMQ group A (comprising narrow terms pertaining to hypersensitivity reactions) showed the smallest reduction in odds and a high degree of uncertainty, with a mean odds ratio of 0.77 and a highest density interval spanning 0.06–3.17.
Posterior distributions from the Bayesian inference of proportions of the odds of serious or severe hypersensitivity reactions occurring on the day or day after administration of intravenous iron for FDI versus FCM. Green dotted line indicates parity (odds ratio of 1); red dashed lines indicate the region of practical equivalence (ROPE). A Narrow terms pertaining to hypersensitivity reactions, B broad terms pertaining to respiratory reactions potentially related to hypersensitivity, C broad terms pertaining to skin reactions potentially related to hypersensitivity, D broad terms pertaining to cardiovascular reactions potentially related to hypersensitivity, FCM ferric carboxymaltose, FDI ferric derisomaltose, HDI highest posterior density interval, ROPE region of practical equivalence
In the naïve pooling approach, with odds ratios derived directly from the event counts, binomial confidence intervals derived using the Clopper Pearson methodology, and p values derived using Fishers exact test, the odds of experiencing any serious or severe hypersensitivity reaction with FDI were 87% lower than with FCM (odds ratio 0.13, 95% confidence interval 0.05–0.34, p < 0.001; Fig. 4).
Naïve pooled analysis of the safety of ferric derisomaltose (FDI) versus ferric carboxymaltose (FCM) with odds ratios and confidence intervals of serious or severe hypersensitivity reactions occurring on the day or day after administration of intravenous iron for FDI versus FCM derived using the Clopper Pearson method, and p values derived using the Fishers exact test. A Narrow terms pertaining to hypersensitivity reactions, B broad terms pertaining to respiratory reactions potentially related to hypersensitivity, C broad terms pertaining to skin reactions potentially related to hypersensitivity, D broad terms pertaining to cardiovascular reactions potentially related to hypersensitivity, FCM ferric carboxymaltose, FDI ferric derisomaltose, MedDRA Medical Dictionary for Regulatory Activities, OR odds ratio
Discussion
This meta-analysis evaluated prospectively collected data from 15 head-to-head RCTs including 10,467 patients with IDA treated with exogenous iron. The Bayesian inference of proportions applied across the pooled data from all trials of FDI versus FCM showed a substantial reduction in the incidence of serious or severe HSRs on the day or day after administration with FDI versus FCM. The key strengths of the analysis lie in the well-specified definition of HSRs in line with that employed by the FDA, and the exclusive use of data from prospective, active comparator, RCTs.
The analysis has some limitations that should be considered when interpreting the findings. While best efforts were made to obtain data from the manufacturer and US licensee of FCM (Vifor Pharma Group and American Regent, respectively), no data were ultimately forthcoming from either party, which restricted the analysis, necessitating the use of data from the CDER report that had already been pooled across multiple trials. Another important limitation was the inability to conduct a more conventional network meta-analysis (NMA) based on the data, despite having limited head-to-head evidence comparing FDI with FCM, FCM with iron sucrose (IS), and FDI with IS. A full NMA was precluded on the grounds that HSR rates across all three IV iron formulations are extremely low and the only two head-to-head trials of FCM and FDI for which HSR incidence data were available enrolled a total of 245 patients, in which only 1 HSR occurred in the FCM arm [45]. The single event in SMQ group B allowed an odds ratio to be calculated for FDI versus FCM, but only by using a correction that results in biasing the estimate towards no difference and overestimating the variance. Utilizing this in an NMA would have resulted in one edge of the network being based on an odds ratio estimate that was intrinsically biased.
Analysis using a Bayesian inference of proportions indicated that FDI would reduce the odds of experiencing serious or severe HSRs in SMQ groups A + B + C + D by 84% relative to FCM (mean odds ratio of 0.16; 95% HDI: 0.05–0.33). The greatest reductions were identified in the analyses of SMQ groups B and D, which reported mean odds ratios of 0.14 and 0.19 respectively, with 95% HDIs that did not cross parity. Our analysis represents, to our knowledge, the most comprehensive effort to synthesize prospectively-gathered evidence on the incidence of serious or severe HSRs in patients treated with modern IV iron formulations conducted to-date, including studies that enrolled over 6000 patients on the two high-dose IV iron formulations. Finally, our analysis is limited to hypersensitivity; other aspects of IV iron safety, particularly risk of infection, hypophosphataemia and cardiovascular events, were not considered.
Despite the limitations inherent in a naively-pooled analysis, the present analysis represents a substantial improvement on approaches relying on combining data from PV and market share data, where there is no guarantee of agreement between the number of patients experiencing HSRs and patient exposure, with market share data additionally not being subject to external scrutiny from peer reviewers, clinicians or marketing authorization holders [13, 14]. The current analysis is not affected by issues of heterogeneous incidence and exposure data, utilizing data exclusively from prospective, active-comparator, RCTs to ensure congruence between exposure and the numbers of patient experiencing events. Furthermore, all serious or severe HSRs were reported using preferred MedDRA terms within the four groups comprising the Anaphylactic Reaction SMQ, ensuring consistency across trials in accordance with validated and pre-specified definitions [48]. Challenges in accurately assessing the safety of IV iron formulations have been documented previously, and we would recommend the use of the Anaphylactic Reaction SMQ groups for the recording and publication of HSR data across future trials of IV iron. Given the rare nature of the serious or severe HSRs with modern IV iron treatments, these data would ideally originate from large-scale head-to-head trials of the modern IV iron formulations, although more data even from smaller trials could still be used to enhance the relative incidence estimates using indirect techniques such as those presented here.
While the present analysis showed a significant reduction in the incidence of HSRs with FDI versus FCM across SMQ groups A + B + C + D, HSRs were uncommon with both iron formulations. In clinical practice, the choice of IV iron formulation should therefore be based on a combination of factors, including the risk of HSRs, risk of other adverse events such as hypophosphatemia, and the maximum allowed single dose, health economic impact, and local availability of each formulation.
Conclusion
This is, to our knowledge, the first systematic review and meta-analysis investigating the incidence of serious or severe hypersensitivity reactions, using MedDRA preferred terms in the four groups of the SMQ for anaphylactic reaction and so giving a robust comparison of the newer IV irons. We have demonstrated that serious or severe hypersensitivity reactions were uncommon with the newer high dose IV iron formulations and that the incidence of serious or severe HSRs is significantly lower with FDI relative to FCM.
References
Macdougall IC, White C, Anker SD, PIVOTAL Investigators and Committees, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):447–58.
Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66(5):863–71.
Breymann C, Auerbach M. Iron deficiency in gynecology and obstetrics: clinical implications and management. Hematol Am Soc Hematol Educ Program. 2017;2017(1):152–9.
Aapro M, Österborg A, Gascón P, et al. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23(8):1954–62.
Anker SD, Comin Colet J, Filippatos G, FAIR-HF Trial Investigators, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–48.
Ponikowski P, van Veldhuisen DJ, Comin-Colet J, CONFIRM-HF Investigators, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657–68.
van Veldhuisen DJ, Ponikowski P, van der Meer P, EFFECT-HF Investigators, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136(15):1374–83.
Ponikowski P, Kirwan BA, Anker SD, AFFIRM-AHF investigators, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396(10266):1895–904.
Charles-Edwards G, Amaral N, Sleigh A, et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency. Circulation. 2019;139(21):2386–98.
Khalafallah AA, Yan C, Al-Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial. Lancet Haematol. 2016;3(9):e415–25.
Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;393(10187):2201–12.
Kim YW, Bae JM, Park YK, FAIRY Study Group, et al. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: the FAIRY randomized clinical trial. JAMA. 2017;317(20):2097–104.
Froessler B, Palm P, Weber I, et al. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264(1):41–6.
Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematol Am Soc Hematol Educ Program. 2010;2010:338–47.
Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematol Am Soc Hematol Educ Program. 2016;2016(1):57–66.
Szebeni J, Fishbane S, Hedenus M, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. Br J Pharmacol. 2015;172:5025–36.
Macdougall IC, Vernon K. Complement activation-related pseudoallergy: a fresh look at hypersensitivity reactions to intravenous iron. Am J Nephrol. 2017;45:60–2.
Garbowski MW, Bansal S, Porter JB, et al. Intravenous iron preparations transiently generate non-transferrin-bound iron from two proposed pathways. Haematologica. 2020;106:2885. https://doi.org/10.3324/haematol.2020.250803.
Avni T, Bieber A, Grossman A, et al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. 2015;90(1):12–23.
Shepshelovich D, Rozen-Zvi B, Avni T, et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68:677–90.
Lewkowitz AK, Gupta A, Simon L, et al. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. J Perinatol. 2019;39(4):519–32.
Schaffalitzky de Muckadell P, Strom CC. Comment on “Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxymaltose and iron(III) isomaltoside 1000 in Europe based on data from EudraVigilance and VigiBase™ between 2014 and 2017.” Drug Saf. 2019;42(5):689–91.
OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 25 Jun 2021.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–10.
Kalra PA, Bhandari S. Safety of intravenous iron use in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25(6):529–35.
US Food and Drug Administration. Center for drug evaluation and research. Application number: 203565Orig1s000. Medical Review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203565Orig1s000MedR.pdf. Accessed 25 Jun 2021.
Pollock R, Pöhlmann J, Achebe M, et al. A systematic review and indirect treatment comparison of serious or severe hypersensitivity reactions after administration of intravenous iron for iron deficiency anemia, reported using the standardized medical dictionary for regulatory activities query for anaphylactic reaction. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=215727. Accessed 22 Jun 2021.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Pollock RF, Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Expert Rev Hematol. 2020;13(2):187–95.
Plummer M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In: Proceedings of the 3rd international workshop on distributed statistical computing (DSC 2003), March 20–22, Vienna, Austria. ISSN 1609-395X.
Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434–55.
Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13.
Onken JE, Bregman DB, Harrington RA, et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant. 2014;29(4):833–42.
Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306–15.
Hussain I, Bhoyroo J, Butcher A, et al. Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia. 2013;2013: 169107.
Barish CF, Koch T, Butcher A, et al. Safety and efficacy of intravenous ferric carboxymaltose (750 mg) in the treatment of iron deficiency anemia: two randomized. Control Trials Anemia. 2012;2012: 172104.
Birgegård G, Henry D, Glaspy J, et al. A randomized noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy: the PROFOUND trial. Pharmacotherapy. 2016;36(4):402–14.
Kalra PA, Bhandari S, Saxena S, et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant. 2016;31(4):646–55.
Bhandari S, Kalra PA, Kothari J, et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant. 2015;30(9):1577–89.
Bhandari S, Kalra PA, Berkowitz M, et al. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant. 2021;36(1):111–20.
Reinisch W, Staun M, Tandon RK, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108(12):1877–88.
Derman R, Roman E, Modiano MR, et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92(3):286–91.
Auerbach M, Henry D, Derman RJ, et al. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol. 2019;94(9):1007–14.
Holm C, Thomsen LL, Norgaard A, et al. Intravenous iron isomaltoside 1000 administered by high single-dose infusions or standard medical care for the treatment of fatigue in women after postpartum haemorrhage: study protocol for a randomised controlled trial. Trials. 2015;16:5.
Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, Thomsen LL, Carpenter TO, Weber T, Brandenburg V, Zoller H. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron- Deficiency Anemia: Two Randomized Clinical Trials. JAMA. 2020;323(5):432–443.
Pharmacosmos A/S. Intravenous iron isomaltoside versus oral iron supplementation for treatment of iron deficiency in pregnancy. https://clinicaltrials.gov/ct2/show/NCT03188445. Accessed 13 Aug 2021.
Pharmacosmos A/S. A trial comparing the incidence of hypophosphatemia in relation to treatment with iron isomaltoside and ferric carboxymaltose in subjects with iron deficiency anaemia due to inflammatory bowel disease. https://clinicaltrials.gov/ct2/show/NCT03466983. Accessed 13 Aug 2021.
Medical Dictionary for Regulatory Activities. Standardised MedDRA queries. https://www.meddra.org/standardised-meddra-queries. Accessed 13 Aug 2021.
Funding
The study was funded by way of an unrestricted grant from Pharmacosmos A/S, as declared in the prospectively-registered PROSPERO protocol (ID: CRD42020215727).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JP is a full-time employee, and RFP is a shareholder, director, and full-time employee of Covalence Research Ltd, which has received consultancy fees from Pharmacosmos A/S to perform the systematic literature review, conduct the pooled analysis, and draft the manuscript. MA has no conflicts of interest to declare. PB declares consultancy fees from Vifor, Fresenius, and Pharmacosmos A/S. NK declares speaker honoraria, support for meetings and/or travel, and participation in an advisory board from Pharmacosmos A/S.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kennedy, N.A., Achebe, M.M., Biggar, P. et al. A systematic literature review and meta-analysis of the incidence of serious or severe hypersensitivity reactions after administration of ferric derisomaltose or ferric carboxymaltose. Int J Clin Pharm 45, 604–612 (2023). https://doi.org/10.1007/s11096-023-01548-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01548-2